Chromium Picolinate Regulates Bone Metabolism and Prevents Bone Loss in Diabetic Rats
Abstract
1. Introduction
2. Results
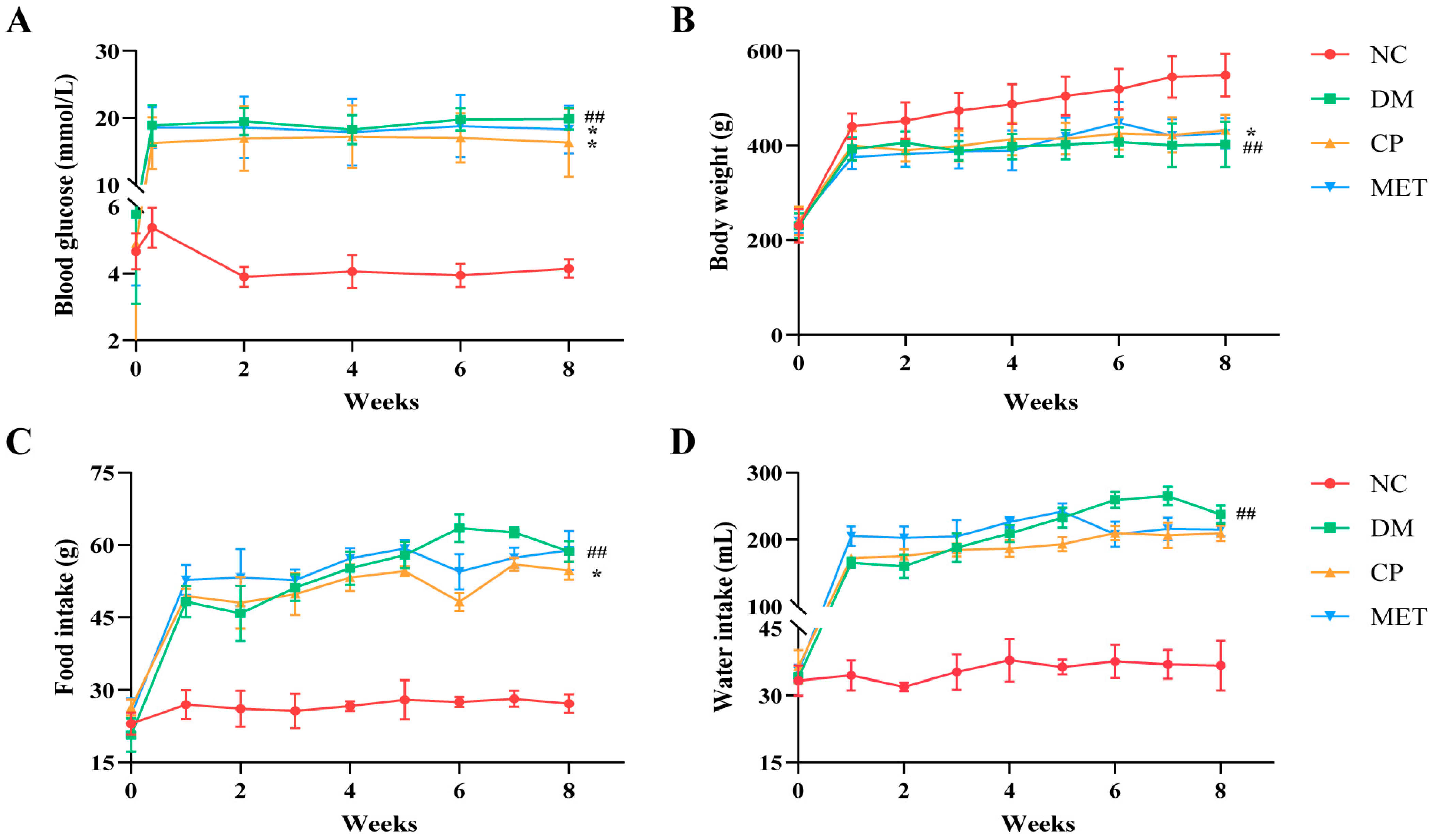
2.1. Chromium Picolinate Relieved Diabetic Symptoms in Diabetic Rats
2.2. Chromium Picolinate Increased BMD and Bone Strength
2.3. micro-CT Test Results
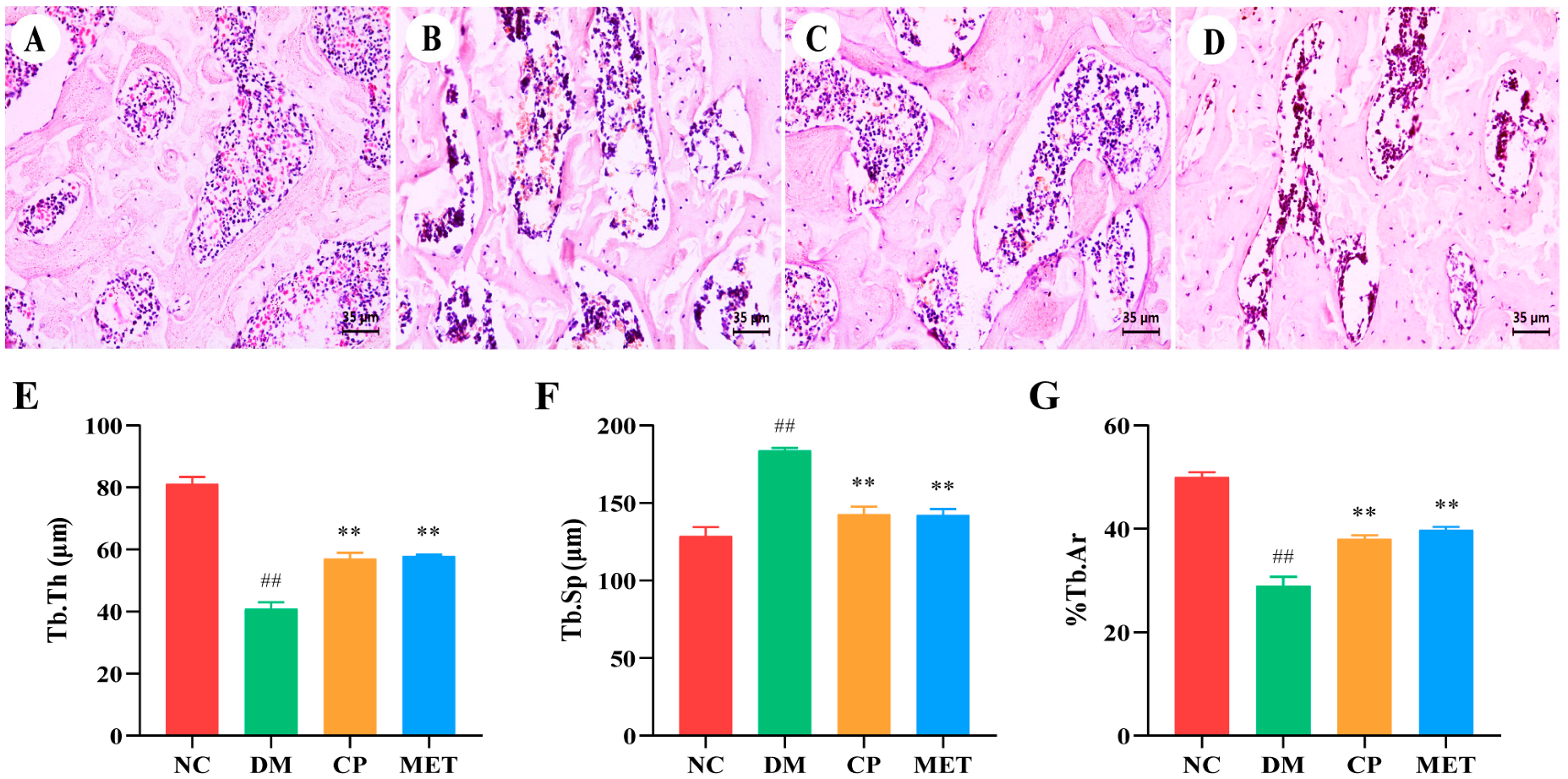
2.4. Chromium Picolinate Repaired Bone Microstructure
2.5. Chromium Picolinate Regulated Bone Turnover
2.6. Chromium Picolinate Alleviated Oxidative Stress
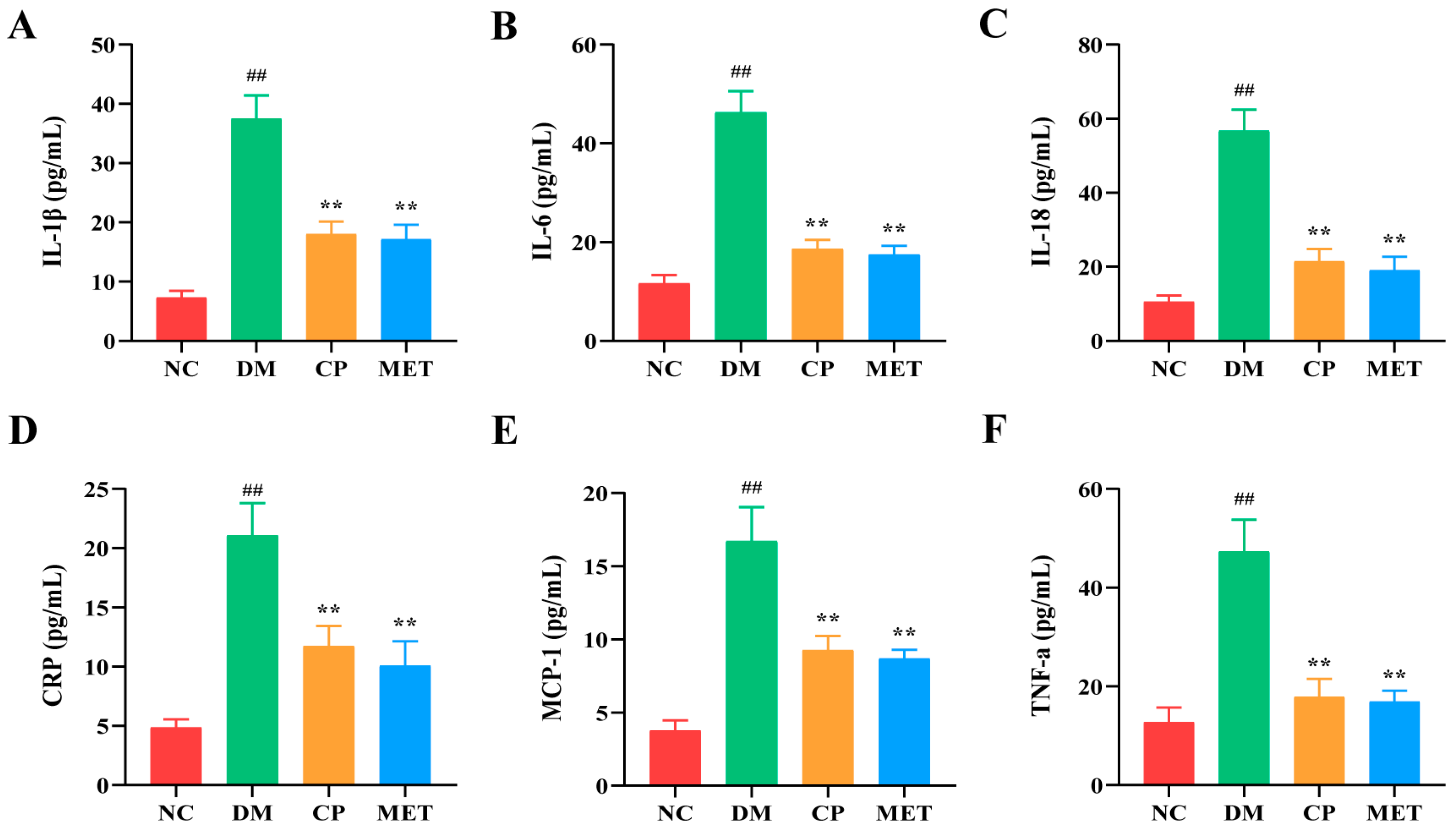
2.7. Chromium Picolinate Lowered Serum Inflammatory Cytokines
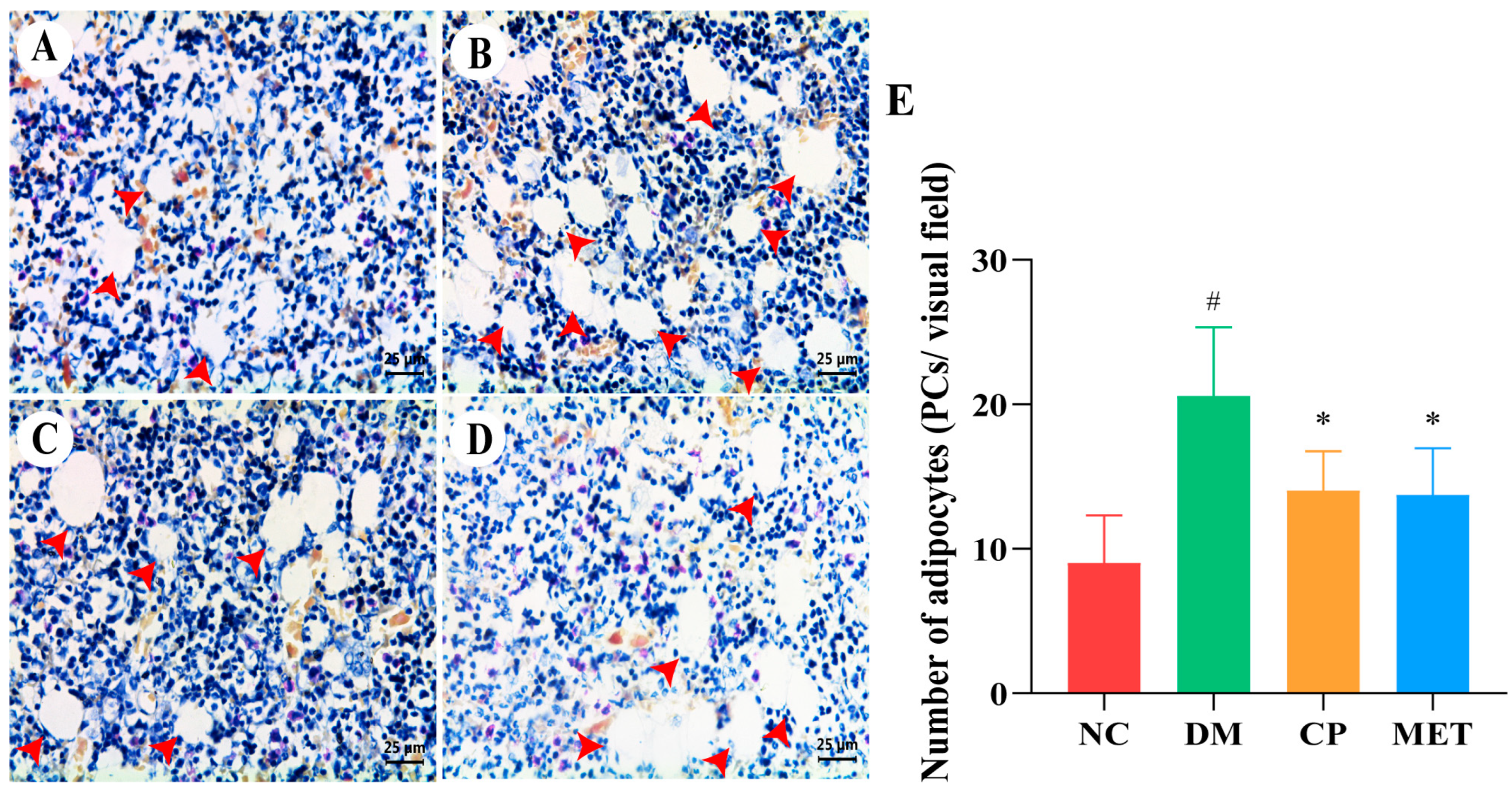
2.8. Chromium Picolinate Inhibited Bone Marrow Adipocyte Differentiation
2.9. Chromium Picolinate Inhibited Osteoclastogenesis
2.10. Chromium Picolinate Promoted Osteoblastogenesis
2.11. Chromium Picolinate Promoted Bone Collagen Fibers Formation
2.12. Effect of Chromium Picolinate on Expression of OPG, RUNX 2 as Well as RANKL in Bone Tissue
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Animal Grouping
4.4. BMD and Bone Strength Measurement
4.5. Bone Micro-CT Detection
4.6. Histopathological Analysis of Femur Sections
4.7. Oxidative Stress, Bone Turnover Markers and Inflammatory Cytokines Detection
4.8. Bone Marrow Adipocytes in the Tibia
4.9. Immunohistochemical (IHC)
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, G. Cellular Senescence: The Villain of Metabolic Disease?: Discovery of a distinct senescent cell population in obesity-induced metabolic dysfunction. Mol. Cells 2022, 45, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease: An update. Hypertension 2001, 37, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B.I.D.F. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Mizumukai, A.I.; Moreira, M.L.M.; Albuquerque, P.F. Diabetes and bone. Arch. Endocrinol. Metab. 2022, 66, 633–641. [Google Scholar]
- Xu, Y.K.; Wu, Q. Trends in osteoporosis and mean bone density among type 2 diabetes patients in the US from 2005 to 2014. Sci. Rep. 2021, 11, 3693. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, F.; Liu, L.; Zhang, Q. Prevalence of osteoporosis in patients with diabetes mellitus: A systematic review and meta-analysis of observational studies. BMC Endocr. Disord. 2023, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Li, J.; Hu, J.; Yang, K.; Tao, L. Oxidative stress: A common pathological state in a high-risk population for osteoporosis. Biomed. Pharmacother. 2023, 163, 114834. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative stress and inflammation in osteoporosis: Molecular mechanisms involved and the relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.W.A. Oxidative stress and osteoporosis. J. Bone Jt. Surg. Am. 2021, 103, 1451–1461. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.L.; Li, T.T.; Yang, F.; Tzeng, C.M. Baicalin ameliorates dexamethasone-induced osteoporosis by regulation of the RANK/RANKL/OPG signaling pathway. Drug Des. Devel. Ther. 2020, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Zhang, N.; Li, H.; Li, F.; Pu, R. Beneficial effects of cuscuta chinensis extract on glucocorticoid-induced osteoporosis through modulation of RANKL/OPG signals. Braz. J. Med. Biol. Res. 2019, 52, e8754. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ma, Y.; Reynolds, J.; Wise, J.P., Sr.; Inzucchi, S.E.; Katz, D.L. Chromium effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocr. Pract. 2011, 17, 16–25. [Google Scholar] [PubMed]
- Vincent, J.B. Effects of chromium supplementation on body composition, human and animal health, and insulin and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Agrawal, R.P.; Choudhary, M.; Jain, S.; Goyal, S.; Agarwal, V. Beneficial effect of chromium supplementation on glucose, HbA1C and lipid variables in individuals with newly onset type-2 diabetes. J. Trace Elem. Med. Biol. 2011, 25, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, B.; Aggarwal, A.; Sandhir, R. Chromium picolinate attenuates hyperglycemia-induced oxidative stress in streptozotocin-induced diabetic rats. J. Trace Elem. Med. Biol. 2013, 27, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Gossa Al-Saadde, D.L.; Haider, A.M.; Ali, A.; Abdu Musad Saleh, E.; Turki Jalil, A.; Abdulelah, F.M.; Romero-Parra, R.M.; Tayyib, N.A.; Ramírez-Coronel, A.A.; Alkhayyat, A.S. The role of chromium supplementation in cardiovascular risk factors; a comprehensive reviews of putative molecular mechanisms. Heliyon 2023, 9, e19826. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Martins, A.D.; Alves, M.G.; de Lourdes Pereira, M.; Oliveira, P.F. A comprehensive review of the impact of chromium picolinate on testicular steroidogenesis and antioxidant balance. Antioxidants 2023, 12, 1572. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Guo, X.; Wang, X.; He, Z.; Sun, R.; Ge, S.; Zhang, Z. Chromium picolinate supplementation for overweight or obese adults. Cochrane Database Syst. Rev. 2013, 11, CD010063. [Google Scholar] [CrossRef]
- Khodavirdipour, A.; Haddadi, F.; Keshavarzi, S. Chromium supplementation; negotiation with diabetes mellitus, hyperlipidemia and depression. J. Diabetes Metab. Disord. 2020, 19, 585–595. [Google Scholar] [CrossRef]
- Qi, S.S.; Zheng, H.X.; Jiang, H.; Yuan, L.P.; Dong, L.C. Protective effects of chromium picolinate against diabetic-induced renal dysfunction and renal fibrosis in streptozotocin-induced diabetic rats. Biomolecules 2020, 10, 398. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.S.; Shao, M.L.; Sun, Z.; Chen, S.M.; Hu, Y.J.; Li, X.S.; Chen, J.; Zheng, H.X.; Yue, T.L. Chondroitin sulfate alleviates diabetic osteoporosis and repairs bone microstructure via anti-oxidation, anti-inflammation, and regulating bone metabolism. Front. Endocrinol. 2021, 12, 759843. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Qi, S.S.; He, J.; Hu, C.Y.; Han, H.; Jiang, H.; Li, X.S. Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor β1/Smad expression. J. Agric. Food Chem. 2020, 68, 4399–4410. [Google Scholar] [CrossRef] [PubMed]
- Orhan, C.; Kucuk, O.; Tuzcu, M.; Sahin, N.; Komorowski, J.R.; Sahin, K. Effect of supplementing chromium histidinate and picolinate complexes along with biotin on insulin sensitivity and related metabolic indices in rats fed a high-fat diet. Food Sci. Nutr. 2018, 7, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Talab, A.T.; Abdollahzad, H.; Nachvak, S.M.; Pasdar, Y.; Eghtesadi, S.; Izadi, A.; Aghdashi, M.A.; Mohammad Hossseini Azar, M.R.; Moradi, S.; Mehaki, B.; et al. Effects of chromium picolinate supplementation on cardiometabolic biomarkers in patients with type 2 diabetes mellitus: A randomized clinical trial. Clin. Nutr. Res. 2020, 9, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Sahu, S.; Ohanyan, V.; Haney, R.; Chavez, R.J.; Shah, S.; Yalamanchili, S.; Raman, P. Oral chromium picolinate impedes hyperglycemia-induced atherosclerosis and inhibits proatherogenic protein TSP-1 expression in STZ-induced type 1 diabetic ApoE−/− mice. Sci Rep. 2017, 7, 45279. [Google Scholar] [CrossRef]
- Farrokhian, A.; Mahmoodian, M.; Bahmani, F.; Amirani, E.; Shafabakhsh, R.; Asemi, Z. The influences of chromium supplementation on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Biol. Trace Elem. Res. 2020, 194, 313–320. [Google Scholar] [CrossRef]
- Aung, M.; Amin, S.; Gulraiz, A.; Gandhi, F.R.; Pena Escobar, J.A.; Malik, B.H. The future of metformin in the prevention of diabetes-related osteoporosis. Cureus 2020, 12, e10412. [Google Scholar] [CrossRef]
- Bahrambeigi, S.; Yousefi, B.; Rahimi, M.; Shafiei-Irannejad, V. Metformin; an old anti-diabetic drug with new potentials in bone disorders. Biomed. Pharmacother. 2019, 109, 1593–1601. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Lu, W.; Xiong, L. Metformin activates Wnt/β-catenin for the treatment of diabetic osteoporosis. BMC Endocr. Disord. 2022, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone turnover markers: Basic biology to clinical applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Branco, J.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Cooper, C.; Cortet, B.; Diez-Perez, A.; Ferrari, S.; Gasparik, A.; et al. Algorithm for the use of biochemical markers of bone turnover in the diagnosis, assessment and follow-up of treatment for osteoporosis. Adv. Ther. 2019, 36, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Lee, S.; Shin, N.; Ko, Y.; Kim, D.; Lee, J.; Lee, W. Bone regeneration with umbilical cord blood mesenchymal stem cells in femoral defects of ovariectomized rats. Osteoporos Sarcopenia 2018, 4, 95–101. [Google Scholar] [CrossRef]
- Verma, S.; Rajaratnam, J.H.; Denton, J.; Hoyland, J.A.; Byers, R.J. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 2002, 55, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, J.; Chen, L. The roles of mesenchymal stem cell-derived exosomes in diabetes mellitus and its related complications. Front. Endocrinol. 2022, 13, 1027686. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, P.; Xue, S.; Xu, Y.; Tan, J.; Liu, G. Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy 2014, 16, 1643–1655. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Styner, M.; Thompson, W.R.; Galior, K.; Uzer, G.; Wu, X.; Kadari, S.; Case, N.; Xie, Z.; Sen, B.; Romaine, A. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 2014, 64, 39–46. [Google Scholar] [CrossRef]
- Bornstein, S.; Moschetta, M.; Kawano, Y.; Sacco, A.; Huynh, D.; Brooks, D.; Manier, S.; Fairfield, H.; Falank, C.; Roccaro, A.M.; et al. Metformin affects cortical bone mass and marrow adiposity in diet-induced obesity in male mice. Endocrinology 2017, 158, 3369–3385. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, W.; Gu, C.; Liu, H.; Hou, M.; Qin, W.; Zhu, X.; Chen, X.; Liu, T.; Yang, H.; et al. Melatonin suppresses bone marrow adiposity in ovariectomized rats by rescuing the imbalance between osteogenesis and adipogenesis through SIRT1 activation. J. Orthop. Translat. 2022, 27, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Martínez Cortizas, A.; López-Costas, O. Linking structural and compositional changes in archaeological human bone collagen: An FTIR-ATR approach. Sci. Rep. 2020, 10, 17888. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, A.M.; van Loon, L.J.C. The impact of collagen protein ingestion on musculoskeletal connective tissue remodeling: A narrative review. Nutr. Rev. 2022, 80, 1497–1514. [Google Scholar] [CrossRef] [PubMed]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine collagen: A promising biomaterial for wound healing, skin anti-aging, and bone regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Kim, T.H.; Kang, K.R.; Lim, H.; Choi, M.C.; Kim, D.K.; Chun, H.S.; Kim, H.J.; Yu, S.K.; Kim, J.S. 7α,25-Dihydroxycholesterol-induced oxiapoptophagic chondrocyte death via the modulation of p53-Akt-mTOR axis in osteoarthritis pathogenesis. Mol. Cells 2023, 46, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cheng, S.; Wang, X.; Zhang, Y.; Chen, L.; Zhang, L. Noncompressible hemostasis and bone regeneration induced by an absorbable bioadhesive self-healing hydrogel. Adv. Funct. Mater. 2021, 31, 2009189. [Google Scholar] [CrossRef]
- Gortázar, A.R.; Ardura, J.A. Osteocytes and diabetes: Altered function of diabetic osteocytes. Curr. Osteoporos Rep. 2020, 18, 796–802. [Google Scholar] [CrossRef]
- Eckhardt, B.A.; Rowsey, J.L.; Thicke, B.S.; Fraser, D.G.; O’Grady, K.L.; Bondar, O.P.; Hines, J.M.; Singh, R.J.; Thoreson, A.R.; Rakshit, K.; et al. Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight 2020, 5, e135236. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular basis of the KEAP1-NRF2 signaling pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Cai, J.; Yan, Z.; Shao, X.; Xie, K.; Guo, X.E.; Luo, E.; Jing, D. Spatiotemporal characterization of microdamage accumulation and its targeted remodeling mechanisms in diabetic fatigued bone. FASEB J. 2020, 34, 2579–2594. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Liu, M.M.; Dong, R.; Hua, Z.; Lv, N.N.; Ma, Y.; Huang, G.C.; Cheng, J.; Xu, H.Y. Therapeutic potential of Liuwei Dihuang pill against KDM7A and Wnt/β-catenin signaling pathway in diabetic nephropathy-related osteoporosis. Biosci. Rep. 2020, 40, BSR20201778. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Xie, J.J.; Hong, R.H.; Pan, H.S.; Zhang, F.G.; Liang, Y.M. Puerarin alleviates streptozotocin (STZ)-induced osteoporosis in rats through suppressing inflammation and apoptosis via HDAC1/HDAC3 signaling. Biomed. Pharmacother. 2019, 115, 108570. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Gambino, R.; Ponzo, V.; Cioffi, I.; Goitre, I.; Evangelista, A.; Ciccone, G.; Cassader, M.; Procopio, M. Effects of resveratrol on bone health in type 2 diabetic patients. A double-blind randomized-controlled trial. Nutr. Diabetes 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Marcadet, L.; Bouredji, Z.; Argaw, A.; Frenette, J. The roles of RANK/RANKL/OPG in cardiac, skeletal, and smooth muscles in health and disease. Front. Cell Dev. Biol. 2022, 10, 903657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, J.; Liu, P.; Wang, Q.; Liu, L.; Zhao, H. The RANK/RANKL/OPG system and tumor bone metastasis: Potential mechanisms and therapeutic strategies. Front. Endocrinol. 2022, 13, 1063815. [Google Scholar] [CrossRef]
- Azizieh, F.Y.; Shehab, D.; Jarallah, K.A.; Gupta, R.; Raghupathy, R. Circulatory levels of RANKL, OPG, and oxidative stress markers in postmenopausal women with normal or low bone mineral density. Biomark. Insights 2019, 14, 1177. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG pathway: A mechanism involved in exercise-induced bone remodeling. Biomed Res. Int. 2020, 19, 6910312. [Google Scholar] [CrossRef]
- Qi, S.S.; Shao, M.L.; Sun, Z.; Chen, S.M.; Hu, Y.J.; Wang, H.T.; Wei, T.K.; Li, X.S.; Zheng, H.X. Lycopene ameliorates diabetic osteoporosis via anti-inflammatory, anti-oxidation, and increasing osteoprotegerin/RANKL expression ratio. J. Funct. Foods 2021, 83, 104539. [Google Scholar] [CrossRef]
- Revell, P.A. Histomorphometry of bone. J. Clin. Pathol. 1983, 36, 1323–1331. [Google Scholar] [CrossRef]



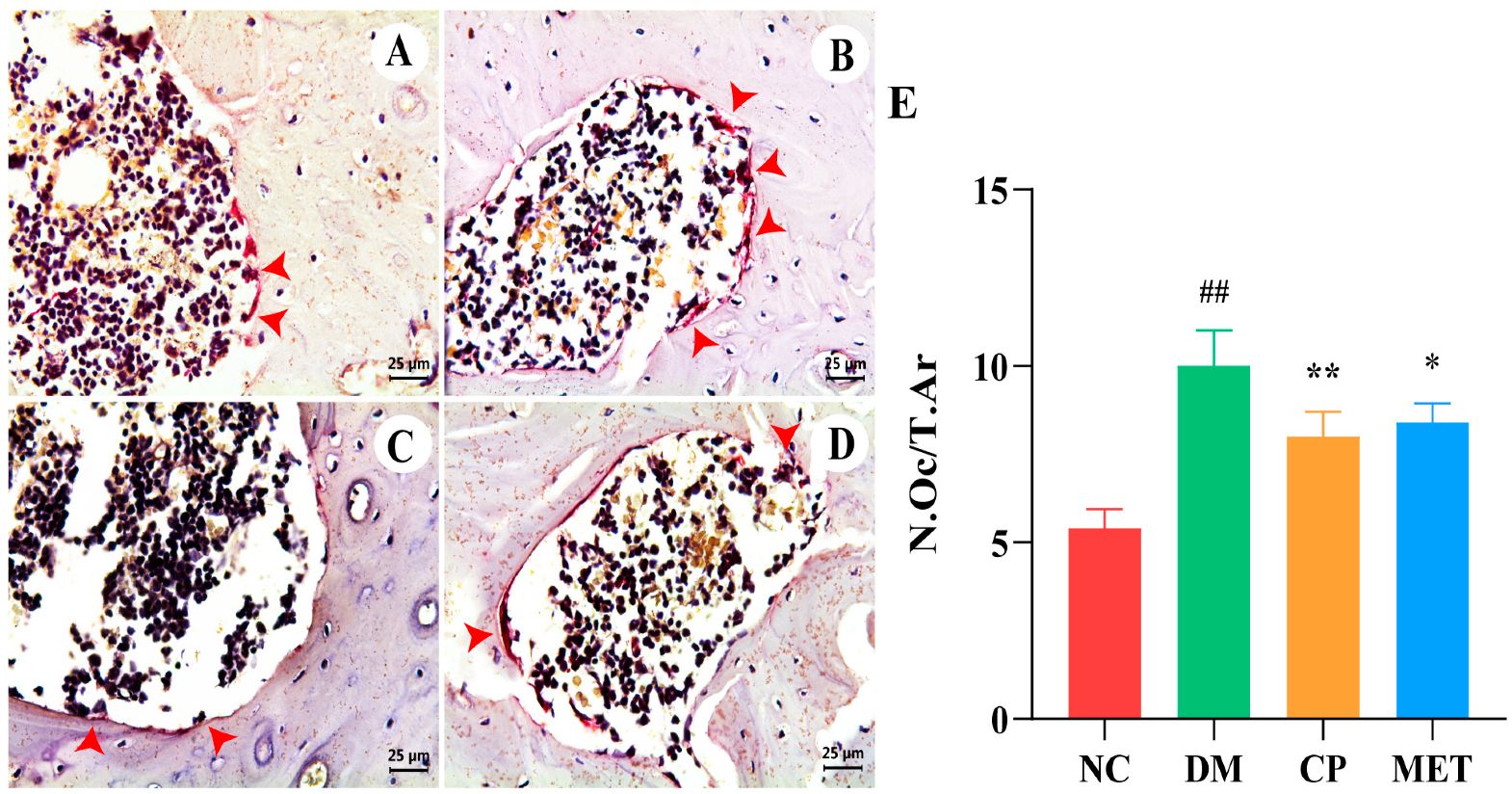

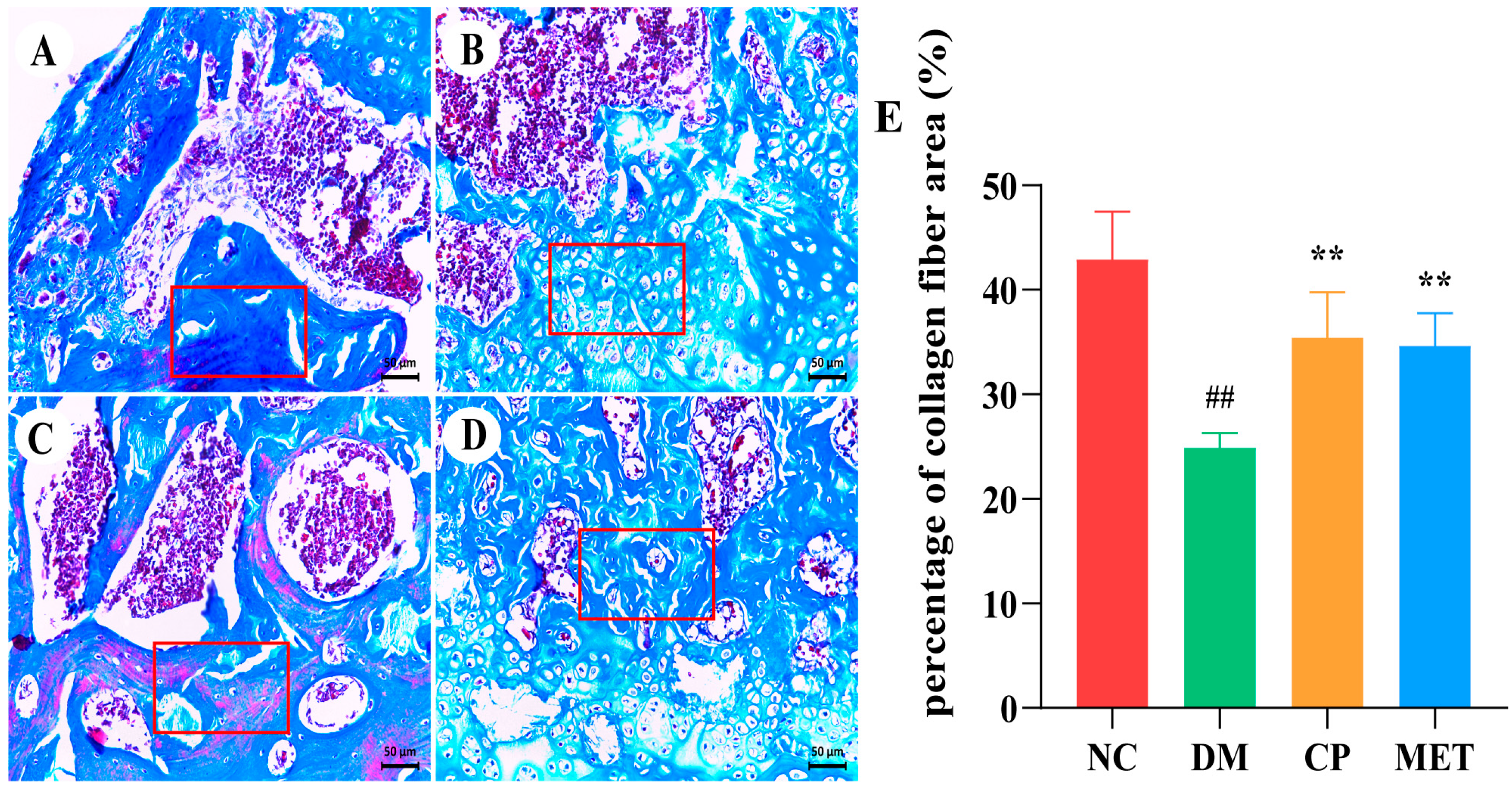
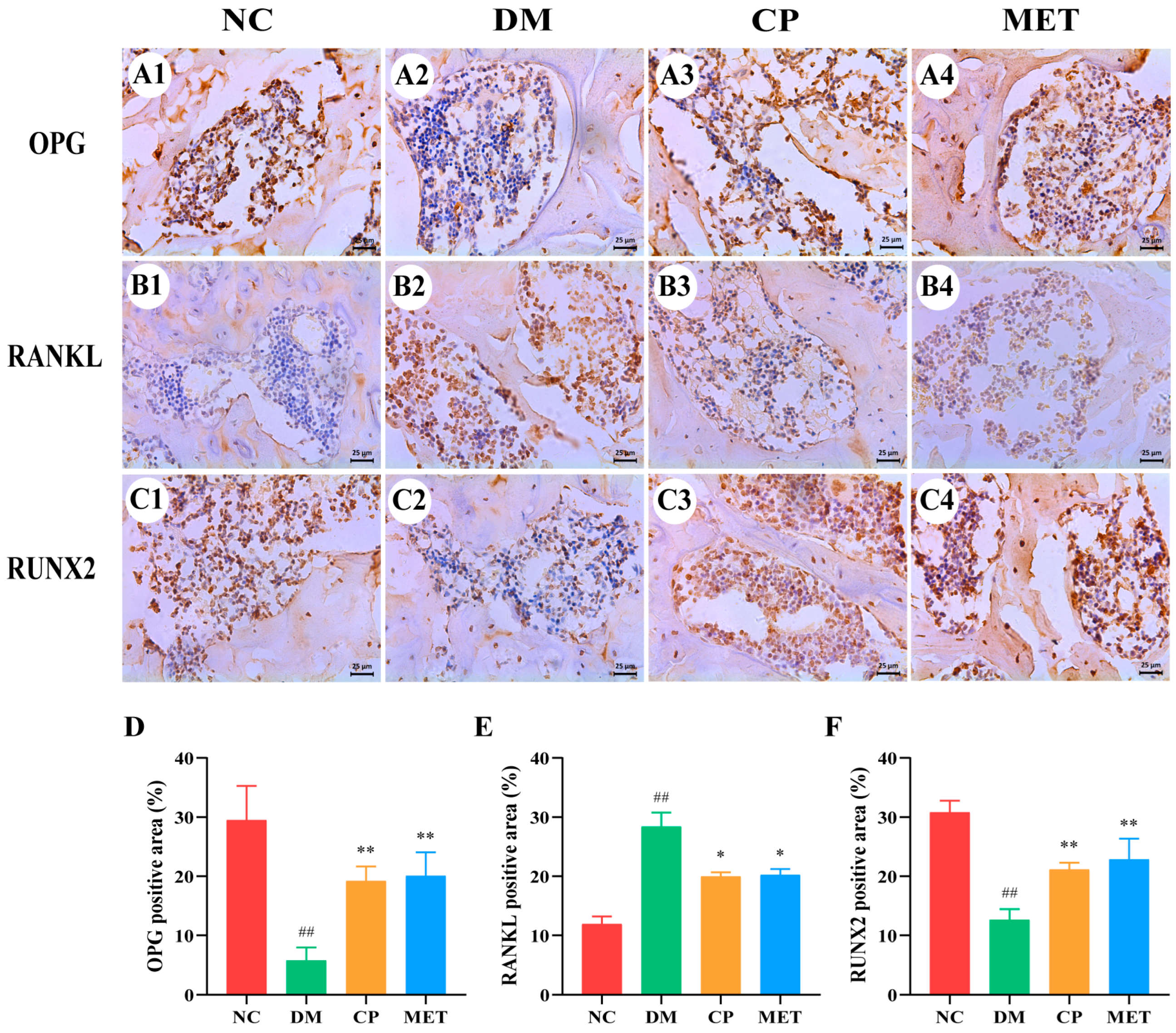
| Parameter | NC | DM | CP | MET |
|---|---|---|---|---|
| OPG (ng mL−1) | 8.93 ± 1.07 | 3.37 ± 0.69 ## | 7.04 ± 1.26 ** | 7.33 ± 0.97 ** |
| RANKL (ng mL−1) | 4.01 ± 0.66 | 11.37 ± 1.97 ## | 5.17 ± 0.74 ** | 5.49 ± 0.67 ** |
| OPG/RANKL ratio | 2.44 ± 0.47 | 0.31 ± 0.11 ## | 1.51 ± 0.39 ** | 1.49 ± 0.37 ** |
| RUNX 2 (ng mL−1) TRACP 5b (U dL−1) | 9.57 ± 1.89 | 3.41 ± 0.79 | 8.46 ± 1.27 ** | 7.96 ± 1.83 ** |
| 2.29 ± 0.63 | 7.32 ± 1.43 ## | 3.37 ± 1.21 ** | 4.01 ± 1.34 * | |
| ALP (U dL−1) CTX-1 (ng mL−1) Osteocalcin (ng mL−1) | 59.64 ± 7.97 | 137.35 ± 14.31 ## | 77.12 ± 8.45 ** | 84.36 ± 10.67 ** |
| 35.47 ± 5.21 | 101.47 ± 11.69 ## | 66.47 ± 8.74 ** | 61.77 ± 9.14 ** | |
| 26.78 ± 4.12 | 8.45 ± 2.17 ## | 19.67 ± 3.64 ** | 21.76 ± 3.99 ** |
| Type of Analysis | Parameter | Unit |
|---|---|---|
| Trabecular bone—3D imaging | Trabecular number (Tb.N) | 1/mm |
| Trabecular thickness (Tb.Th) | mm | |
| Trabecular separation (Tb.Sp) | mm | |
| Relative bone volume (Tb.BV/TV) | % | |
| Trabecular bone pattern factor (Tb.Pf) | 1/mm | |
| Structural model index (SMI) | ||
| Bone mineral content (BMC) | mg | |
| Cortical bone—3D imaging | Cortical bone thickness (Ct.Th) | mm |
| Cortical bone density (Ct.TMD) | mg/cm3 | |
| Cortical bone area (Ct.Ar) | mm2 | |
| Total cortical bone area (Tt.Ar) | mm2 | |
| Percentage of bone marrow cavity area (%Ma.Ar) | % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Yan, W.; Shao, M.; Qi, S. Chromium Picolinate Regulates Bone Metabolism and Prevents Bone Loss in Diabetic Rats. Molecules 2024, 29, 924. https://doi.org/10.3390/molecules29050924
Zheng H, Yan W, Shao M, Qi S. Chromium Picolinate Regulates Bone Metabolism and Prevents Bone Loss in Diabetic Rats. Molecules. 2024; 29(5):924. https://doi.org/10.3390/molecules29050924
Chicago/Turabian StyleZheng, Hongxing, Wenrui Yan, Mengli Shao, and Shanshan Qi. 2024. "Chromium Picolinate Regulates Bone Metabolism and Prevents Bone Loss in Diabetic Rats" Molecules 29, no. 5: 924. https://doi.org/10.3390/molecules29050924
APA StyleZheng, H., Yan, W., Shao, M., & Qi, S. (2024). Chromium Picolinate Regulates Bone Metabolism and Prevents Bone Loss in Diabetic Rats. Molecules, 29(5), 924. https://doi.org/10.3390/molecules29050924






