Synthesis, Crystal Structures, Genotoxicity, and Antifungal and Antibacterial Studies of Ni(II) and Cd(II) Pyrazole Amide Coordination Complexes
Abstract
1. Introduction
2. Results and Discussion
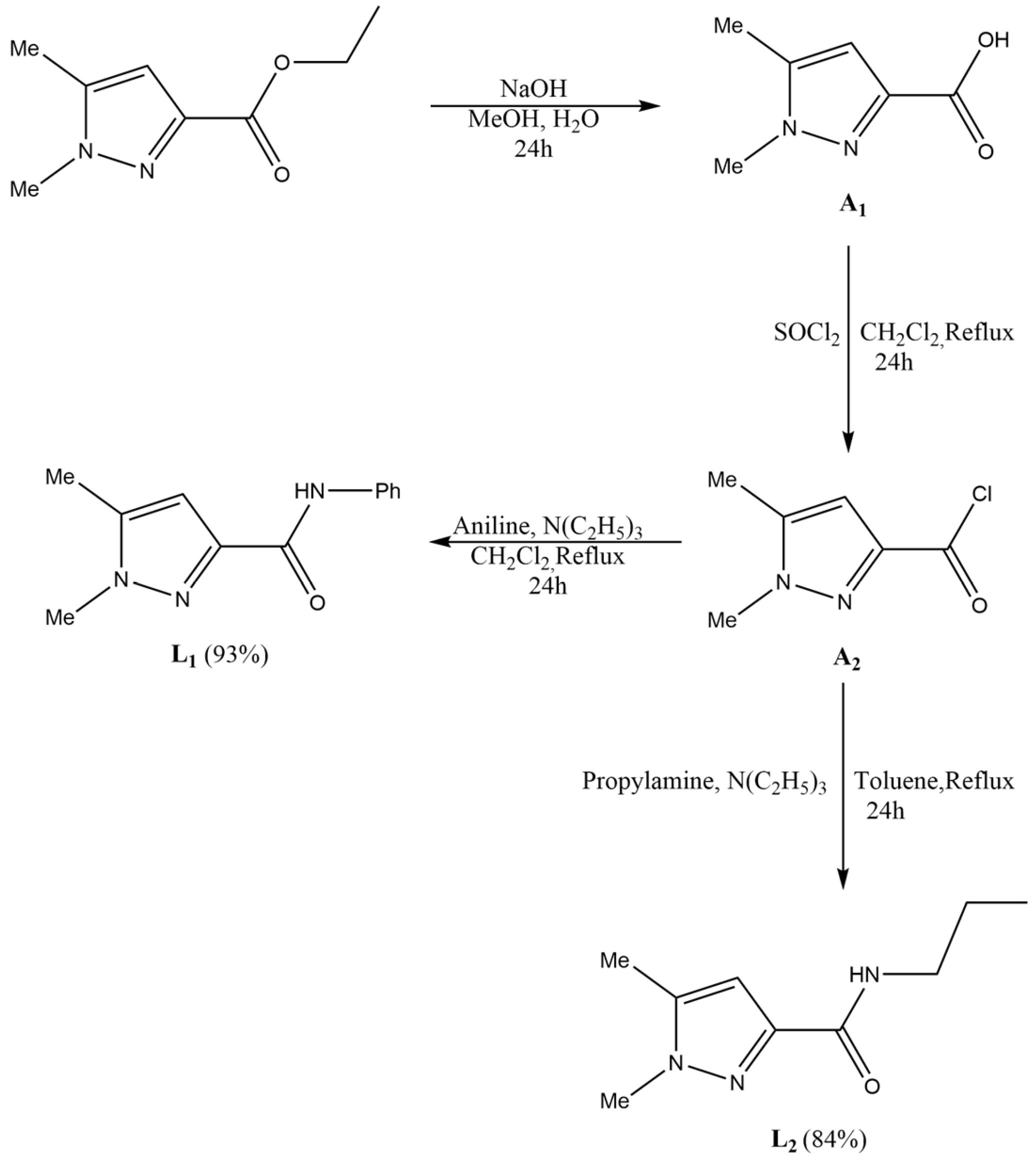
2.1. Synthesis of the Ligands
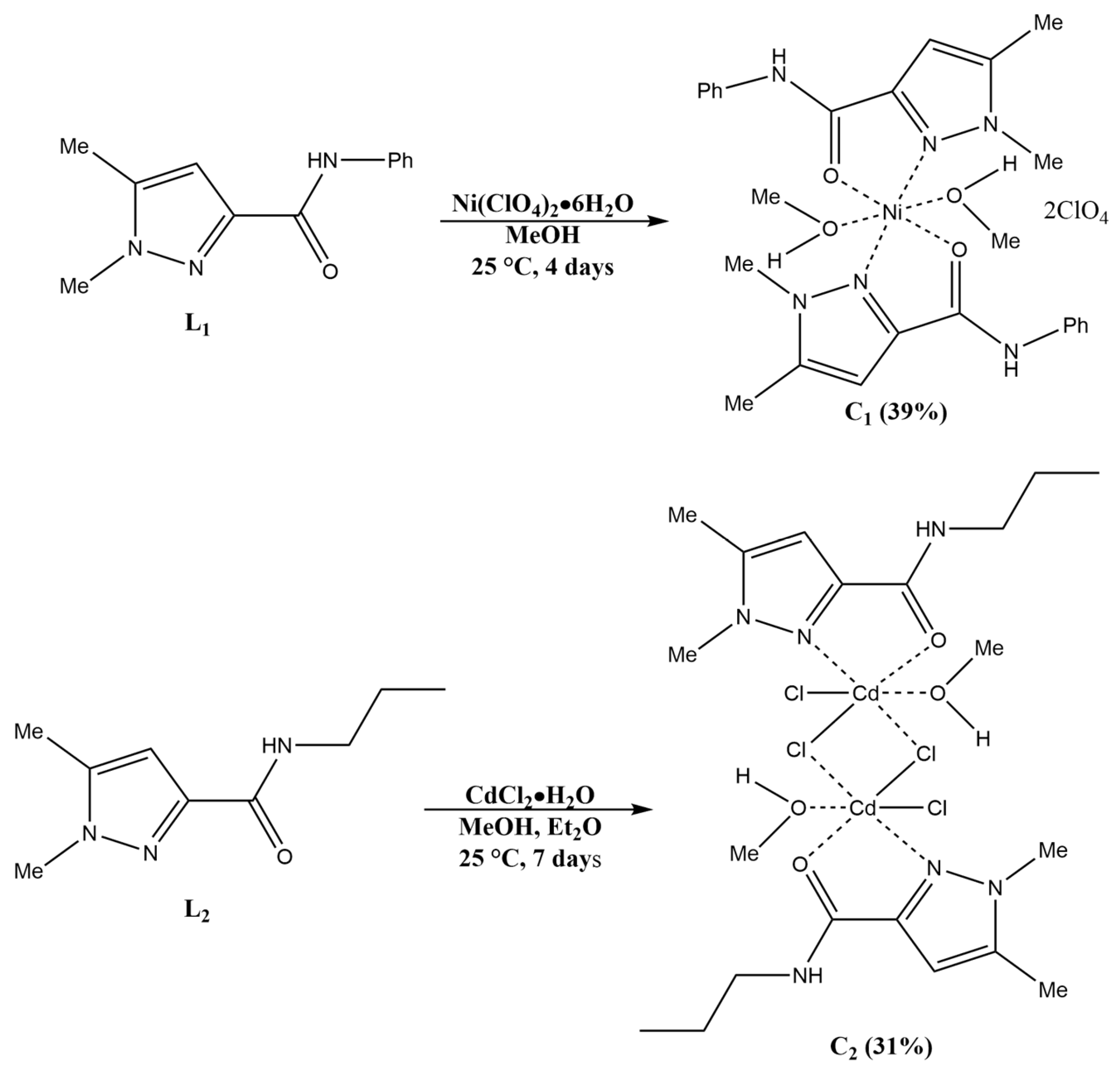
2.2. Synthesis of the Complexes
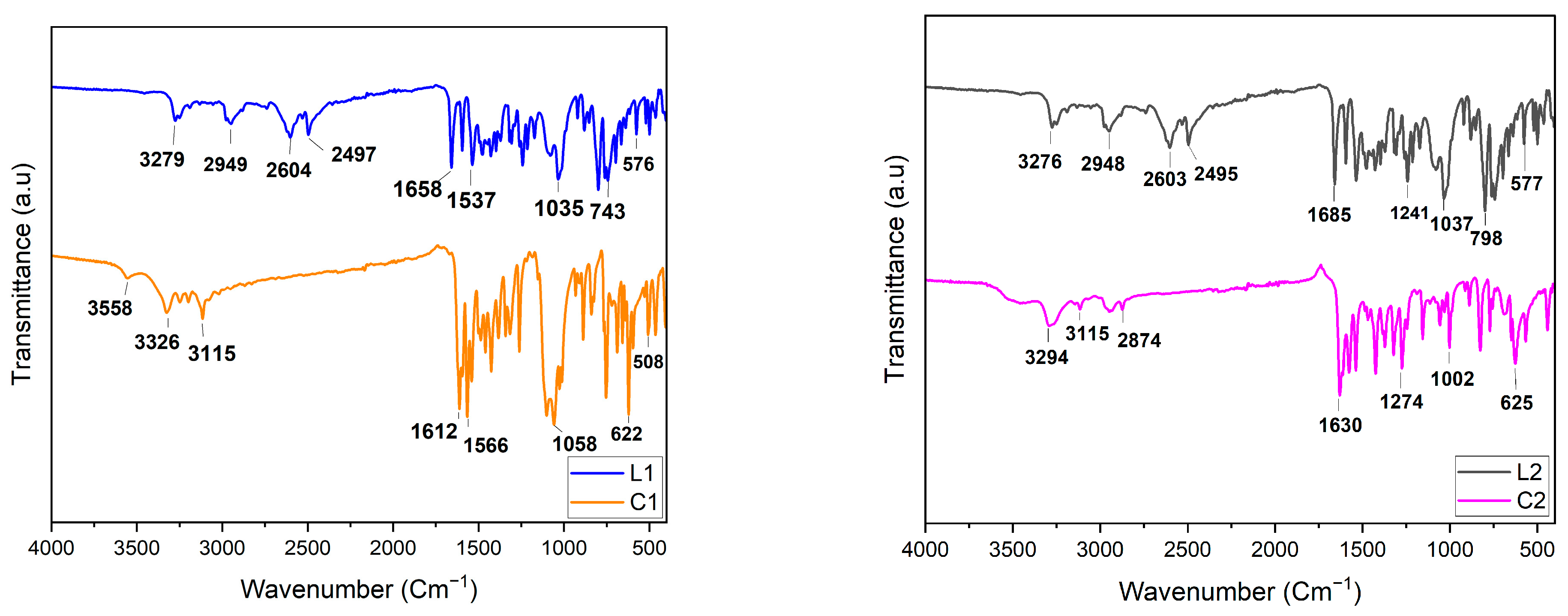
2.3. FT-IR Spectroscopy
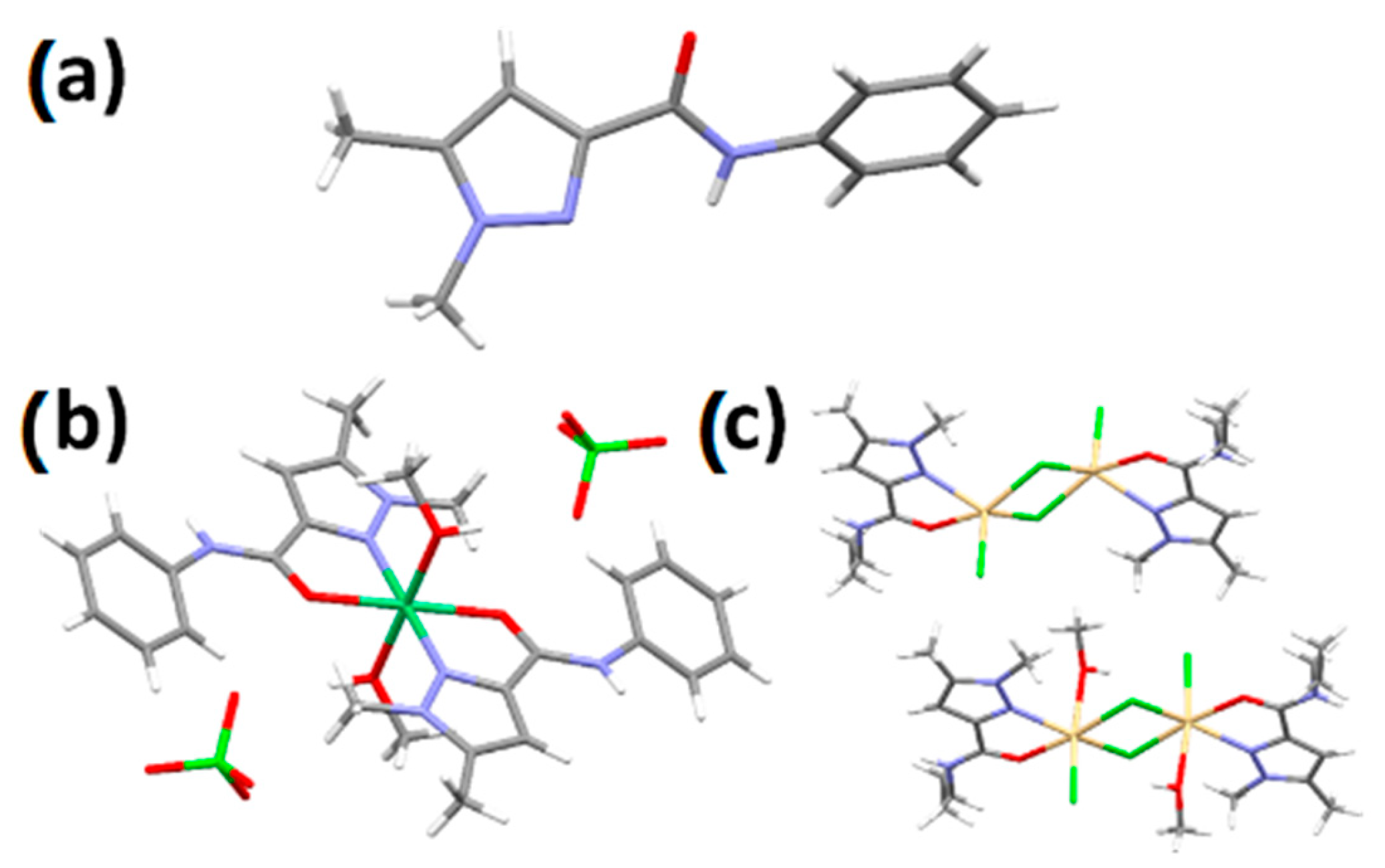
2.4. X-ray Crystallography
2.5. Antibacterial Activity
2.6. Antifungal Activity
2.7. Antifungal Activity on Mycelial Growth of Fusarium oxysporum f. sp. Albinidis
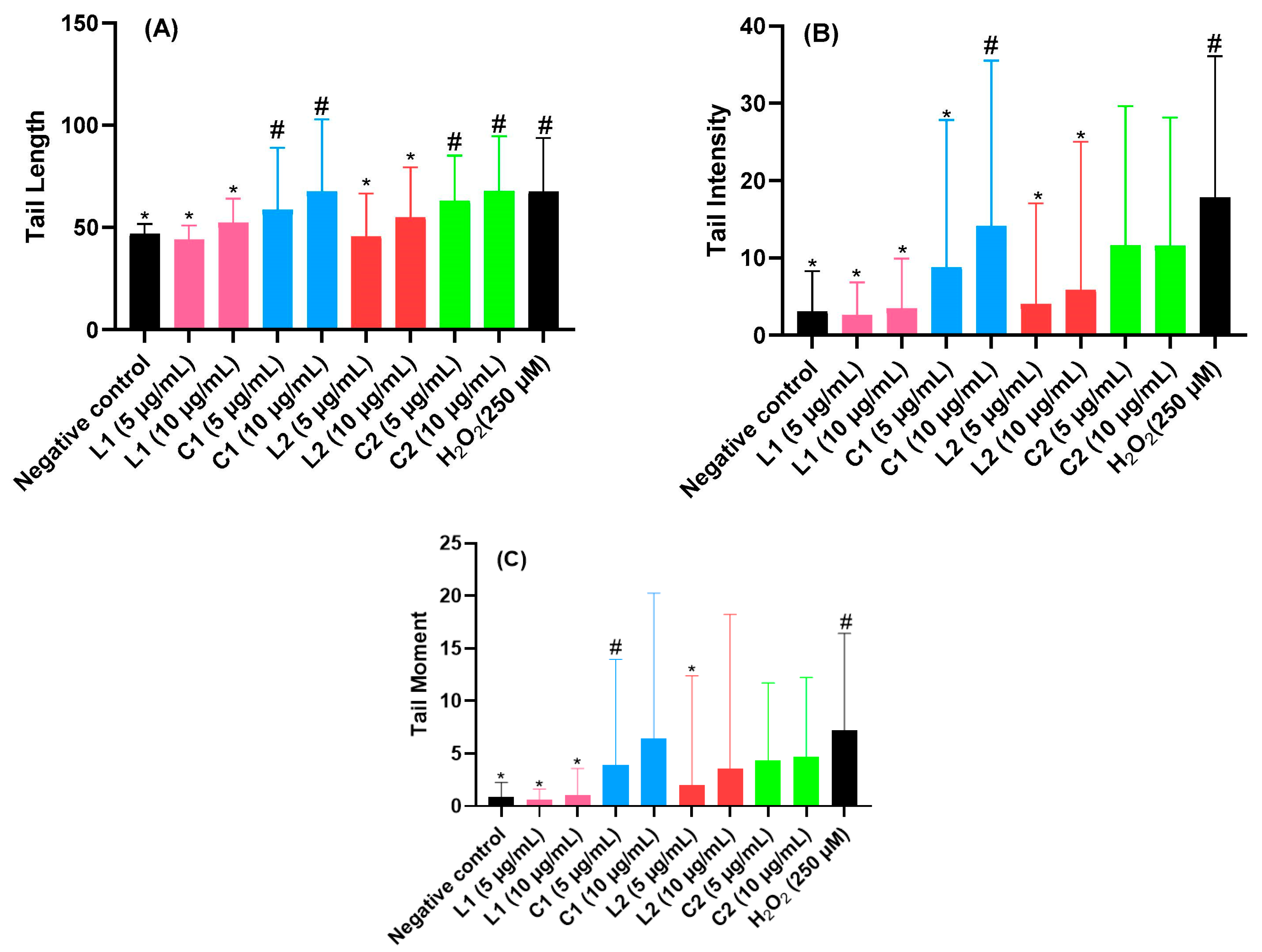
2.8. Genotoxicity
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis Section
3.2.1. Synthesis of Ethyl 1,5-Dimethyl-1H-pyrazole-3-carboxylate
3.2.2. Synthesis of 1,5-Dimethyl-1H-pyrazole-3-carboxylic Acid (A1)
3.2.3. Synthesis of 1,5-Dimethyl-1H-pyrazol-3-carbonyl Chloride (A2)
3.2.4. Synthesis of 1,5-Dimethyl-N-phenyl-1H-pyrazole-3-carboxamide (L1)
3.2.5. Synthesis of 1,5-Dimethyl-N-propyl-1H-pyrazole-3-carboxamide (L2)
3.2.6. Synthesis of [Ni(L1)2](ClO4)2 (C1)
3.2.7. Synthesis of [Cd2(L2)2]Cl4 (C2)
3.3. X-ray Crystallographic Studies
4. Biological Activity
4.1. Microorganisms and Culture Media
4.2. Measurement of Antibacterial and Antifungal Activities
4.3. Antifungal Activity on Mycelial Growth of Fusarium oxysporum
4.4. Genotoxicity Effect
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cattoir, V.; Felden, B. Future Antibacterial Strategies: From Basic Concepts to Clinical Challenges. J. Infect. Dis. 2019, 220, 350–360. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2021 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal Complexes as a Promising Source for New Antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Swiatoslaw, T. Coordination Chemistry of Pyrazole-Derived Ligands. Chem. Rev. 1972, 72, 497–509. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Draoui, Y.; Radi, S.; Tanan, A.; Oulmidi, A.; Miras, H.N.; Benabbes, R.; Ouahhoudo, S.; Mamri, S.; Rotaru, A.; Garcia, Y. Novel Family of Bis-Pyrazole Coordination Complexes as Potent Antibacterial and Antifungal Agents. RSC Adv. 2022, 12, 17755–17764. [Google Scholar] [CrossRef]
- Oulmidi, A.; Rotaru, A.; Radi, S.; Garcia, Y. Pyrazole’s Substituents Effect on the Spin State of [Fe(Bpp)2]2+complexes. Hyperfine Interact. 2021, 242, 8. [Google Scholar] [CrossRef]
- Oulmidi, A.; Radi, S.; Miras, H.N.; Adarsh, N.N.; Garcia, Y. New Bis-Pyrazole-Bis-Acetate Based Coordination Complexes: Influence of Counter-Anions and Metal Ions on the Supramolecular Structures. Sustainability 2020, 13, 288. [Google Scholar] [CrossRef]
- Radi, S.; El-Massaoudi, M.; Benaissa, H.; Adarsh, N.N.; Ferbinteanu, M.; Devlin, E.; Sanakis, Y.; Garcia, Y. Crystal Engineering of a Series of Complexes and Coordination Polymers Based on Pyrazole-Carboxylic Acid Ligands. New J. Chem. 2017, 41, 8232–8241. [Google Scholar] [CrossRef]
- Chkirate, K.; Karrouchi, K.; Dege, N.; Kheira Sebbar, N.; Ejjoummany, A.; Radi, S.; Adarsh, N.N.; Talbaoui, A.; Ferbinteanu, M.; Essassi, E.M.; et al. Co(II) and Zn(II) Pyrazolyl-Benzimidazole Complexes with Remarkable Antibacterial Activity. New J. Chem. 2020, 44, 2210–2221. [Google Scholar] [CrossRef]
- Chkirate, K.; Karrouchi, K.; Chakchak, H.; Mague, J.T.; Radi, S.; Adarsh, N.N.; Li, W.; Talbaoui, A.; Essassi, E.M.; Garcia, Y. Coordination Complexes Constructed from Pyrazole–Acetamide and Pyrazole–Quinoxaline: Effect of Hydrogen Bonding on the Self-Assembly Process and Antibacterial Activity. RSC Adv. 2022, 12, 5324–5339. [Google Scholar] [CrossRef]
- Chkirate, K.; Fettach, S.; Karrouchi, K.; Sebbar, N.K.; Essassi, E.M.; Mague, J.T.; Radi, S.; El Abbes Faouzi, M.; Adarsh, N.N.; Garcia, Y. Novel Co(II) and Cu(II) Coordination Complexes Constructed from Pyrazole-Acetamide: Effect of Hydrogen Bonding on the Self Assembly Process and Antioxidant Activity. J. Inorg. Biochem. 2019, 191, 21–28. [Google Scholar] [CrossRef]
- Malek, F.; Ramdani, A.; Zidane, I.; Yahyi, A.; Radi, S. Tetrapyrazolic Tripods. Synthesis and Preliminary Use in Metal Ion Extraction. Tetrahedron 2005, 61, 2995–2998. [Google Scholar] [CrossRef]
- Malek, F.; Ramdani, A.; Radi, S. Pyrazolic Tripods Synthesis and Cation Binding Properties. J. Chem. Res. 2004, 2004, 640–641. [Google Scholar] [CrossRef]
- Radi, S.; Ramdani, A.; Lekchiri, Y.; Morcellet, M.; Crini, G.; Janus, L.; Martel, B. Extraction of Metal Ions from Water with Tetrapyrazolic Macrocycles Bound to Merrifield Resin and Silica Gel. J. Appl. Polym. Sci. 2000, 78, 2495–2499. [Google Scholar] [CrossRef]
- Mouadili, A.; Attayibat, A.; Kadiri, S.E.; Radi, S.; Touzani, R. Catecholase Activity Investigations Using in Situ Copper Complexes with Pyrazole and Pyridine Based Ligands. Appl. Catal. A Gen. 2013, 454, 93–99. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Toubi, Y.; Bacquet, M. Polysiloxane Surface Modified with Bipyrazolic Tripodal Receptor for Quantitative Lead Adsorption. J. Hazard. Mater. 2011, 185, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Janus, L.; Mabkhot, Y.N. Fabrication and Covalent Modification of Highly Chelated Hybrid Material Based on Silica-Bipyridine Framework for Efficient Adsorption of Heavy Metals: Isotherms, Kinetics and Thermodynamics Studies. RSC Adv. 2016, 6, 82505–82514. [Google Scholar] [CrossRef]
- Radi, S.; El Abiad, C.; Carvalho, A.P.; Santos, S.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. An Efficient Hybrid Adsorbent Based on Silica-Supported Amino Penta-Carboxylic Acid for Water Purification. J. Mater. Chem. A 2018, 6, 13096–13109. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, Y.; El-Massaoudi, M.; Bacquet, M.; Degoutin, S.; Mabkhot, Y.N. Efficient Extraction of Heavy Metals from Aqueous Solution by Novel Hybrid Material Based on Silica Particles Bearing New Schiff Base Receptor. J. Mol. Liq. 2016, 223, 112–118. [Google Scholar] [CrossRef]
- Radi, S.; Attayibat, A.; Ramdani, A.; Bacquet, M. Synthesis and Characterization of Novel Silica Gel Supported N-Pyrazole Ligand for Selective Elimination of Hg(II). Eur. Polym. J. 2008, 44, 3163–3168. [Google Scholar] [CrossRef]
- Cherrak, K.; Belghiti, M.E.; Berrissoul, A.; El Massaoudi, M.; El Faydy, M.; Taleb, M.; Radi, S.; Zarrouk, A.; Dafali, A. Pyrazole Carbohydrazide as Corrosion Inhibitor for Mild Steel in HCl Medium: Experimental and Theoretical Investigations. Surf. Interfaces 2020, 20, 100578. [Google Scholar] [CrossRef]
- Belghiti, M.; Tighadouini, S.; Karzazi, Y.; Dafali, A.; Hammouti, B.; Radi, S.; Solmaz, R. New Hydrazine Derivatives as Corrosion Inhibitors for Mild Steel Protection in Phosphoric Acid Medium. Part A: Experimental Study. J. Mater. Environ. Sci. 2016, 7, 337–346. [Google Scholar]
- Oulmidi, A.; Radi, S.; Idir, A.; Zyad, A.; Kabach, I.; Nhiri, M.; Robeyns, K.; Rotaru, A.; Garcia, Y. Synthesis and Cytotoxicity against Tumor Cells of Pincer N-Heterocyclic Ligands and Their Transition Metal Complexes. RSC Adv. 2021, 11, 34742–34753. [Google Scholar] [CrossRef]
- Ronco, C.; Martin, A.R.; Demange, L.; Benhida, R. ATM, ATR, CHK1, CHK2 and WEE1 Inhibitors in Cancer and Cancer Stem Cells. Med. Chem. Commun. 2017, 8, 295–319. [Google Scholar] [CrossRef]
- Radi, S.; Salhi, S.; Radi, A. Synthesis and Preliminary Biological Activity of Some New Pyrazole Derivatives as Acyclonucleoside Analogues. Lett. Drug Des. Discov. 2010, 7, 27–30. [Google Scholar] [CrossRef]
- Mabkhot, Y.; Al-Majid, A.; Barakat, A.; Al-Showiman, S.; Al-Har, M.; Radi, S.; Naseer, M.; Hadda, T. Synthesis and Biological Evaluation of 2-Aminobenzamide Derivatives as Antimicrobial Agents: Opening/Closing Pharmacophore Site. Int. J. Mol. Sci. 2014, 15, 5115–5127. [Google Scholar] [CrossRef]
- Karrouchi, K.; Fettach, S.; Anouar, E.H.; Tüzün, B.; Radi, S.; Alharthi, A.I.; Ghabbour, H.A.; Mabkhot, Y.N.; Faouzi, M.E.A.; Ansar, M.; et al. Synthesis, Crystal Structure, DFT, α-Glucosidase and α-Amylase Inhibition and Molecular Docking Studies of (E)-N’-(4-Chlorobenzylidene)-5-Phenyl-1H-Pyrazole-3-Carbohydrazide. J. Mol. Struct. 2021, 1245, 131067. [Google Scholar] [CrossRef]
- Cetin, A.; Kurt, H. Synthesis, Antibacterial Activity and Molecular Docking Studies of New Pyrazole Derivatives. Lett. Drug Des. Discov. 2020, 17, 745–756. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.; Al-aizari, F.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Mukherjee, R. Coordination Chemistry with Pyridine/Pyrazine Amide Ligands. Some Noteworthy Results. Coord. Chem. Rev. 2013, 257, 350–368. [Google Scholar] [CrossRef]
- Draoui, Y.; Radi, S.; El Massaoudi, M.; Bahjou, Y.; Ouahhoud, S.; Mamri, S.; Ferbinteanu, M.; Benabbes, R.; Wolff, M.; Robeyns, K.; et al. Coordination Complexes Built from a Ditopic Triazole-Pyrazole Ligand with Antibacterial and Antifungal Performances. Molecules 2023, 28, 6801. [Google Scholar] [CrossRef]
- Preti, C.; Tosi, G.; Filippo, D.D.; Verani, G. Cobalt(II) and Nickel(II) Complexes with Nitrogen, Sulfur, and Selenium Containing Heterocyclic Ligands. Can. J. Chem. 1974, 52, 2021–2028. [Google Scholar] [CrossRef]
- Popović, Z.V.; Stanišić, G.; Stojanović, D.; Kostić, R. Infrared and Raman Spectra of CdO. Phys. Status Solidi B 1991, 165, K109–K112. [Google Scholar] [CrossRef]
- Kamath, P.V.; Ganguly, S. Infrared Spectroscopic Studies of the Oxide-Hydroxides of Ni, Co and Mn. Mater. Lett. 1991, 10, 537–539. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Khan, A.; Haque, A.; Ahmad, A.; Saleem, K.; Manzoor, N. Synthesis and Synergistic Antifungal Activities of a Pyrazoline Based Ligand and Its Copper(II) and Nickel(II) Complexes with Conventional Antifungals. Microb. Pathog. 2012, 53, 66–73. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Zhu, H. Syntheses, Crystal Structures, and Antibacterial Activities of Four Schiff Base Copper(II), Zinc(II), and Cadmium(II) Complexes Derived from 2-[(2-Dimethylaminoethylimino)Methyl]Phenol. Z. Anorg. Allge Chem. 2006, 632, 140–146. [Google Scholar] [CrossRef]
- Bahjou, Y.; Radi, S.; El Massaoudi, M.; Draoui, Y.; Adarsh, N.N.; Miras, H.N.; Ferbinteanu, M.; Robeyns, K.; Ouahhoud, S.; Benabbes, R.; et al. High Inhibition for a Co II Tetrazole Bi-pyrazole Dinuclear Complex against Fusarium Oxysporum f. Sp. Albedinis. Eur. J. Inorg. Chem. 2023, e202300634. [Google Scholar] [CrossRef]
- Biswas, F.B.; Roy, T.G.; Rahman, M.A.; Emran, T.B. An in Vitro Antibacterial and Antifungal Effects of Cadmium(II) Complexes of Hexamethyltetraazacyclotetradecadiene and Isomers of Its Saturated Analogue. Asian Pac. J. Trop. Med. 2014, 7, S534–S539. [Google Scholar] [CrossRef] [PubMed]
- Dal Pizzol, M.; Freitas, E.C.; Locatelli, C.; Guareze, F.; Reginatto, P.; Machado, G.; Fuentefria, A.; Marinho, D. Antifungal Efficacy and Safety of Cycloheximide as a Supplement in Optisol-GS. Lett. Drug Des. Discov. 2021, 15, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.; Hassan, F.; Hossainy, A.; Khidr, M. Coordination Behaviour and Biological Activity Studies of Transition Metal Complexes with Indapamide and Mixed Ligands of Indapamide and Glycine. J. Pharm. 2012, 20125, 3753–3763. [Google Scholar]
- Montazerozohori, M.; Zahedi, S.; Nasr-Esfahani, M.; Naghiha, A. Some New Cadmium Complexes: Antibacterial/Antifungal Activity and Thermal Behavior. J. Ind. Eng. Chem. 2014, 20, 2463–2470. [Google Scholar] [CrossRef]
- Chandra, S.; Vandana; Kumar, S. Synthesis, Spectroscopic, Anticancer, Antibacterial and Antifungal Studies of Ni(II) and Cu(II) Complexes with Hydrazine Carboxamide, 2-[3-Methyl-2-Thienyl Methylene]. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 356–363. [Google Scholar] [CrossRef]
- Bahjou, Y.; Draoui, Y.; Radi, S.; Robeyns, K.; Li, W.; Miras, H.N.; Ferbinteanu, M.; Bentouhami, N.E.; Asehraou, A.; Rotaru, A.; et al. A Cu(II) Coordination Polymer as a Robust Inhibitor of Fusarium Oxysporum f. Sp. Albedinis; LCAE, Department of Chemistry, Faculty of Sciences, University Mohammed I: Oujda, Morocco, 2024; submitted. [Google Scholar]
- Islam, M.R.; Islam, S.M.R.; Noman, A.S.M.; Khanam, J.A.; Ali, S.M.M.; Alam, S.; Lee, M.-W. Biological Screening of a Novel Nickel (II) Tyrosine Complex. Mycobiology 2007, 35, 25–29. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Abrigach, F.; Benabbes, R.; Eddike, D.; Tillard, M. Novel β-Keto–Enol Pyrazolic Compounds as Potent Antifungal Agents. Design, Synthesis, Crystal Structure, DFT, Homology Modeling, and Docking Studies. J. Chem. Inf. Model. 2019, 59, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Kaddouri, Y.; Abrigach, F.; Ouahhoud, S.; Benabbes, R.; El Kodadi, M.; Alsalme, A.; Al-Zaqri, N.; Warad, I.; Touzani, R. Mono-Alkylated Ligands Based on Pyrazole and Triazole Derivatives Tested against Fusarium Oxysporum f. Sp. Albedinis: Synthesis, Characterization, DFT, and Phytase Binding Site Identification Using Blind Docking/Virtual Screening for Potent Fophy Inhibitors. Front. Chem. 2020, 8, 559262. [Google Scholar] [CrossRef] [PubMed]
- Benhusein, G.; Mutch, E.; Aburawi, S.; Williams, F. Genotoxic Effect Induced by Hydrogen Peroxide in Human Hepatoma Cells Using Comet Assay. Libyan J. Med. 2010, 5, 4637. [Google Scholar] [CrossRef]
- El-Habit, O.H.; Abdel Moneim, A.E. Testing the Genotoxicity, Cytotoxicity, and Oxidative Stress of Cadmium and Nickel and Their Additive Effect in Male Mice. Biol. Trace Elem. Res. 2014, 159, 364–372. [Google Scholar] [CrossRef]
- Permenter, M.G.; Lewis, J.A.; Jackson, D.A. Exposure to Nickel, Chromium, or Cadmium Causes Distinct Changes in the Gene Expression Patterns of a Rat Liver Derived Cell Line. PLoS ONE 2011, 6, e27730. [Google Scholar] [CrossRef]
- Cameron, K.S.; Buchner, V.; Tchounwou, P.B. Exploring the Molecular Mechanisms of Nickel-Induced Genotoxicity and Carcinogenicity: A Literature Review. Rev. Environ. Health 2011, 26, 81–92. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysAlisPro, V 1.171. 37.35; Agilent Technologies: Santa Clara, CA, USA, 2014.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Nelson, P.E.; Toussoun, T.A.; Cook, R.J. (Eds.) Fusarium: Diseases, Biology, and Taxonomy; Pennsylvania State University Press: University Park, PA, USA, 1981; ISBN 978-0-271-00293-4. [Google Scholar]
- Booth, C. The Genus Fusarium; Commonwealth Agricultural Bureaux [for the] Commonwealth Mycological Institute: Farnham Royal, UK, 1971; ISBN 978-0-85198-046-1. [Google Scholar]
- Rossi, R.; Ciofalo, M. An Updated Review on the Synthesis and Antibacterial Activity of Molecular Hybrids and Conjugates Bearing Imidazole Moiety. Molecules 2020, 25, 5133. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. MB 2004, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Banáth, J.P.; Durand, R.E. Heterogeneity in Radiation-Induced DNA Damage and Repair in Tumor and Normal Cells Measured Using the “Comet” Assay. Radiat. Res. 2012, 178, AV35–AV42. [Google Scholar] [CrossRef] [PubMed]
- Ouahhoud, S.; Khoulati, A.; Kadda, S.; Bencheikh, N.; Mamri, S.; Ziani, A.; Baddaoui, S.; Eddabbeh, F.-E.; Lahmass, I.; Benabbes, R.; et al. Antioxidant Activity, Metal Chelating Ability and DNA Protective Effect of the Hydroethanolic Extracts of Crocus Sativus Stigmas, Tepals and Leaves. Antioxidants 2022, 11, 932. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Strubbia, S.; Lyons, B.P.; Lee, R.J. Spatial and Temporal Variation of Three Biomarkers in Mytilus Edulis. Mar. Pollut. Bull. 2019, 138, 322–327. [Google Scholar] [CrossRef]
| Compound | C (mmol/L) | Inhibition Zone (mm) | Ref. | |||
|---|---|---|---|---|---|---|
| E. coli [n] | Li. Innocua [p] | Ps. Aeruginosa [n] | S. Aureus [p] | |||
| L1 | 27.8 | 9.20 ± 0.53 * | 7.94 ± 0.40 * | 9.17 ± 1.04 * | 8.17 ± 0.76 * | This work |
| C1 | 7.98 | 9.65 ± 0.28 * | 10.20 ± 0.28 * | 10.15 ± 0.28 * | 10.6 ± 0.28 * | This work |
| L2 | 33.1 | 9.30 ± 0.17 * | 8.33 ± 1.15 * | 9.17 ± 0.85 * | 7.10 ± 1.56 * | This work |
| C2 | 7.88 | 16.10 ± 0.28 * | 14 ± 0.28 * | 14.7 ± 0.57 * | 12.8 ± 0.28 * | This work |
| Gentamicin | 1.25 | 29.9 ± 0.28 | 30.10 ± 0.28 | 27.75 ± 0.35 | 32.85 ± 0.70 | This work |
| [Cd2(dmamp)2 (NCS)2]n | --- | 9.2 | -- | 13.1 | -- | [37] |
| [Ni(btbepa)CH3OH(H2O)](ClO4)2 | -- | 28 | -- | -- | 27 | [6] |
| H3O[Ni(dmptr)3](ClO4)3 | 4.62 | 12 ± 0.38 | -- | -- | 12 ± 0.25 | [32] |
| [Cd(dmptr)2 Cl2] | 7.85 | 27 ± 0.25 | -- | -- | 27 ± 0.19 | [32] |
| [Cd(htcd)(SCN)]SCN | -- | 22 | -- | -- | 24 | [39] |
| [NiL3](ClO4)2 | 3.49 | 8 ± 0.8 | 9 ± 0.35 | 9 ± 0.95 | 8 ± 0.35 | [38] |
| CdL2Cl2 | 5.25 | 14 ± 0.75 | 11 ± 0.5 | 12 ± 0.3 | 11 ± 0.5 | [38] |
| Ni(ClO4)2·6H2O | 8.2 | 0 | 0 | 0 | 0 | [38] |
| CdCl2·H2O | 14.9 | 11 ± 0.3 | 0 | 8 ± 0.2 | 0 | [38] |
| Compound | C (mmol/L) | Inhibition Zone (mm) | Ref. | ||
|---|---|---|---|---|---|
| G.candidum | A. niger | P. crustosum | |||
| L1 | 27.87 | 13.9 ± 0.1 * | 10.25 ± 0.75 * | 8.8 ± 0.2 * | This work |
| C1 | 7.98 | 15.5 ± 0.71 * | 19 ± 1.41 * | 22 ± 1.41 | This work |
| L2 | 33.11 | 12.5 ± 0.6 * | 9.85 ± 0.65 * | 8.25 ± 0.25 * | This work |
| C2 | 7.88 | 20 ± 1.41 * | 24.5 ± 0.71 * | 26 ± 2.83 * | This work |
| Cycloheximide | 21.33 | 22.8 ± 0.2 | 22.7 ± 0.5 | 22.6 ± 0.6 | This work |
| Cycloheximide (lower concentration) | 10.66 | 22 ± 0.8 | 22 ± 0.7 | 22 ± 0.6 | This work |
| [Ni(IND)2(Gly)(H2O) 2]Cl | -- | 22.7 ± 0.03 | -- | -- | [41] |
| Cd(bdade)(NCS)2 | -- | -- | 18.44 | -- | [42] |
| [Ni(psi)2SO4] | -- | -- | 10 | -- | [43] |
| [Ni(CH3COO)2(Tyr)2] Tyr = Tyrosine | -- | -- | 23 | -- | [45] |
| [NiL3](ClO4)2 | 3.49 | 0 | 9 | 9 ± 0.1 | [38] |
| CdL2Cl2 | 5.25 | 14 ± 0.2 | 14 ± 0.8 | 15 ± 0.45 | [38] |
| Ni(ClO4)2·6H2O | 8.2 | 0 | 0 | 0 | [38] |
| CdCl2·H2O | 14.9 | 10 ± 0.1 | 6 ± 0.3 | 5 ± 0.2 | [38] |
| Compound | Sample Volumes (µL) | C (µmol/L) | Inhibition (%) | Ref. |
|---|---|---|---|---|
| L1 | 50 | 92.9 | 46.3 ± 2.5 * | This work |
| 150 | 278.7 | 50 ± 4.5 | ||
| C1 | 50 | 26.6 | 43.8 ± 3.3 | This work |
| 150 | 79.8 | 62.5 ± 2.2 * | ||
| L2 | 50 | 110.35 | 50 ± 7.8 * | This work |
| 150 | 331.05 | 57.5 ± 6.6 | ||
| C2 | 50 | 26.3 | 87.5 ± 3.3 * | This work |
| 150 | 78.9 | 96.3 ± 1.3 * | ||
| Cycloheximide | 50 | 71.1 | 35 ± 3.3 | This work |
| 150 | 213.3 | 55 ± 2.5 | ||
| 250 | 355.5 | 66.7 ± 2.9 | ||
| Cd(dmptr)2 Cl2 | 200 | 104.6 | 50 | [32] |
| (H3O)[Ni(dmptr)3](ClO4)3 | 200 | 29.41 | 38 | [32] |
| [Ni(btbepa)CH3OH(H2O)](ClO4)2 | 200 | 68.8 | 44 | [6] |
| NiL3(ClO4)2 | 150 | 66.42 | 35 | [38] |
| CdL2Cl2 | 150 | 97.75 | 51 | [38] |
| Co2(HL2)L2Cl3 | 150 | 81.1 | 97 | [38] |
| Cu2(HL’2)Cl2 | 150 | 158.02 | 97 | [44] |
| Ni(ClO4)2·6H2O | 150 | 164.07 | 8 | [38] |
| CdCl2·H2O | 150 | 298.01 | 12 | [38] |
| Compound | L1 | C1 | C2 |
|---|---|---|---|
| Empirical formula | C12 H13 N3 O | C26 H34 Cl2 N6 Ni O12 | C38 H68 Cd4 Cl8 N12 O6 |
| Formula weight | 215.25 | 752.20 | 1522.24 |
| T (K) | 293(2) | 293(2) | 293(2) |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Orthorhombic | Monoclinic | Orthorhombic |
| Space group | Pbca | P21/n | Pca21 |
| Unit cell dimensions (Å,°) | a = 10.4982(4) | a = 10.6244(6) | a = 16.7846(3) |
| b = 9.8653(4) | b = 13.0110(7) | b = 16.4470(3) | |
| c = 21.4838(9) | c = 11.8231(7) | c = 20.9709(3) | |
| α = 90 | α = 90 | α = 90 | |
| β = 90 | β = 96.503(6) | β = 90 | |
| γ = 90 | γ = 90 | γ = 90 | |
| V (Å3) | 2225.04(15) | 1623.84(16) | 5789.16(16) |
| Z | 8 | 2 | 4 |
| Density (calculated) (g/cm3) | 1.285 | 1.538 | 1.747 |
| Absorption coefficient (mm−1) | 0.085 | 0.832 | 1.869 |
| F(000) | 912 | 780 | 3024 |
| Crystal size (mm3) | 0.32 × 0.15 × 0.12 | 0.35 × 0.30 × 0.20 | 0.40 × 0.25 × 0.20 |
| θrange for data collection (°) | 2.988 to 26.193 | 2.902 to 26.232 | 2.893 to 26.187 |
| Reflections collected | 16410 | 11040 | 39165 |
| Independent reflections | 2215 [R(int) = 0.0480] | 3199 [R(int) = 0.0253] | 11,407 [R(int) = 0.0185] |
| Completeness to θ 25.242° (%) | 99.2 | 98.9 | 98.9 |
| Max. and min. transmission | 1.00000 and 0.82569 | 1.00000 and 0.88273 | 1.00000 and 0.66350 |
| Data/restraints/parameters | 2215/0/147 | 3199/99/273 | 11407/43/721 |
| Goodness-of-fit on F2 | 1.086 | 1.045 | 1.070 |
| Final R indices [I > 2s(I)] | R1 = 0.0441, wR2 = 0.1216 | R1 = 0.0374, wR2 = 0.1059 | R1 = 0.0188, wR2 = 0.0486 |
| R indices (all data) | R1 = 0.0488, wR2 = 0.1254 | R1 = 0.0398, wR2 = 0.1079 | R1 = 0.0197, wR2 = 0.0490 |
| Absolute structure parameter | . | . | 0.31(2) |
| Δρ(max, min)(e.Å−3) | 0.187, −0.170 | 0.411, −0.289 | 0.528, −0.371 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mahdaoui, A.; Radi, S.; Draoui, Y.; El Massaoudi, M.; Ouahhoud, S.; Asehraou, A.; Bentouhami, N.E.; Saalaoui, E.; Benabbes, R.; Robeyns, K.; et al. Synthesis, Crystal Structures, Genotoxicity, and Antifungal and Antibacterial Studies of Ni(II) and Cd(II) Pyrazole Amide Coordination Complexes. Molecules 2024, 29, 1186. https://doi.org/10.3390/molecules29051186
El Mahdaoui A, Radi S, Draoui Y, El Massaoudi M, Ouahhoud S, Asehraou A, Bentouhami NE, Saalaoui E, Benabbes R, Robeyns K, et al. Synthesis, Crystal Structures, Genotoxicity, and Antifungal and Antibacterial Studies of Ni(II) and Cd(II) Pyrazole Amide Coordination Complexes. Molecules. 2024; 29(5):1186. https://doi.org/10.3390/molecules29051186
Chicago/Turabian StyleEl Mahdaoui, Amal, Smaail Radi, Youssef Draoui, Mohamed El Massaoudi, Sabir Ouahhoud, Abdeslam Asehraou, Nour Eddine Bentouhami, Ennouamane Saalaoui, Redouane Benabbes, Koen Robeyns, and et al. 2024. "Synthesis, Crystal Structures, Genotoxicity, and Antifungal and Antibacterial Studies of Ni(II) and Cd(II) Pyrazole Amide Coordination Complexes" Molecules 29, no. 5: 1186. https://doi.org/10.3390/molecules29051186
APA StyleEl Mahdaoui, A., Radi, S., Draoui, Y., El Massaoudi, M., Ouahhoud, S., Asehraou, A., Bentouhami, N. E., Saalaoui, E., Benabbes, R., Robeyns, K., & Garcia, Y. (2024). Synthesis, Crystal Structures, Genotoxicity, and Antifungal and Antibacterial Studies of Ni(II) and Cd(II) Pyrazole Amide Coordination Complexes. Molecules, 29(5), 1186. https://doi.org/10.3390/molecules29051186









