Extraction and Purification of Nicotine from Tobacco Rhizomes by Supercritical CO2
Abstract
1. Introduction
2. Results
2.1. Factors Affecting Nicotine Yield
2.2. Design and Analysis of Orthogonal Experiments
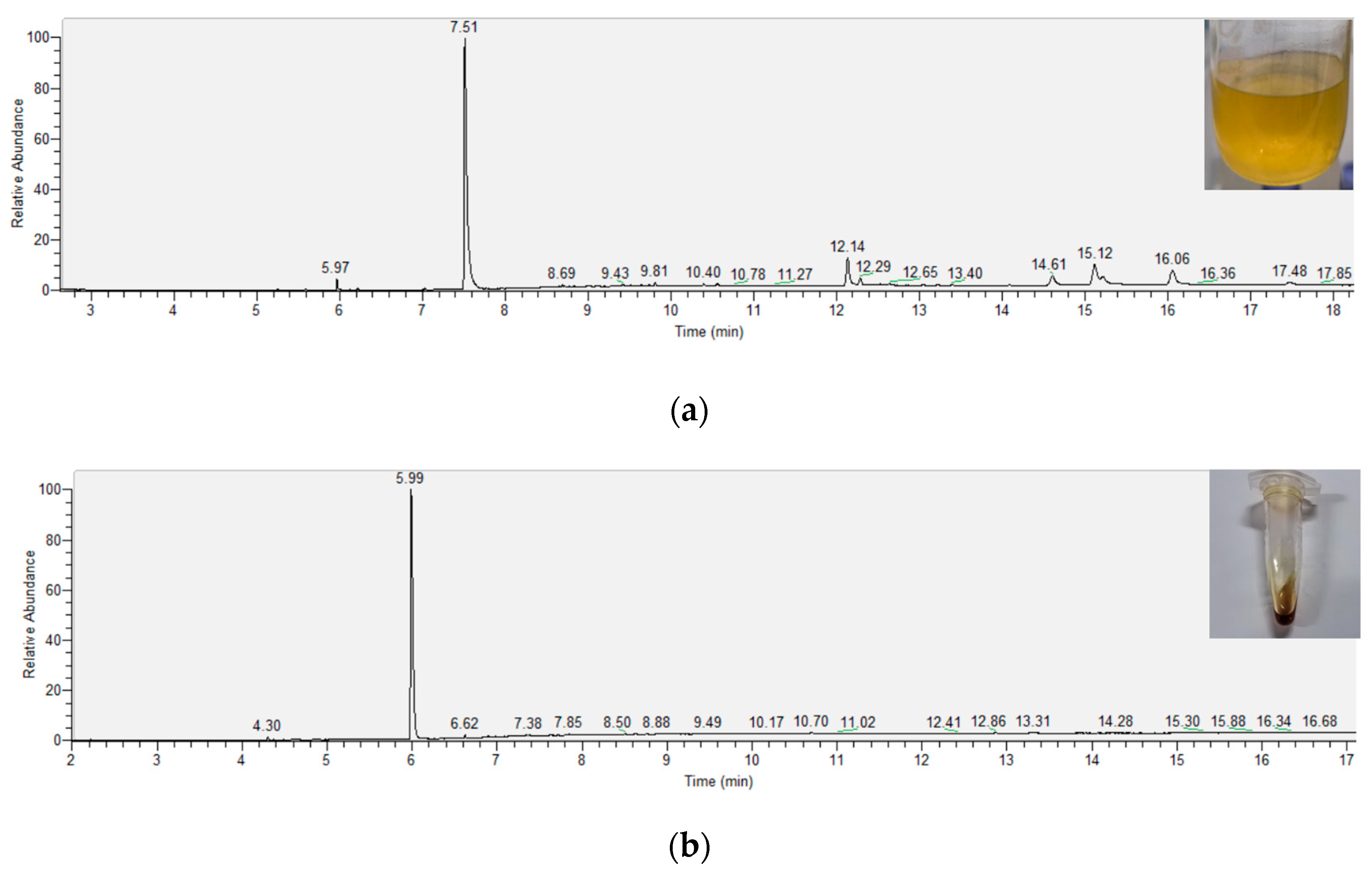
2.3. Component Analysis of Crude Extracts and Purified Products
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Tobacco Materials
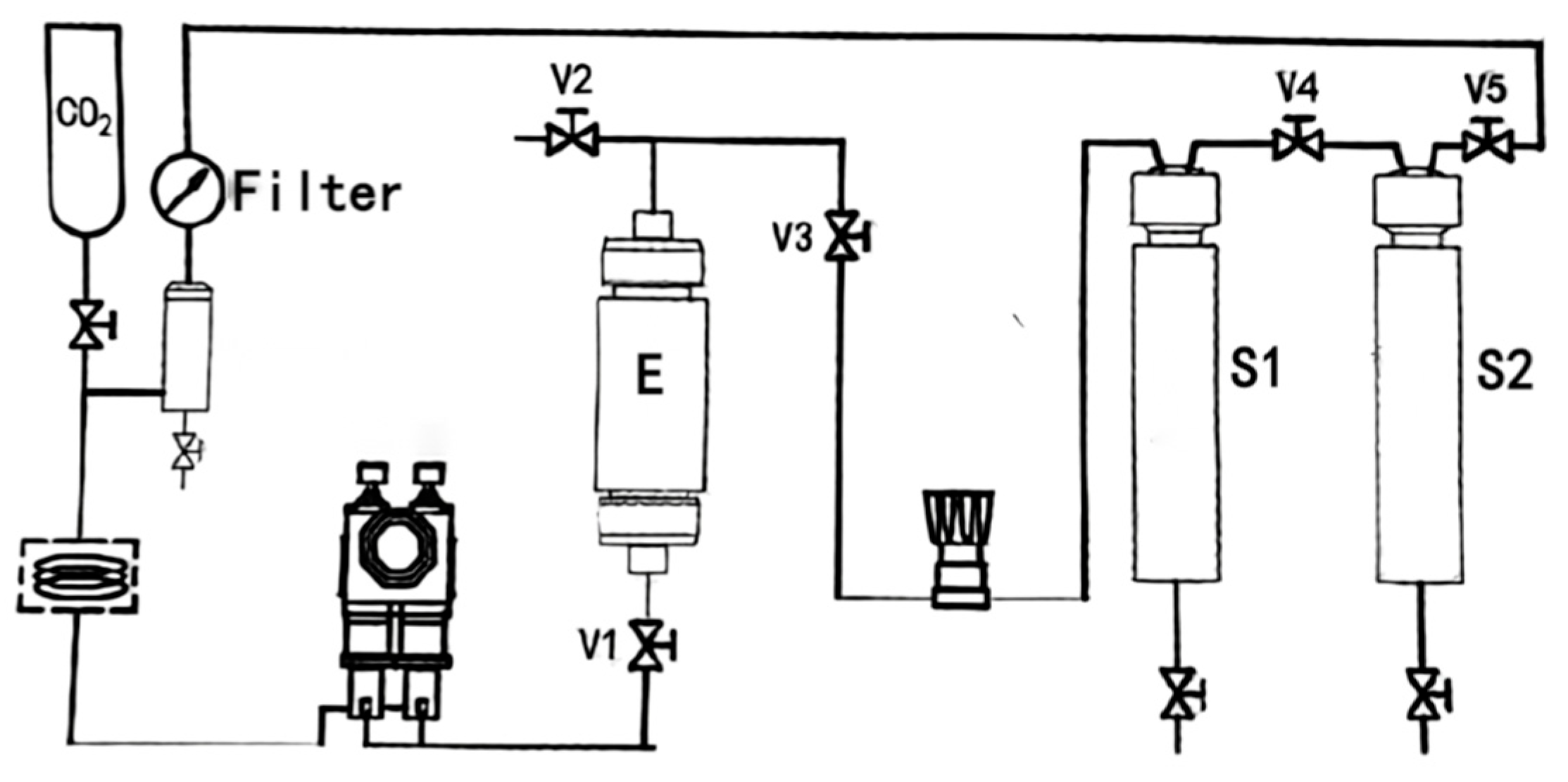
4.3. Extraction Process
4.4. GC-MS Analysis
4.5. Purification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.; Picciotto, M.R. Nicotine addiction: More than just dopamine. Curr. Opin. Neurobiol. 2023, 83, 102797. [Google Scholar] [CrossRef] [PubMed]
- Stolerman, I.P.; Jarvis, M.J. The scientific case that nicotine is addictive. Psychopharmacology 1995, 117, 2–10. [Google Scholar] [CrossRef]
- Thompson, G.H.; Hunter, D.A. Nicotine replacement therapy. Ann. Pharmacother. 1998, 32, 1067–1075. [Google Scholar] [CrossRef]
- Newhouse, P.A.; Potter, A.; Kelton, M.; Corwin, J. Nicotinic treatment of Alzheimer’s disease. Biol. Psychiatry 2001, 49, 268–278. [Google Scholar] [CrossRef]
- Lu, W.; Ferguson, S.G.; Nichols, D.; Patel, R.; Jacobson, G.A. Determination of Nicotine in Cartridge-Based Electronic Cigarettes. Anal. Lett. 2015, 48, 2715–2722. [Google Scholar] [CrossRef]
- Etter, J.F. An 8-year longitudinal study of long-term, continuous users of electronic cigarettes. Addict. Behav. 2024, 149, 107891. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, L.; Yang, H.; Wu, X.; Luo, X.; Shen, J.; Xiao, Z.; Zhao, Y.; Du, F.; Chen, Y.; et al. Dysregulation of immunity by cigarette smoking promotes inflammation and cancer: A review. Environ. Pollut. 2023, 339, 122730. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W. Study on supercritical fluid extraction of solanesol from industrial tobacco waste. J. Supercrit. Fluids 2018, 138, 228–237. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, M.; Yang, B.; Jiang, Y.; Rao, G. Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem. 2008, 107, 1399–1406. [Google Scholar] [CrossRef]
- Briski, F.; Gomzi, Z.; Horgas, N.; Vukovic, M. Aerobic composting of tobacco solid waste. Acta Chim. Slov. 2003, 50, 715–729. [Google Scholar]
- Vuković, M.; Ćosić, I.; Kolačko, K.; Briški, F. Kinetics of Organic Matter Biodegradation in Leachate from Tobacco Waste. Kem. U Ind./J. Chem. Chem. Eng. 2012, 61, 417–425. [Google Scholar]
- Tayoub, G.; Sulaiman, H.; Alorfi, M. Determination of nicotine levels in the leaves of some Nicotiana tabacum varieties cultivated in Syria. Herba Pol. 2015, 61, 23–30. [Google Scholar] [CrossRef]
- Djapic, N. Supercritical Carbon Dioxide Extraction of Nicotiana tabacum Leaves: Optimization of Extraction Yield and Nicotine Content. Molecules 2022, 27, 8328. [Google Scholar] [CrossRef]
- Rincón, J.; De Lucas, A.; García, M.A.; García, A.; Alvarez, A.; Carnicer, A. Preliminary Study on the Supercritical Carbon Dioxide Extraction of Nicotine from Tobacco Wastes. Sep. Sci. Technol. 1998, 33, 411–423. [Google Scholar] [CrossRef]
- Purwono, S.; Murachman, B.; Wintoko, J.; Simanjuntak, B.A.; Sejati, P.P.; Permatasari, N.E.; Lidyawati, D. The Effect of Solvent for Extraction for Removing Nicotine on the Development of Charcoal Briquette from Waste of Tobacco Stem. J. Sustain. Energy Environ. 2011, 2, 11–13. [Google Scholar]
- Williams, J.R.; Clifford, A.A.; Al-Saidi, S.H.R. Supercritical fluids and their applications in biotechnology and related areas. Mol. Biotechnol. 2002, 22, 263–286. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.K.; Hupé, M. Effects of moisture content in cigar tobacco on nicotine extraction similarity between Soxhlet and focused open-vessel microwave-assisted techniques. J. Chromatogr. A 2003, 1011, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.N.; Rossa, G.E.; de Castro, J.H.; Cavassa, A.F.; Vargas, R.M.F.; Cassel, E. Extraction and fractionation of long pepper essential oil: Process modeling and simulation. Braz. J. Chem. Eng. 2023, 40, 1103–1113. [Google Scholar] [CrossRef]
- Banožić, M.; Babić, J.; Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste—A review. Ind. Crops Prod. 2020, 144, 112009. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Baldino, L.; Adami, R.; Reverchon, E. Concentration of Ruta graveolens active compounds using SC-CO2 extraction coupled with fractional separation. J. Supercrit. Fluids 2018, 131, 82–86. [Google Scholar] [CrossRef]
- da Silva, R.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TRAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Todd, R.; Baroutian, S. A techno-economic comparison of subcritical water, supercritical CO2 and organic solvent extraction of bioactives from grape marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Fischer, M.; Jefferies, T.M. Optimization of Nicotine Extraction from Tobacco Using Supercritical Fluid Technology with Dynamic Extraction Modeling. J. Agric. Food Chem. 1996, 44, 1258–1264. [Google Scholar] [CrossRef]
- Patino, R.; Gutierrez, H.; Bravo, D.; Cova, A. Supercritical Extraction of Nicotine from Tobacco Using as Solvent Dioxide of Carbonin Supercritical Phase. Ing. Quim. 2009, 36, 10–13. [Google Scholar]
- Iwai, Y.; Uno, M.; Nagano, H.; Arai, Y. Measurement of solubilities of palmitic acid in supercritical carbon dioxide and entrainer effect of water by FTIR spectroscopy. J. Supercrit. Fluids 2004, 28, 193–200. [Google Scholar] [CrossRef]
- Wang, B.H.; Li, Q.S.; Zhang, Z.T.; Chang, Q.L. Preparation of benzonic-acid in supercritical carbon dioxide with alcohol entrainer. In Proceedings of the Asian International Conference on Advanced Materials, Beijing, China, 3–5 November 2005. [Google Scholar]
- Iwai, Y.; Koujina, Y.; Arai, Y.; Watanabe, I.; Mochida, I.; Sakanishi, K. Low temperature drying of low rank coal by supercritical carbon dioxide with methanol as entrainer. J. Supercrit. Fluids 2002, 23, 251–255. [Google Scholar] [CrossRef]
- Iwai, Y.; Okamoto, N.; Ohta, S.; Arai, Y.; Sakanishi, K. Extraction of iron and calcium from low rank coal by supercritical carbon dioxide with entrainers. J. Supercrit. Fluids 2007, 40, 227–231. [Google Scholar] [CrossRef]
- Hu, R.-S.; Wang, J.; Li, H.; Ni, H.; Chen, Y.-F.; Zhang, Y.-W.; Xiang, S.-P.; Li, H.-H. Simultaneous extraction of nicotine and solanesol from waste tobacco materials by the column chromatographic extraction method and their separation and purification. Sep. Purif. Technol. 2015, 146, 1–7. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, A.; Bronze, M.-R.; da Ponte, M.N. Supercritical fluid extraction of tobacco leaves: A preliminary study on the extraction of solanesol. J. Supercrit. Fluids 2008, 45, 171–176. [Google Scholar] [CrossRef]
- Nerome, H.; Ito, M.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Extraction of phytochemicals from saffron by supercritical carbon dioxide with water and methanol as entrainer. J. Supercrit. Fluids 2016, 107, 377–383. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, X.-H.; Jiang, W.-J. Theoretical models for supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Tita, G.J.; Navarrete, A.; Martín, A.; Cocero, M.J. Model assisted supercritical fluid extraction and fractionation of added-value products from tobacco scrap. J. Supercrit. Fluids 2021, 167, 105046. [Google Scholar] [CrossRef]

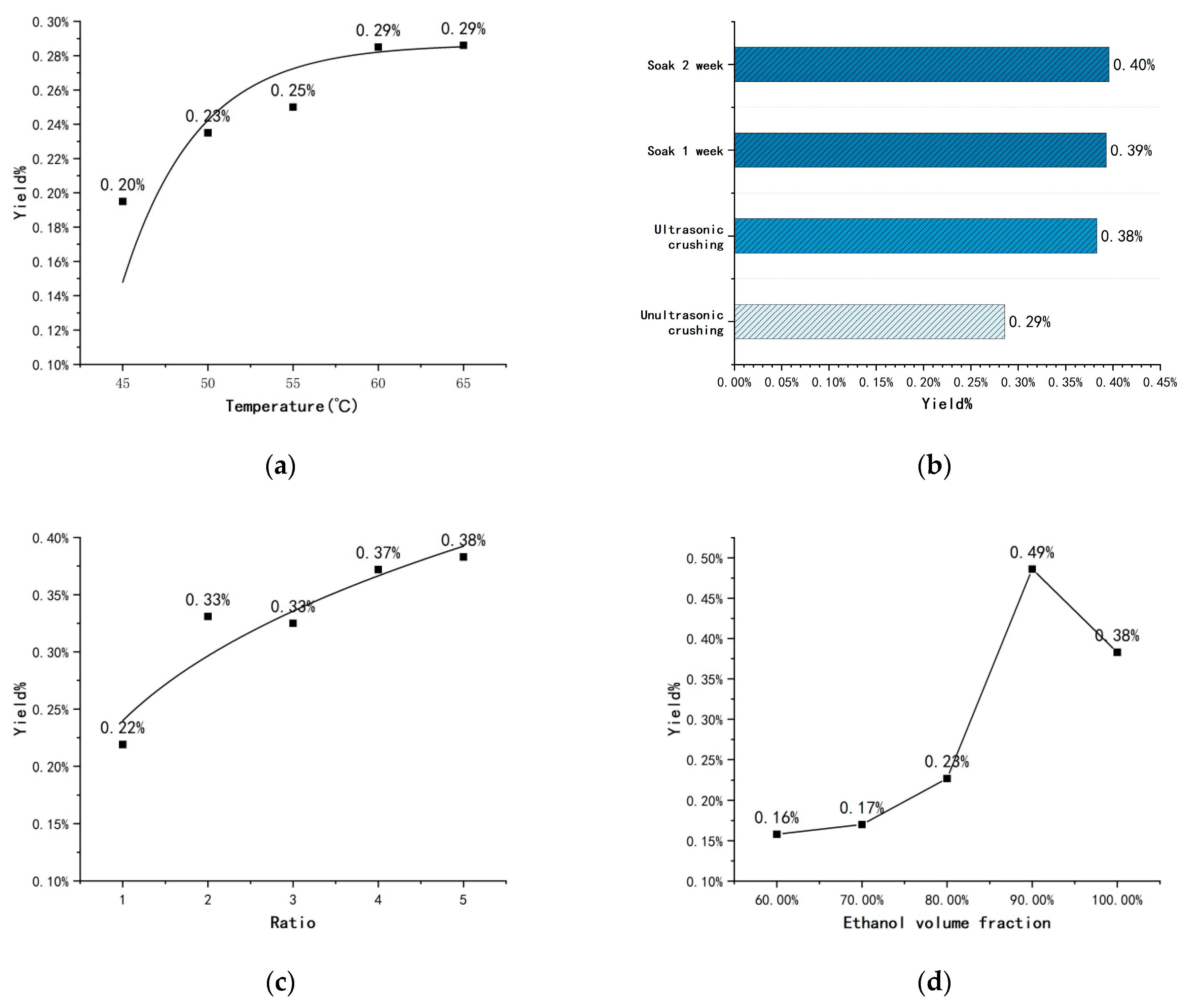
| No. | Temperature | Ratio | Ethanol Fraction of Entrainer | Yield |
|---|---|---|---|---|
| 1 | 45 | 1:1 | 80 | 0.26% |
| 2 | 45 | 1:3 | 90 | 0.29% |
| 3 | 45 | 1:5 | 100 | 0.20% |
| 4 | 55 | 1:1 | 90 | 0.21% |
| 5 | 55 | 1:3 | 100 | 0.30% |
| 6 | 55 | 1:5 | 80 | 0.27% |
| 7 | 65 | 1:1 | 100 | 0.22% |
| 8 | 65 | 1:3 | 80 | 0.24% |
| 9 | 65 | 1:5 | 90 | 0.49% |
| 10 | 65 | 1:5 | 90 | 0.48% |
| 11 | 65 | 1:5 | 90 | 0.49% |
| Term | Level | Temperature | Ration | Volume Fraction |
|---|---|---|---|---|
| K-Value | 1 | 0.74 | 0.69 | 0.77 |
| 2 | 0.78 | 0.83 | 0.99 | |
| 3 | 0.95 | 0.95 | 0.71 | |
| K avg-Value | 1 | 0.25 | 0.23 | 0.26 |
| 2 | 0.26 | 0.28 | 0.33 | |
| 3 | 0.32 | 0.32 | 0.24 | |
| Best level | 3 | 3 | 2 | |
| R | 0.07 | 0.09 | 0.09 | |
| Account of Level | 3 | 3 | 3 | |
| Number of repetitions per level (r) | 3 | 3 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Xie, Q.; Ren, Q.; Kong, J. Extraction and Purification of Nicotine from Tobacco Rhizomes by Supercritical CO2. Molecules 2024, 29, 1147. https://doi.org/10.3390/molecules29051147
Zheng F, Xie Q, Ren Q, Kong J. Extraction and Purification of Nicotine from Tobacco Rhizomes by Supercritical CO2. Molecules. 2024; 29(5):1147. https://doi.org/10.3390/molecules29051147
Chicago/Turabian StyleZheng, Fangyuan, Qishan Xie, Qingguang Ren, and Jilie Kong. 2024. "Extraction and Purification of Nicotine from Tobacco Rhizomes by Supercritical CO2" Molecules 29, no. 5: 1147. https://doi.org/10.3390/molecules29051147
APA StyleZheng, F., Xie, Q., Ren, Q., & Kong, J. (2024). Extraction and Purification of Nicotine from Tobacco Rhizomes by Supercritical CO2. Molecules, 29(5), 1147. https://doi.org/10.3390/molecules29051147





