Abstract
In the literature, the chemical composition of Rhododendron tomentosum is mainly represented by the study of isoprenoid compounds of essential oil. In contrast, the study of the content of flavonoids will contribute to the expansion of pharmacological action and the use of the medicinal plant for medical purposes. The paper deals with the technology of extracts from Rh. tomentosum shoots using ethanol of various concentrations and purified water as an extractant. Extracts from Rh. tomentosum were obtained by a modified method that combined the effects of ultrasound and temperature to maximize the extraction of biologically active substances from the raw material. Using the method of high-performance thin-layer chromatography in a system with solvents ethyl acetate/formic acid/water (15:1:1), the following substances have been separated and identified in all the extracts obtained: rutin, hyperoside, quercetin, and chlorogenic acid. The total polyphenol content (TPC) and total flavonoid content (TFC) were estimated using spectrophotometric methods involving the Folin-Ciocalteu (F-C) reagent and the complexation reaction with aluminum chloride, respectively. A correlation analysis was conducted between antioxidant activity and the polyphenolic substance content. Following the DPPH assay, regression analysis shows that phenolic compounds contribute to about 80% (r2 = 0.8028, p < 0.05) of radical scavenging properties in the extract of Rh. tomentosum. The extract of Rh. tomentosum obtained by ethanol 30% inhibits the growth of test cultures of microorganisms in 1:1 and 1:2 dilutions of the clinical strains #211 Staphylococcus aureus and #222 Enterococcus spp. and the reference strain Pseudomonas aeruginosa ATCC 10145.
1. Introduction
Rhododendron tomentosum (formerly Ledum palustre) is an evergreen, squat shrub that has a significant distribution area on the territory of Ukraine throughout its northern part [1]. Rh. tomentosum is widespread mainly in wet and swampy coniferous and deciduous forests, sphagnum marshes, and peatlands in the northern regions of Europe, North America, including the northern parts of Canada and Alaska, Siberia, and North Asia [2]. The popular names of Rh. tomentosum are marsh labrador tea, northern labrador tea, and wild rosemary [3,4,5]. As a raw material of Rh. tomentosum, only young shoots of the first year up to 10.0 cm long are harvested. They are crushed together with flowers and leaves. The Rh. tomentosum has a low level of regeneration, so after cutting the shoots, the biomass is restored only after 3 years. Rh. tomentosum is a pharmacopeial plant raw material in France and Germany (French Pharmacopoeia, 2007; German Homoeopathic Pharmacopoeia, 2000).
In traditional Ukrainian medicine, the shoots of Rh. Tomentosum are used in the form of an aqueous infusion or decoction. Historically, this infusion has been used to treat various respiratory and pulmonary ailments, such as bronchitis, tuberculosis, pertussis (whooping cough), and asthma. On the other hand, decoction and oil infusion are used as an ointment formulated with an animal fat base, which is applied topically to address issues such as eczema, scabies, and insect stings. And the shoots of Rh. Tomentosum have long been used as an insecticide to repel moths and other insects by placing the shoots indoors. In the pharmaceutical market of Ukraine, there is a plant blend containing the shoots of Rh. Tomentosum, “Fitobronkhol” (Liktravy, Zhytomyr), and separately, the shoots of Rh. tomentosum (PJSC Pharmaceutical Factory “VIOLA”, Zaporizhzhia) can be purchased at a pharmacy to treat diseases that are accompanied by cough in the form of an infusion (10 g in 200 mL of water at the dose of ¼ of a glass, 2–3 times per day).
In the traditional medicine of other peoples of the world, Rh. tomentosum has been used to treat headaches, toothache, pain, and shingles. The leaves are also used as marsh tea, which is considered to act as an abortifacient, diaphoretic, diuretic, emetic, expectorant, and galactagogue. Rh. tomentosum leaves and shoots are used in China to treat coughs and asthma, to decrease blood pressure, and as an antifungal agent [6,7,8].
Despite the long tradition of its use, this plant has always been treated with caution due to its essential oil content. The fragrance of Rh. tomentosum is so intense that it can cause a headache. Drinking excessive amounts of Rh. tomentosum tea is not recommended, as it is very strong and can lead to serious side effects, including intestinal disturbances, drowsiness, and a strong diuretic effect. Rh. tomentosum has a high content of volatile compounds in the essential oil; one of the more toxic compounds involved is the sesquiterpenoid ledol, which has an effect on the nervous system [6].
Table 1 shows collected information on the results of the study of the pharmacological activity of shoots, which are presented in scientific publications and placed in publicly accessible reference databases.

Table 1.
Summary of pharmacological studies of Rh. tomentosum.
Rh. tomentosum is considered by scientists primarily as an ethereal medicinal plant, but it also contains other groups of biologically active substances, such as phenol carboxylic acids, flavonoids, polysaccharides, and pectin substances [5,8]. The chemical composition of the plant in the literature is represented mainly by the study of isoprenoid compounds of essential oil. In contrast, the content of polyphenolic compounds has not been studied enough. Given the wide biological activity of polyphenolic compounds as flavonoids, it is important to study the technology of extracts from the shoots of Rh. tomentosum and carry out a qualitative and quantitative study of their composition [26]. Thus, extraction of dried plant material with different solvents was performed, and characterization of the obtained extracts was achieved by chromatographic (HPTLC), spectrophotometric, and microbiological methods.
2. Results
For the research, 10 samples of extracts of Rh. tomentosum were prepared, nine of which were obtained by extraction with ethanol of various concentrations from 10% to 90% in steps of 10% (EtOH10, EtOH20, etc.) and one with purified water (EtOH0). On the one hand, this approach was chosen due to the fact that ethanol is the most acceptable compound in pharmaceutical drug technology due to its relatively safe toxic profile, and on the other hand, the studied group of biologically active substances, polyphenolic compounds, are soluble in a wide range of ethanol concentrations [27,28,29]. The liquid extracts were obtained by the method of threefold fractional maceration in combination with the effect of ultrasound in the ratio of raw materials/extractant 1:20. The first portion of the extract was collected separately, the second and third portions were combined and evaporated to dryness, and the resulting dry residue was dissolved in the first portion of the extract. Thus, the extracts to be studied were prepared in the ratio of raw material to extractant 1:5.
2.1. HPTLC-UV/Vis Method Development
The purpose of the chromatographic study was to identify flavonoids in Rh. tomentosum using active markers, which were subsequently used as standard samples for the quantitative determination of the total flavonoid content by the UV–Vis method. For the chromatographic separation of polyphenol compounds, several eluent systems were tested: ethyl acetate/methanol/water/formic acid (50:10:7:1), ethyl acetate/methyl ethyl ketone/formic acid/water (50:30:10:10), and ethyl acetate/water/formic acid/acetic acid (68:18:7:7). The highest resolution of flavonoids and phenolic acids was obtained using the solvent system ethyl acetate/formic acid/water (15:1:1); the obtained HPTLC chromatograms are shown in Figure 1 and Figure 2.
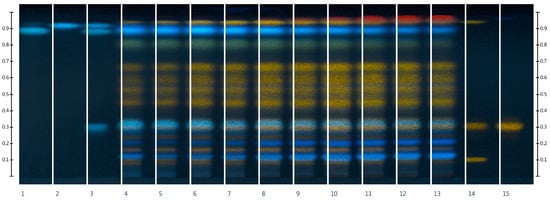
Figure 1.
HPTLC fingerprints of the extracts of Rh. tomentosum and standards with increasing Rf at λ 366 nm after derivatization: 1—rosmarinic acid, 2—caffeic acid, 3—chlorogenic acid + rosmarinic acid + caffeic acid, 4—EtOH0, 5—EtOH10, 6—EtOH20, 7—EtOH30, 8—EtOH40, 9—EtOH50, 10—EtOH60, 11—EtOH70, 12—EtOH80, 13—EtOH90, 14—rutin + hyperoside + quercetin, 15—hyperoside.
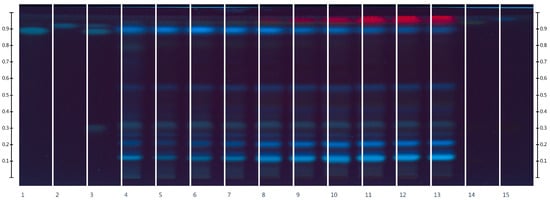
Figure 2.
HPTLC fingerprints of the extracts of Rh. tomentosum and standards with increasing Rf at λ 254 nm before derivatization: 1—rosmarinic acid, 2—caffeic acid, 3—chlorogenic acid + rosmarinic acid + caffeic acid, 4—EtOH0, 5—EtOH10, 6—EtOH20, 7—EtOH30, 8—EtOH40, 9—EtOH50, 10—EtOH60, 11—EtOH70, 12—EtOH80, 13—EtOH90, 14—rutin + hyperoside + quercetin, 15—hyperoside.
In the process of HPTLC fingerprint analysis, the identification of substances is based on the Rf value (position of the bands and color background) of the standard substances and the studied extracts [30,31]. HPTLC chromatograms visually show similarities and differences in the composition of the test samples. As can be seen from the results, on the chromatographic strips of the test samples scanned after derivatization in UV light at 366 nm, there are zones that correspond in color and Rf to the standard samples of rutin Rf (0.12), hyperoside Rf (0.32), and quercetin Rf (0.94) [32,33]. As can be seen from Figure 1, rutin, hyperoside, and quercetin are present in all extract samples regardless of the extractant, but in terms of the intensity of color and the size of the colored zones, it can be argued that samples in which the extractants were purified water and ethanol 10% are somewhat inferior. In addition to flavonoids, standards of phenolic acids, namely chlorogenic acid, rosmarinic acid, and caffeic acid, were applied to the chromatographic plate [34,35].
After scanning chromatographic zones before derivatization in UV light at 254 nm (Figure 2) and after detection in UV light at 366 nm (Figure 1), chlorogenic acid Rf (0.32) was identified. The substances rutin, hyperoside, quercetin, and chlorogenic acid were also detected by the authors [3,36] in the raw material of Rh. tomentosum. The zones of rosmarinic acid and caffeic acid standard substances differ in color in UV light at 254 nm, which may indicate their absence in the raw material.
2.2. Determination of Total Phenolic Content
The Folin-Ciocalteu (F-C) assay is a colorimetric method based on single-electron transfer reactions between the F-C reagent and phenolic compounds. Phenolic compounds act as effective oxygen radical scavengers because phenolic radicals’ electron reduction potential is lower than oxygen radicals, and phenoxyl radicals are less reactive than oxygen radicals. Thus, phenolic compounds’ scavenging of reactive oxygen radicals halts further oxidative reactions [37]. The results of determining the total phenolic compounds in the Rh. tomentosum extracts are presented in Figure 3. The total phenolic content was expressed as gallic acid equivalents (mg of GAE/g).
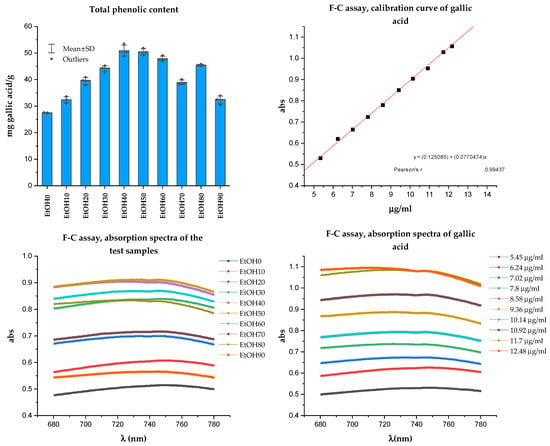
Figure 3.
Result of Folin-Ciocalteu assay.
2.3. Determination of Total Flavonoid Content
The most commonly used method for quantifying the total flavonoid content (TFC) is the method of differential spectrophotometry after the complexation reaction with AlCl3 [38,39]. Due to the multifactorial dependence of the results of spectrophotometric quantification of the TFC, the reproducibility of the technique receives special attention. Therefore, at the stage of the methodology development, the electronic absorption spectra of extracts and the spectra obtained by three methods that differed in conditions were taken (Figure 4). All samples were tested under the same sample preparation conditions: a reaction time of 30 min at room temperature without direct sunlight [40].
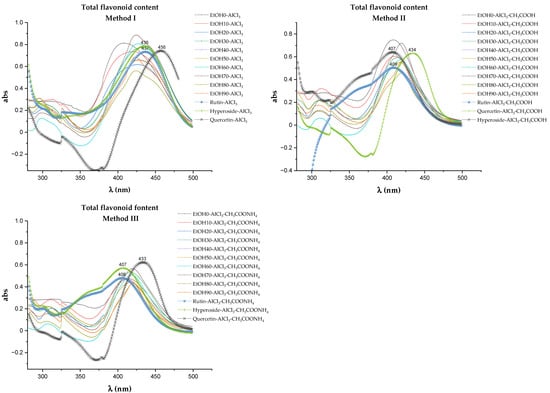
Figure 4.
Absorption spectra of the test samples and aluminum chloride complexes.
The study of the TFC in the analyzed samples, depending on the extractant used, was carried out in comparison with such standard samples as rutin, hyperoside, and quercetin. The TFC of Rh. tomentosum determined in extracts under the conditions of three methods and expressed as rutin, hyperoside, and quercetin equivalents are shown in Figure 5.
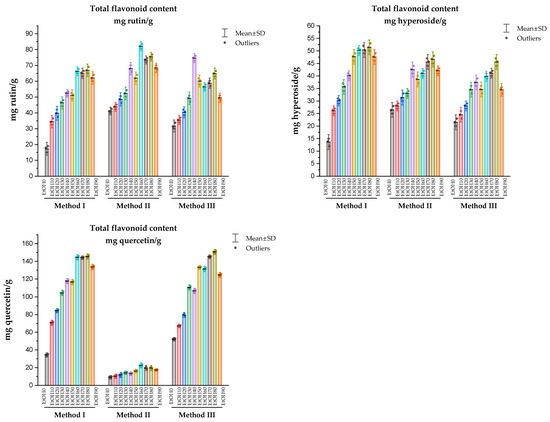
Figure 5.
The total content of flavonoids of Rh. tomentosum extracts determined using three methods, expressed as equivalents of rutin, hyperoside, and quercetin.
2.4. DPPH Radical Scavenging Activity
The DPPH radical is known for its remarkable stability due to the delocalization of the radical in aromatic rings. The radical is neutralized in assays by accepting either a hydrogen atom or an electron from an antioxidant species (or reducing agent). It is converted into a reduced form (DPPH or DPPH-H) during this process. The unpaired electron of the DPPH radical absorbs strongly at 517 nm, resulting in a deep purple color. However, when the odd electron pairs up with another electron, the initial color gradually fades to pale yellow [37]. The results of radical scavenging activity of the Rh. tomentosum extracts are presented in Figure 6. The antioxidant activity was expressed as quercetin equivalent antioxidant capacity (mg QEAC/mL).
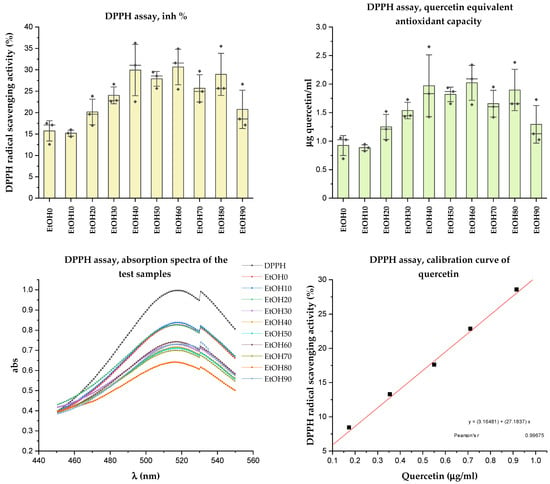
Figure 6.
Result of DPPH assay.
2.5. Determining Antimicrobial Activity In Vitro
By the well agar diffusion method, there were no growth inhibition zones of the tested microorganisms around the wells with the water extract. However, when the extract was dripped onto the inoculated agar, there was growth inhibition. It could be explained by the slow diffusion of the extract into the agar. Ethanol extracts inhibited the growth of Gram-positive bacteria such as staphylococci (#223, #221, and #b2) and enterococci and did not inhibit the growth of Gram-negative microorganisms and fungi (Figure 7). Extracts EtOH70 and EtOH60 showed the best antimicrobial activity against staphylococci and the reference Pseudomonas. Despite this, EtOH30-90 extracts inhibited the growth of Pseudomonas aeruginosa. It was often possible to observe the secondary growth of microorganisms in the zone of growth retardation of the culture, which indicates an insufficient concentration of the active substance for a bactericidal effect on some microorganisms. Ethanol control in all dilutions did not inhibit the growth of the tested microorganisms.
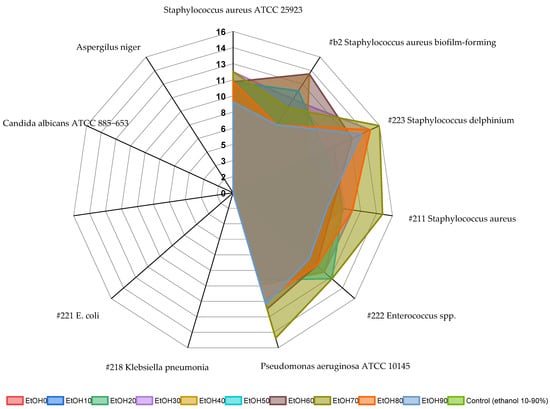
Figure 7.
Antimicrobial activity of Rh. tomentosum extracts determined by the well diffusion method, shown as diameter of growth inhibition. Extracts EtOH70 and EtOH60 showed the best antimicrobial activity against staphylococci and the reference Pseudomonas. Color area = size of growth retardation zone.
The water extract EtOH0 partially inhibited the growth of all tested microorganisms in the first dilution (1:1). In the second dilution (1:2), there was no inhibition. A bactericidal effect was observed against Enterococcus spp. in the first dilution (the colonies were pale and small in contrast to the colonies from the second dilution) (Table 2).

Table 2.
Antimicrobial effect of Rh. tomentosum extracts by the method of serial dilutions (1:1; 1:2), number of colony-forming units (CFU).
Ethanol extracts EtOH10 and EtOH20 inhibit the growth of bacteria in dilutions 1:1 and partially in 1:2. The EtOH10 extract practically did not inhibit the clinical strain of biofilm-forming Staphylococcus aureus #b2 and the museum strain of Pseudomonas aeruginosa ATCC 10145, while the EtOH20 extract inhibited these strains at 1:1 dilution. Ethanol extract EtOH30 completely inhibits the growth of test cultures of microorganisms in 1:1 and 1:2 dilutions of the clinical strains #211 Staphylococcus aureus and #222 Enterococcus spp. and the reference strain Pseudomonas aeruginosa ATCC 10145. Ethanol extracts from EtOH40 to EtOH90 completely suppress the growth of test cultures of microorganisms in 1 and 2 dilutions, as well as ethyl alcohol (control).
3. Discussion
Polyphenols are a numerous class of secondary metabolites of plant raw materials, which today has about 10,000 identified individual compounds [41]. They are produced by plants to protect them from abiotic and biotic factors. They can be classified into five different subgroups based on their structure: phenolic acids (which are further subdivided into hydroxybenzoic, and hydroxycinnamic acids), flavonoids (subdivided into flavonols, flavan-3-ols, flavones, flavanones, isoflavones, flavanonols, and anthocyanidins), coumarins, lignans, and stilbenes. Among them, flavonoids are the most abundant in plants [38].
Using the HPTL method (Figure 1 and Figure 2), flavonols such as rutin, hyperoside, quercetin, and one hydroxycinnamic acid, chlorogenic acid, were identified in all studied extracts of Rh. tomentosum. The presence of these substances in the raw materials of Rh. tomentosum causes a wide spectrum of pharmacotherapeutic action characteristic of these substances. This may partly explain the effectiveness of infusions and decoctions in folk medicine. At the same time, the presence of various groups of biologically active substances in the raw material may lead to a synergistic pharmacotherapeutic effect. Therefore, appropriate methods are employed to assess the total content of some groups of substances.
Several analytical techniques can determine polyphenol content. An alternative method for evaluating the content of total polyphenols in various samples is to use a spectrophotometric method such as Folin-Ciocalteu (F-C) or a method that utilizes an aluminum complexation reaction for total flavonoids. These methods are not specific and are typically used to assess the total content of all polyphenols or some subgroup of polyphenols. The F-C method is based on single-electron transfer reactions between the F-C reagent and phenolic compounds [37]. The TPC was expressed as gallic acid equivalents. As can be seen from Figure 3, all studied extracts of Rh. tomentosum have substances of polyphenol structure; however, the maximum amount is observed in extracts extracted with ethanol at a concentration of 40% and 50% (50.91 ± 2.78 and 50.45 ± 1.60 mgGAE/g, respectively, for EtOH40 and EtOH50). The wide range of ethanol concentrations at which the maximum extraction of polyphenolic substances is observed may be due to the fact that, for example, glycoside and aglycone forms of flavonoids have different solubility in ethanol [28].
During the determination of TFC using the complexation reaction with aluminum chloride in various reaction media, variations in the values of the bathochromic shift of the electronic absorption spectrum were noted (Figure 4). Quercetin, rutin, and hyperoside were used as flavonoid markers, which were identified in the extracts of Rh. tomentosum by the HTPLC method. Correct determination of absorption maxima affects the final result of calculations of TFC (Figure 5). Numerous scientific publications are devoted to the study of the spectrophotometric behavior of flavonoids in reactions with AlCl3, which demonstrate that the differential spectra of flavonoids (wavelength and intensity of maximum absorption) depend on the chemical nature of the flavonoids, the stoichiometric ratio of AlCl3 molecules and flavonoids, the pH of the medium, the time and conditions (in darkness or daylight) of the reaction, etc. [42,43,44,45,46,47].
According to the scientific literature, the reaction of complexation with AlCl3 can potentially have several centers for the course, the localization of which is determined by the presence of a nearby hydroxyl and carbonyl group in the C-4 position of the C-ring [38,39,41,45]. The 3-OH and C=O group (“3-4 site”), 5-OH and C=O group (“5-4 site”), or a pair of 3′-OH and 4′-OH groups of ring B (“3′-4′ site”) can participate in the binding of metal ions. Figure 8 shows possible ways of complexation using the example of the quercetin molecule [38].
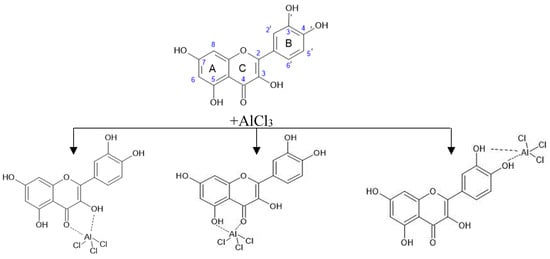
Figure 8.
Diagram of possible focal points of binding between the quercetin molecule and AlCl3.
A possible simultaneous substitution along the rings A and B leads to a bathochromic shift of bands and an increase in the intensity of absorption [38]. Rutin and hyperoside flavonoid molecules differ from quercetin in the presence of a glycosidic substituent attached to the oxygen atom at position 3, so rutin and hyperoside have a “5-4 site” and a “3′-4′ site” to form complexes with aluminum ions.
The bathochromic shift of absorption maxima in the electronic spectra of both the test samples and the standard substances of rutin, hyperoside, and quercetin compared to the published data in scientific articles [39,43,44] can be explained by the use of ammonium acetate instead of potassium or sodium acetates (Method III) and a different, twice greater concentration of AlCl3 in all three methods.
Under the conditions of an increase in the concentration of AlCl3 compared to those described in the literature [44], a significant bathochromic shift is observed as a possible result of interaction at several binding coordination centers in the flavonoid molecule. According to the authors [46], the interaction of flavonoid molecules with aluminum ions occurs sequentially in the order of binding “3′-4′ site”—“3-4 site”—“5-4 site” [46,48]. This means that the binding of aluminum ions to the site “3-4 site” will take place when all “3′-4′ sites” are occupied.
Analysis of the obtained data (Figure 4) indicates that the introduction of donor substituents into the reaction mixture leads to a bathochromic shift in all test samples. The value of bathochromic shift for extracts obtained by Method I is in the range of 65–85 nm and maxima are observed at 420–431 nm. The values of bathochromic shift for test samples obtained by methods II and III practically coincide and are slightly less than 65–80 nm, and the absorption maxima are in the wavelength range of 405–420 nm. The difference in the spectral behavior of flavonoids can be explained by the fact that flavones and flavonols from AlCl3, which contain hydroxyl groups at C-3 and/or C-5 and C-4 ketol groups, form acid-resistant complexes and acid-labile complexes with ortho-dihydroxyl systems (3′-OH and 4′-OH groups of ring B) [38,46]. The complexes formed between AlCl3 and the ortho-dihydroxyl groups of ring B decompose in the presence of acid. Thus, by performing the complexation reaction of flavonoids with AlCl3 in various reaction media under these experimental conditions, it can be assumed that there are such groups of flavonoids as flavones and flavonols with a potential antioxidant effect [49].
The TFC of Rh. tomentosum determined in liquid extracts under the conditions of three methods and expressed as equivalents of rutin, hyperoside, and quercetin are shown in Figure 5. As can be seen from the data, the use of quercetin as a standard substance to recalculate the total amount of flavonoids in Rh. tomentosum extracts under these experimental conditions can give both false-positive and false-negative results. The question of the dependence of the results of determining the total flavonoid content on the choice of the standard of flavonoids, experimental conditions, and selected wavelengths for calculations is of critical importance and is discussed by the authors in publications [43,44,45]. Thus, the question of the dependence of the results of determining the amount of flavonoids on the experiment conditions and the selected wavelengths for calculations is of critical importance and affects the final result of the determination.
For the raw material of Rh. tomentosum shoots, according to the results of chromatographic and spectrophotometric studies, it is advisable to use rutin or hyperoside as a standard substance. Regardless of the selected markers and method, the largest amount of flavonoids was found in the samples obtained using 60%, 70%, and 80% ethyl alcohol as an extractant.
Analyzing the antioxidant activity of the Rh. tomentosum extracts using the DPPH assay (Figure 6), it was observed that the extracts obtained by extraction with ethanol concentrations ranging from 30% to 90% exhibit slightly higher values of radical scavenging activity, falling within the range of 15.19 ± 1.14%–30.65 ± 6.75%. There is a correlation between the values obtained during the determination of TPC and antioxidant capacity. Quercetin was used as a standard antioxidant, positive control, and its calibration curve was determined in the range of concentration from 0.1 to 1.0 µg/mL. Quercetin equivalent antioxidant capacity was calculated using the regression equation between radical scavenging activity (%) of the extracts of Rh. tomentosum and mg QEAC/mL (Pearson’s r 0.996, p < 0.05). The quercetin equivalent antioxidant capacity of the extracts of Rh. tomentosum ranged from 0.88 ± 0.08 to 2.02 ± 0.49 mgQEAC/mL. However, no significant difference in the antioxidant activity of the samples was found, and the obtained values were within the standard deviation.
The relationship between total flavonoid content under different methods and total phenolic and antioxidant activity using the DPPH assay extract of Rh. tomentosum is shown in Figure 9.
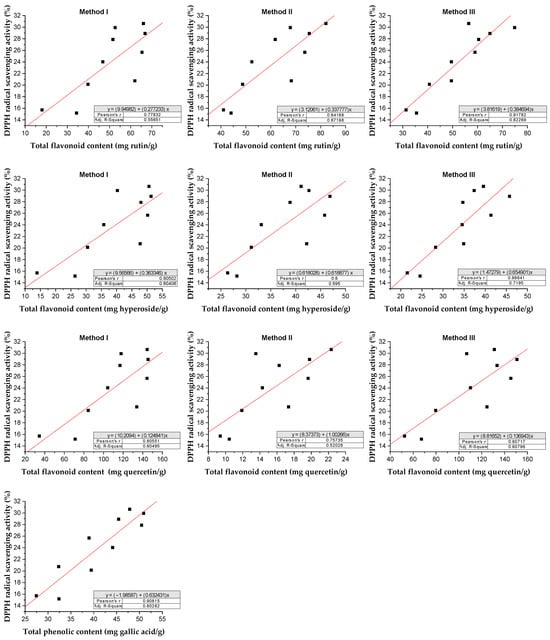
Figure 9.
Relationship between total flavonoid content and total phenolic content and antioxidant activity of Rh. tomentosum by DPPH assay.
Following the DPPH assay, regression analysis shows that phenolic compounds contribute to about 80% (r2 = 0.8028, p < 0.05) of the radical scavenging properties in the extract of Rh. tomentosum [50,51]. Similarly, flavonoids contribute to about 55–82% (r2 = 0.5565, p < 0.05; r2 = 0.6719, p < 0.05; r2 = 0.8226, p < 0.05 under the methods I, II, and II, respectively) expressed as rutin equivalents, about 60–72% (r2 = 0.60406, p < 0.05; r2 = 0.595, p < 0.05; r2 = 0.7195, p < 0.05 under the methods I, II, and II, respectively) expressed as hyperoside equivalents, and about 52–61% (r2 = 0.6049, p < 0.05; r2 = 0.5202, p < 0.05; r2 = 0.6079, p < 0.05 under the methods I, II, and II, respectively) expressed as quercetin equivalents. Evidently, the rest of the proportion of antioxidant activity comes from nonphenolic compounds. Phenolics and flavonoids, in general, constitute a major group of compounds that act as primary antioxidants and are known to react with superoxide anion radicals, hydroxyl radicals, and lipid peroxyl radicals. They are also known to protect DNA from oxidative damage, inhibit the growth of tumor cells, and possess anti-inflammatory and antimicrobial properties [52,53].
When conducting microbiological studies, unexpected results were obtained, which require detailed further studies of the chemical composition of Rh. tomentosum extracts obtained during extraction with water and low-concentration ethanol. Despite the quantitative analysis showing a lower presence of polyphenols and flavonoids in extracts obtained with water and lower ethanol concentrations, ethanol extracts at 10%, 20%, and 30%, and especially at 60% and 70%, concentrations demonstrated significant antimicrobial effectiveness against both Gram-positive and Gram-negative bacteria, with water extracts also displaying this activity, albeit to a reduced degree. As can be seen from the data in Table 2, ethanol extract EtOH30 completely inhibits the growth of test cultures of microorganisms in 1:1 and 1:2 dilutions of the clinical strains #211 Staphylococcus aureus and #222 Enterococcus spp. and the reference strain Pseudomonas aeruginosa ATCC 10145. In a solid nutrient medium (agar diffusion method), extracts EtOH70 and EtOH 60 showed the best antimicrobial activity, but the presence of secondary growth of bacteria may indicate an insufficient amount of active substances in these extracts. On the other hand, water extract and EtOH30 did not show any effect on solid agar, in contrast to liquid medium, which may indicate poor diffusion of active substances.
4. Materials and Methods
4.1. Chemicals
All the chemicals and reagents used were of analytical grade. Ethanol, methanol, ethyl acetate, formic acid, ammonium acetate, glacial acetic acid, aluminum chloride hexahydrate, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), Folin-Ciocalteu reagent, and sodium carbonate were purchased from Poch S.A. (Gliwice, Poland). The standards of rutin, hyperoside, quercetin, rosmarinic acid, caffeic acid, gallic acid, and chlorogenic acid were purchased from Sigma-Aldrich (Poznań, Poland). HPTLC analyses were performed on 20 cm × 10 cm HPTLC silica gel 60 F254 plates (Merck, Darmstadt, Germany).
4.2. Plant Material
The shoots of Rh. tomentosum (Ledum palustre) were collected at the fruiting stage in the forest belts of the Rivne region (Ukraine, 51.302381° N, 26.509154° E). The identity of the raw material was established by Nadiia Kovalska, Assoc. Prof. of the Department of Pharmacognosy and Botany of Bogomolets National Medical University. The voucher specimens of the plant have been deposited in the herbarium of the department [54,55,56]. The shoots were shade-dried at 25–35 °C and stored in tightly closed containers.
4.3. Extracts Preparation
Extracts from Rh. tomentosum shoots were obtained using a modified method that combined the effects of ultrasound and temperature to maximize the extraction of biologically active substances from the raw material [28,57,58,59]. Extracts were prepared using different concentrations of hydroalcoholic mixture and purified water to determine the effect of ethanol concentration on the extraction of flavonoids in the form of aglycones and glycosides since glycosidic and aglyconic forms of flavonoids have different solubility [27,60,61]. The use of ethanol as an extractant is the most acceptable in the pharmaceutical industry for the manufacture of herbal medicines and does not require a test for residual amounts of extractants in plant extracts. About 5.0 g of raw material (exact weight) was crushed to the size of particles passing through a 60 mesh sieve, placed in a flask with a thin section with a capacity of 100 mL, and 50 mL of the corresponding extractant was added to it. An extractant ethanol of various concentrations was used: 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, or purified water. The flask was sealed and left to infuse for 24 h at room temperature (21 ± 1) °C. After infusion, ultrasonic extraction was performed by placing the flasks in an ultrasonic bath (Elmasonic S30H, Elma Schrnidbauer GmbH) for 30 min. Under the influence of ultrasound, there is an unauthorized increase in temperature, which at the end of the ultrasonic extraction of raw materials was (45 ± 0.5) °C. The resulting extract was filtered through cotton wool so that the particles of raw materials did not fall on the filter. Extraction was carried out twice more under the conditions described above with 25 mL of fresh extractant, filtering the extracts into the same flask. The first portion of the extract was collected separately, the second and third portions were combined and evaporated to dryness (rotary evaporator Heating Bath B-100, BUCHI) at a temperature of (45 ± 0.2) °C and a vacuum of 0.01 MPa; the resulting dry residue was dissolved in the first portion of the extract. Thus, the extracts to be studied were prepared in the ratio of raw material to extractant 1:5.
4.4. Chromatographic Analyses
The test samples and standard substances were applied to the plates using an automatic HPTLC application device (Linomat 5, CAMAG, Muttenz, Switzerland). For chromatographic studies, extracts (2 mL) were additionally filtered through a Millipore filter with a pore size of 0.45 µm. The sample application volume was 5 µL. Methanol solutions of standard substances were prepared at the following concentrations: rutin 1 mg/mL, hyperoside 1 mg/mL, quercetin 0.2 mg/mL, rosmarinic acid 1 mg/mL, caffeic acid 1 mg/mL, and chlorogenic acid 0.2 mg/mL.
Chromatographic separation was performed on HPTLC plates in a vertical glass chamber (CAMAG). In the mobile phase, the ethyl acetate/formic acid/water ratio was 15:1:1 [31,62,63]. A total of 70 mL of the mobile phase was used, and the chamber saturation time with the mobile phase was 40 min. After the eluent covered the distance from the start line to the finish line, the plates were removed from the chamber and dried in an oven at (105 ± 2) °C. Detection was based on natural fluorescence before and after derivatization by sequentially spraying 2-aminoethyl diphenylborate (10 g/L) and macrogol 400 (50 g/L) in UV light at 254 and 366 nm. The obtained chromatographic images were analyzed using HPTLC software (visionCATS, CAMAG).
4.5. Method for Determination of Total Phenolic Content
Total phenolic content (TPC) was determined by using the Folin-Ciocalteu method [64]. Briefly, 0.25 mL of the diluted sample was added to 0.25 mL 1:1 diluted Folin-Ciocalteu reagent, and then 0.5 mL of saturated sodium carbonate solution (200 g/L) was added after 4 min, along with 4.0 mL of water. After incubation at room temperature (23–25 °C) for 45 min, the absorbance of the mixture was measured at 760 nm using a U-3900/3900H Hitachi UV–Vis spectrophotometer (Hitachi, Tokyo, Japan). The compensation solution was water. All analyses were performed in triplicate. The total phenolic content was expressed as gallic acid equivalents (mg of GAE/g) according to the formula:
where X—the total phenolic content in terms of gallic acid, mg/g;
K—dilution factor;
A—absorption of diluted sample at 760 nm;
Ast—absorption of solutions of gallic acid at 760 nm.
The calibration range of gallic acid was from 5.45 to 12.48 µg/mL.
4.6. Method for Determining the Total Flavonoid Content
The content of flavonoids was determined using differential spectrophotometry based on the formation of complexes of aluminum ions with flavonoids in a different reaction medium according to the method described in article [45]. Determination was carried out in methanol according to three methods that differed in the reaction medium—the first with the addition of only AlCl3, the second with AlCl3 and CH3COOH, and the third with AlCl3 and CH3COONH4 [44,65,66]. For the UV–Vis analysis, the original test samples were diluted with methanol so that the maximum absorption at the wavelengths of interest (λmax) was within the range of 0.6–0.8.
Method I: 2 mL of methanol, 0.5 mL of the test extract sample (or standard), and 0.20 mL of a 10% solution of AlCl3 in methanol were successively added to a 5.0 mL flask and held for 3 min; the volume of methanol was adjusted to 5.0 mL.
Method II: 2 mL of methanol, 0.5 mL of the test extract sample (or standard). and 0.20 mL of a 10% solution of AlCl3 in methanol were successively added to a 5.0 mL flask and held for 3 min. Next, 0.2 mL of a 1M solution of glacial acetic acid in methanol was added. and the volume was adjusted to 5.0 mL with methanol.
Method III: 2 mL of methanol, 0.5 mL of the test extract sample (or standard). and 0.20 mL of a 10% solution of AlCl3 in methanol were successively added to a 5.0 mL flask and held for 3 min. Next, 0.2 mL of a 1M solution of ammonium acetate in methanol was added. and the volume was adjusted to 5.0 mL with methanol.
Compensation solutions were prepared similarly but without the addition of AlCl3.
The reaction mixtures were thoroughly stirred, held for 30 min at room temperature, and subjected to spectrophotometric analysis in the range of 280 to 500 nm (U-3900/3900H Hitachi). All analyses were performed in triplicate.
The total content of flavonoids in the studied extracts was calculated by the standard method in terms of rutin, hyperoside, or quercetin according to the formula as in the Section 4.5.
Where X—the total content of flavonoids in terms of rutin, hyperoside, or quercetin, mg/g;
K—dilution factor;
A—absorption at 437 nm (Method I), 409 nm (Method II), or 406 nm (Method III) for conversion to rutin; at 436 nm (Method I), 407 nm (Method II), or 407 nm (Method III) for conversion to hyperoside; at 456 nm (Method I), 434 nm (Method II), or 433 nm (Method III) for conversion to quercetin;
Ast—absorption of solutions of standard samples of flavonoids at the same wavelengths.
4.7. DPPH Radical Scavenging Activity
Antioxidant activities of the samples were analyzed by investigating their abilities to scavenge the DPPH% [67]. Briefly, a 76 µM solution of DPPH (1,1-diphenyl-2-picrylhydrazyl) was prepared using 96% ethanol. Then, 0.05 mL of the tested extract was added to 1.95 mL of the DPPH solution in 2 mL tubes. The blank consisted of the same volume of the extract and 1.95 mL of 96% ethanol. The mixtures were then mixed vigorously and allowed to stand at room temperature in the dark for 40 min. The absorbance of the resulting mixtures was read at a wavelength of 517 nm in 40 min, using the spectrophotometer U-3900/3900H Hitachi. The DPPH radical scavenging activity was computed according to the following equation:
where Acontrol is the absorbance of the solution of DPPH against 96% ethanol;
Asample is the absorbance of the reaction mixtures of the extract with DPPH at a wavelength of 517 nm against the same volume of the extract and 1.95 mL of 96% ethanol.
The reaction mixture for measuring Acontrol consisted of 1.95 mL of 76 µM solution of DPPH and 0.05 mL of 96% ethanol. All analyses were performed in triplicate. Quercetin was used as a reference standard, and the results were expressed as mg quercetin/mL extract of Rh. tomentosum.
4.8. Antimicrobial Activity In Vitro
The studied extracts were tested for antimicrobial activity by the method of diffusion in agar and the method of serial dilutions [68,69,70]. For the agar diffusion method, 100 μL of the extract was sprinkled into a well in an agar plate (MPA, Sabouraud), where a bacterial suspension (McFarland 0.5) was inoculated. For the broth serial dilutions assay, we added 50 μL of meat-peptone broth and 100 μL of the studied extract to the first well, mixed, and 50 μL was transferred to the second well (second dilution), which contained 50 μL of the broth, and so on. Then, 50 μL of pure bacterial suspension (0.5 McFarland) was added to each well. The bacterial control contained 50 μL of broth + 50 μL of pure bacterial suspension. The plate was incubated for 24 h (37 °C). To count the number of colonies from each well, a sterile disposable loop (1 μL) was applied to a sector of an agar plate (MPA). After 24 h of incubation, colonies were counted on each sector. The control of test microorganisms was the total bacterial growth.
Ten reference and clinical microbial and fungal strains were used, previously identified by the MALDI TOF system (Bruker, Bremen, Germany) and 16S rRNA gene sequences. Reference strains: Candida albicans ATCC 885-653, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 10145, Aspergilus niger. All clinical strains (b2 Staphylococcus aureus, biofilm-forming; 223 Staphylococcus delphinium; 211 Staphylococcus aureus; 222 Enterococcus spp, 218 Klebsiella pneumonia, and 221 E. coli) were multidrug-resistant or extensively drug-resistant with different antibiotic resistance patterns. Clinical strains were isolated from a patient with healthcare-associated infections from regional hospitals. All testing was repeated in triplicate.
4.9. Statistical Analysis
The mean and standard deviation (SD) were calculated according to the monograph “Statistical Analysis of the Results of a Chemical Experiment” of the State Pharmacopoeia of Ukraine [71]. The average value was established based on 3 measurements. Values of the confidence interval were calculated using Student’s criterion limit. The data are presented as the mean ± SD [46].
5. Conclusions
Using an inexpensive, fast, and reproducible technique (HPTLC) and test markers, the presence of substances of flavonoid structure, rutin, hyperoside, and quercetin, and hydroxycinnamic acids, chlorogenic acid, were established in all the studied samples. The obtained results show that the HPTLC is the method of choice for the analysis of plant herbal extracts. The TPC and TFC were estimated using spectrophotometric methods involving the Folin-Ciocalteu reagent and the complexation reaction with aluminum chloride. The TPC expressed as gallic acid equivalents ranged from 27.42 ± 0.13 to 50.91 ± 2.78 mg GAE/g. Spectrophotometric techniques are proposed based on the reaction of the complexation of flavonoids with aluminum chloride using appropriate standard samples of flavonoid rutin, hyperoside, and quercetin. It has been proven that the course of the complexation reaction, according to the quantification of the flavonoid structure substances by the spectrophotometric method, is influenced by some factors, including the environment, the structure of the complexes, etc. Regardless of the chosen method, for the further production of Rh. tomentosum extracts with a higher content of flavonoid structure substance, it is advisable to use 60%, 70%, and 80% ethyl alcohol as the extractant. Following the DPPH assay, regression analysis shows that phenolic compounds contribute to about 80% (r2 = 0.8028, p < 0.05) of the radical scavenging properties in the extract of Rh. tomentosum. The microbiological evaluation demonstrated that ethanol extracts, particularly those with concentrations of 60% and 70%, as well as those with 10%, 20%, and 30%, were effective against both Gram-positive (clinical and reference staphylococci) and Gram-negative bacteria (reference Pseudomonas aeruginosa). The water-based extract also displayed antimicrobial properties, though its efficacy was comparatively lower.
Author Contributions
Conceptualization, I.J.-M. and H.K.; methodology, H.K., N.B., Y.K. and I.J.-M.; software, O.K.; validation, N.B.; formal analysis, I.J.-M.; investigation H.K., N.B., Y.K. and I.J.-M.; resources, H.K., N.B. and I.J.-M.; data curation, I.J.-M. and N.B.; writing original draft preparation, H.K. and N.B.; writing—review and editing, I.J.-M.; visualization, O.K.; supervision, I.J.-M.; project administration, H.K. and I.J.-M.; funding acquisition, I.J.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project “Supporting the cooperation of the University of Opole with Ukrainian universities within the FORTHEM Alliance 2024”. Publishing this paper was financed by the Polish Agency for Academic Exchange, agreement No. BNI-UE-2023-9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skliar, V.; Sherstuk, M. Size structure of phytopopulations and its quantitative evaluation. Eureka Life Sci. 2016, 1, 9–15. [Google Scholar] [CrossRef]
- Ledum Palustre—Labrador-Tea—Discover Life. Available online: https://www.discoverlife.org/mp/20q?search=Ledum+palustre (accessed on 1 November 2023).
- Dampc, A.; Luczkiewicz, M. Rhododendron Tomentosum (Ledum palustre). A Review of Traditional Use Based on Current Research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef]
- Upyr, T.V.; Tolmachova, K.S.; Koshovyi, O.M.; Komisarenko, A.M.; Kireyev, I.V. The Study of the Chemical Composition and the Pharmacological Activity of the Polysaccharide Complex Obtained from Ledum Palustre. News Pharm. 2016, 3, 32–35. [Google Scholar] [CrossRef]
- Judzentiene, A.; Butkiene, R.; Budiene, J.; Tomi, F.; Casanova, J. Composition of Seed Essential Oils of Rhododendron Tomentosum. Nat. Prod. Commun. 2012, 7, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.; Palmer, J.; McKenna, E.B.; Gendron, F. A Traditional Elder’s Anti-Aging Cornucopia of North American Plants. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; Academic Press: Cambridge, MA, USA, 2015; pp. 3–11. [Google Scholar] [CrossRef]
- Han, M.H.; Han, M.-H.; Kim, K.Y. Research Progress on the Identification and Pharmacological Activity of the Active Compo-Nents of the Rhododendron Species. Biosci. Biotechnol. Res. Asia 2021, 18, 543–565. [Google Scholar] [CrossRef]
- Judžentienė, A. Review: Marsh Rosemary (Rhododendron Tomentosum Harmaja (Ex Ledum Palustre Linn) Growing in Lithuania) Essential Oils and Their Properties. Chemija 2020, 31, 269–277. [Google Scholar] [CrossRef]
- McGill, C.M.; Tomco, P.L.; Ondrasik, R.M.; Belknap, K.C.; Dwyer, G.K.; Quinlan, D.J.; Kircher, T.A.; Andam, C.P.; Brown, T.J.; Claxton, D.F.; et al. Therapeutic Effect of Northern Labrador Tea Extracts for Acute Myeloid Leukemia. Phytother. Res. PTR 2018, 32, 1636–1641. [Google Scholar] [CrossRef]
- Baldea, L.A.N.; Martineau, L.C.; Benhaddou-Andaloussi, A.; Arnason, J.T.; Lévy, É.; Haddad, P.S. Inhibition of Intestinal Glucose Absorption by Anti-Diabetic Medicinal Plants Derived from the James Bay Cree Traditional Pharmacopeia. J. Ethnopharmacol. 2010, 132, 473–482. [Google Scholar] [CrossRef]
- Harbilas, D.; Martineau, L.C.; Harris, C.S.; Adeyiwola-Spoor, D.C.; Saleem, A.; Lambert, J.; Caves, D.; Johns, T.; Prentki, M.; Cuerrier, A.; et al. Evaluation of the Antidiabetic Potential of Selected Medicinal Plant Extracts from the Canadian Boreal Forest Used to Treat Symptoms of Diabetes: Part II. Can. J. Physiol. Pharmacol. 2009, 87, 479–492. [Google Scholar] [CrossRef]
- Kim, S.G. Antioxidant and Anti-Inflammatory Activities of Extracts from Ledum palustre L. Food Sci. Preserv. 2017, 24, 1025–1033. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Zhao, J.-j.; Wu, W.-b.; Zhao, Y.; Zhu, X.-l.; Qu, W.-j. Analgesic and Anti-Inflammatory Activities of Extracts from Ledum palustre L. in Mice. Nat. Prod. Res. Dev. 2010, 22, 326. [Google Scholar]
- Liu HaiJun, L.H.; Sun JinMin, S.J.; Jia LanLing, J.L. Experimental Study of Extract from Ledum Inhibiting the Growth of Tumor-Bearing Mice H22. Chin. J. Gerontol. 2011, 31, 3743–3744. [Google Scholar]
- Tunón, H.; Olavsdotter, C.; Bohlin, L. Evaluation of Anti-Inflammatory Activity of Some Swedish Medicinal Plants. Inhibition of Prostaglandin Biosynthesis and PAF-Induced Exocytosis. J. Ethnopharmacol. 1995, 48, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Judzentiene, A.; Budiene, J.; Svediene, J.; Garjonyte, R. Toxic, Radical Scavenging, and Antifungal Activity of Rhododendron Tomentosum H. Essential Oils. Molecules 2020, 25, 1676. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-M.; Nam, B.-W. Extracts and Essential Oil of Ledum Palustre L. Leaves and Their Antioxidant and Antimicrobial Activities. J. Food Sci. Nutr. 2006, 11, 100–104. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, Q.; Wu, Y.; Zhong, Z.; Ouyang, J. Comparison of the Chemical Compounds and Antioxidant Activities of Essential Oil and Ethanol Extract from Rhododendron Tomentosum Harmaja. J. Essent. Oil Bear. Plants 2017, 20, 927–936. [Google Scholar] [CrossRef]
- Korpinen, R.I.; Välimaa, A.-L.; Liimatainen, J.; Kunnas, S. Essential Oils and Supercritical CO2 Extracts of Arctic Angelica (Angelica archangelica L.), Marsh Labrador Tea (Rhododendron tomentosum) and Common Tansy (Tanacetum vulgare)—Chemical Compositions and Antimicrobial Activities. Molecules 2021, 26, 7121. [Google Scholar] [CrossRef]
- Zhang, L.; Internationals, O. Chemical Composition and Antibacterial Activity of the Essential Oil from Chinese Wild Ledum palustre L. on Vibrio parahaemolyticus. Int. J. Food Nutr. Sci. 2017, 4, 8–12. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Sender, J.; Danuta, U.; Maślanko, W.; Canale, A.; Barboni, L.; Petrelli, R.; Zeppa, L.; et al. Ascaridole-Rich Essential Oil from Marsh Rosemary (Ledum palustre) Growing in Poland Exerts Insecticidal Activity on Mosquitoes, Moths and Flies without Serious Effects on Non-Target Organisms and Human Cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 138, 111184. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Pålsson, K.; Borg-Karlson, A.-K. Evaluation of Extracts and Oils of Mosquito (Diptera: Culicidae) Repellent Plants from Sweden and Guinea-Bissau. J. Med. Entomol. 2006, 43, 113–119. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Pålsson, K.; Borg-Karlson, A.-K. Evaluation of Extracts and Oils of Tick-Repellent Plants from Sweden. Med. Vet. Entomol. 2005, 19, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Ahmed, S.; Rehman, T.; Mumtaz, W.; Anwar, H.; Hussain, G. Anti-Hyperuricemic Potential of Rhododendron Tomentosum Harmaja Syn. Ledum palustre L. 30c and 1M in Potassium Oxonate Induced Rat Model. Indian J. Tradit. Knowl. 2018, 17, 724–731. [Google Scholar]
- Ahmad, M.; Faraazi, A.A.; Aamir, M. The Effect of Ocimum Sanctum and Ledum Palustre on Serum Uric Acid Level in Patients Suffering from Gouty Arthritis and Hyperuricaemia. Bull. Chem. Soc. Ethiop. 2013, 27, 469–473. [Google Scholar] [CrossRef]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. SI Extr. Encapsulation 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Charegaonkar, D. High-Performance Thin-Layer Chromatography: Excellent Automation. In High-Performance Thin-Layer Chromatography (HPTLC); Springer: Berlin/Heidelberg, Germany, 2010; pp. 55–65. [Google Scholar]
- de Rijke, E.; Out, P.; Niessen, W.M.A.; Ariese, F.; Gooijer, C.; Brinkman, U.A.T. Analytical Separation and Detection Methods for Flavonoids. Plant Anal. 2006, 1112, 31–63. [Google Scholar] [CrossRef]
- Orfali, R.; Perveen, S.; Aati, H.Y.; Alam, P.; Noman, O.M.; Palacios, J.; Al-Kurbi, B.S.S.; Al-Taweel, A.M.; Khan, A.; Mehmood, R.; et al. High-Performance Thin-Layer Chromatography for Rutin, Chlorogenic Acid, Caffeic Acid, Ursolic Acid, and Stigmasterol Analysis in Periploca Aphylla Extracts. Separations 2021, 8, 44. [Google Scholar] [CrossRef]
- Scotti, F.; Löbel, K.; Booker, A.; Heinrich, M.S. John’s Wort (Hypericum Perforatum) Products—How Variable Is the Primary Material? Front. Plant Sci. 2018, 9, 1973. [Google Scholar] [CrossRef]
- Larit, F.; León, F.; Jasika-Misiak, I.; Wieczorek, P.P.; Cutler, S.J. Phenolic Content and Antioxidant Activity of Cytisus Villosus and Hypericum Afrum Extracts. Planta Med. 2016, 82, PB21. [Google Scholar] [CrossRef]
- Shanaida, M.; Golembiovska, O.; Jasicka-Misiak, I.; Oleshchuk, O.; Beley, N.; Kernychna, I.; Wieczorek, P.P. Sedative Effect and Standardization Parameters of Herbal Medicinal Product Obtained from the Ocimum americanum L. Herb. Eur. Pharm. J. 2021, 68, 1–9. [Google Scholar] [CrossRef]
- Upyr, T.V.; Zaitsev, G.P.; Koshovyi, O.M.; Komisarenko, A.M. Phenolic composition of the ledum palustre shoots liquid extrac. Coliection Sci. Work. Staff. Memb. Shupyk NHU Ukr. 2015, 24, 239–243. [Google Scholar]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Marby, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1970. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colometric Methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Ramos, R.T.M.; Bezerra, I.C.F.; Ferreira, M.R.A.; Soares, L.A.L. Spectrophotometric Quantification of Flavonoids in Herbal Material, Crude Extract, and Fractions from Leaves of Eugenia Uniflora Linn. Pharmacogn. Res. 2017, 9, 253. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Petry, R.D.; Ortega, G.G.; Silva, W.B. Flavonoid Content Assay: Influence of the Reagent Concentration and Reaction Time on the Spectrophotometric Behavior of the Aluminium Chloride—Flavonoid Complex. Pharm 2001, 56, 465–470. [Google Scholar]
- Mammen, D.; Daniel, M. A Critical Evaluation on the Reliability of Two Aluminum Chloride Chelation Methods for Quantification of Flavonoids. Food Chem. 2012, 135, 1365–1368. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of Total Flavonoid Content by Aluminum Chloride Assay: A Critical Evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Cornard, J.P.; Merlin, J.C. Spectroscopic and Structural Study of Complexes of Quercetin with Al(III). J. Inorg. Biochem. 2002, 92, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and Pharmacological Research in Agrimonia eupatoria L. Herb Extract with Anti-Inflammatory and Hepatoprotective Properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef]
- Struchkov, P. Comparison of Spectrophotometric Methods of Total Flavonoid Assay Based on Complex Formation with Aluminum Chloride as Applied to Multicomponent Herbal Drug Angionorm. J. Pharm. Negat. Results 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Dj, G.; Hl, C.; Sw, C.; Ph, Y. Antioxidative Activities and the Total Phenolic Contents of Tonic Chinese Medicinal Herbs. Inflammopharmacology 2008, 16, 201–207. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Kovalska, N.; Karpiuk, U.; Minarchenko, V.; Cholak, I.; Zaimenko, N.; Skrypchenko, N.; Liu, D. Comparative Analysis of the Content of Sum of Hydroxycinnamic Acids from Leaves of Actinidia Arguta Lindl. Collected in Ukraine and China. J. Chem. 2023, 2023, 2349713. [Google Scholar] [CrossRef]
- Minarchenko, V.; Tymchenko, I.; Pidchenko, V.; Dvirna, T.; Makhynia, L.; Karpiuk, U.; Kovalska, N. Diagnostic Features of Raw Materials of Related Equisetum Species of Ukrainian Flora. J. Res. Pharm. 2022, 26, 1780–1788. [Google Scholar] [CrossRef]
- Minarchenko, V.; Karpiuk, U.; Kovalska, N.; Tymchenko, I.; Dvirna, T.; Cholak, I. Diagnostic Micromorphological Features of Leaf Surface of Selected Species of the Genus L. (Asteraceae). Hacquetia 2023, 22, 131–141. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Horčinová Sedláčková, V.; Kukhtenko, H.; Svydenko, L. Facets of the Elaboration of the Salvia sclarea L. Extracts. Agrobiodivers. Improv. Nutr. Health Life Qual. 2023, 7, 61–69. [Google Scholar]
- Kukhtenko, H.; Bevz, N.; Hudz, N.; Datkhayev, U.; Kukhtenko, O. Alhagi Kirghisorum: Technological Aspects of Its Thick Extract for the Pharmaceutical Application. Agrobiodivers. Improv. Nutr. Health Life Qual. 2022, 6. [Google Scholar] [CrossRef]
- Monagas, M.; Brendler, T.; Brinckmann, J.; Dentali, S.; Gafner, S.; Giancaspro, G.; Johnson, H.; Kababick, J.; Ma, C.; Oketch-Rabah, H.; et al. Understanding Plant to Extract Ratios in Botanical Extracts. Front. Pharmacol. 2022, 13, 981978. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of Phenolics and Essential Oil from Dried Sage (Salvia officinalis) Using Ethanol–Water Mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic Fingerprint, Antioxidant Activity, and Colour Characteristic of Polish Goldenrod (Solidago virgaurea L.) Honey and Flower. Eur. Food Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Shanaida, M.; Hudz, N.; Jasicka-Misiak, I.; Wieczorek, P.P. Polyphenols and Pharmacological Screening of a Monarda fistulosa L. Dry Extract Based on a Hydrodistilled Residue By-Product. Front. Pharmacol. 2021, 12, 563436. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Elena, D.L. Pharmacognostic Methods for Analysis of Herbal Drugs, According to European Pharmacopoeia. In Promising Pharmaceuticals; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef][Green Version]
- Shinkovenko, I.L.; Kashpur, N.V.; Ilyina, T.V.; Kovalyova, A.M.; Goryacha, O.V.; Koshovyi, O.M.; Toryanyk, E.L.; Kryvoruchko, O.V. The Immunomodulatory Activity of the Extracts and Complexes of Biologically Active Compounds of Galium verum L. Herb. Ceska Slov. Farm. Cas. Ceske Farm. Spolecnosti Slov. Farm. Spolecnosti 2018, 67, 25–29. [Google Scholar]
- Yezerska, O.; Hudz, N.; Kobylinska, L.; Filipska, A.; Turkina, V.; Brindza, J.; Wieczorek, P.P. Methodical approach to the determination of the antioxidant activity of the echinacea purpurea and monarda fistulosa tinctures as a quality criterion of the development of their composition and laboratory technology. Acta Medica Leopoliensia 2022, 28, 89–98. [Google Scholar] [CrossRef]
- Eucast: Disk Diffusion Methodology. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology (accessed on 2 November 2023).
- Eucast: Eucast Broth Microdilution Reading Guide Updated. Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=362&cHash=f2196d399c8618b7313ccb7f048f7af5 (accessed on 11 November 2023).
- Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [CrossRef]
- State Pharmacopoeia of Ukraine; Ukrainian Scientific Pharmacopoeial Center of Drugs Quality: Kharkiv, Ukraine, 2015.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).