Zn-Doped MnCO3/CS Composite Photocatalyst for Visible-Light-Driven Decomposition of Organic Pollutants
Abstract
1. Introduction
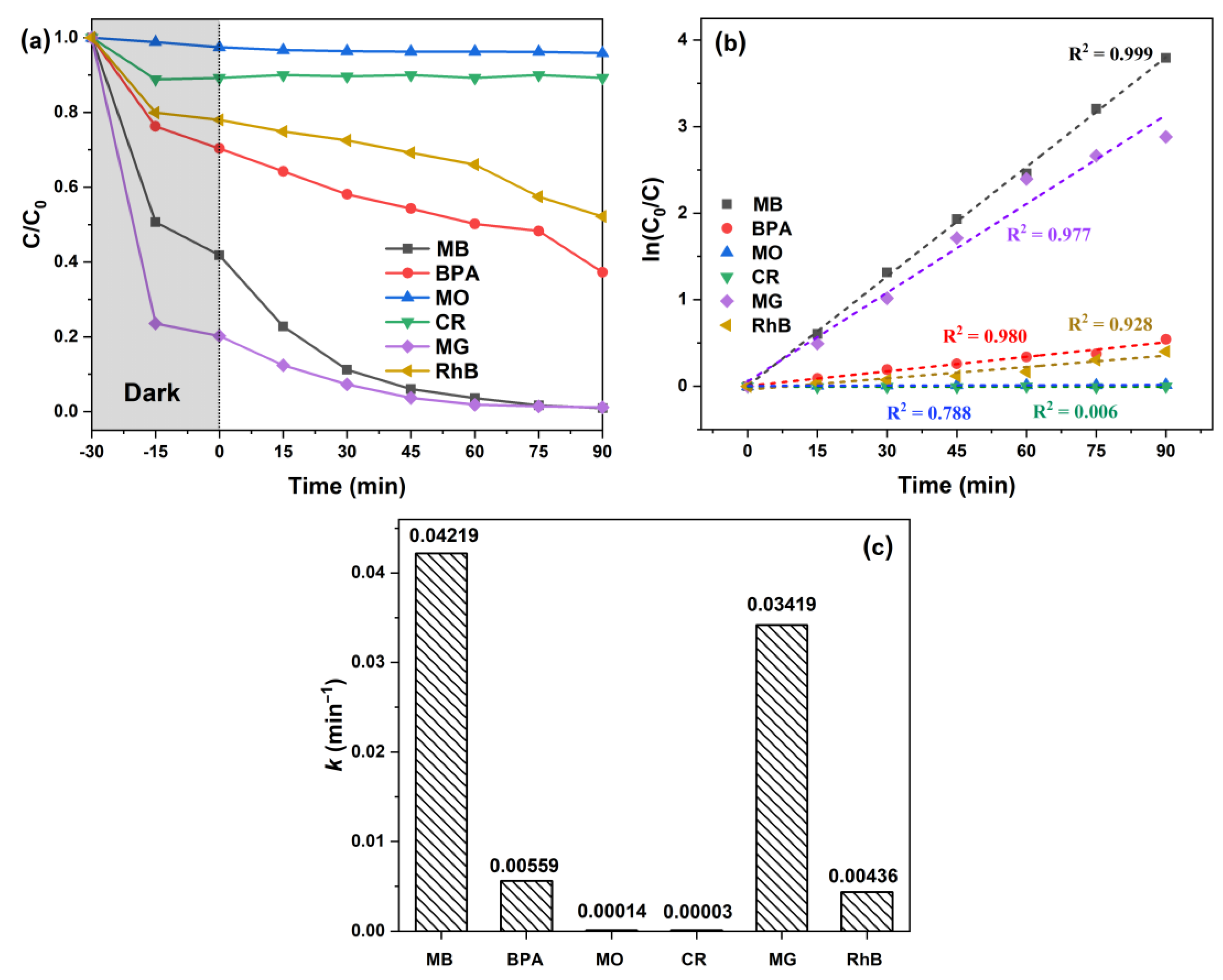
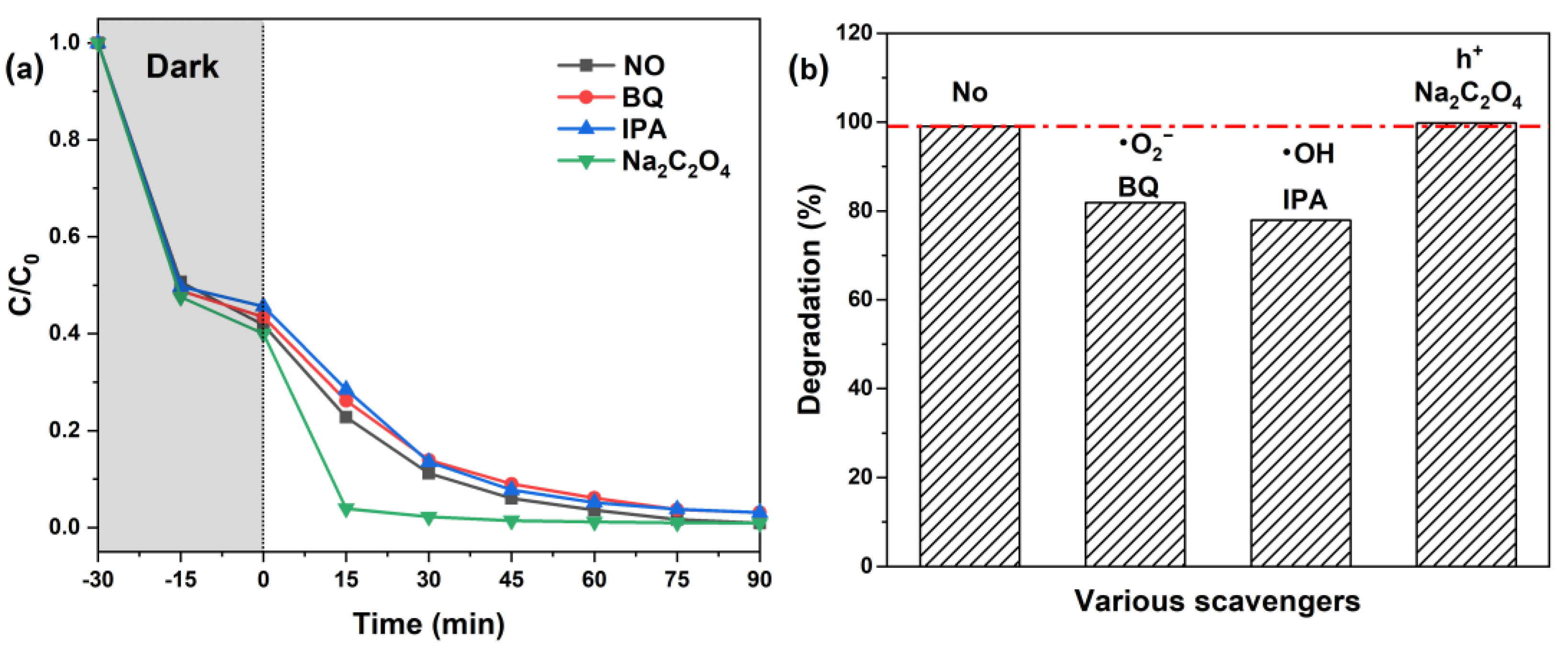
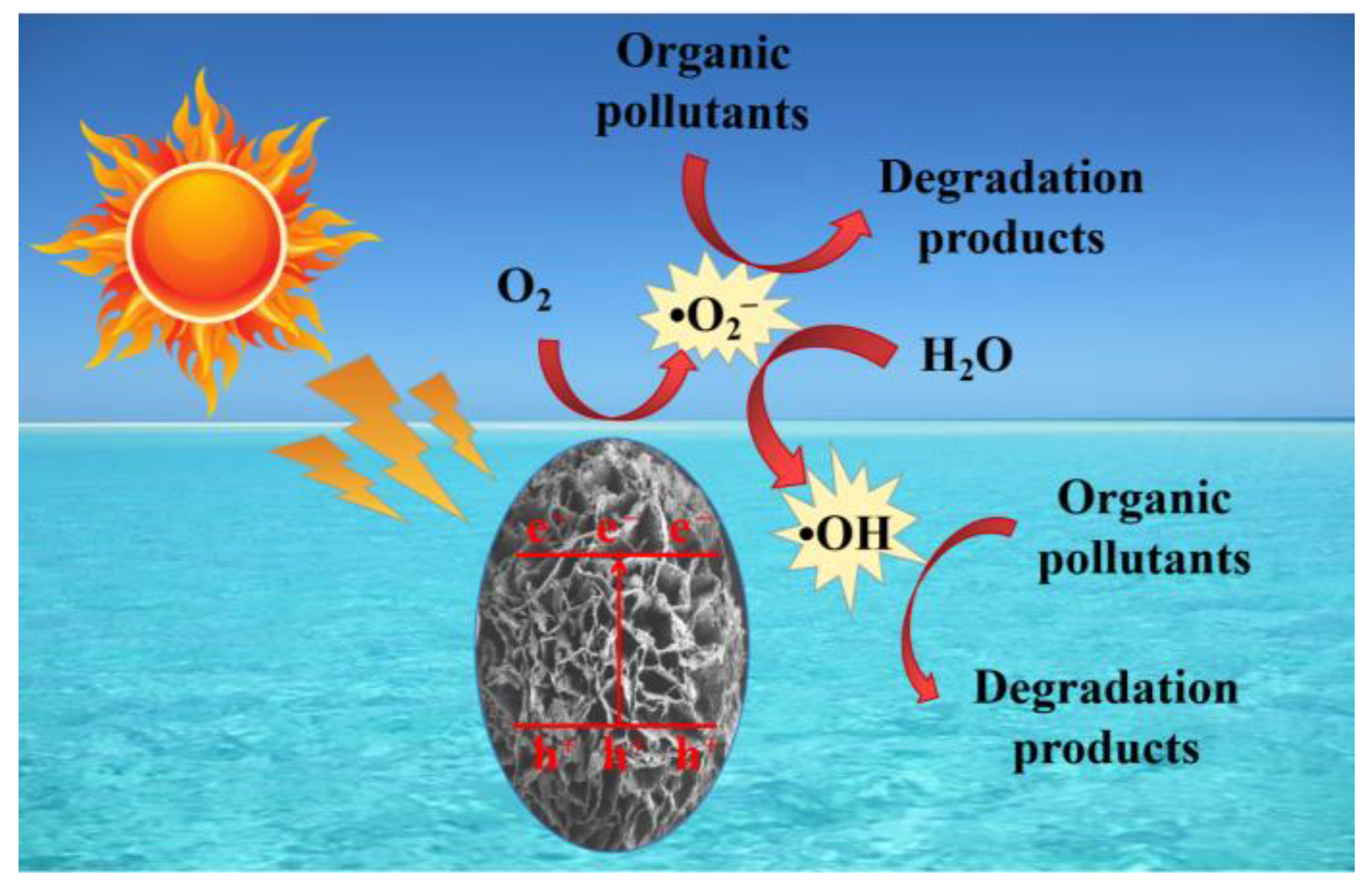
2. Results
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.2.1. Fabrication of Zn-Doped MnCO3
3.2.2. Fabrication of Zn-Doped MnCO3/CS Composites
3.3. Characterization
3.4. Photocatalytic Activity (PCA) Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raza, A.; Ikram, M.; Aqeel, M.; Imran, M.; Ul-Hamid, A.; Riaz, K.N.; Ali, S. Enhanced industrial dye degradation using Co doped in chemically exfoliated MoS2 nanosheets. Appl. Nanosci. 2020, 10, 1535–1544. [Google Scholar] [CrossRef]
- Yi, H.; Huang, D.; Qin, L.; Zeng, G.; Lai, C.; Cheng, M.; Ye, S.; Song, B.; Ren, X.; Guo, X. Selective prepared carbon nanomaterials for advanced photocatalytic application in environmental pollutant treatment and hydrogen production. Appl. Catal. B Environ. 2018, 239, 408–424. [Google Scholar] [CrossRef]
- Li, Y.H.; Lai, Z.; Huang, Z.J.; Wang, H.Y.; Zhao, C.X.; Ruan, G.H.; Du, F.Y. Fabrication of BiOBr/MoS2/graphene oxide composites for efficient adsorption and photocatalytic removal of tetracycline antibiotics. Appl. Surf. Sci. 2021, 550, 149342. [Google Scholar] [CrossRef]
- Kumar, G.; Kumar, J.; Bag, M.; Dutta, R.K. Solar light induced photocatalytic process for reduction of hexavalent chromium and degradation of tetracycline and methylene blue by heterostructures made of SnS2 nanoplates surface modified by ZnWO4 nanorods. Sep. Purif. Technol. 2022, 292, 121040. [Google Scholar] [CrossRef]
- Zheng, J.H.; Liu, X.Y.; Zhang, L. Design of porous double-shell Cu2O@CuCo2O4 Z-Scheme hollow microspheres with superior redox property for synergistic photocatalytic degradation of multi-pollutants. Chem. Eng. J. 2020, 389, 124339. [Google Scholar] [CrossRef]
- Aghasiloo, P.; Yousefzadeh, M.; Latifi, M.; Jose, R. Highly porous TiO2 nanofibers by humid-electrospinning with enhanced photocatalytic properties. J. Alloys Compd. 2019, 790, 257–265. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, X.Y.; Wang, Y.M.; Zhang, X.T.; Wang, X.M. Length Carbon quantum dots from tea enhance z-type BiOBr/C3N4 heterojunctions for RhB degradation: Catalytic effect, mechanisms, and intermediates. Appl. Surf. Sci. 2023, 639, 158254. [Google Scholar] [CrossRef]
- Wang, C.C.; Yi, X.H.; Wang, P. Powerful combination of MOFs and C3N4 for enhanced photocatalytic performance. App. Catal. B Environ. 2019, 247, 24–48. [Google Scholar] [CrossRef]
- Xing, S.T.; Li, W.Q.; Liu, B.; Wu, Y.S.; Gao, Y.Z. Removal of ciprofloxacin by persulfate activation with CuO: A pH-dependent mechanism. Chem. Eng. J. 2020, 382, 122837. [Google Scholar] [CrossRef]
- Tian, N.; Giannakis, S.; Akbarzadeh, L.; Hasanvandian, F.; Dehghanifard, E.; Kakavandi, B. Improved catalytic performance of ZnO via coupling with CoFe2O4 and carbon nanotubes: A new, photocatalysis-mediated peroxymonosulfate activation system, applied towards Cefixime degradation. J. Environ. Manag. 2023, 329, 117022. [Google Scholar] [CrossRef]
- Wei, Z.D.; Li, Y.; Luo, S.L.; Liu, C.B.; Meng, D.S.; Ding, M.Y.; Zeng, G.S. Hierarchical heterostructure of CdS nanoparticles sensitized electrospun TiO2 nanofibers with enhanced photocatalytic activity. Sep. Purif. Technol. 2014, 122, 60–66. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, Y.C.; Huang, Q.L. Solvothermal synthesis of N-doped ZnO microcrystals from commercial ZnO powder with visible light-driven photocatalytic activity. Mater. Lett. 2014, 119, 104–106. [Google Scholar] [CrossRef]
- Zhao, Y.; Mu, Y.L.; Wang, L.; Liu, M.J.; Lai, X.; Bi, J.; Gao, D.J.; Chen, Y.F. MnCO3-RGO composite anode materials: In-situ solvothermal synthesis and electrochemical performances. Electrochim. Acta 2019, 317, 786–794. [Google Scholar] [CrossRef]
- Liu, M.J.; Wang, Q.; Liu, Z.Y.; Zhao, Y.; Lai, X.; Bi, J.; Gao, D.J. In-situ N-doped MnCO3 anode material via one-step solvothermal synthesis: Doping mechanisms and enhanced electrochemical performances. Chem. Eng. J. 2020, 383, 123161. [Google Scholar] [CrossRef]
- Sudrajat, H.; Babel, S.; Ta, A.T.; Nguyen, T.K. Mn-doped TiO2 photocatalysts: Role, chemical identity, and local structure of dopant. J. Phys. Chem. Solids. 2020, 144, 109517. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.F.; Li, Q.; Xin, C.H.; Tian, Y.J.; Zhang, W.P.; Yu, X. Design of porous ZrO2 with well-tuned band structures and strong visible-light harvesting via Zn doping for enhanced visible-light photocatalysis. Chem. Eng. J. 2024, 481, 148489. [Google Scholar] [CrossRef]
- Bhatia, V.; Ray, A.K.; Dhir, A. Enhanced photocatalytic degradation of ofloxacin by co-doped titanium dioxide under solar irradiation. Sep. Purif. Technol. 2016, 161, 1–7. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, J.; Tian, L.J.; Ge, S.J.; Zhai, Z.Y. Photocatalytic degradation of gaseous benzene using Cu/Fe-doped TiO2 nanocatalysts under visible light. Molecules 2024, 29, 144. [Google Scholar] [CrossRef]
- Bahadi, S.A.; Drmosh, Q.A.; Onaizi, S.A. Adsorptive removal of organic pollutants from aqueous solutions using novel GO/bentonite/MgFeAl-LTH nanocomposite. Environ. Res. 2024, 248, 118218. [Google Scholar] [CrossRef]
- Chen, F.; An, W.; Liu, L.; Liang, Y.; Cui, W. Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/ photocatalysis synergy. Appl. Catal. B Environ. 2017, 217, 65–80. [Google Scholar] [CrossRef]
- Zou, W.X.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Yang, K.; Wei, X.H.; Ding, S.; Tian, F.; Li, F. One-pot solvothermal synthesis of carbon dots/Ag nanoparticles/TiO2 nanocomposites with enhanced photocatalytic performance. Ceram. Int. 2018, 44, 22481–22488. [Google Scholar] [CrossRef]
- Chen, Y.X.; Ji, X.B.; Vadivel, S.; Paul, B. Anchoring carbon spheres on BiOBr/g-C3N4 matrix for high-performance visible light photocatalysis. Ceram. Int. 2018, 44, 23320–23323. [Google Scholar] [CrossRef]
- Yao, C.C.; Qin, Y.A.; Li, Y.Y.; An, Q.D.; Xiao, Z.Y.; Wang, C.H.; Zhai, S.R. Activation of peroxymonosulfate by cobalt-embedded carbon aerogels: Preparation and singlet oxygen-dominated catalytic degradation insight. Sep. Purif. Technol. 2023, 307, 122728. [Google Scholar] [CrossRef]
- Hintsho, N.; Petrik, L.; Nechaev, A.; Titinchi, S.; Ndungu, P. Photo-catalytic activity of titanium dioxide carbon nanotube nano-composites modified with silver and palladium nanoparticles. Appl. Catal. B Environ. 2014, 156, 273–283. [Google Scholar] [CrossRef]
- Zhang, H.R.; Zhang, D.; Zhang, D.; Shao, X.Z.; Zhang, T.L.; Wu, R.; Ji, X.H. High-Performance Integrated rGO- Polymeric Ionic Liquid Heteropolyanions for Catalytic Degradation of Azo Dyes. Langmuir 2023, 39, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.F.; Ren, M.; Yue, D.T.; Zhu, Y.; Han, Y.; Bian, Z.F.; Zhao, Y.X. Mesoporous TiO2 films coated on carbon foam based on waste polyurethane for enhanced photocatalytic oxidation of VOCs. Appl. Catal. B Environ. 2017, 212, 1–6. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, J.; Yang, E.; Li, H.; Wang, H. Magnetic Fe3O4-N-doped carbon sphere composite for tetracycline degradation by enhancing catalytic activity for peroxymonosulfate: A dominant non-radical mechanism. Chemosphere 2021, 263, 128011. [Google Scholar] [CrossRef]
- Sun, J.C.; Fan, H.; Wang, N.; Ai, S.Y. Controlled synthesis of Sn doped ZnO microspheres stringed on carbon fibers with enhanced visible-light photocatalytic activities. Sep. Purif. Technol. 2016, 160, 67–72. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Wang, J.; Dong, F.; Chu, P.K.; Zhang, T.; Zhang, Y. Anionic group self-doping as a promising strategy: Band-gap engineering and multi-functional applications of high-performance CO32−doped Bi2O2CO3. ACS Catal. 2015, 5, 4094–4103. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, Z.D.; Ma, Z. Facile formation of Bi2O2CO3/Bi2MoO6 nanosheets for visible light driven photocatalysis. ACS Omega 2019, 4, 3871–3880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Wang, J.J.; Shen, X.Q.; Wang, J.J.; Wang, B.Y.; Gao, D.Z. Br-doped Bi2O2CO3 nanosheets with improved electronic structure and accelerated charge migration for outstanding photocatalytic behavior. Appl. Surf. Sci. 2019, 470, 63–73. [Google Scholar] [CrossRef]
- Cen, W.L.; Xiong, T.; Tang, C.Y.; Yuan, S.D.; Dong, F. Effects of morphology and crystallinity on the photocatalytic activity of (BiO)2CO3 nano/microstructures. Ind. Eng. Chem. Res. 2014, 53, 15002–15011. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wang, Y.X.; Hou, F.L.; Li, H.X.; Yang, Y.; Zhang, X.X.; Yang, Y.Q. Effects of Ag loading on structural and photocatalytic properties of flower-like ZnO microspheres. Appl. Surf. Sci. 2016, 391, 476–483. [Google Scholar] [CrossRef]
- Guo, G.J.; Yan, H. Zn-doped Bi2O2CO3: Synthesis, characterization and photocatalytic properties. Chem. Phys. 2020, 538, 110920. [Google Scholar] [CrossRef]
- Karuppaiah, M.; Sakthivel, P.; Asaithambi, S.; Murugan, R.; Yuvakkumar, R.; Ravi, G. Formation of one dimensional nanorods with microsphere of MnCO3 using Ag as dopant to enhance the performance of pseudocapacitors. Mater. Chem. Phys. 2019, 228, 1–8. [Google Scholar] [CrossRef]
- Sun, D.T.; Bai, H.Y.; Zhao, Y.; Zhang, Q.X.; Bai, Y.J.; Liu, Y.; Pang, X.L.; Wang, F.G.; Ding, J.R.; Xu, D.B.; et al. Amorphous MnCO3/C double layers decorated on BiVO4 photoelectrodes to boost nitrogen reduction. ACS Appl. Mater. Interfaces 2020, 12, 52763–52770. [Google Scholar] [CrossRef]
- Zhang, Z.; Lang, J.; Hua, Z.; Song, Y.; Wang, D. Template free N-doped 3D porous carbon@Co3O4: Towards highly efficient catalysis for peroxymonosulfate degradation of antibiotics. Opt. Mater. 2021, 111, 110538. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zeng, Z.; Zeng, G.; Xiong, W. Metal-organic frameworks derived Bi2O2CO3/porous carbon nitride: A nanosized Z-scheme systems with enhanced photocatalytic activity. Appl. Catal. B Environ. 2020, 267, 118700. [Google Scholar] [CrossRef]
- Li, J.; Zhan, G.; Yu, Y.; Zhang, L. Superior visible light hydrogen evolution of Janus bilayer junctions via atomic-level charge flow steering. Nat. Commun. 2016, 7, 11480. [Google Scholar] [CrossRef]
- Zhao, H.P.; Li, G.F.; Tian, F.; Jia, Q.T.; Liu, Y.L.; Chen, R. g-C3N4 surface-decorated Bi2O2CO3 for improved photocatalytic performance: Theoretical calculation and photodegradation of antibiotics in actual water matrix. Chem. Eng. J. 2019, 36, 468–479. [Google Scholar] [CrossRef]
- Dong, J.R.; Li, P.; Ji, X.Y.; Kang, Y.; Yuan, X.; Tang, J.C.; Shen, B.X.; Dong, H.J.; Lyu, H. Electrons of d-orbital (Mn) and p-orbital (N) enhance the photocatalytic degradation of antibiotics by biochar while maintaining biocompatibility: A combined chemical and biological analysis. J. Hazard. Mater. 2023, 451, 131083. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, N.; Yaseen, M.; Rehan, Z.A.; Zahid, M.; Shakoor, R.A.; Jilani, A.; Iqbal, J.; Rasul, S.; Shahid, I. Coal fly ash supported CoFe2O4 nanocomposites: Synergetic Fenton-like and photocatalytic degradation of methylene blue. Environ. Res. 2022, 206, 112280. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, C.B.; Yuan, Y.X.; Jin, Y.J.; Liu, Y.; Jiang, Z.H.; Li, X.T.; Dai, J.J.; Zhang, Y.W.; Siyal, A.A.; et al. Catalytic degradation of crystal violet and methyl orange in heterogeneous Fenton-like processes. Chemosphere 2023, 344, 140406. [Google Scholar] [CrossRef]
- Hou, M.F.; Ma, C.X.; Zhang, W.D.; Tang, X.Y.; Fan, Y.N.; Wan, H.F. Removal of rhodamine B using iron-pillared bentonite. J. Hazard. Mater. 2011, 186, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wang, H.R.; Jiang, X.Y.; Yuan, G.L.; Wang, X. Enhanced photocatalytic efficiency in degrading organic dyes by coupling CdS nanowires with ZnFe2O4 nanoparticles. Sol. Energy 2020, 195, 271–277. [Google Scholar] [CrossRef]
- Ganiyu, S.A.; Suleiman, M.A.; Al-Amrani, W.A.; Usman, A.K.; Onaizi, S.A. Adsorptive removal of organic pollutants from contaminated waters using zeolitic imidazolate framework composites: A comprehensive and Up-to-date review. Sep. Purif. Technol. 2023, 318, 123765. [Google Scholar] [CrossRef]
- Li, H.X.; Su, L.Y.; Zheng, J.T.; Lu, S.; Yang, Z.X.; Wang, C.H.; Xu, S.D.; Zhou, Q.W.; Tang, J.H.; Huang, M.Z. MOFs derived carbon supporting CuCo nanospheres as efficient catalysts of peroxymonosulfate for rapid removal of organic pollutant. Chem. Eng. J. 2023, 451, 139114. [Google Scholar] [CrossRef]
- Wen, Y.Z.; Shen, C.S.; Ni, Y.Y.; Tong, S.P.; Yu, F. Glow discharge plasma in water: A green approach to enhancing ability of chitosan for dye removal. J. Hazard. Mater. 2012, 201–202, 162–169. [Google Scholar] [CrossRef]
- Xu, L.L.; Li, X.F.; Ma, J.Q.; Wen, Y.Z.; Liu, W.P. Nano-MnOx on activated carbon prepared by hydrothermal process for fast and highly efficient degradation of azo dyes. Appl. Catal. A Gen. 2014, 485, 91–98. [Google Scholar] [CrossRef]
- Hirani, R.A.K.; Hannan, A.; Rafique, N.; Shi, L.; Tian, W.J.; Wang, H.T.; Sun, H.Q. Three-dimensional rGO/CNT/g-C3N4 macro discs as an efffcient peroxymonosulfate activator for catalytic degradation of sulfamethoxazole. J. Hazard. Mater. 2023, 460, 132400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Z.; Khan, S.; Naz, F.; Noman, A.; Nawaz, A.; Ali, S.; Saeed, K.; Ali, N.; Ge, M. Robust iron-doped manganese oxide nanoparticles from facile fabrication to photo-catalytic degradation application of binary dyes mixture. Environ. Res. 2024, 240, 117384. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.J.; Huang, D.L.; Li, J.; Zeng, G.M.; Deng, R.; Yang, Y.; Chen, S.; Li, Z.H.; Gong, X.M.; Li, B.; et al. Assembly of AgI nanoparticles and ultrathin g-C3N4 nanosheets codecorated Bi2WO6 direct dual Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced photocatalytic performance. Chem. Eng. J. 2019, 373, 1144–1157. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhou, X.J.; Shao, C.L.; Li, X.H.; Liu, Y.C. Graphitic carbon nitride/BiOI loaded on electrospun silica nanofifibers with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 455, 952–962. [Google Scholar] [CrossRef]
- Molla, A.; Sahu, M.; Hussain, S. Under dark and visible light: Fast degradation of methylene blue in the presence of Ag-In-Ni-S nanocomposites. J. Mater. Chem. A 2015, 3, 15616–15625. [Google Scholar] [CrossRef]
- Cabrera, A.F.; Rodríguez Torres, C.E.; Marchetti, S.G.; Stewart, S.J. Degradation of methylene blue dye under dark and visible light conditions in presence of hybrid composites of nanostructured MgFe2O4 ferrites and oxygenated organic compounds. J. Environ. Chem. Eng. 2020, 8, 104274. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Li, X.; Yi, X.; Wang, C.C. Facile fabrication of BUC-21/Bi24O31Br10 composites for enhanced photocatalytic Cr(VI) reduction under white light. Chem. Eng. J. 2020, 389, 123431. [Google Scholar] [CrossRef]

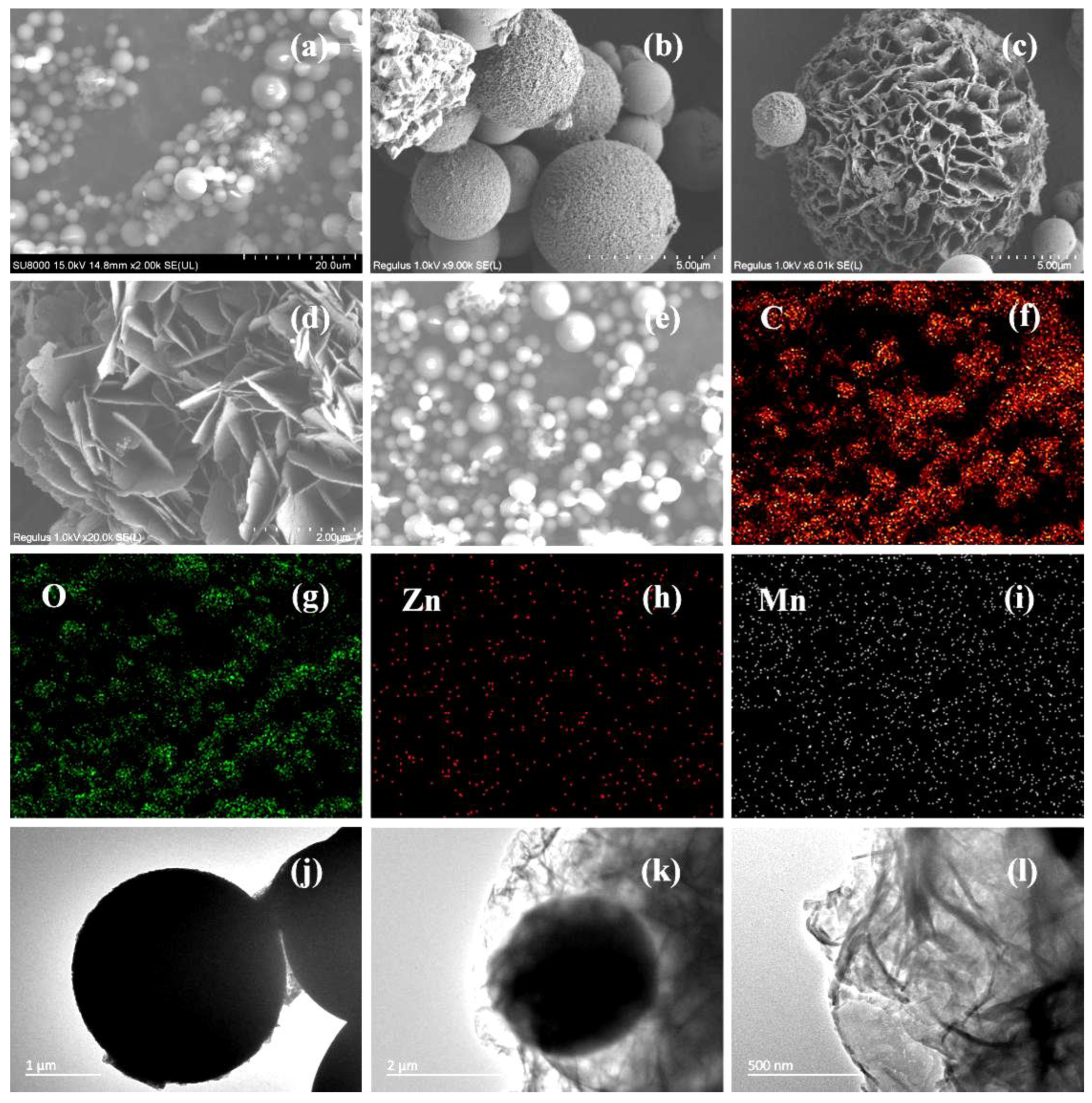
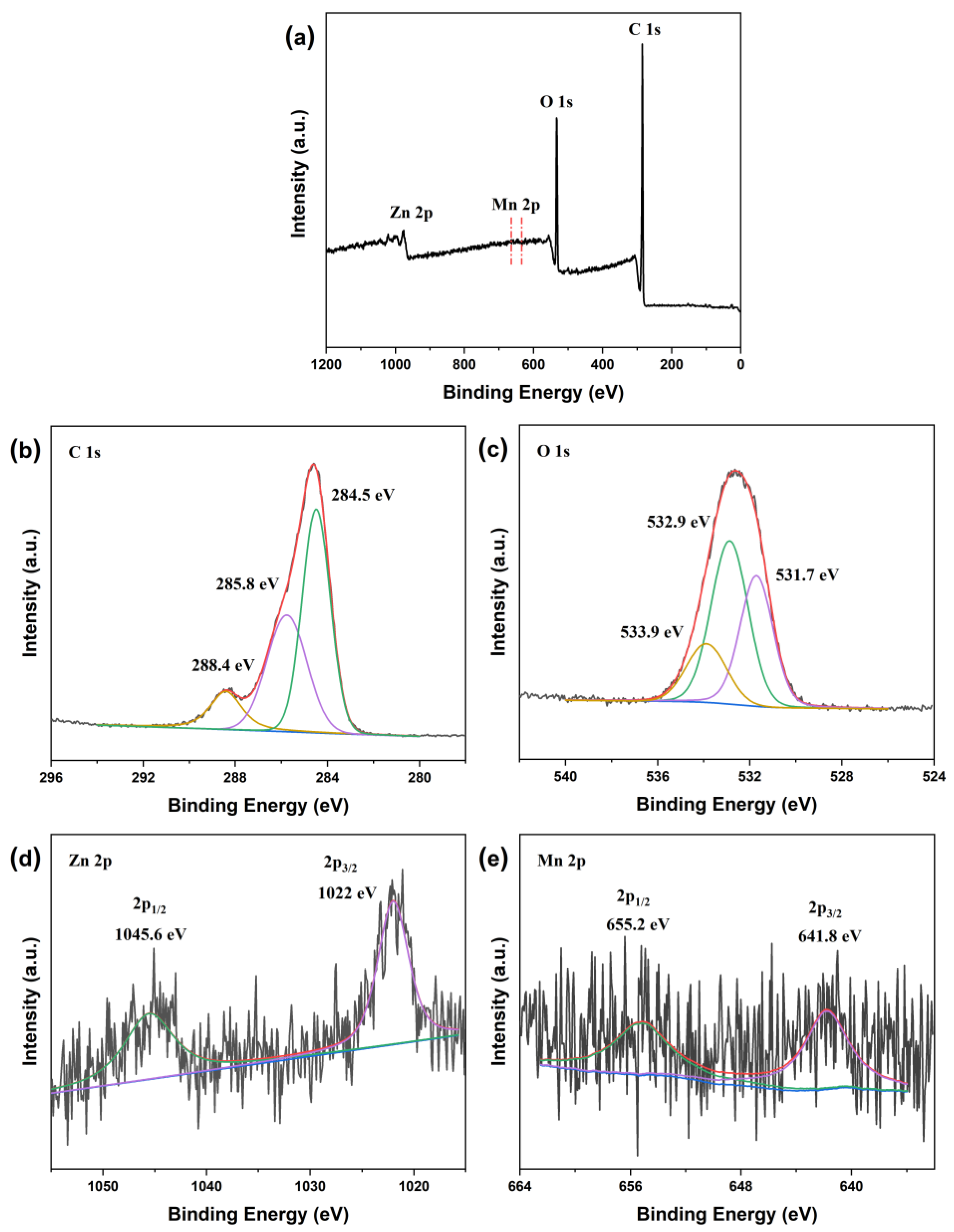
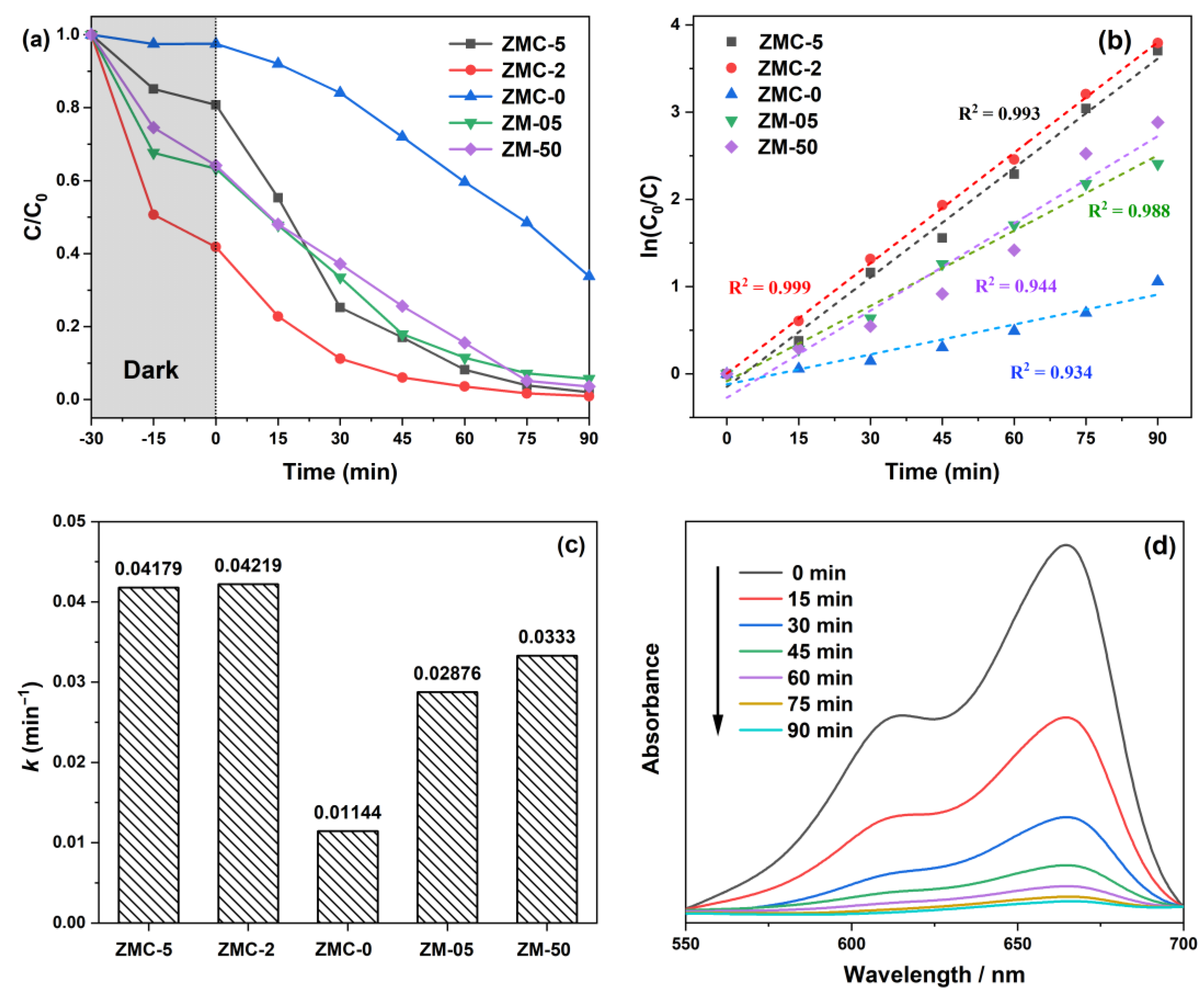
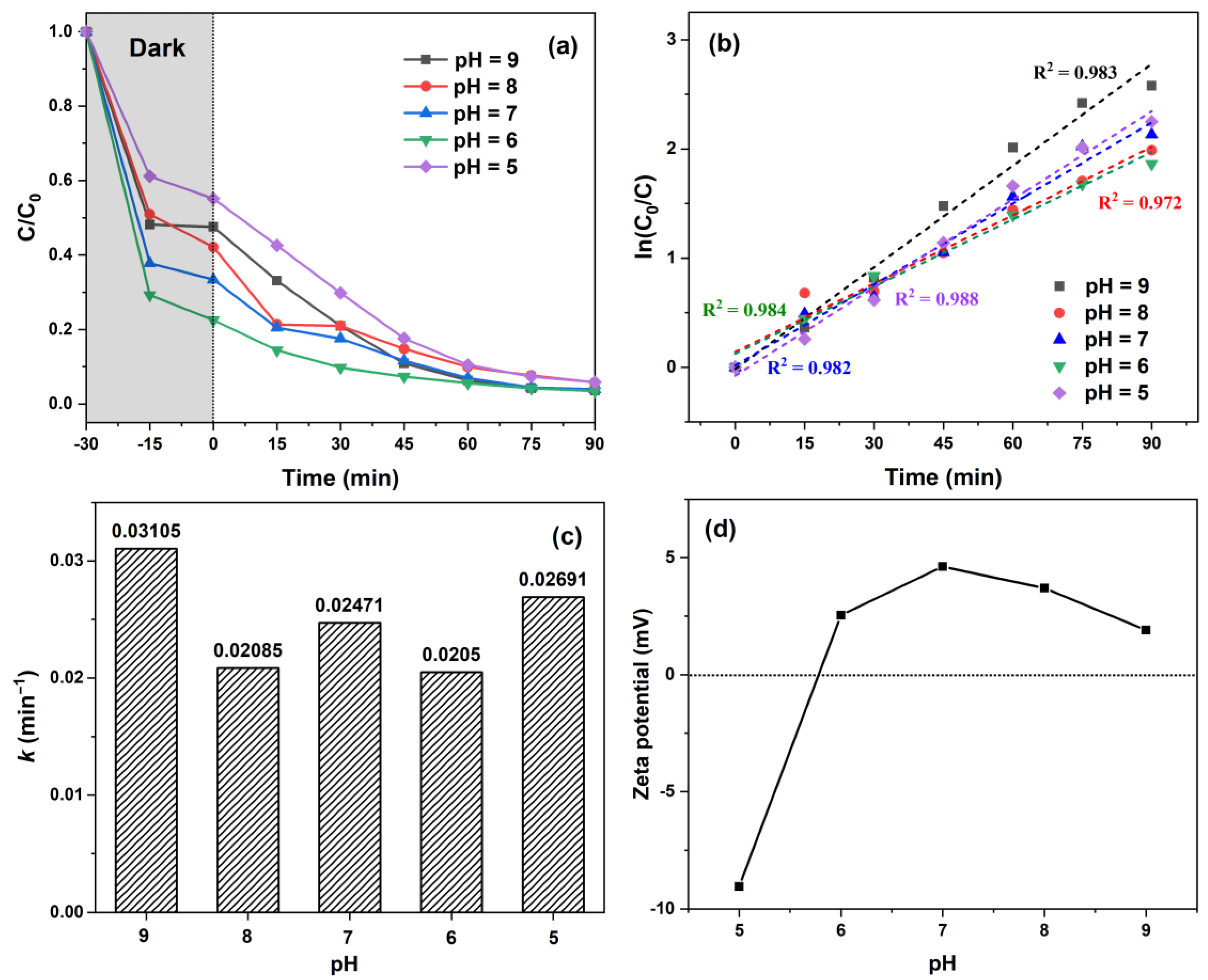

| Samples | Molar Ratio of Zn/Mn | Amount of C6H12O6 (g) |
|---|---|---|
| ZMC-5 | 2:3 | 5 |
| ZMC-2 | 2:3 | 2 |
| ZMC-0 | 2:3 | 0 |
| ZM-05 | 0:5 | 2 |
| ZM-50 | 5:0 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Zhao, Y.; Liu, T.; Li, R.; Li, R.; Zhu, Y.; Fang, F. Zn-Doped MnCO3/CS Composite Photocatalyst for Visible-Light-Driven Decomposition of Organic Pollutants. Molecules 2024, 29, 1094. https://doi.org/10.3390/molecules29051094
Liang H, Zhao Y, Liu T, Li R, Li R, Zhu Y, Fang F. Zn-Doped MnCO3/CS Composite Photocatalyst for Visible-Light-Driven Decomposition of Organic Pollutants. Molecules. 2024; 29(5):1094. https://doi.org/10.3390/molecules29051094
Chicago/Turabian StyleLiang, Hui, Yongxin Zhao, Tongjin Liu, Ruijuan Li, Rumei Li, Yuxiao Zhu, and Feng Fang. 2024. "Zn-Doped MnCO3/CS Composite Photocatalyst for Visible-Light-Driven Decomposition of Organic Pollutants" Molecules 29, no. 5: 1094. https://doi.org/10.3390/molecules29051094
APA StyleLiang, H., Zhao, Y., Liu, T., Li, R., Li, R., Zhu, Y., & Fang, F. (2024). Zn-Doped MnCO3/CS Composite Photocatalyst for Visible-Light-Driven Decomposition of Organic Pollutants. Molecules, 29(5), 1094. https://doi.org/10.3390/molecules29051094




