Abstract
Conjugated polymers (CPs) have attracted much attention in recent years due to their structural abundance and tunable energy bands. Compared with CP-based materials, the inorganic semiconductor TiO2 has the advantages of low cost, non-toxicity and high photocatalytic hydrogen production (PHP) performance. However, studies on polymeric-inorganic heterojunctions, composed of D-A type CPs and TiO2, for boosting the PHP efficiency are still rare. Herein, an elucidation that the photocatalytic hydrogen evolution activity can actually be improved by forming polymeric-inorganic heterojunctions TFl@TiO2, TS@TiO2 and TSO2@TiO2, facilely synthesized through efficient in situ direct C–H arylation polymerization, is given. The compatible energy levels between virgin TiO2 and polymeric semiconductors enable the resulting functionalized CP@TiO2 heterojunctions to exhibit a considerable photocatalytic hydrogen evolution rate (HER). Especially, the HER of TSO2@TiO2 heterojunction reaches up to 11,220 μmol g−1 h−1, approximately 5.47 and 1260 times higher than that of pristine TSO2 and TiO2 photocatalysts. The intrinsic merits of a donor-acceptor conjugated polymer and the interfacial interaction between CP and TiO2 account for the excellent PHP activity, facilitating the separation of photo-generated excitons. Considering the outstanding PHP behavior, our work discloses that the coupling of inorganic semiconductors and suitable D-A conjugated CPs would play significant roles in the photocatalysis community.
1. Introduction
The efficient use of solar energy by conserving it in the form of chemical bonds is considered to be one of the most effective ways to build sustainable society. Hydrogen energy, featured by high energy density, high calorific value and zero pollution, has been widely regarded as a clean secondary energy [1,2,3,4]. Solar-driven hydrogen evolution has been developed as a promising means for obtaining renewable energy; meanwhile, the seek for highly active photocatalysts that are capable of efficiently absorbing sunlight to drive the hydrogen evolution reaction (HER) has aroused tremendous interest from scientists. From this perspective, the first discovery of titanium dioxide (TiO2) as an inorganic semiconductor photocatalyst, dating back to 1972, reported by Fujishima and Honda [5], laid the foundations and brings infinite opportunities in the field of photocatalysis. Benefiting from the advantages of low cost, non-toxicity, high stability, n-type semiconducting properties, and strong oxidizing ability under light irradiation, TiO2 has been considered as the most representative semiconductor photocatalyst and has been widely used in molecular photocatalysis [5] and photocatalyzed hydrogen production (PHP) [6,7,8]. However, the intrinsic narrow photo-response range of TiO2 severely limits the light-harvesting ability and causes the rapid recombination of photogenerated electron-hole (e−-h+) pairs, which has greatly hindered the development of titanium dioxide-based photovoltaic conversion technologies. In order to overcome the abovementioned drawbacks, organic semiconductors have gradually emerged as promising candidates for photocatalysis [9,10,11,12]. Among these, conjugated polymers (CPs), including the g-C3N4 derivatives [13,14,15], conjugated microporous polymers (CMP) [16,17,18,19,20,21], frameworks of covalent triazines (CTFs) [22,23,24], covalent organic frameworks (COF) [25,26,27,28], and linear conjugated polymers (LCPs) [29,30,31,32], are showcasing the enormous potentials for PHP.
The broad designability of CPs at the molecular-level allows the regulation of structural and electronic properties, which results in narrower bandgap (Eg) and wider absorption range compared to TiO2 [33,34]. However, the CP-based photocatalysts still suffer from lower charge carrier mobility because of their shorter exciton diffusion length. In order to settle shortcomings of the single-component photocatalysts, the reasonable exploitation of heterojunctions between CPs and TiO2 is desired to be explored, wherein the formed CP@TiO2 heterojunctions might take the advantages of CPs and TiO2 [35]. As a result, an extended visible-light responsive and the enhanced photoexcited e−-h+ pairs separation would be expected for the CP@TiO2 heterojunctions, thus accelerating the migration of photogenerated charge carriers between CPs and TiO2. Such CP@TiO2 heterojunctions, in which CPs are mainly g-C3N4, CMP and COF structures [36,37,38,39,40,41,42,43,44,45,46,47], have showed great potentials in PHP fields.
Donor-acceptor (D-A) conjugated polymers with alternating structures have emerged as promising photocatalysts for hydrogen generation [48,49,50], owing to the intramolecular pull-push effect of D-A structure that would promote electron (e−)/hole (h+) separation in the presence of light irradiation [51,52,53]. To our knowledge, organic–inorganic heterojunctions composed of D-A type CPs and TiO2 have rarely been investigated to date. For example, Chen and Xiang et al. reported that CMP@TiO2 heterojunction with D-A motif, in which 1,3,5-triethynylbenzene and thiadiazole derivatives act as D and A unit, respectively, exhibited dramatically increased PHP performance with Pt-cocatalyst [46]. Lately, the same group designed a series of linear conjugated poly(benzothiadiazole) and incorporated onto the surface of TiO2 in a similar in situ polycondensation strategy, the resulting heterojunctions consisting of electron-donor and electron-acceptor units embodied superior PHP activity in the absence of a Pt-cocatalyst [54]. In 2019, Xiang and Huang et al. introduced benzene (D) and 2-fluorobenzene(A) to prepare a library of functionalized D-A type heterojunctions in the presence of TiO2; the results showed that the HER of functionalized 4% BFBA-TiO2 was up to 228.2 μmol h−1 [55]. Therefore, it is still challenge to build CP@TiO2 heterojunctions involving D-A architecture whilst little progress has been made. Recently, Wang and Zhang et al. described the fabrication of homopolymer poly (dibenzothiophene-S, S-dioxide) (PDBTSO)/TiO2 composite composed of A-A structure, exhibiting considerable HER [47]. These meaningful achievements inspired us to explore whether the strategy of introduction D-A conjugated polymers to form TiO2-based heterojunction is applicable for improving the PHP performance or not.
Given our consideration in this work, LCPs TFl, TS and TSO2 were employed as the polymeric “partner” to couple with pristine TiO2, and the resulting CP@TiO2 heterojunctions TFl@TiO2, TS@TiO2 and TSO2@TiO2 were prepared with in situ direct C–H arylation polymerization processes. The structural information, opt-electronic and the photocatalytic H2 production were investigated. Benefiting from the D-A merits, TSO2@TiO2 heterojunction exhibited broader light responsiveness and more efficient photo-excited charge separation and transfer. HER of TSO2@TiO2 heterojunction reaches 11,220 μmol h−1 g−1 under full arc light irradiation, which is 5.47 and 1260 times higher than that of pure polymeric TSO2 and TiO2, respectively. Our findings offer an alternative way to fabricate a polymeric heterojunction photocatalyst consisting of a D-A building block for boosting PHP performance.
2. Results and Discussion
2.1. Synthesis and Characterization of the CPs and CP@TiO2 Heterojunctions
On the basis of our previous well-developed Pd-catalyzed direct C–H arylation polymerization strategy (DArP) [32,56,57], three pristine LCPs denoted as TFl, TS and TSO2 showed in Scheme 1a were prepared by treatment of corresponding dibromo-precursors, 2,2-biothiophene via DArP methods, as detailed in the supporting information. Accordingly, the CP@TiO2 heterojunctions (TFl@TiO2, TS@TiO2 and TSO2@TiO2, Scheme 1b) were fabricated via in situ DArP between appropriate dibromides, 2,2-biothiophene in the presence of TiO2. Taking TSO2@TiO2 as an example, anhydrous toluene (8 mL) was added to a Schlenk tube containing 3,7-dibromobenzothiophene-S, S-dioxide (40.0 mg, 1 eq), 2,2-biothiophene (19.7 mg, 1 eq), Cs2CO3 (77.1 mg, 2 eq), PivOH (3.6 mg, 30 mol%), P(o-MeOPh)3 (2.5 mg, 6 mol%), Pd2(dba)3 (3.2 mg, 3 mol%) and TiO2 (81.0 mg). Then, the resulting mixture was stirred in an oil bath at 100 °C under an argon atmosphere for 48 h. After the reaction was completed, the desired TSO2@TiO2 heterojunction was obtained by filtration, followed by continuous washing with dichloromethane-methanol-water and finally drying at 80 °C under vacuum conditions for 24 h.
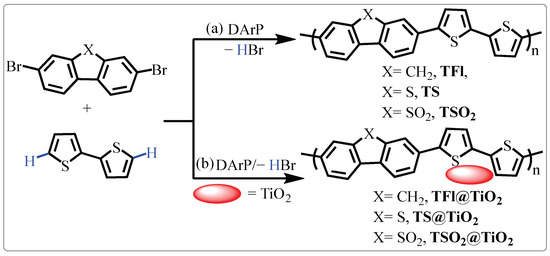
Scheme 1.
(a) Synthesis of LCPs TFl, TS and TSO2 by DArP polymerization; (b) in situ preparation of TFl@TiO2, TS@TiO2 and TSO2@TiO2 heterojunctions.
The structures of the as-prepared LCPs and CP@TiO2 heterojunctions were initially determined by Fourier transform infrared (FT-IR) spectra. As shown in Figure 1a, the broad peaks falling in the range of 500–800 cm−1 observed for TFl@TiO2, TS@TiO2 and TSO2@TiO2 are assigned for the Ti–O–Ti stretching vibrations [37,39], which firmly provides evidence for the successful heterojunction interactions between the as-prepared LCPs and TiO2. The characteristic signals located at ~1630 cm−1, attributing to the stretching vibration of aromatic rings, can be found in the LCPs and CP@TiO2 heterojunctions. The characteristic signals displayed at ~1466 cm−1 and ~796 cm−1 are attributable to the thiophene unit indicate that the bithiophene building block has been introduced into six samples. Additionally, the typical peaks appeared at ~1301 cm−1 and ~1157 cm−1, belonging to characteristic peaks that for the sulfonyl group (O=S=O), are found in both TSO2 and TSO2@TiO2, respectively.
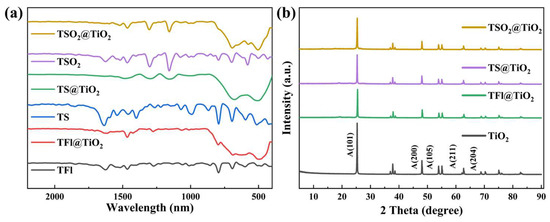
Figure 1.
(a): FT-IR spectra and (b): XRD patterns of CPs (TFl, TS, TSO2) and CP/TiO2 heterojunctions (TFl@TiO2, TS@TiO2, TSO2@TiO2).
The formation of CP@TiO2 heterojunctions was also determined by powder X-ray diffraction (XRD) patterns. As shown in Figure 1b, the pristine TiO2 exhibits representative high and narrow diffraction peaks located at 2θ = 25.3°, 37.8°, 48.0°, 53.9°, 55.1° and 62.7°, corresponding to the (101), (004), (200), (105), (211) and (204) planes of TiO2, respectively, which are consistent with the tetragonal anatase TiO2 standard card (JCPDS No. 21-1272). In contrast, the TFl@TiO2, TS@TiO2 and TSO2@TiO2 heterojunctions show identical XRD patterns to those of pure TiO2, which reveals that the in situ polymerization has a negligible impact on the crystallinity of TiO2.
X-ray photoelectron spectroscopy (XPS) measurement was performed to analyze the elemental composition and chemical state of pristine TiO2 and the obtained TSO2 and TSO2@TiO2 (Figure 2 and Figure S1). The XPS survey spectrum illustrated in Figure 2a elucidated the existence of Ti, O, C, and S elements in the chemical composition of TSO2@TiO2, suggesting the successful construction of the expected heterojunction. Meanwhile, the corresponding high resolution XPS (HR-XPS) spectra of Ti 2p shown in Figure 2b indicate two similar peaks, which are ascribed to Ti 2p1/2 and Ti 2p3/2, respectively, for pure TSO2 and TSO2@TiO2. Simultaneously, the HR-XPS spectra of O 1s demonstrates the characteristic peaks for OH and Ti-O-Ti on the surface of TiO2 with a slight energy shift for TSO2@TiO2 compared to those of TSO2 and TiO2 (Figure 2c). Similarly, the HR-XPS C 1s spectra of TSO2@TiO2 shows a slightly up-shifted binding energy compared with the polymeric TSO2 (Figure 2d). The change in binding energy indicates the involvement of an interfacial interaction between the TSO2 and TiO2, which could accelerate the charge transfer.
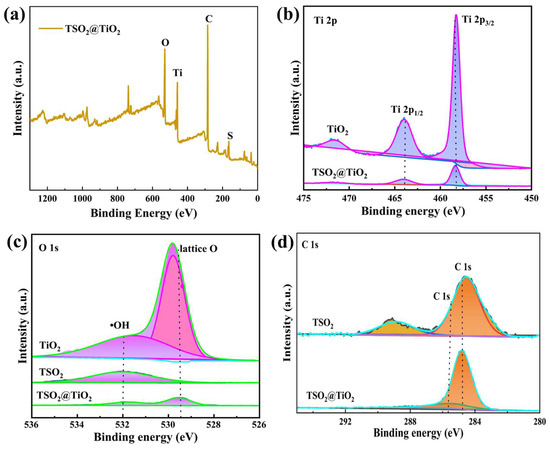
Figure 2.
XPS spectra of TSO2@TiO2. (a) Survey spectrum, (b) Ti 2p, (c) O 1s, (d) C 1s.
To further investigate the heterojunction morphology of CP@TiO2, transmission electron microscopy (TEM) and EDS measurements were carried out next (Figure 3, Figures S2 and S3). Typically, pure TiO2 is in the shape of microspheres; however, when composites are formed by coupling with the conjugated polymers, small particles and many folds are observed on the surface (Figure 3a–c). In addition, a high-resolution TEM was carried out on TSO2@TiO2 and it was evident that the lattice striations were anatase (101) with a crystalline surface spacing of 0.35 nm (Figure 3d). The EDS elemental analysis of the TSO2@TiO2 composite shows the existence of Ti, S, C and O atoms (Figures S2 and S3). Therefore, both high-resolution TEM and EDS clearly prove the successful formation of the TSO2@TiO2 heterojunction.
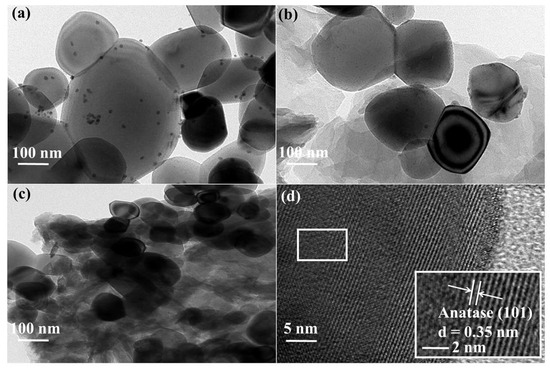
Figure 3.
TEM images of (a) TFl@TiO2, (b) TS@TiO2 and (c,d) TSO2@TiO2.
The specific Brunauer–Emmett–Teller (BET) surface area of LCPs, CP@TiO2 heterojunctions and TiO2 was obtained by nitrogen adsorption–desorption isotherms methods (Figure 4), which indicates a descending order in a sequence of TSO2@TiO2 > TS@TiO2 > TFl@TiO2. The relatively high surface area of TS@TiO2, about six times than of pristine TiO2, is favorable for the adsorption of reactant molecules and provides a wider range of sites for photocatalytic reactions.
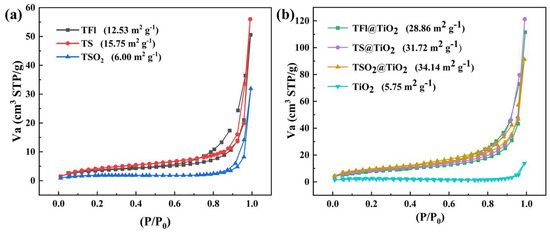
Figure 4.
N2 adsorption-desorption isothermal profiles of (a) TFl, TS and TSO2, and (b) TFl@TiO2, TS@TiO2, TSO2@TiO2, and TiO2.
2.2. Opt-Electronic Properties
The optical properties of TiO2, LCPs and CP@TiO2 heterojunctions were first characterized by UV-vis diffuse reflectance spectroscopy (DRS) (Figure 5a), which reveals that both LCPs and CPs@TiO2 heterojunctions have wide and strong light absorption, located in the range of 300 to 600 nm, compared to pristine TiO2, enabling them to act as suitable light-harvesting materials. To our delight, TSO2 exhibits a redshift phenomenon compared to those of TFl and TS among the three LCPs, which is probably due to the fact that TSO2 has an intramolecular donor-acceptor (D-A) interaction induced by the D-A structure. In contrast, TFl and TS contain D-D structures, thus proving the push–pull electron effect of the D-A interaction is of great significance for broadening the light-absorption. Particularly, the TSO2@TiO2 heterojunction possesses the most red-shifted absorption between 300–600 nm, with an extended absorption peak at ~700 nm compared to those of TFl@TiO2 and TS@TiO2, could be attributable to the tighter connection of the polymer TSO2 to TiO2. As can be seen in Figure 5b, the optical band gaps (Eg) of TFl, TS and TSO2 are, as calculated by Tauc plots, 2.2, 2.19 and 2.18 eV, respectively. In addition, the Eg values of TiO2 and the CP@TiO2 heterojunctions are 3.2 (TiO2), 2.25 (TFl@TiO2), 2.28 (TS@TiO2) and 2.17 eV (TSO2@TiO2), among which the narrowest Eg of TSO2@TiO2 is consistent with the wide light absorption property.
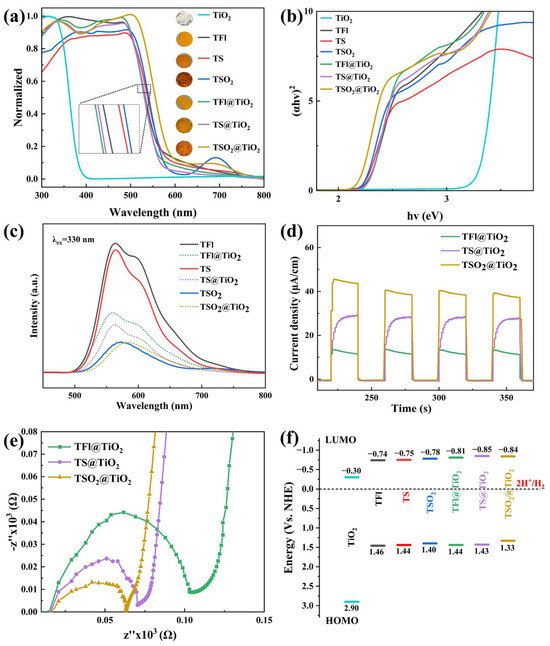
Figure 5.
(a) UV-vis diffuse reflectance spectra of TiO2, TFl, TS, TSO2 and CP@TiO2. (b) Kubelka-Munk functions of TiO2, TFl, TS, TSO2 and CP@TiO2 with Tauc plots. (c) PL spectra. The PL spectra of TFl, TS, TSO2 and CP@TiO2 were measured in the range of 400–850 nm at an excitation wavelength of 330 nm (λex = 330 nm); (d) transient photocurrent-time (i-t) curve; (e) electrical impedance spectra; (f) energy band diagrams of TiO2, TFl, TS, TSO2 and CP@TiO2 and their proton reduction potentials.
To evaluate the abilities of photogenerated carrier migration and separation for the obtained LCPs and CP@TiO2 heterojunctions, we then carried out the steady-state PL (Figure 5c), TPR (Figure 5d) and EIS (Figure 5e) measurements. The PL intensities for D-A type TSO2 and TSO2@TiO2 heterojunction are comparative and relatively lower than those of the remaining four samples, indicating an immense tendency for photo-to-current conversion, thereby resulting in superior separation of the e−/h+ pairs. In addition, the PL intensities of TFl@TiO2 and TS@TiO2 heterojunctions are significantly lower than the behaviors of their parent conjugated polymers, which are in accordance with the DRS results. Furthermore, the TPR curves showcase that the instantaneous photocurrent-time response of three heterojunctions is in a sequence of TSO2@TiO2 > TS@TiO2 > TFl@TiO2, implying that more light-induced excitons can be generated for the D-A motif containing TSO2@TiO2 heterojunction [58,59]. Consistent with the PL and TPR results, the EIS experiments demonstrate that the order of the Nyquist circle radius is TSO2@TiO2 < TS@TiO2 < TFl@TiO2. Therefore, the smallest arc radius of the Nyquist plot for TSO2@TiO2 means it has lower interfacial resistance and excellent charge mobility characters [60,61,62].
In combination with cyclic voltammetry (CV) (Figure S4) curves and the equation EHOMO = ELUMO − Eg, the HOMO and LUMO levels of LCPs and CP@TiO2 heterojunctions are estimated. As shown in Figure 5f, the HOMO/LUMO levels of LCPs are 1.46/−0.74 (TFl), 1.44/−0.75 (TS) and 1.40/−0.78 eV (TSO2), while their corresponding TiO2-based heterojunctions are 1.44/−0.81 (TFl@TiO2), 1.43/−0.85 (TS@TiO2) and 1.33/−0.84 eV (TSO2@TiO2), respectively. In contrast, the HOMO/LUMO levels of pristine TiO2 are 2.90/−0.30 eV. Consequently, the TSO2@TiO2 heterojunction exhibits the narrowest Eg with a value of 2.17 eV, agreeing with the Eg calculated using Tauc plots, which provides many opportunities for the TSO2@TiO2 heterojunction to achieve the reductive transformation of H+ to H2. Overall, the opt-electronic properties suggest that TSO2@TiO2 heterojunction can serve as a potential efficient photocatalyst for water splitting.
2.3. Photocatalytic Hydrogen Production Performances
Based on the opt-electronic properties of LCPs and CP@TiO2 heterojunctions, the PHP performances were spontaneously tested under visible or full-arc light irradiation by utilizing the relevant photocatalysts (10 mg) dispersed in N-methyl pyrrolidone (NMP)/H2O (30 mL) aqueous solutions, together with the ascorbic acid (AA) as a sacrificial electron donor (SED) [20,32]. As shown in Figure 5a, apparent differences are observed for the normalized hydrogen evolution rates (HERs) of three LCPs under full-arc light irradiation, among which the polymeric TSO2 photocatalyst exhibits the highest PHP activity with HER of 2050 μmol/g−1 h−1 due to the introduction of the D-A structure. However, the raw material TiO2 only shows weak PHP capacity (8.9 μmol/g−1 h−1). Impressively, enormous improvements in HERs are found when TiO2 is coupled with LCPs for CP@TiO2 heterojunctions compared to those of TiO2 and their parent LCPs (Figure 6b). Specifically, the HERs in the full-arc band are 1430 (TFl@TiO2), 2000 (TS@TiO2) and 11,220 μmol/g−1 h−1 (TSO2@TiO2), respectively, which indicates that the formation of heterojunction would improve the performance of polymeric photocatalysts because of the charge transfer between the polymer and TiO2. In comparison, we also test the PHP activities of CP@TiO2 heterojunctions under the irradiation of visible light (λ > 420 nm) for their intense absorption in this range. As displayed in Figure 6b, the HERs of TFl@TiO2, TS@TiO2 and TSO2@TiO2 are still far exceeded by the corresponding LCPs and TiO2, albeit with inferior performance compared with the full-arc band irradiation, proving that the combination of conjugated polymer and TiO2 can significantly extend the visible light response. Notably, the HERs of TSO2@TiO2 heterojunction are 1260 and 5.47 times higher than those of pristine TiO2 and the linear polymeric photocatalyst TSO2, respectively, which suggests that building D-A architecture-type polymeric and forming organic polymer@TiO2 heterojunctions is an effective strategy for improving the performance of PHP for the reason that the interaction between the D-A motif and TiO2 could synergistically facilitate exciton diffusion and enhance charge separation for proton reduction.
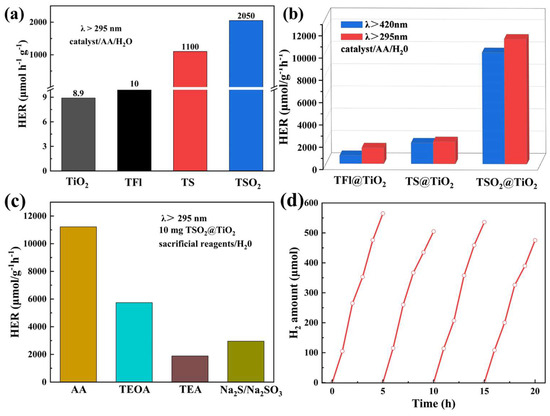
Figure 6.
(a) Hydrogen production data of TiO2, TFl, TS and TSO2 under UV-vis light; (b) Hydrogen production data for TFl@TiO2, TS@TiO2 and TSO2@TiO2 under visible light and UV-vis light; (c) HER of TSO2@TiO2 in different sacrificial agents; (d) TSO2@TiO2 photocatalytic hydrogen production cycle experiments.
To explore the effect of different SEDs on the PHP activities, as illustrated in Figure 6c, TEOA, TEA and Na2S/Na2SO3 were used instead of AA. As a result, a better performance was achieved when AA was employed as the SED. With the optimized condition in hand, the cycling experiments were studied to assess the long-term stability of TSO2@TiO2 heterojunction toward the PHP reaction. As depicted in Figure 6d, steady photocatalytic dihydrogen gas generation could be observed through 20 h cycling test with four successive cycles, implying the photochemical stability of the TSO2@TiO2 heterojunction. Meanwhile, the TSO2@TiO2 was recovered for structural investigation, and no discernible differences were observed from both the UV-vis and FT-IR spectra compared to the as-prepared one (Figure 7a,b), demonstrating the long-term photo-stability of the TSO2@TiO2 during the PHP process.
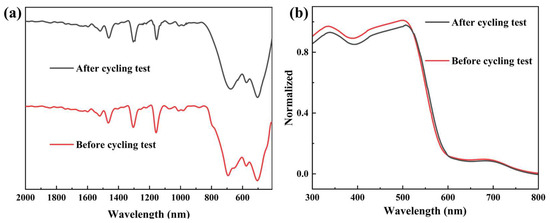
Figure 7.
(a) FT-IR and (b) UV-vis DRS spectra of TSO2@TiO2 before and after cycling tests.
According to the above experimental results, the plausible mechanism for the increased photocatalytic H2 evolution of TSO2@TiO2 heterojunction is proposed in Figure 8. Since the pure polymeric D-A type TSO2 exhibits more negative LUMO value and less positive value than those of pristine TiO2 (Figure 5f), TSO2 rather than TiO2 is photo-activated under the illumination of full-arc or visible light (λ > 420 nm). As a result, hole-electron pairs were generated from the TSO2 phase. Meanwhile, some of the photo-generated electrons were inclined to jump from the LUMO energy level of TSO2 to the conduction band of TiO2, driven by the built-in electric field, via the interfacial heterojunction, thus subsequently reacting with the protons derived from water to produce H2. Besides, the photo-generated holes can easily transfer from the valence band of TiO2 to the HOMO energy level of TSO2, which in turn contributes to oxidizing the AA to AA+. Therefore, the effective photo-excited e−-h+ separation of TSO2@TiO2 heterojunction results in enhanced photocatalytic H2 generation.
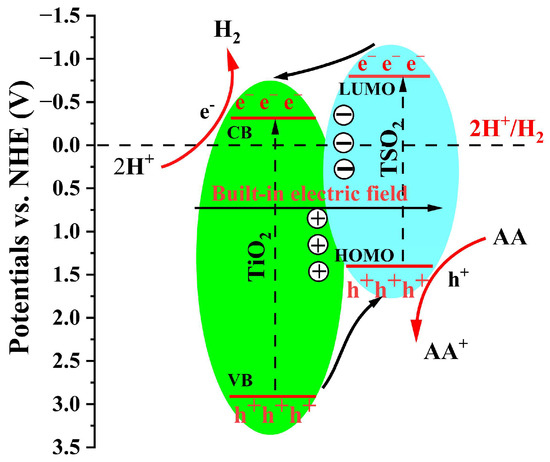
Figure 8.
TSO2@TiO2 mechanism diagram.
3. Materials and Methods
3.1. Materials and Methods
All of the starting materials and reagents were purchased from commercial suppliers and used directly without further purification. Anhydrous toluene was pretreated with calcium hydride (CaH2) and freshly distilled.
FT-IR spectra were collected on a FT-IR spectrometer (Bruker ALPHA, Billerica, MA, USA) and the KBr was mixed with the sample for sample preparation. The morphology of heterojunction photocatalysts was obtained by scanning electron microscopy (SEM, MLA650F, Hillsboro, OR, USA) and transmission electron microscopy (TEM, FEI Tecnai G2 F20, Thermo Fisher Scientific, Waltham, MA, USA). We recorded polymer photoluminescence (PL) conductivity by employing a HORIBA FL-1000 fluorescence spectrometer for solid powders. EDS uses TESCAN MIRA LMS + Quantax 200 X Flash 6|60. X-ray diffraction (XRD) was measured by the Thermo Fisher NexsaI instrument. X-ray photoelectron spectroscopy (XPS) was measured by the Thermo Fisher NexsaI instrument. The solid UV-visible absorption spectra of the synthesized polymers were detected by a UV-2600 spectrophotometer using BaSO4 as a substrate reference. The water contact angle was measured using a JCY type contact angle measuring instrument. Transient photo-response tests were performed using a three-electrode configuration electrochemical workstation (CHI650E/700E, Huachen Co., Ltd., Shanghai, China) to measure transient photocurrents using a Pt electrode as the auxiliary electrode and an Ag/AgCl electrode containing a saturated KCl solution as the reference electrode. The polymer was ultrasonically dispersed with ethanol to form a suspension, which was then dripped onto ITO conductive glass to form a sample with an effective area of 0.6 cm × 0.6 cm, and tested in a 0.1 M aqueous sodium sulfate solution as the electrolyte. Cyclic voltammetry tests were carried out with cyclic voltammetry using an electrochemical workstation (CHI650E/700E, Huachen Co., Ltd., Shanghai, China) with a three-electrode system using a glassy carbon electrode as the working electrode, an Ag/AgCl electrode as the reference electrode, and a Pt electrode as the auxiliary electrode, and an electrolyte was prepared by dissolving the TBAPF6 in acetonitrile solution, and the electrolyte was then used as an electrophoretic solution according to the equation: EHOMO = −(EOX + 4.8 eV(νs Ag/Ag+) − EOXFc/Fc+), EHOMO was calculated from the curve. The volume of nitrogen adsorption was recorded over a relative pressure range between 0.01 and 0.99 points in the relative pressure range of 0.05–0.2 were used for the calculation of the surface area according to the Brunauer-Emmet-Teller (BET) theory.
3.2. Synthesis of 3,7-Dibromodibenzothiophene-S,S-dioxide [63]
NBS (2.47 g, 13.87 mmol) was added into this solution of dibenzothiophene-S, S-dioxide (1.5 g, 6.94 mmol) in concentrated H2SO4 (90 mL) in several portions, and the resulting mixture was stirred at 0–5 °C for 24 h. The mixture was carefully poured into ice/water. The off-white solid was filtered off, washed with 20% aqueous sodium hydrogen carbonate, water and dried to an afford white solid. The product was further recrystallized from chloroform to gain a white crystal with a 60% yield. 1H-NMR (400 MHz, CDCl3) δ 7.93 (s, 2H), 7.78 (d, 2H), 7.65 (d, 2H). 13CNMR (400 MHz, CDCl3) δ 138.96, 137.25, 129.68, 125.70, 123.02.
3.3. Synthesis of TFl, TS, TSO2, TiO2, TFl@TiO2, TS@TiO2 and TSO2@TiO2
TFl: A mixture of 2,7-dibromofluorene (100.0 mg, 1.0 eq), 2,2-biothiophene (51.3 mg, 1.0 eq), Cs2CO3 (100.6 mg, 2.0 eq), PivOH (4.7 mg, 30 mol%), P(o-MeOPh)3 (2.2 mg, 4 mol%) and Pd2(dba)3 (2.8 mg, 2 mol%) was added to 5 mL of anhydrous toluene under an argon atmosphere, and the mixture was degassed in three freeze-vacuum-thaw cycles. The reaction was then stirred in an oil bath at 100 °C for 48 h. After the reaction was completed and cooled, the product was washed and filtered using dichloromethane–methane-water in sequence to remove inorganic salts and residual small molecules. The reaction was then dried in a vacuum oven at 80 °C for 24 h, 115.8 mg, yield: 91%.
TS: A mixture of 3,7-dibromodibenzothiophene (100.0 mg, 1.0 eq), 2,2-biothiophene (48.6 mg, 1.0 eq), Cs2CO3 (190.5 mg, 2.0 eq), PivOH (8.9 mg, 30 mol%), P(o-MeOPh)3 (2.2 mg, 4 mol%) and Pd2(dba)3 (2.8 mg, 2 mol%) was added to 5 mL of anhydrous toluene under an argon atmosphere and the mixture was degassed in three freeze-vacuum-thaw cycles. The reaction was then stirred in an oil bath at 100 °C for 48 h. After the reaction was completed and cooled, the product was washed and filtered using dichloromethane-methane-water in sequence to remove inorganic salts and residual small molecules. The reaction was then dried in a vacuum oven at 80 °C for 24 h, 118.3 mg, yield: 95%.
TSO2: A mixture of 3,7-dibromodibenzothiophene-5,5-dioxide (100.0 mg, 1.0 eq), 2,2-biothiophene (44.4 mg, 1.0 eq), Cs2CO3 (174.2.5 mg, 2.0 eq), PivOH (8.2 mg, 30 mol%), P(o-MeOPh)3 (3.8 mg, 4 mol%) and Pd2(dba)3 (4.9 mg, 2 mol%) was added to 5 mL of anhydrous toluene under an argon atmosphere, and the mixture was degassed in three freeze-vacuum-thaw cycles. The reaction was then stirred in an oil bath at 100 °C for 48 h. After the reaction was completed and cooled, the product was washed and filtered using dichloromethane–methane-water in sequence to remove inorganic salts and residual small molecules. The reaction was then dried in a vacuum oven at 80 °C for 24 h, 115.7 mg, yield: 94%.
TFl@TiO2: A mixture of 2,7-dibromofluorene (40.0 mg, 1.0 eq), 2,2-biothiophene (20.5 mg, 1.0 eq), Cs2CO3 (80.4 mg, 2.0 eq), PivOH (3.8 mg, 30 mol%), P(o-MeOPh)3 (2.6 mg, 6 mol%), Pd2(dba)3 (3.4 mg, 3 mol%) and TiO2 (81.0 mg) was added to 8 mL of anhydrous toluene under an argon atmosphere, and the mixture was degassed in three freeze-vacuum-thaw cycles. The reaction was then stirred in an oil bath at 110 °C for 48 h. After the reaction was completed and cooled, the product was washed and filtered using dichloromethane–methane-water in sequence to remove inorganic salts and residual small molecules. The reaction was then dried in a vacuum oven at 80 °C for 24 h, 113.9 mg, yield: 87%.
TS@TiO2: A mixture of 3,7-dibromodibenzothiophene (40.0 mg, 1.0 eq), 2,2-biothiophene (19.4 mg, 1.0 eq), Cs2CO3 (76.2 mg, 2.0 eq), PivOH (3.6 mg, 30 mol%), P(o-MeOPh)3 (2.4 mg, 6 mol%), Pd2(dba)3 (3.2 mg, 3 mol%) and TiO2 (81.0 mg) was added to 8 mL of anhydrous toluene under an argon atmosphere, and the mixture was degassed in three freeze-vacuum-thaw cycles. The reaction was then stirred in an oil bath at 110 °C for 48 h. After the reaction was completed and cooled, the product was washed and filtered using dichloromethane–methane-water in sequence to remove inorganic salts and residual small molecules. The reaction was then dried in a vacuum oven at 80 °C for 24 h, 119.2 mg, yield: 91%.
TSO2@TiO2: A mixture of 3,7-dibromobenzothiophene-5,5-dioxide (40.0 mg, 1.0 eq), 2,2-biothiophene (19.7 mg, 1.0 eq), Cs2CO3 (77.1 mg, 2.0 eq), PivOH (3.6 mg, 30 mol%), P(o-MeOPh)3 (2.5 mg, 6 mol%), Pd2(dba)3 (3.2 mg, 3 mol%) and TiO2 (81.0 mg) was added to 8 mL of anhydrous toluene under an argon atmosphere, and the mixture was degassed in three freeze-vacuum-thaw cycles. The reaction was then stirred in an oil bath at 110 °C for 48 h. After the reaction was completed and cooled, the product was washed and filtered using dichloromethane–methane-water in sequence to remove inorganic salts and residual small molecules. The reaction was then dried in a vacuum oven at 80 °C for 24 h, 116.5 mg, yield: 89%.
3.4. PHP Tests
A gas chromatograph (GC9790, FuLi, Wenzhou, China) was linked with the photocatalytic online analysis system (LabSolar-III AG, Beijing Perfect Light, Beijing, China) for the typical PHP test; it was equipped with the thermal conductive detector (TCD) and used argon as the carrier gas. A mixed aqueous solution containing 30 mL H2O and 5 g AA as the sacrificial agent was prepared for the photocatalysts TFl, TS, TSO2, TiO2, TFl@TiO2, TS@TiO2 and TSO2@TiO2 (10 mg) ultrasonic dispersal. The oil pump was used to remove the dissolved air form the mixture and maintain it in vacuum. To irradiate the reaction vessel, a 300 W Xe lamp (Beijing Perfect Light, PLS-SXE300) under full-arc light irradiation was used. To fix the reaction temperature at 25 °C, a flow of cooling water was used.
4. Conclusions
In summary, three LCPs entitled TFl, TS and TSO2 were facile synthesized via the atom-economic DArP methodology, featuring D-D (for TFl and TS) and D-A (for TSO2) architectures, respectively. Additionally, compatible CP@TiO2 heterojunctions TFl@TiO2, TS@TiO2 and TSO2@TiO2 were successfully constructed through in situ DArP routes in the presence of TiO2. The chemical structures and compositions of the expected LCPs and CP@TiO2 heterojunctions were characterized by FT-IR, XRD, XPS and TEM. The investigation, including DRS, PL, TPR, EIS and CV measurements, on the opt-electronic properties revealed that the TSO2@TiO2 heterojunction can serve as an ideal polymeric photocatalyst candidate due to its increased photo-responsive and separated electron-hole pairs, inducing the intramolecular D-A interaction. Impressively, the PHP tests showed that the desired photocatalyst TSO2@TiO2 heterojunction presented a remarkable photocatalytic HER of 11,220 μmol g−1 h−1 under full arc irradiation, which is 1260 and 5.47 times higher than that of pristine TiO2 and TSO2 photocatalysts, respectively. A plausible mechanism involving electron and hole transfer between polymeric TSO2 and TiO2 was raised to elucidate the enhancement of PHP performance. Our results not only demonstrate that the D-A conjugated polymer and TiO2 heterostructures bring tremendous opportunities for PHP, but also provide a feasible avenue to seek novel photocatalysts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29051103/s1, Scheme S1: Synthesis of 3,7-dibromodibenzothiophene-S, S-dioxide; Figure S1: XPS plots of TiO2, TSO2 and TSO2@TiO2; Figure S2: TEM plots of TSO2@TiO2; Figure S3: EDS plots of TSO2@TiO2; Figure S4: CV plots of (a) TFl (b) TS (c) TSO2 (d) TFl@TiO2 (e) TS@TiO2 (f) TSO2@TiO2.
Author Contributions
Methodology and synthesis, H.G. and Y.X.; manuscript drafting, H.G. and J.L.; PHP test, Y.X.; data analysis, H.G.; conceptualization, S.L.; writing—review and editing, J.L. and S.L.; supervision, S.L.; project administration, S.L.; funding acquisition, J.L. and S.L.; S.L. provided guidance during all stages. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (no. 22169009), the Jiangxi Provincial Natural Science Foundation (nos. 20212ACB204007 and 20232BAB213007), the High-level Talents Research Initiation Project of Jiangxi University of Science and Technology (no. 205200100676), and the Jiangxi Key Laboratory of Functional Molecular Materials Chemistry (no. 205200100543).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Carrillo, A.; González-Aguilar, J.; Romero, M.; Coronado, J. Solar energy on demand: A review on high temperature thermochemical heat storage systems and materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef]
- Wang, Y.O.; Suzuki, H.; Xie, J.J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J.W. Mimicking natural photosynthesis: Solar to renewable H2 fuel synthesis by z-scheme water splitting systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef]
- Xu, M.-L.; Li, D.-D.; Sun, K.; Jiao, L.; Xie, C.-F.; Ding, C.-M.; Jiang, H.-L. Interfacial microenvironment modulation boosting electron transfer between metal nanoparticles and MOFs for enhanced photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 16372–16376. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent developments of TiO2-based photocatalysis in the hydrogen evolution and photodegradation: A review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-K.; Mei, S.-B.; Jiang, H.-P.; Wang, L.-L.; Tang, H.; Liu, Q.-Q. Recent advances in TiO2-based S-scheme heterojunction photocatalysts. Chin. J. Catal. 2023, 5, 137–158. [Google Scholar] [CrossRef]
- Rafique, M.; Hajra, S.; Irshad, M.; Usman, M.; Imran, M.; Assiri, M.-A.; Ashraf, W.-M. Hydrogen production using TiO2-based photocatalysts: A comprehensive review. ACS Omega 2023, 8, 25640–25648. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-P.; Guo, R.-T.; Bi, Z.-X.; Zhang, Z.-R.; Li, C.-F.; Pan, W.-G. Recent progress of covalent organic frameworks-based materials in photocatalytic applications: A review. Small 2023, 19, 2303632. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-Q.; Xie, J.; Chen, X.-B.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Park, J. Visible and near infrared light active photocatalysis based on conjugated polymers. J. Ind. Eng. Chem. 2017, 51, 27–43. [Google Scholar] [CrossRef]
- Mansha, M.; Ahmad, T.; Ullah, N.; Khan, S.-A.; Ashraf, M.; Ali, S.; Tan, B.; Khan, I. Photocatalytic water-splitting by organic conjugated polymers: Opportunities and challenges. Chem. Rec. 2022, 22, e202100336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.-M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-H.; Chen, X.-R.; Lin, L.-H.; Fang, Y.-X.; Wang, X.-C. Biomimetic donor–acceptor motifs in conjugated polymers for promoting exciton splitting and charge separation. Angew. Chem. Int. Ed. 2018, 57, 8729–8733. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Lin, Z.-Y.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.-Y.; Wang, X.-C. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Han, C.-Z.; Dong, P.-H.; Tang, H.-R.; Zheng, P.-Y.; Zhang, C.; Wang, F.; Huang, F.; Jiang, J.-X. Realizing high hydrogen evolution activity under visible light using narrow band gap organic photocatalysts. Chem. Sci. 2021, 12, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-M.; Shu, C.; Zhang, C.; Ma, W.-Y.; Ren, S.-B.; Wang, F.; Chen, Y.; Zeng, J.-H.; Jiang, J.X. Substituent effect of conjugated microporous polymers on the photocatalytic hydrogen evolution activity. J. Mater. Chem. A 2020, 8, 2404–2411. [Google Scholar] [CrossRef]
- Ru, C.-L.; Zhou, T.; Zhang, J.; Wu, X.; Sun, P.-Y.; Chen, P.-Y.; Zhou, L.; Zhao, H.; Wu, J.-C.; Pan, X.-B. Introducing secondary acceptors into conjugated polymers to improve photocatalytic hydrogen evolution. Macromolecules 2021, 54, 8839–8848. [Google Scholar] [CrossRef]
- Wang, J.-L.; Ouyang, G.-C.; Wang, D.-W.; Li, J.; Yao, J.-H.; Li, W.-S.; Li, H.-X. Enhanced photocatalytic performance of donor-acceptor-type polymers based on a thiophene-contained polycyclic aromatic Unit. Macromolecules 2021, 54, 2661–2666. [Google Scholar] [CrossRef]
- Cheng, J.-Z.; Liu, L.-L.; Liao, G.-F.; Shen, Z.-Q.; Tan, Z.-R.; Xing, Y.-Q.; Li, X.-X.; Yang, K.; Chen, L.; Liu, S.-Y. Achieving an unprecedented hydrogen evolution rate by solvent exfoliated CPP-based photocatalysts. J. Mater. Chem. A 2020, 8, 5890–5899. [Google Scholar] [CrossRef]
- Cheng, J.-Z.; Tan, Z.-R.; Xing, Y.-Q.; Shen, Z.-Q.; Zhang, Y.-J.; Liu, L.-L.; Yang, K.; Chen, L.; Liu, S.-Y. Exfoliated conjugated porous polymer nanosheets for highly efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2021, 9, 5787–5795. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Huang, Q.; Wang, S.-L.; Li, Z.-Y.; Li, B.-Y.; Jin, S.-B.; Tan, B.-E. Crystalline covalent triazine frameworks by in situ oxidation of alcohols to aldehyde monomers. Angew. Chem. Int. Ed. 2018, 57, 11968–11972. [Google Scholar] [CrossRef]
- Wang, K.-W.; Yang, L.-M.; Wang, X.; Guo, L.-P.; Cheng, G.; Zhang, C.; Jin, S.-B.; Tan, B.-E.; Cooper, A. Covalent triazine frameworks via a low-temperature polycondensation approach. Angew. Chem. Int. Ed. 2017, 56, 14149–14153. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.-B.; Clowes, R.; Berardo, E.; Jelfs, K.-E.; Zwijnenburg, M.-A.; Sprick, R.; Cooper, A.-I. Structurally diverse covalent triazine-based framework materials for photocatalytic hydrogen evolution from water. Chem. Mater. 2019, 31, 8830–8838. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, C.; Liu, J.; Zeng, Y.-F.; Wang, D.-D.; Zhou, W.-Q.; Gu, L.; Wu, H.-W.; Liu, G.-F.; Zhao, Y.-L. Integrating suitable linkage of covalent organic frameworks into covalently bridged inorganic/organic hybrids toward efficient photocatalysis. J. Am. Chem. Soc. 2020, 142, 4862–4871. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nakada, A.; Springer, M.-A.; Kawaguchi, T.; Suzuki, K.; Kaji, H.; Baburin, I.; Kuc, A.; Heine, T.; Suzuki, H.; et al. Identification of prime factors to maximize the photocatalytic hydrogen evolution of covalent organic frameworks. J. Am. Chem. Soc. 2020, 142, 9752–9762. [Google Scholar] [CrossRef]
- Chen, W.-B.; Wang, L.; Mo, D.-Z.; He, F.; Wen, Z.-L.; Wu, X.-J.; Xu, H.-X.; Chen, L. Modulating benzothiadiazole-based covalent organic frameworks via halogenation for enhanced photocatalytic water splitting. Angew. Chem. Int. Ed. 2020, 59, 16902–16909. [Google Scholar] [CrossRef]
- Zhao, Z.-F.; Zheng, Y.-L.; Wang, C.; Zhang, S.-N.; Song, J.; Li, Y.-F.; Ma, S.-Q.; Cheng, P.; Zhang, Z.-J.; Chen, Y. Fabrication of robust covalent organic frameworks for enhanced visible-light-driven H2 evolution. ACS Catal. 2021, 11, 2098–2107. [Google Scholar] [CrossRef]
- Hu, Z.-C.; Wang, Z.-F.; Zhang, X.; Tang, H.-R.; Liu, X.-C.; Huang, F.; Cao, Y. Conjugated polymers with oligoethylene glycol side chains for improved photocatalytic hydrogen evolution. iScience 2019, 13, 33–42. [Google Scholar] [CrossRef]
- Wang, W.-H.; Ting, L.-Y.; Ayakumar, J.; Chang, J.-L.; Lin, W.-C.; Chung, C.-C.; Elsayed, M.-H.; Lu, C.-Y.; Elewa, A.-M.; Chou, H.-H. Design and synthesis of phenylphosphine oxide-based polymer photocatalysts for highly efficient visible-light-driven hydrogen evolution. Sustain. Energy Fuels 2020, 4, 5264–5270. [Google Scholar] [CrossRef]
- Lin, W.-C.; Jayakumar, J.; Chang, C.-L.; Ting, L.-Y.; Elsayed, M.-H.; Abdellah, M.; Zheng, K.-B.; Elewa, A.-M.; Lin, Y.-T.; Liu, J.-J.; et al. Effect of energy bandgap and sacrificial agents of cyclopentadithiophene-based polymers for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2021, 298, 120577. [Google Scholar] [CrossRef]
- Tan, Z.-R.; Xing, Y.-Q.; Cheng, J.-Z.; Zhang, G.; Shen, Z.-Q.; Zhang, Y.-J.; Liao, G.; Chen, L.; Liu, S.-Y. EDOT-based conjugated polymers accessed via C-H direct arylation for efficient photocatalytic hydrogen production. Chem. Sci. 2022, 13, 1725–1733. [Google Scholar] [CrossRef]
- Zhang, G.-G.; Lan, Z.-A.; Wang, X.-C. Conjugated polymers: Catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef]
- Do, H.-H.; Nguyen, D.-L.T.; Nguyen, X.-C.; Le, T.-H.; Nguyen, T.-P.; Trinh, Q.-T.; Ahn, S.-H.; Vo, D.-V.N.; Kim, S.-Y.; Le, Q.-V. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Ioravanti, A.; Amato, F.-S.; Maddalena, P. Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.-Q.; Wang, W.-K.; Wang, L.-L.; Tang, H.; Hu, J.; Liu, Q.-Q. Organic-inorganic heterojunction photocatalysts: From organic molecules to frameworks. Mater. Sci. Semicond. Process. 2023, 164, 107623. [Google Scholar] [CrossRef]
- Wei, G.; Niu, F.; Wang, Z.; Liu, X.; Feng, S.; Hu, K.; Gong, X.; Hua, J. Enhanced photocatalytic H2 evolution based on a polymer/TiO2 film heterojunction. Mater. Today Chem. 2022, 26, 101075. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Han, W.; Yang, Y.; Zhang, H.-Y.; Wang, Y.; Wang, L.; Sun, X.-J.; Zhang, F.-M. S-scheme heterojunction of black TiO2 and covalent-organic framework for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 446, 137213. [Google Scholar] [CrossRef]
- Xing, Y.-Q.; Tan, Z.-R.; Cheng, J.-Z.; Shen, Z.-Q.; Zhang, Y.-J.; Chen, L.; Liu, S.-Y. In situ C-H activation-derived polymer@TiO2 p-n heterojunction for photocatalytic hydrogen evolution. Sustain. Energy Fuels 2021, 5, 5166–5174. [Google Scholar] [CrossRef]
- Ye, H.-N.; Wang, Z.-Q.; Yu, F.-T.; Zhang, S.-C.; Kong, K.-Y.; Gong, X.-Q.; Hua, J.-L.; Tian, H. Fluorinated conjugated poly(benzotriazole)/g-C3N4 heterojunctions for significantly enhancing photocatalytic H2 evolution. Appl. Catal. B Environ. 2020, 267, 118577. [Google Scholar] [CrossRef]
- Shen, H.-Q.; Shang, D.-D.; Li, L.-H.; Li, D.; Shi, W.-D. Rational design of 2D/2D covalent-organic framework/TiO2 nanosheet heterojunction with boosted photocatalytic H2 evolution. Appl. Surf. Sci. 2022, 578, 152024. [Google Scholar] [CrossRef]
- Liu, S.-J.; Zou, Q.-C.; Ma, Y.; Chi, D.-J.; Chen, R.; Fang, H.-X.; Hu, W.; Zhang, K.; Chen, L.-F. Metal-organic frameworks derived TiO2/carbon nitride heterojunction photocatalyst with efficient catalytic performance under visible light. Inorg. Chem. 2022, 536, 120918. [Google Scholar] [CrossRef]
- Tatykayev, B.; Chouchene, B.; Balan, L.; Gries, T.; Medjahdi, G.; Girot, M.; Uralbekov, B.; Schneider, R. Heterostructured g-CN/TiO2 photocatalysts prepared by thermolysis of g-CN/MIL-125(Ti) composites for efficient pollutant degradation and hydrogen production. Nanomaterials 2020, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Parida, K. A review on TiO2/g-C3N4 visible-light- responsive photocatalysts for sustainable energy generation and environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103896. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Yang, H.-G.; Xu, S.-B.; Yang, L.; Song, Y.-Q.; Jiang, L.; Dan, Y. Dramatic enhancement of visible light photocatalysis due to strong interaction between TiO2 and end-group functionalized P3HT. Appl. Catal. B Environ. 2015, 174–175, 193–202. [Google Scholar] [CrossRef]
- Hou, H.-J.; Zhang, X.-H.; Huang, D.-K.; Ding, X.; Wang, S.-Y.; Yang, X.-L.; Li, S.-Q.; Xiang, Y.-G.; Chen, H. Conjugated microporous poly(benzothiadiazole)/TiO2 heterojunction for visible-light-driven H2 production and pollutant removal. Appl. Catal. B Environ. 2017, 203, 563–571. [Google Scholar] [CrossRef]
- Shu, G.; Wang, Y.; Li, Y.-D.; Zhang, S.; Jiang, J.-X.; Wang, F. A high performance and low-cost poly (dibenzothiophene-S, S-dioxide) @TiO2 composite with hydrogen evolution rate up to 51.5 mmol h−1 g−1. J. Mater. Chem. A 2020, 8, 18292–18301. [Google Scholar] [CrossRef]
- Sheng, Z.Q.; Xing, Y.Q.; Chen, Y.; Zhang, G.; Liu, S.Y.; Chen, L. Nanoporous and nonporous conjugated donor-acceptor polymer semiconductors for photocatalytic hydrogen production. Beilstein J. Nanotechnol. 2021, 12, 607–623. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Sheng, J.-F.; Zhao, X.-B.; Mo, J.; Wang, J.-L.; Chen, Z.; Dong, H.-J.; Li, C.M. Recent progress in conjugated polymers-based donor–acceptor semiconductor materials for photocatalytic hydrogen evolution from water splitting. Catalysts 2023, 13, 850. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, B.; Xu, J.-S.; Yu, J.-G.; Cao, S.-W. Donor-acceptor-based conjugated polymers for photocatalytic energy conversion. EnergyChem 2023, 29, 100116. [Google Scholar] [CrossRef]
- Pati, P.-B.; Damas, G.; Tian, L.; Fernandes, D.-L.A.; Zhang, L.; Pehlivan, I.-B.; Edvinsson, T.; Araujo, C.-M.; Tian, H.-N. An experimental and theoretical study of an efficient polymer nano-photocatalyst for hydrogen evolution. Energy Environ. Sci. 2017, 10, 1372–1376. [Google Scholar] [CrossRef]
- Reyes, Y.I.A.; Ting, L.-Y.; Tu, X.; Chen, H.-Y.T.; Chou, H.-H.; Coluccini, C. Mechanistic studies of hydrogen evolution reaction on donor-acceptor conjugated polymer photocatalysts. Appl. Sci. 2020, 10, 7017. [Google Scholar] [CrossRef]
- Brédas, J.-L.; Beljonne, D.; Coropceanu, V.; Cornil, J. Charge-transfer and energy-transfer processes in π-conjugated oligomers and polymers: A molecular picture. Chem. Rev. 2004, 104, 4971–5004. [Google Scholar] [CrossRef]
- Xiang, Y.-G.; Wang, X.-P.; Zhang, X.-H.; Hou, H.-J.; Dai, K.; Huang, Q.-Y.; Chen, H. Enhanced visible light photocatalytic activity of TiO2 assisted by organic semiconductors: A structure optimization strategy of conjugated polymers. J. Mater. Chem. A 2018, 6, 153–159. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.-P.; Dong, W.-B.; Zhang, X.-H.; Rao, L.; Chen, H.; Huang, D.-K.; Xiang, Y.-G. Enhanced light-driven hydrogen-production activity induced by accelerated interfacial charge transfer in donor–acceptor conjugated polymers/TiO2 hybrid. Chem. Eur. J. 2019, 25, 3362–3368. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Shen, Z.-Q.; Cheng, J.-Z.; Liu, L.-L.; Yang, K.; Wen, H.; Liu, S.-Y. C-H activation derived CPPs for photocatalytic hydrogen production excellently accelerated by a DMF cosolvent. J. Mater. Chem. A 2019, 7, 24222–24229. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.; Huang, X.-M.; Yang, L.-J.; Liu, P.; Chen, N.; Li, C.-Z.; Liu, S.-Y. Synthesis of Long-Chain Oligomeric Donor and Acceptors via Direct Arylation for Organic Solar Cells. Chin. J. Chem. 2024, 42, 523–532. [Google Scholar] [CrossRef]
- Yu, F.-T.; Wang, Z.-Q.; Zhang, S.-C.; Ye, H.-N.; Kong, K.-Y.; Gong, X.-Q.; Hua, J.-L.; Tian, H. Molecular engineering of donor–acceptor conjugated polymer/g-C3N4 heterostructures for significantly enhanced hydrogen evolution under visible-light irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Guo, Y.-X.; Sun, J.-Y.; Guo, T.; Liu, Y.; Yao, Z.-Y. Emerging Light-Harvesting Materials Based on Organic Photovoltaic D/A Heterojunctions for Efficient Photocatalytic Water Splitting. Angew. Chem. Int. Ed. 2024, 19, e202319664. [Google Scholar]
- Hu, N.-N.; Cai, Y.-L.; Li, L.; Wang, X.-S.; Gao, J.-K. Amino-functionalized titanium-based metal-organic framework for photocatalytic hydrogen production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef]
- Ye, D.-N.; Liu, L.; Peng, Q.-M.; Qiu, J.-B.; Gong, H.; Zhong, A.-G.; Liu, S.-Y. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.-N.; Liu, L.; Zhang, Y.-J.; Qiu, J.-B.; Tan, Z.-R.; Xing, Y.-Q.; Liu, S.-Y. Tunable donor-acceptor linear conjugated polymers involving cyanostyrylthiophene linkages for visible-light-driven hydrogen production. Molecules 2023, 28, 2203. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-Z.; Xiang, S.-H.; Jin, S.-L.; Zhang, C.; Jiang, J.-X. Rational design of conjugated microporous polymer photocatalysts with definite D−π—A structures for ultrahigh photocatalytic hydrogen evolution activity under natural sunlight. ACS Catal. 2023, 13, 204–212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).