Fabrication of Eco-Friendly Hydrolyzed Ethylene–Maleic Anhydride Copolymer–Avermectin Nanoemulsion with High Stability, Adhesion Property, pH, and Temperature-Responsive Releasing Behaviors
Abstract
1. Introduction
2. Results and Discussion
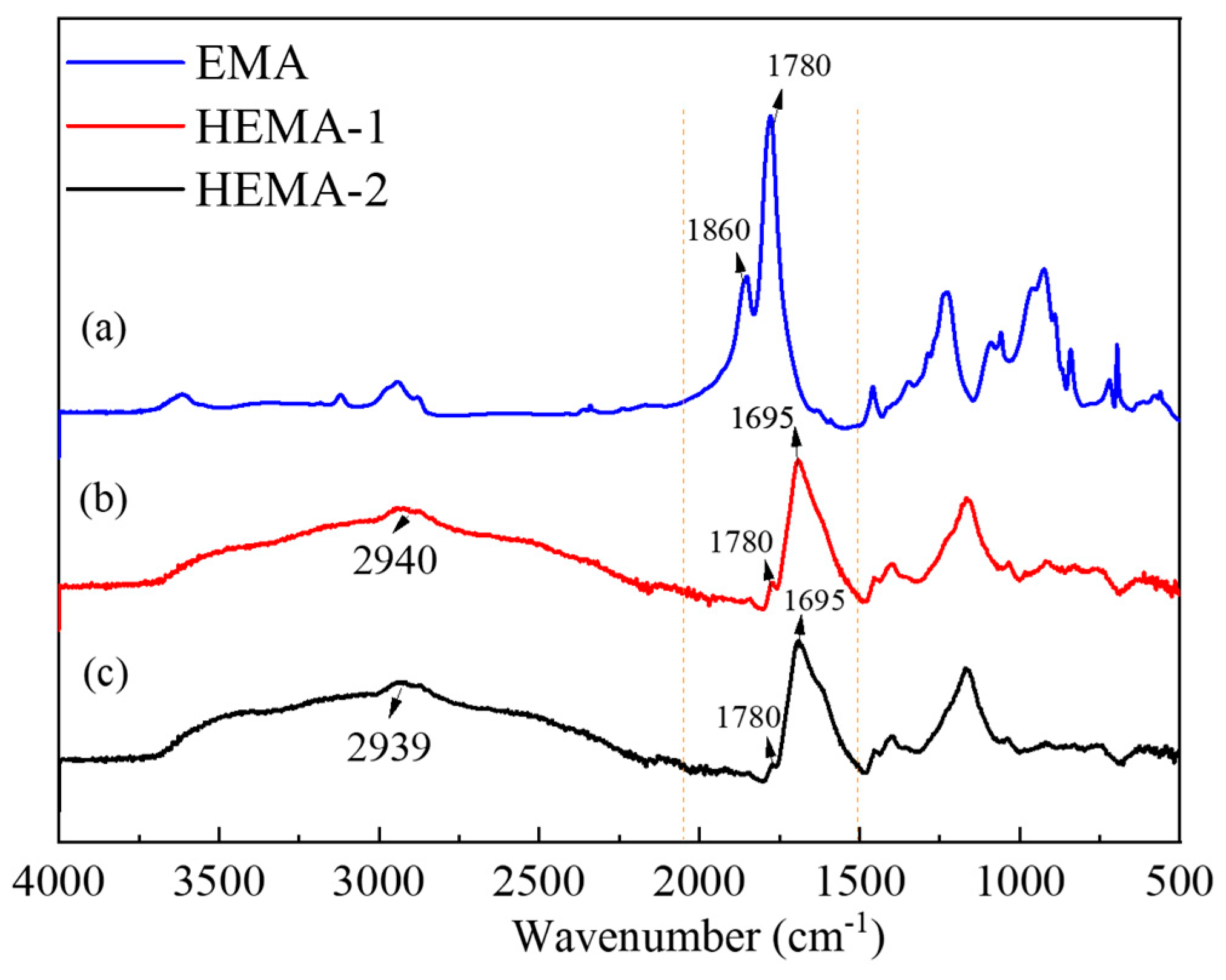
2.1. Preparation of Emulsifier and Fabrication of Avm-Nanoemulsion
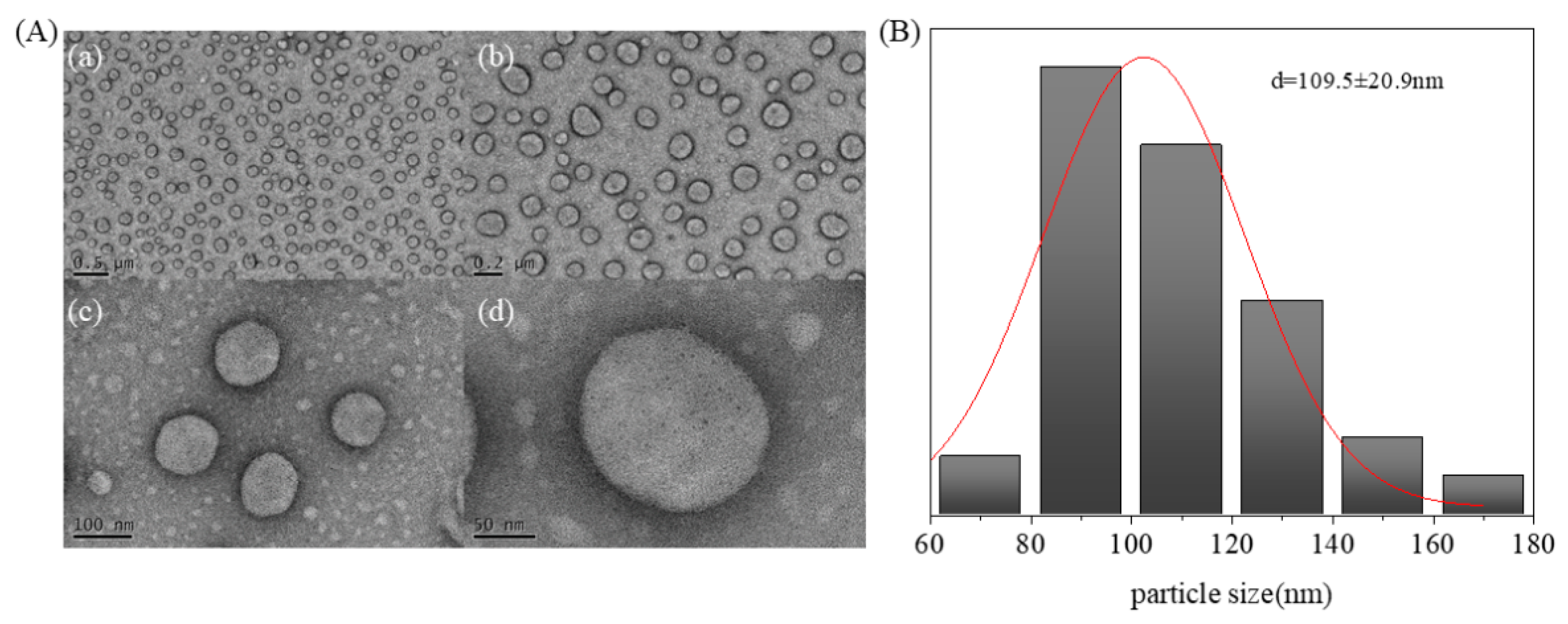
2.2. Morphology of Avm@HEMA
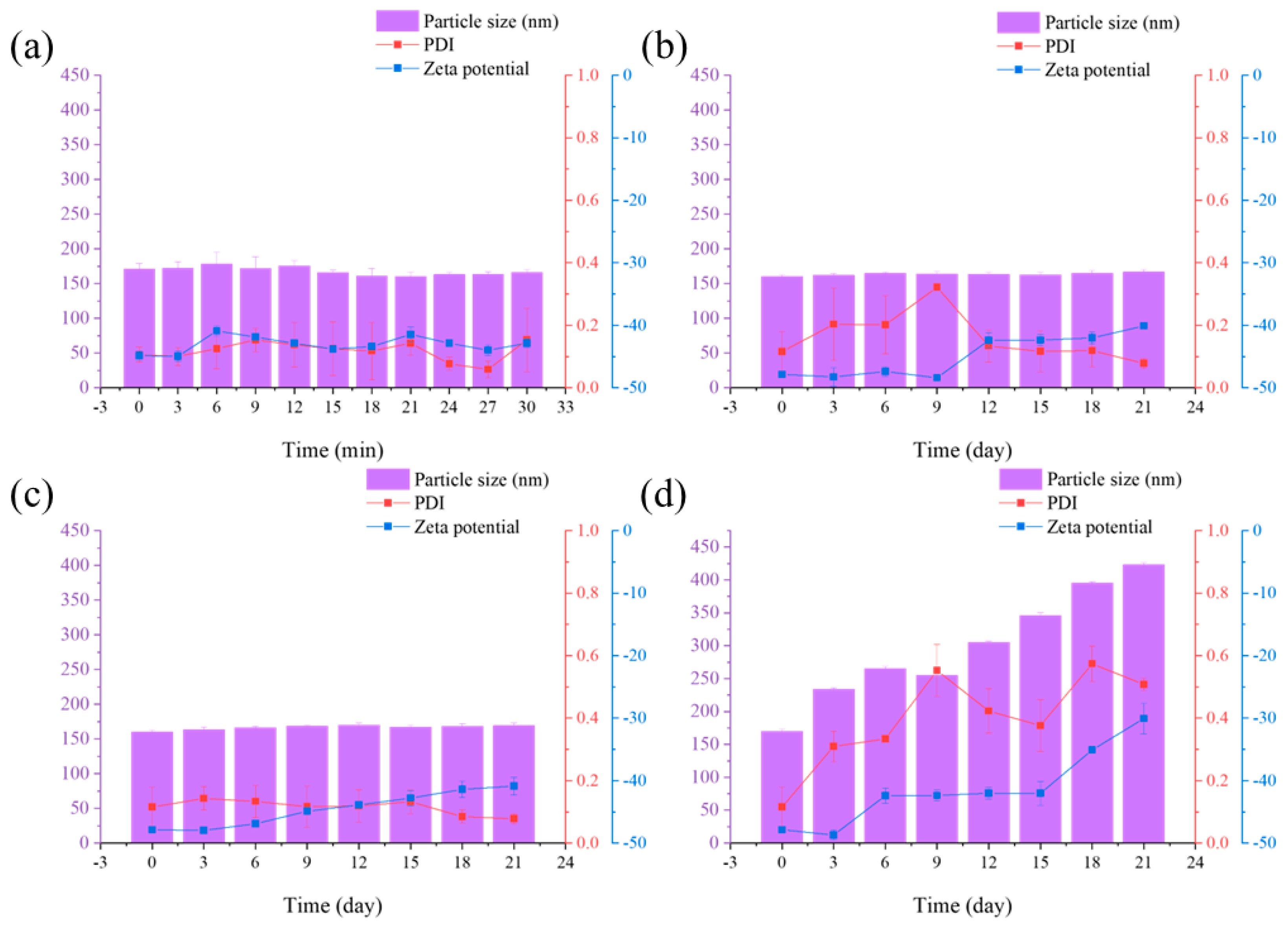
2.3. Stability of Avm@HEMA
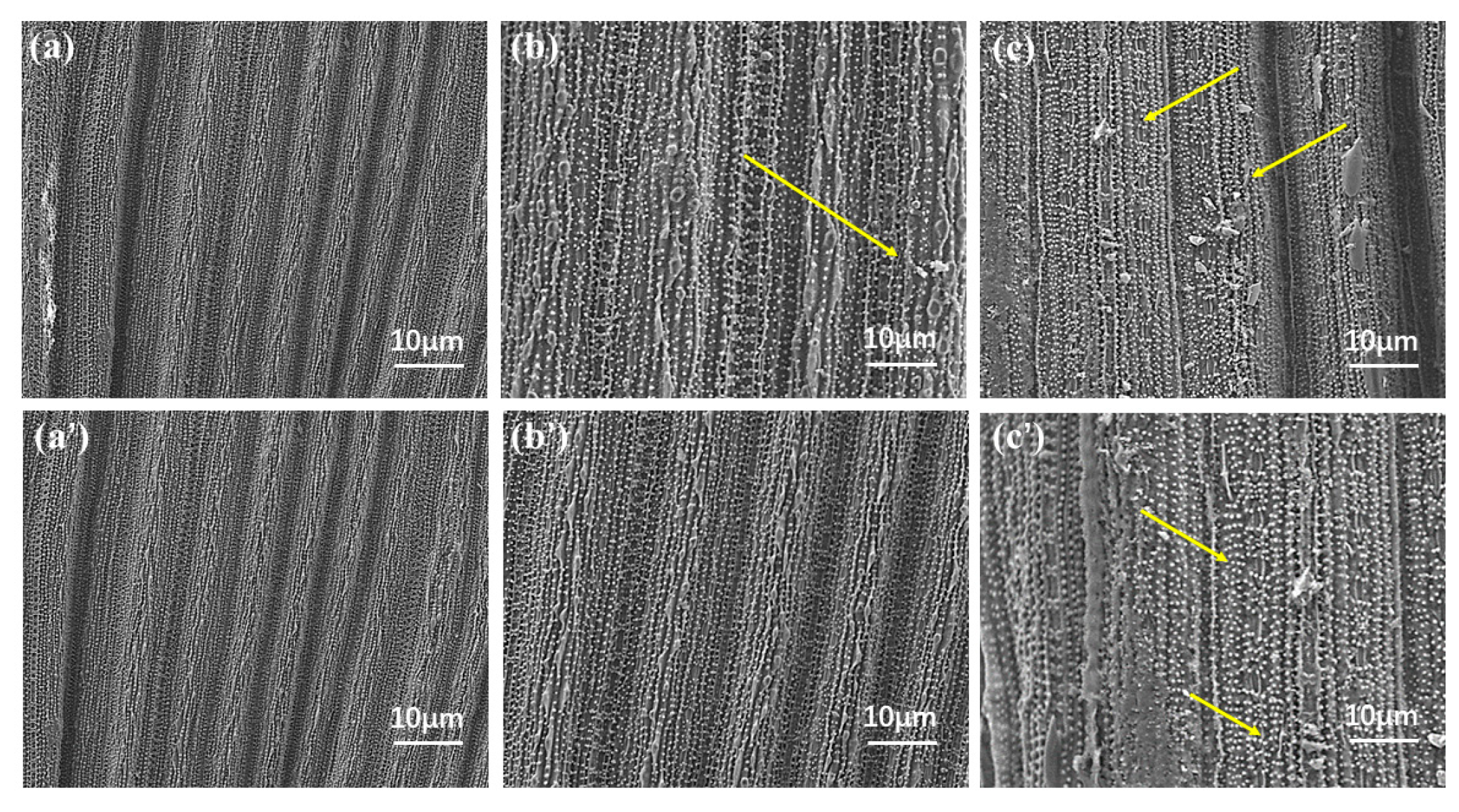
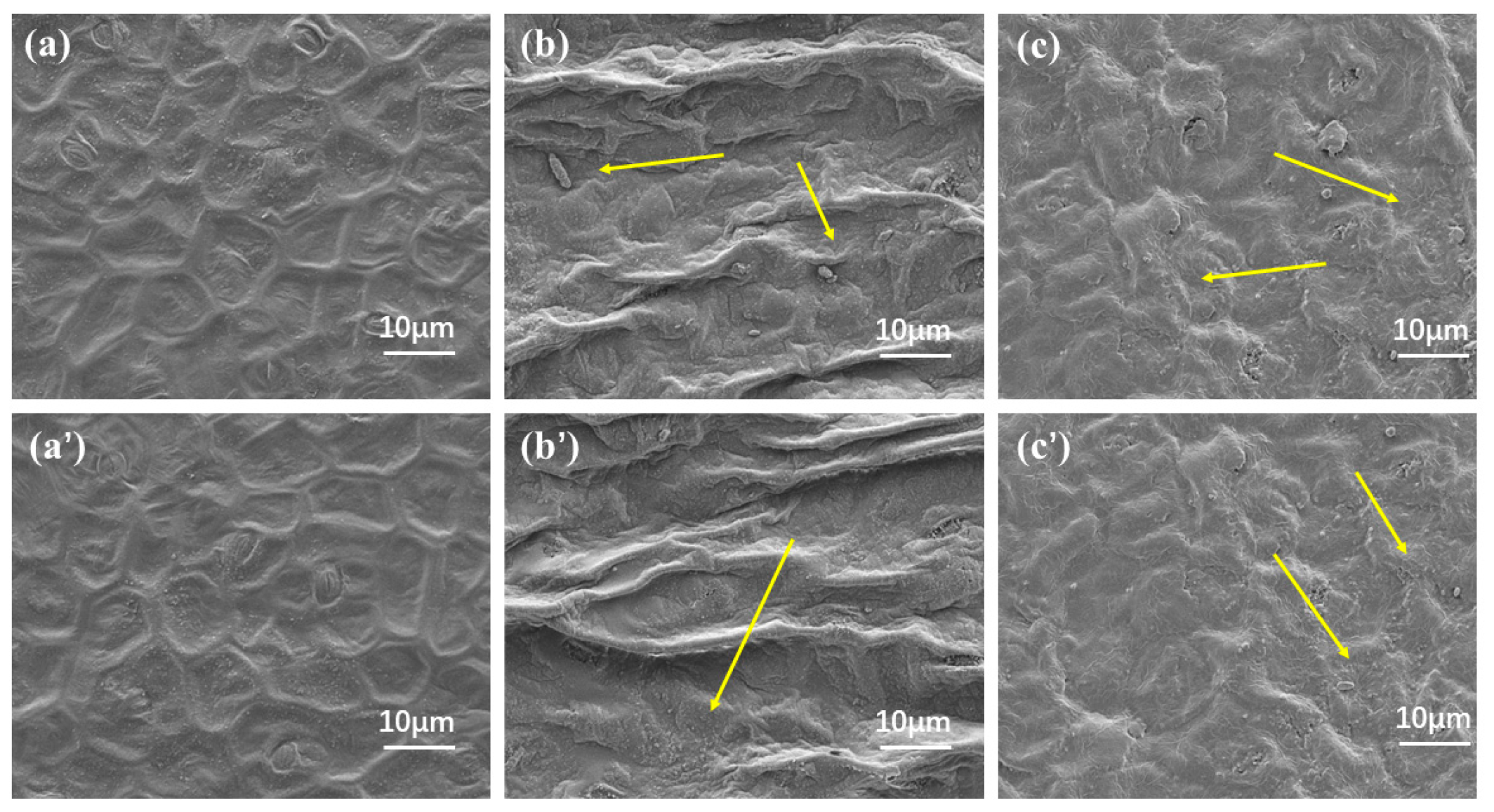
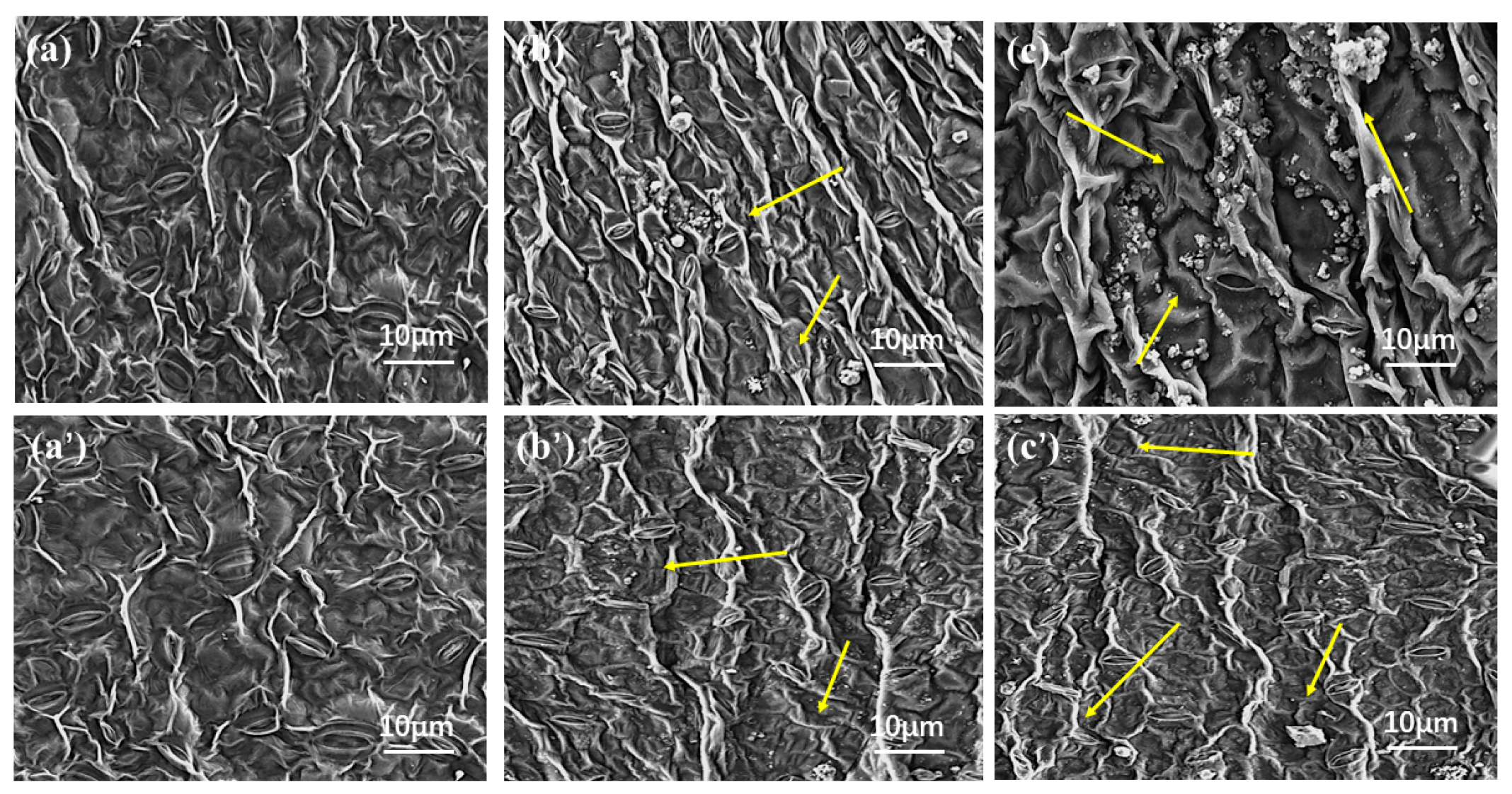
2.4. Wetting and Adhesion Behavior of Avm@HEMA on Leaves
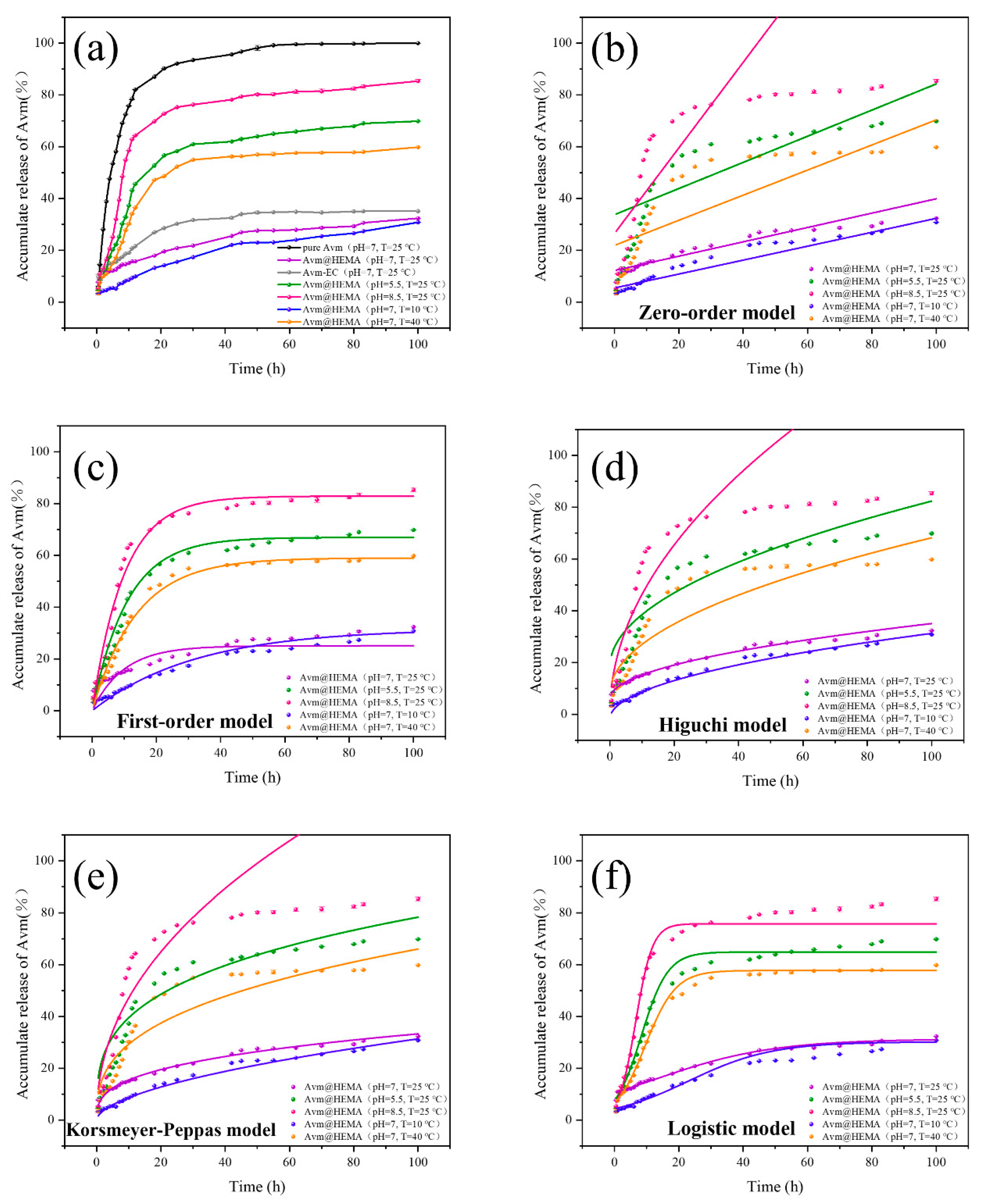
2.5. Releasing Behavior of Avm@HEMA
3. Materials and Methods
3.1. Materials
3.2. Hydrophilic Modification of EMA
3.3. Fabrication of Avm-Nanoemulsion
3.4. Characterization of EMA and the Modified EMA
3.5. Characterization of Avm-Nanoemulsion
3.5.1. Particle Size and Particle Size Distribution
3.5.2. Morphology
3.5.3. Solid Content, Drug Loading, and Encapsulation Efficiency
3.5.4. Stability
- (1)
- Centrifugal stability. The Avm-nanoemulsions were separated and transferred to identical centrifuge tubes. Z-average particle size, PDI, and Zeta potential in the supernatant were measured using a dynamic light scattering (DLS) instrument (3000HS Malvern Zetasizer) after centrifugation at 10,000 rpm for 3, 6, 9, 12, 15, 18, 21, 24, 27, and 30 min.
- (2)
- (3)
- Dilution stability. Avm-nanoemulsions were diluted 20 times (World Health Organization standard), 200 times (GB/T 1603-2001 [66]), and 2000 times (Beijing Green Agricultural Science and Technology Group; active ingredient content: 1.8%, diluting 2000-fold) using deionized water and the Z-average particle size, PDI, and Zeta potential were measured.
- (4)
- Storage stability. The Avm-nanoemulsion was placed in T = 25 °C (T represents temperature) for one month and then the Z-average particle size, PDI, and Zeta potential were tested.
- (5)
- Photostability. Pure Avm and Avm-nanoemulsion containing 10 mg Avm was weighed and mixed with ethanol into a 100 mL glass bottle. After vacuum drying, they were exposed to a 400 W UV lamp (Emax = 365 nm) at a distance of 20 cm for 20 min. Dried samples were removed and dissolved in ethanol at 2 min intervals and analyzed by UV–Vis spectrophotometer (UV–Vis) to determine the concentration of undecomposed Avm. The concentration of Avm was measured using a UV-Vis Spectrometer (UV-3200, Mapada Instruments) and calculated by the standard curve (A = 0.03323 C + 0.09497, R2 = 0.99) at .
3.5.5. Surface Tension, Contact Angle, Retention, and Leaf Adhesion
- (1)
- Surface tension. The pendant drop method was used to measure the surface tension of Avm-nanoemulsion by a series of aqueous solutions of emulsifier with a concentration gradient (0.001–100 g/L), and the surface tension curves were measured by a surface tension meter (DCAT21, dataphysics, Filderstadt, Germany).
- (2)
- Contact angle. Fresh plant leaves were collected and fixed on clean slides to avoid damaging the leaf structure and keep the leaves in their natural state. Using water as a blank control, 10 μL of Avm-EC and Avm-nanoemulsion (field use concentration 0.02%) was applied to the rice, cabbage, and cucumber leaves via microinjector drops with a video optical contact angle measuring instrument (OCA 25). After 30 s of dropping, the droplets were photographed on the leaves with the CCD camera on the contact angle meter, and the contact angle of the droplets on the experimental leaves was calculated by a five-point fit analysis. Given the data reliability and complexity of leaf surfaces, measurements were repeated at least five times, and the average values were reported.
- (3)
- Leaf retention and leaching loss. The leaves were immersed in deionized water for 20 min after an ultrasonic cleaning instrument was applied to separate the attached dust, washed three times, and dried. Using water as a blank control, retention and adhesion of Avm-EC and Avm-nanoemulsion (field use concentration 0.02%) on plant leaf surfaces were measured by the Wilhelmy [67] methodl leaf area was measured with a handheld leaf area meter (S, cm2). The liquid sample and slender-tipped forceps were placed in a beaker and weighed on a balance, which was then zeroed. A small piece of leaf was fully immersed into the test solution for 30 s and then removed vertically and hang on to the liquid until no droplets drip off. The leaf was then set aside, the forceps were placed back into the beaker and the balance reading W1 (g) was recorded. The leaf retention was calculated as R1 = W1/S (mg/cm2). Then, to test the leaching loss, after evaporation of the solvent, the leaves were washed with a continuous stream of water for 0.5 h and again dried naturally for 20 min, at which point the leaves were weighed and their mass recorded as W2. The leaf retention was calculated as R2 = W2/S (mg/cm2). Finally, based on the leaf retention before and after leaching, the leaching loss was calculated by 100 × (R1 − R2)/R1 (%). On the other hand, the leaf images were recorded with a field emission scanning electron microscope (FESEM, JSM-7900F, JEOL, Tokyo, Japan) at an accelerating voltage of 5 KV to observe the leaf surface before and after leaching.
3.5.6. Releasing Behavior
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yusoff, S.N.M.; Kamari, A.; Aljafree, N.F.A. A Review of Materials Used as Carrier Agents in Pesticide Formulations. Int. J. Environ. Sci. Technol. 2016, 13, 2977–2994. [Google Scholar] [CrossRef]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and Technology of Nanoemulsion-Based Pesticide Formulation. Nanomaterials 2020, 10, 1608. [Google Scholar] [CrossRef]
- Morris, J.; Willis, J.; De Martinis, D.; Hansen, B.; Laursen, H.; Sintes, J.R.; Kearns, P.; Gonzalez, M. Science Policy Considerations for Responsible Nanotechnology Decisions. Nat. Nanotechnol. 2011, 6, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Maestro, A.; Solè, I.; González, C.; Solans, C.; Gutiérrez, J.M. Influence of the Phase Behavior on the Properties of Ionic Nanoemulsions Prepared by the Phase Inversion Composition Method. J. Colloid Interface Sci. 2008, 327, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Investigation of Factors Influencing Formation of Nanoemulsion by Spontaneous Emulsification: Impact on Droplet Size, Polydispersity Index, and Stability. Bioengineering 2022, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.T.; Alexandre, B.; Cacciatore, F.A.; Magedans, Y.V.d.S.; Fett-Neto, A.G.; Contri, R.V.; Malheiros, P.d.S. Carvacrol-Loaded Nanoemulsions Produced with a Natural Emulsifier for Lettuce Sanitization. Food Res. Int. 2023, 168, 112748. [Google Scholar] [CrossRef]
- Yan, H.; Bao, C.; Chen, X.; Yu, C.; Kong, D.; Shi, J.; Lin, Q. Preparation of Biodiesel Oil-in-Water Nanoemulsions by Mixed Surfactants for Bifenthrin Formulation. RSC Adv. 2019, 9, 11649–11658. [Google Scholar] [CrossRef]
- Dong, X.; Shao, H.; Chang, J.; Qin, S. Tailoring the Dual Role of Styrene-Maleic Anhydride Copolymer in the Fabrication of Polysulfone Ultrafiltration Membranes: Acting as a Pore Former and Amphiphilic Surface Modifier. Sep. Purif. Technol. 2022, 283, 120219. [Google Scholar] [CrossRef]
- Hou, S.-S.; Kuo, P.-L. Synthesis and Characterization of Amphiphilic Graft Copolymers Based on Poly(Styrene-Co-Maleic Anhydride) with Oligo(Oxyethylene) Side Chains and Their GPC Behavior. Polymer 2001, 42, 2387–2394. [Google Scholar] [CrossRef]
- Qiang, X.; Ma, L.; Yan, Z.; Zhang, H. Preparation of Comb-Like Amphiphilic Styrene Maleic Anhydride Copolymer Derivatives and Their Modification to Surface of Chrome-Tanned Collagen Fiber. J. Surfact. Deterg. 2013, 16, 321–326. [Google Scholar] [CrossRef]
- Xue, T.; Yu, C.; Zhou, Z.; Zhang, F. Imidization of Styrene–Maleic Anhydride Copolymer for Dispersing Nano-SiO2 in Water. J. Coat. Technol. Res. 2023, 89, 320–323. [Google Scholar] [CrossRef]
- Duan, X.; Xiao, J.; Yin, Q.; Zhang, Z.; Mao, S.; Li, Y. Amphiphilic Graft Copolymer Based on Poly(Styrene-Co-Maleic Anhydride) with Low Molecular Weight Polyethylenimine for Efficient Gene Delivery. Int. J. Nanomed. 2012, 7, 4961–4972. [Google Scholar]
- Ruland, A.; Schenker, S.; Schirmer, L.; Friedrichs, J.; Meinhardt, A.; Schwartz, V.B.; Kaiser, N.; Konradi, R.; MacDonald, W.; Helmecke, T.; et al. Amphiphilic Copolymers for Versatile, Facile, and In Situ Tunable Surface Biofunctionalization. Adv. Mater. 2021, 33, 2102489. [Google Scholar] [CrossRef]
- Yi, C.; Liu, N.; Zheng, J.; Jiang, J.; Liu, X. Dual-Responsive Poly(Styrene-Alt-Maleic Acid)-Graft-Poly(N-Isopropyl Acrylamide) Micelles as Switchable Emulsifiers. J. Colloid Interface Sci. 2012, 380, 90–98. [Google Scholar] [CrossRef]
- Larson, N.; Greish, K.; Bauer, H.; Maeda, H.; Ghandehari, H. Synthesis and Evaluation of Poly(Styrene-Co-Maleic Acid) Micellar Nanocarriers for the Delivery of Tanespimycin. Int. J. Pharm. 2011, 420, 111–117. [Google Scholar] [CrossRef]
- Henry, S.M.; El-Sayed, M.E.H.; Pirie, C.M.; Hoffman, A.S.; Stayton, P.S. pH-Responsive Poly(Styrene-Alt-Maleic Anhydride) Alkylamide Copolymers for Intracellular Drug Delivery. Biomacromolecules 2006, 7, 2407–2414. [Google Scholar] [CrossRef]
- Sun, T.T.; Zhang, S.Q. Effect of Styrene-Maleic Anhydride Copolymer on the Dispersibility of 20% Diflubenzuron Suspension Concentrate. Chin. J. Appl. Chem. 2012, 29, 1046–1052. [Google Scholar] [CrossRef]
- Komber, H. The 1H and 13C NMR Spectra of an Alternating Ethene/Maleic Anhydride Copolymer and the Corresponding Acid and Sodium Salt. Macromol. Chem. Phys. 1995, 196, 669–678. [Google Scholar] [CrossRef]
- Feltz, E.T.; Regelson, W. Ethylene Maleic Anhydride Copolymers as Viral Inhibitors. Nature 1962, 196, 642–645. [Google Scholar] [CrossRef]
- Centeno, E.R.; Sehon, A.H. The Use of Ethylene Maleic Anhydride for the Preparation of Versatile Immunosorbents. Immunochemistry 1971, 8, 887–900. [Google Scholar] [CrossRef]
- Goldstein, L. Immobilized Enzymes: The Coupling of Biologically Active Proteins to Ethylene-Maleic Anhydride Copolymers of Different Anhydride Content. Anal. Biochem. 1972, 50, 40–46. [Google Scholar] [CrossRef]
- Chen, G.C. Synthesis of Ethylene Maleic Anhydride Copolymer Containing Fungicides and Evaluation of Their Effect for Wood Decay Resistance. Holzforschung 2008, 62, 488–493. [Google Scholar] [CrossRef]
- Liang, X.-R.; Chen, X.-Y.; Chen, F.-Y.; Su, W.-K. Solubility of Avermectin B 1a in Some Pure and Mixed Solvents from (278.2 to 318.2) K. J. Chem. Eng. Data 2010, 55, 2340–2342. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alqahtani, A.; Ilesanmi, O.B.; Saati, A.A.; El-Mleeh, A.; Hetta, H.F.; Magdy Beshbishy, A. Avermectin Derivatives, Pharmacokinetics, Therapeutic and Toxic Dosages, Mechanism of Action, and Their Biological Effects. Pharmaceuticals 2020, 13, 196. [Google Scholar] [CrossRef]
- Liang, W.; Yu, A.; Wang, G.; Zheng, F.; Jia, J.; Xu, H. Chitosan-Based Nanoparticles of Avermectin to Control Pine Wood Nematodes. Int. J. Biol. Macromol. 2018, 112, 258–263. [Google Scholar] [CrossRef]
- Lin, G.; Zhou, H.; Lian, J.; Chen, H.; Xu, H.; Zhou, X. Preparation of pH-Responsive Avermectin/Feather Keratin-Hyaluronic Acid with Anti-UV and Sustained-Release Properties. Colloids Surf. B Biointerfaces 2019, 175, 291–299. [Google Scholar] [CrossRef]
- Guan, W.; Tang, L.; Wang, Y.; Cui, H. Fabrication of an Effective Avermectin Nanoemulsion Using a Cleavable Succinic Ester Emulsifier. J. Agric. Food Chem. 2018, 66, 7568–7576. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bai, B.; Wang, H.; Suo, Y. A Near-Infrared and Temperature-Responsive Pesticide Release Platform through Core–Shell Polydopamine@PNIPAm Nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 6424–6432. [Google Scholar] [CrossRef] [PubMed]
- Oishi, R.; Maki, K.; Mizuta, T.; Sueyoshi, K.; Endo, T.; Hisamoto, H. Enzyme-Responsive Fluorescent Nanoemulsion Based on Lipophilic Dye Liquid. Analyst 2021, 146, 4121–4124. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Zhang, W.; Tang, L.; Wang, Y.; Cui, H. Fabrication of Novel Avermectin Nanoemulsion Using a Polyurethane Emulsifier with Cleavable Disulfide Bonds. J. Agric. Food Chem. 2018, 66, 6569–6577. [Google Scholar] [CrossRef]
- Marcu, A.; Pop, S.; Dumitrache, F.; Mocanu, M.; Niculite, C.M.; Gherghiceanu, M.; Lungu, C.P.; Fleaca, C.; Ianchis, R.; Barbut, A.; et al. Magnetic Iron Oxide Nanoparticles as Drug Delivery System in Breast Cancer. Appl. Surf. Sci. 2013, 281, 60–65. [Google Scholar] [CrossRef]
- Regueiro, J.; Llompart, M.; Garcia-Jares, C.; Garcia-Monteagudo, J.C.; Cela, R. Ultrasound-Assisted Emulsification–Microextraction of Emergent Contaminants and Pesticides in Environmental Waters. J. Chromatogr. A 2008, 1190, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Anton, N.; Gayet, P.; Benoit, J.-P.; Saulnier, P. Nano-Emulsions and Nanocapsules by the PIT Method: An Investigation on the Role of the Temperature Cycling on the Emulsion Phase Inversion. Int. J. Pharm. 2007, 344, 44–52. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. The Universality of Low-Energy Nano-Emulsification. Int. J. Pharm. 2009, 377, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and Stability of Nano-Emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of Oil Type on Nanoemulsion Formation and Ostwald Ripening Stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef] [PubMed]
- Onuki, Y.; Morishita, M.; Takayama, K. Formulation Optimization of Water-in-Oil-Water Multiple Emulsion for Intestinal Insulin Delivery. J. Control. Release 2004, 97, 91–99. [Google Scholar] [CrossRef]
- Su, R.; Yang, L.; Wang, Y.; Yu, S.; Guo, Y.; Deng, J.; Zhao, Q.; Jin, X. Formulation, Development, and Optimization of a Novel Octyldodecanol-Based Nanoemulsion for Transdermal Delivery of Ceramide IIIB. Int. J. Nanomed. 2017, 12, 5203–5221. [Google Scholar] [CrossRef]
- Pongsumpun, P.; Iwamoto, S.; Siripatrawan, U. Response Surface Methodology for Optimization of Cinnamon Essential Oil Nanoemulsion with Improved Stability and Antifungal Activity. Ultrason. Sonochem. 2020, 60, 104604. [Google Scholar] [CrossRef]
- Homayoonfal, M.; Khodaiyan, F.; Mousavi, M. Modelling and Optimising of Physicochemical Features of Walnut-Oil Beverage Emulsions by Implementation of Response Surface Methodology: Effect of Preparation Conditions on Emulsion Stability. Food Chem. 2015, 174, 649–659. [Google Scholar] [CrossRef]
- Niknam, S.M.; Escudero, I.; Benito, J.M. Formulation and Preparation of Water-In-Oil-In-Water Emulsions Loaded with a Phenolic-Rich Inner Aqueous Phase by Application of High Energy Emulsification Methods. Foods 2020, 9, 1411. [Google Scholar] [CrossRef] [PubMed]
- Jm, M.; Pa, R.-F.; Dj, B. Influence of Phase Inversion on the Formation and Stability of One-Step Multiple Emulsions. Langmuir ACS J. Surf. Colloids 2009, 25, 7954–7961. [Google Scholar]
- Drews, J.; Launay, H.; Hansen, C.M.; West, K.; Hvilsted, S.; Kingshott, P.; Almdal, K. Hydrolysis and Stability of Thin Pulsed Plasma Polymerised Maleic Anhydride Coatings. Appl. Surf. Sci. 2008, 254, 4720–4725. [Google Scholar] [CrossRef]
- Leal, M.; Leiva, Á.; Villalobos, V.; Palma, V.; Carrillo, D.; Edwards, N.; Maine, A.; Cauich-Rodriguez, J.V.; Tamayo, L.; Neira-Carrillo, A.; et al. Blends Based on Amino Acid Functionalized Poly (Ethylene-Alt-Maleic Anhydride) Polyelectrolytes and PEO for Nanofiber Elaboration: Biocompatible and Angiogenic Polyelectrolytes. Eur. Polym. J. 2022, 173, 111269. [Google Scholar] [CrossRef]
- Bali, A.P.; Sahu, I.D.; Craig, A.F.; Clark, E.E.; Burridge, K.M.; Dolan, M.T.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Structural Characterization of Styrene-Maleic Acid Copolymer-Lipid Nanoparticles (SMALPs) Using EPR Spectroscopy. Chem. Phys. Lipids 2019, 220, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Salanci, E.; Malík, I.; Šandrik, R.; Pecher, D.; Andriamainty, F. Determination of the Critical Micelle Concentration and Thermodynamic Parameters of Phenylcarbamic Acid Derivatives Using a Fluorescence Method. Chem. Pap. 2021, 75, 3081–3090. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the Determination of the Critical Micelle Concentration by the Pyrene 1:3 Ratio Method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Feng, J.; Sun, L.; Zhai, T.; Liang, Q.; Jiang, T.; Chen, Z. Preparation of Cinnamaldehyde Nanoemulsions: Formula Optimization, Antifungal Activity, Leaf Adhesion, and Safety Assessment. Ind. Crops Prod. 2023, 200, 116825. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Bertoncini, P.; Cathala, B.; Capron, I. Cellulosic Nanorods of Various Aspect Ratios for Oil in Water Pickering Emulsions. Soft Matter 2012, 9, 952–959. [Google Scholar] [CrossRef]
- Bai, L.; McClements, D.J. Development of Microfluidization Methods for Efficient Production of Concentrated Nanoemulsions: Comparison of Single- and Dual-Channel Microfluidizers. J. Colloid Interface Sci. 2016, 466, 206–212. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Tang, C.-H.; Liu, T.-X.; Liu, R. Ovalbumin as an Outstanding Pickering Nanostabilizer for High Internal Phase Emulsions. J. Agric. Food Chem. 2018, 66, 8795–8804. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Sheng, J.J. Properties, Preparation, Stability of Nanoemulsions, Their Improving Oil Recovery Mechanisms, and Challenges for Oil Field Applications—A Critical Review. Geoenergy Sci. Eng. 2023, 221, 211360. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, S.; Yu, B.; Gao, C.; Wang, J. Hydrophobically Modified Inulin Based Nanoemulsions for Enhanced Stability and Transdermal Delivery of Retinyl Propionate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129883. [Google Scholar] [CrossRef]
- Tan, X.-Y.; Liu, L.; Wu, D.-Y. Relationship between Leaf Dust Retention Capacity and Leaf Microstructure of Six Common Tree Species for Campus Greening. Int. J. Phytoremediation 2022, 24, 1213–1221. [Google Scholar] [CrossRef]
- Wen, H.; Zhou, H.; Hao, L.; Chen, H.; Xu, H.; Zhou, X. Enzyme Cum pH Dual-Responsive Controlled Release of Avermectin from Functional Polydopamine Microcapsules. Colloid Surf. B 2020, 186, 110699. [Google Scholar] [CrossRef]
- Cheng, Y.; Guan, W.; Tang, L.; Huang, Y.; Yang, W. Optimization of Multifunctional Polymer Emulsifiers Properties for Lambda-Cyhalothrin Nanoemulsion Fabrication. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133319. [Google Scholar] [CrossRef]
- Costa, F.O.; Sousa, J.J.S.; Pais, A.A.C.C.; Formosinho, S.J. Comparison of Dissolution Profiles of Ibuprofen Pellets. J. Control. Release 2003, 89, 199–212. [Google Scholar] [CrossRef]
- Tahara, K.; Yamamoto, K.; Nishihata, T. Application of Model-Independent and Model Analysis for the Investigation of Effect of Drug Solubility on Its Release Rate from Hydroxypropyl Methylcellulose Sustained Release Tablets. Int. J. Pharm. 1996, 133, 17–27. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Chang, T.; Qi, H.; Liang, C.; Huang, Y.; Yang, W. A Facile Approach for the Preparation of Poly(Benzothiophene-Alt-Maleic Anhydride) Microspheres by Self-Stabilized Precipitation Polymerization. Polym. Chem. 2022, 13, 4054–4063. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, X.; Zhang, L. Hole Structure and Its Formation in Thin Films of Hydrolyzed Poly(Styrene Maleic Anhydride) Alternating Copolymers. J. Appl. Polym. Sci. 2000, 75, 267–274. [Google Scholar] [CrossRef]
- Kopf, A.H.; Koorengevel, M.C.; van Walree, C.A.; Dafforn, T.R.; Killian, J.A. A Simple and Convenient Method for the Hydrolysis of Styrene-Maleic Anhydride Copolymers to Styrene-Maleic Acid Copolymers. Chem. Phys. Lipids 2019, 218, 85–90. [Google Scholar] [CrossRef] [PubMed]
- GB/T 19137-2003; Testing Method for the Storage Stability at Low Temperature of Pesticides. National Standardization Administration: Beijing, China, 2003.
- NY/T 1427-2016; Guidelines for Storage Stability Testing of Pesticides at Ambient Temperature. Ministry of Agriculture: Beijing, China, 2016.
- GB/T 19136-2003; Testing Method of the Accelerated Storage Stability at Elevated Temperature for Pesticides. National Standardization Administration: Beijing, China, 2003.
- GB/T 1603-2001; Determination Method of Emulsion Stability for Pesticide. National Standardization Administration: Beijing, China, 2001.
- Zhao, K.; Hu, J.; Ma, Y.; Wu, T.; Gao, Y.; Du, F. Topology-Regulated Pesticide Retention on Plant Leaves through Concave Janus Carriers. ACS Sustain. Chem. Eng. 2019, 7, 13148–13156. [Google Scholar] [CrossRef]
- Ren, G.; Liu, D.; Guo, W.; Wang, M.; Wu, C.; Guo, M.; Ai, X.; Wang, Y.; He, Z. Docetaxel Prodrug Liposomes for Tumor Therapy: Characterization, in Vitro and in Vivo Evaluation. Drug Deliv. 2016, 23, 1272–1281. [Google Scholar] [CrossRef]




| Sample | Mn (g/mol) | Mw (g/mol) | PDI | C (%) | H (%) | O (%) |
|---|---|---|---|---|---|---|
| EMA | 5978 | 10,913 | 1.83 | 54.81 ± 0.66 | 4.15 ± 0.23 | 40.63 ± 0.22 |
| HEMA-1 | 8623 | 17,619 | 2.04 | 44.97 ± 0.56 | 5.95 ± 0.20 | 45.26 ± 0.42 |
| HEMA-2 | 7680 | 14,502 | 1.89 | 44.52 ± 0.44 | 5.67 ± 0.10 | 45.14 ± 0.37 |
| Emulsifier | Z-Average Particle Size/nm | PDI | Zeta Potential/mV |
|---|---|---|---|
| HEMA-1 | 563.1 ± 22.5 | 0.109 ± 0.102 | −32.0 ± 1.0 |
| HEMA-3 | 231.0 ± 3.4 | 0.069 ± 0.053 | −50.4 ± 1.5 |
| Releasing Model | Releasing Condition | Parameter | |||
|---|---|---|---|---|---|
| k1 | k2 | k3 | R2 | ||
| Zero-order y = k1t + k2 | pH = 7.0, T = 25 °C | 0.003 | 0.122 | 0.903 | |
| pH = 8.5, T = 25 °C | 0.005 | 0.337 | 0.584 | ||
| pH = 5.5, T = 25 °C | 0.017 | 0.265 | 0.637 | ||
| pH = 7.0, T = 10 °C | 0.003 | 0.055 | 0.941 | ||
| pH = 7.0, T = 40 °C | 0.005 | 0.219 | 0.718 | ||
| First-order y = k2[1 − exp(−k1t)] | pH = 7.0, T = 25 °C | 0.102 | 0.251 | 0.741 | |
| pH = 8.5, T = 25 °C | 0.092 | 0.669 | 0.982 | ||
| pH = 5.5, T = 25 °C | 0.101 | 0.828 | 0.936 | ||
| pH = 7.0, T = 10 °C | 0.031 | 0.317 | 0.986 | ||
| pH = 7.0, T = 40 °C | 0.073 | 0.590 | 0.992 | ||
| Higuchi y = k1+ k2 | pH = 7.0, T = 25 °C | 0.029 | 0.003 | 0.985 | |
| pH = 8.5, T = 25 °C | 0.064 | 0.032 | 0.770 | ||
| pH = 5.5, T = 25 °C | 0.146 | 0.050 | 0.796 | ||
| pH = 7.0, T = 10 °C | 0.033 | 0.002 | 0.992 | ||
| pH = 7.0, T = 40 °C | 0.060 | 0.029 | 0.881 | ||
| Korsmeyer-Peppas y = k2 | pH = 7.0, T = 25 °C | 0.333 | 0.072 | 0.995 | |
| pH = 8.5, T = 25 °C | 0.297 | 0.200 | 0.855 | ||
| pH = 5.5, T = 25 °C | 0.460 | 0.164 | 0.803 | ||
| pH = 7.0, T = 10 °C | 0.558 | 0.024 | 0.994 | ||
| pH = 7.0, T = 40 °C | 0.353 | 0.130 | 0.917 | ||
| Logistic | pH = 7.0, T = 25 °C | 12.315 | 0.054 | 0.313 | 0.992 |
| pH = 8.5, T = 25 °C | 8.324 | 0.243 | 0.649 | 0.983 | |
| pH = 5.5, T = 25 °C | 6.574 | 0.382 | 0.758 | 0.995 | |
| pH = 7.0, T = 10 °C | 23.037 | 0.077 | 0.302 | 0.989 | |
| pH = 7.0, T = 40 °C | 9.594 | 0.212 | 0.577 | 0.995 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Pan, Z.; Tang, L.; Huang, Y.; Yang, W. Fabrication of Eco-Friendly Hydrolyzed Ethylene–Maleic Anhydride Copolymer–Avermectin Nanoemulsion with High Stability, Adhesion Property, pH, and Temperature-Responsive Releasing Behaviors. Molecules 2024, 29, 1148. https://doi.org/10.3390/molecules29051148
Cheng Y, Pan Z, Tang L, Huang Y, Yang W. Fabrication of Eco-Friendly Hydrolyzed Ethylene–Maleic Anhydride Copolymer–Avermectin Nanoemulsion with High Stability, Adhesion Property, pH, and Temperature-Responsive Releasing Behaviors. Molecules. 2024; 29(5):1148. https://doi.org/10.3390/molecules29051148
Chicago/Turabian StyleCheng, Yuxin, Zeyu Pan, Liming Tang, Yanbin Huang, and Wantai Yang. 2024. "Fabrication of Eco-Friendly Hydrolyzed Ethylene–Maleic Anhydride Copolymer–Avermectin Nanoemulsion with High Stability, Adhesion Property, pH, and Temperature-Responsive Releasing Behaviors" Molecules 29, no. 5: 1148. https://doi.org/10.3390/molecules29051148
APA StyleCheng, Y., Pan, Z., Tang, L., Huang, Y., & Yang, W. (2024). Fabrication of Eco-Friendly Hydrolyzed Ethylene–Maleic Anhydride Copolymer–Avermectin Nanoemulsion with High Stability, Adhesion Property, pH, and Temperature-Responsive Releasing Behaviors. Molecules, 29(5), 1148. https://doi.org/10.3390/molecules29051148






