Near-Infrared Light-Excited Quinolinium-Carbazole Small Molecule as Two-Photon Fluorescence Nucleic Acid Probe
Abstract
1. Introduction
2. Results and Discussion
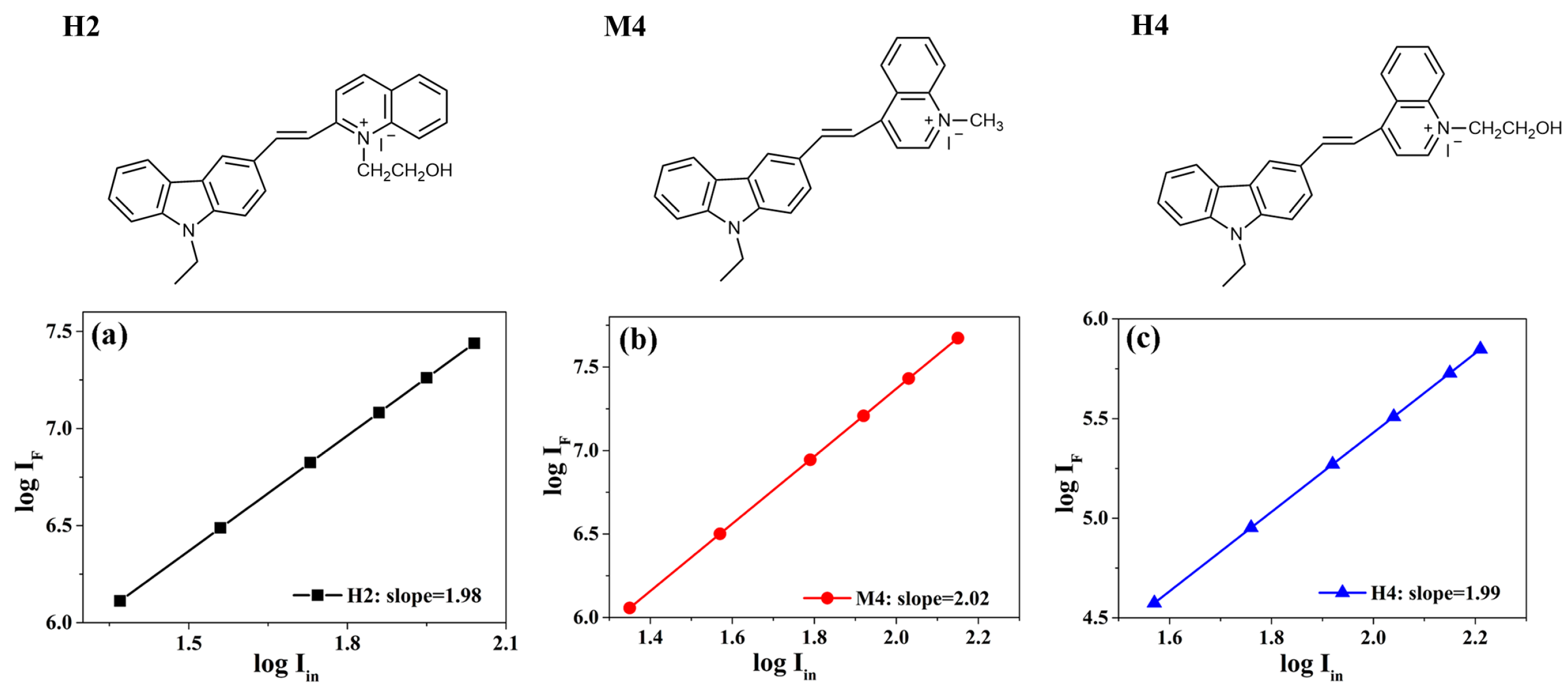
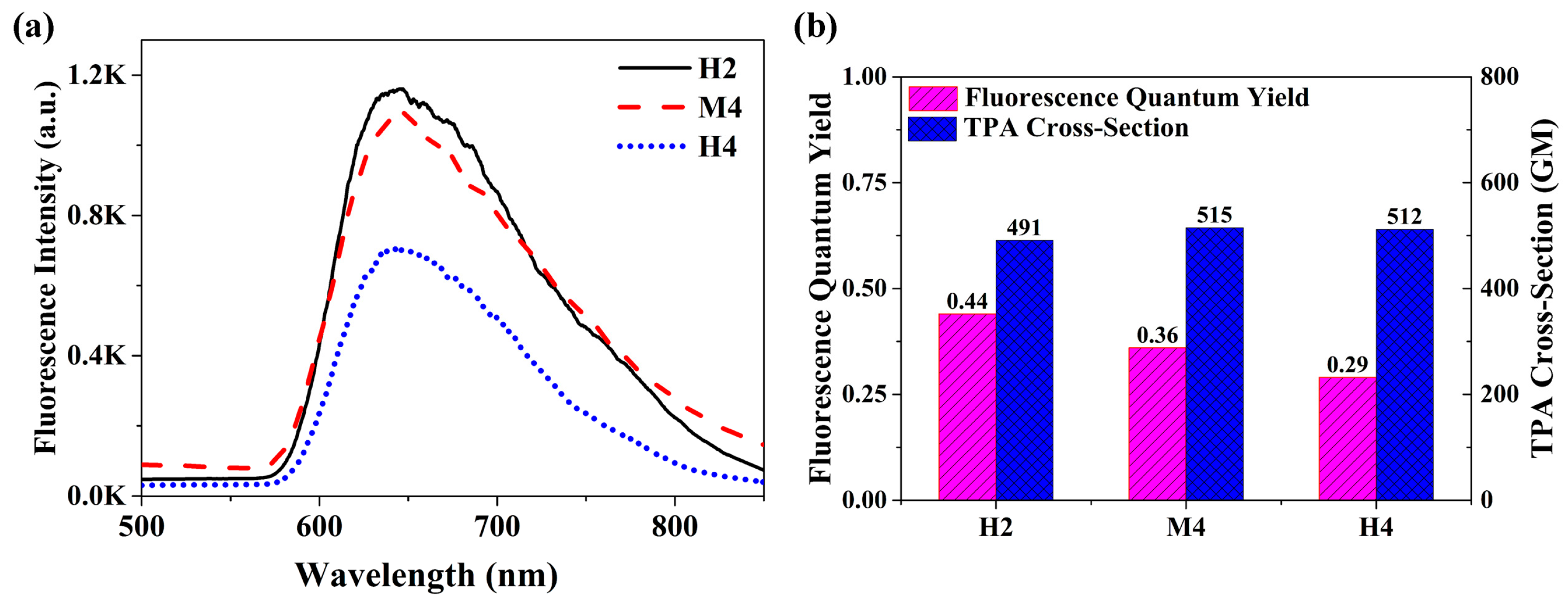
2.1. Two-Photon Optical Properties
2.2. DNA Optical On–Off Effect
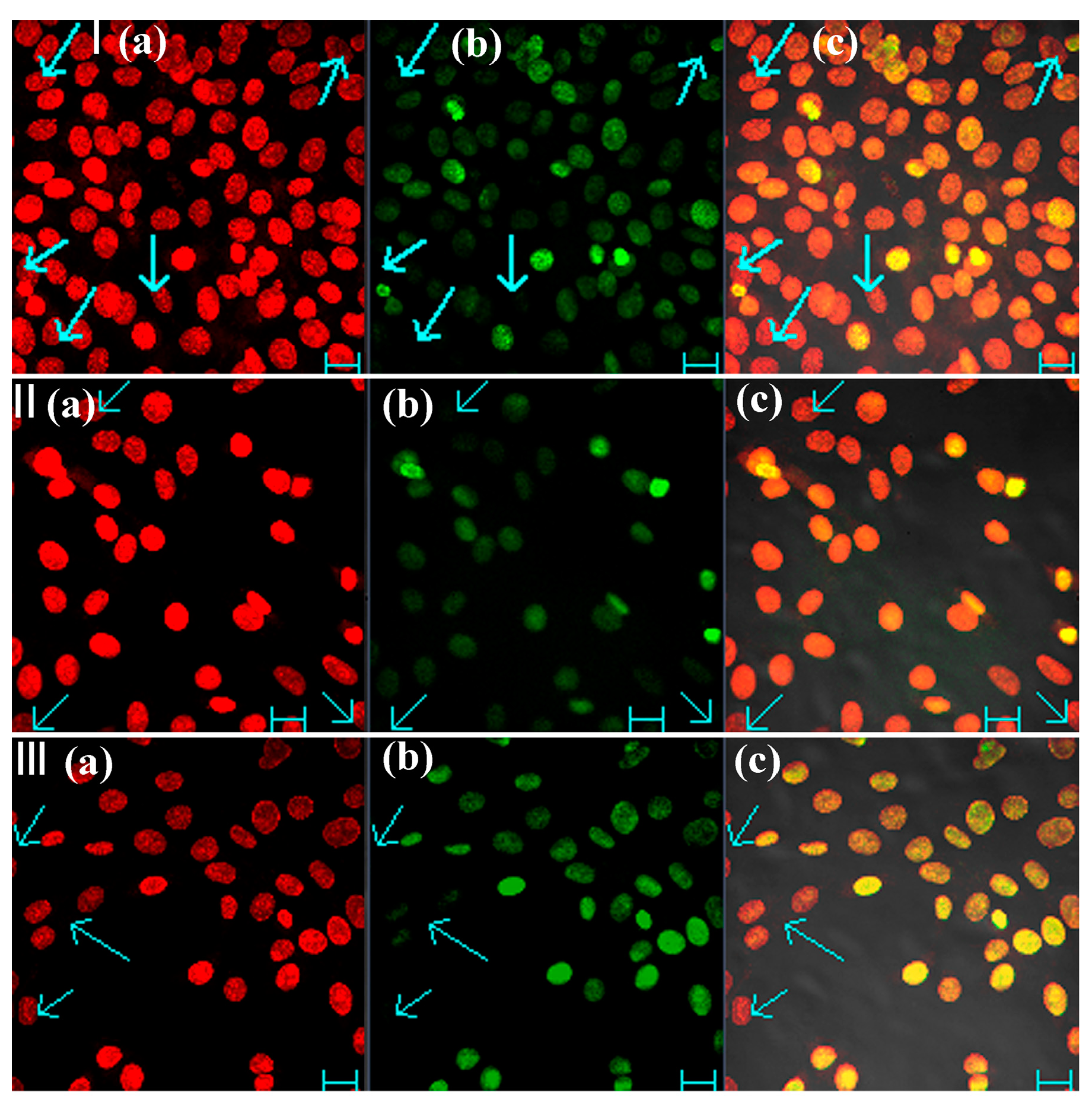
2.3. Bio-Imaging of Colocalization Experiment
2.4. One- and Two-Photon Bio-Imaging
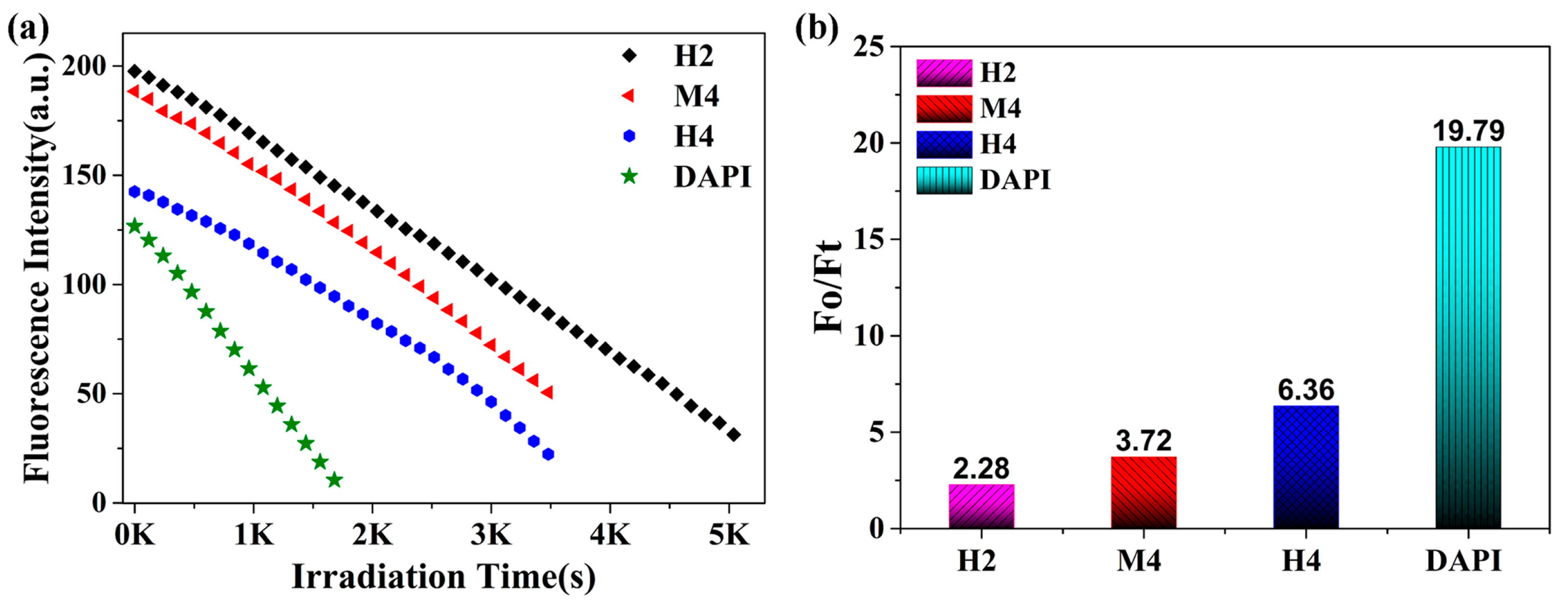
2.5. Photo-Stability Experiment
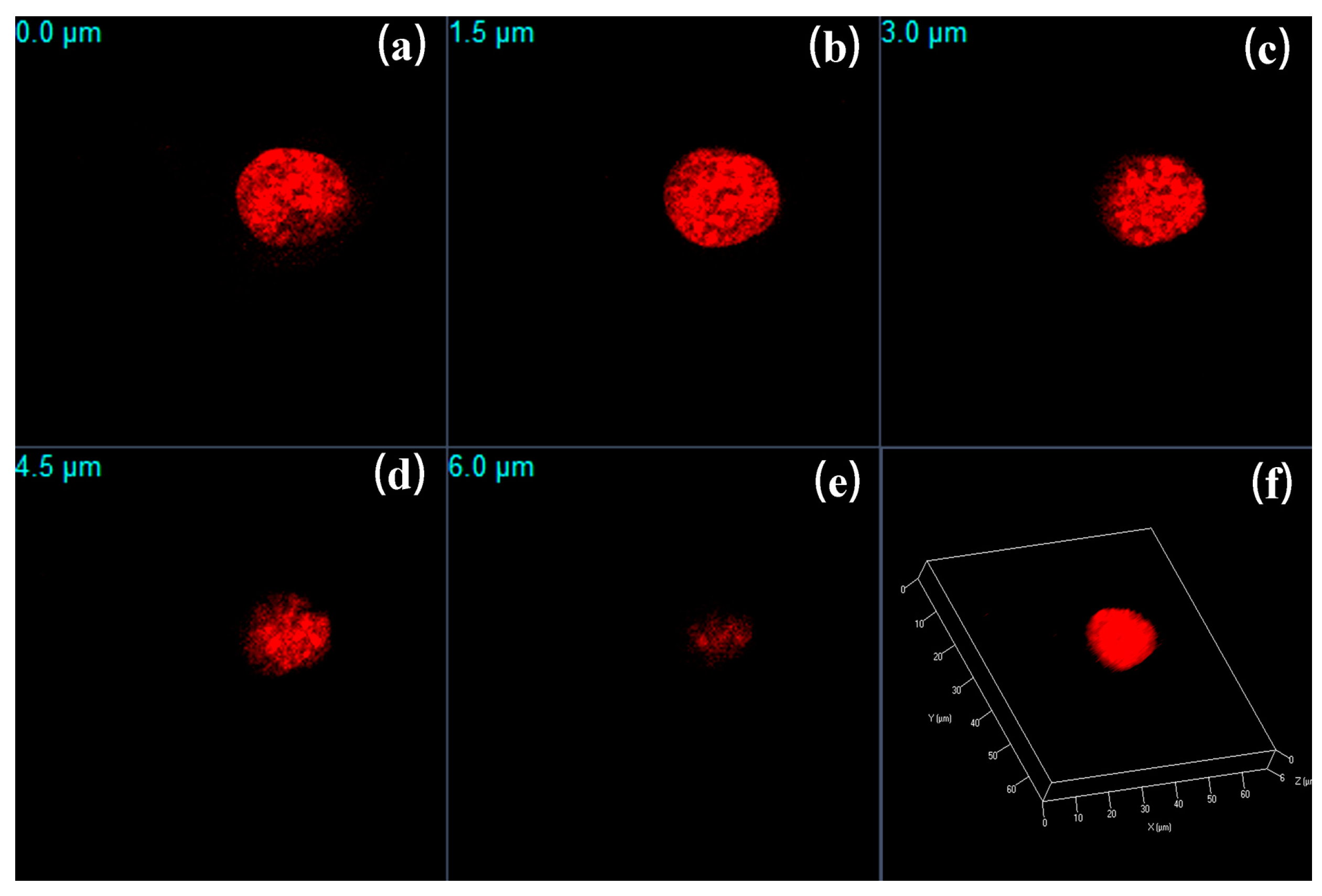
2.6. Cell 3D Bio-Imaging
3. Experimental Section
3.1. Materials and Chemicals
3.2. Synthesis
3.3. Spectroscopic Measurements
3.4. Cell Culture and Bio-Imaging Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; He, S.; Gao, X.; Ye, P.; Lei, L.; Dong, W.; Zhang, X.; Xu, P. Enhanced Optical Nonlinearity in a Silicon–Organic Hybrid Slot Waveguide for All-Optical Signal Processing. Photonics Res. 2022, 10, 50. [Google Scholar] [CrossRef]
- Chang, L.; Boes, A.; Shu, H.; Xie, W.; Huang, H.; Qin, J.; Shen, B.; Wang, X.; Mitchell, A.; Bowers, J.E. Second Order Nonlinear Photonic Integrated Platforms for Optical Signal Processing. IEEE J. Select. Top. Quantum Electron. 2021, 27, 1–11. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Wu, J.; Li, X.; Zeng, H. Nonlinear Optics in Lead Halide Perovskites: Mechanisms and Applications. ACS Photonics 2021, 8, 113–124. [Google Scholar] [CrossRef]
- Liu, K.; Han, J.; Baiheti, T.; Li, F.; Wei, Z.; Yang, Z.; Mutailipu, M.; Pan, S. Finding a Series of BaBOF3 Fluorooxoborate Polymorphs with Tunable Symmetries: A Simple but Flexible Case. Chem. Mater. 2021, 33, 7905–7913. [Google Scholar] [CrossRef]
- Hernandez-Rueda, J.; Noordam, M.L.; Komen, I.; Kuipers, L. Nonlinear Optical Response of a WS2 Monolayer at Room Temperature upon Multicolor Laser Excitation. ACS Photonics 2021, 8, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Li, Y.; Wang, C.; Zhang, H.; Li, D. Recent Investigations on Nonlinear Absorption Properties of Carbon Nanotubes. Nanophotonics 2020, 9, 761–781. [Google Scholar] [CrossRef]
- Lee, C.; Xu, E.Z.; Liu, Y.; Teitelboim, A.; Yao, K.; Fernandez-Bravo, A.; Kotulska, A.M.; Nam, S.H.; Suh, Y.D.; Bednarkiewicz, A.; et al. Giant Nonlinear Optical Responses from Photon-Avalanching Nanoparticles. Nature 2021, 589, 230–235. [Google Scholar] [CrossRef]
- Stachelek, P.; MacKenzie, L.; Parker, D.; Pal, R. Circularly Polarised Luminescence Laser Scanning Confocal Microscopy to Study Live Cell Chiral Molecular Interactions. Nat. Commun. 2022, 13, 553. [Google Scholar] [CrossRef]
- Ismail, M.Y.; Patanen, M.; Kauppinen, S.; Kosonen, H.; Ristolainen, M.; Hall, S.A.; Liimatainen, H. Surface Analysis of Tissue Paper Using Laser Scanning Confocal Microscopy and Micro-Computed Topography. Cellulose 2020, 27, 8989–9003. [Google Scholar] [CrossRef]
- Tran, T.; Denimal, E.; Lafarge, C.; Journaux, L.; Lee, J.A.; Winckler, P.; Perrier-Cornet, J.-M.; Pradelles, R.; Loupiac, C.; Cayot, N. Effect of High Hydrostatic Pressure on Extraction of B-Phycoerythrin from Porphyridium Cruentum: Use of Confocal Microscopy and Image Processing. Algal Res. 2019, 38, 101394. [Google Scholar] [CrossRef]
- Deroubaix, A.; Moahla, B.; Penny, C. Monitoring of Intracellular Localization of Hepatitis B Virus P22 Protein Using Laser Scanning Confocal Microscopy and Airyscan. Microsc. Res. Tech. 2020, 83, 499–506. [Google Scholar] [CrossRef]
- Bohn, S.; Sperlich, K.; Stolz, H.; Guthoff, R.F.; Stachs, O. In Vivo Corneal Confocal Microscopy Aided by Optical Coherence Tomography. Biomed. Opt. Express 2019, 10, 2580. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, K.; Suman, G.; Dhyani, V.; Swain, S.; Giri, L.; Samavedi, S. Three-dimensional Imaging and Quantification of Real-time Cytosolic Calcium Oscillations in Microglial Cells Cultured on Electrospun Matrices Using Laser Scanning Confocal Microscopy. Biotechnol. Bioeng. 2020, 117, 3108–3123. [Google Scholar] [CrossRef]
- Reichhardt, C.; Parsek, M.R. Confocal Laser Scanning Microscopy for Analysis of Pseudomonas aeruginosa Biofilm Architecture and Matrix Localization. Front. Microbiol. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Huo, F.; Wen, W.; Yin, C. Engineering an ESIPT-Based Fluorescent Probe for Dual-Channel (Vis/NIR) Ratiometric Monitoring of Intracellular Sulfur Dioxide by Single Wavelength Excitation. Dye. Pigment. 2022, 199, 110111. [Google Scholar] [CrossRef]
- Qin, J.; Liang, Q.; Wang, G.; Hao, L.; Liu, X.; Wang, X.; Hu, Z.; Fang, G.; Xue, L.; Zhao, Y.; et al. Targeted Delivery of Nuclear Targeting Probe for Bladder Cancer Using Cyclic Pentapeptide c(RGDfK) and Acridine Orange. Clin. Transl. Oncol. 2022, 25, 375–383. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, K.; He, M.; Gao, Y.; Zhang, D.; Bai, Y.; Lai, Y.; Liu, M.; Han, X.; Xu, S.; et al. The Effects of Different Fluorescent Indicators in Observing the Changes of the Mitochondrial Membrane Potential during Oxidative Stress-Induced Mitochondrial Injury of Cardiac H9c2 Cells. J. Fluoresc. 2020, 30, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Lu, W.; Zhao, X.; Guan, X.; Ji, Y.; Zhang, X.; Pan, J.; Ning, J.; Zhou, H.; Wang, C. Real-Time Temperature Measurement of Living Cells Exposed to Microwaves Using a Temperature-Dependent Fluorescent Dye. Chem. Phys. 2021, 547, 111190. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-Photon Laser Scanning Fluorescence Microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef]
- Kaur, G.; Lewis, J.; van Oijen, A. Shining a Spotlight on DNA: Single-Molecule Methods to Visualise DNA. Molecules 2019, 24, 491. [Google Scholar] [CrossRef]
- Drobizhev, M.; Molina, R.S.; Franklin, J. Multiphoton Bleaching of Red Fluorescent Proteins and the Ways to Reduce It. Int. J. Mol. Sci. 2022, 23, 770. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, K.; Nienhaus, G.U. Genetically encodable fluorescent protein markers in advanced optical imaging. Methods Appl. Fluoresc. 2022, 10, 042002. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.S.; King, J.; Franklin, J.; Clack, N.; McRaven, C.; Goncharov, V.; Flickinger, D.; Svoboda, K.; Drobizhev, M.; Hughes, T.E. High Throughput Instrument to Screen Fluorescent Proteins under Two-Photon Excitation. Biomed. Opt. Express 2020, 11, 7192. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Li, L.; Wu, Y. Photostability Enhancement of Fluorenone-Based Two-Photon Fluorescent Probes for Cellular Nucleus Monitoring and Imaging. J. Phys. Chem. C 2016, 120, 13706–13715. [Google Scholar] [CrossRef]
- Zaleśny, R.; Murugan, N.A.; Tian, G.; Medved’, M.; Ågren, H. First-Principles Simulations of One- and Two-Photon Absorption Band Shapes of the Bis(BF2) Core Complex. J. Phys. Chem. B 2016, 120, 2323–2332. [Google Scholar] [CrossRef]
- Chi, S.; Li, L.; Wu, Y. A Series of Novel Dibenzothiophene-Based Two-Photon Fluorescent Probes for Cellular Nucleus Imaging. Sens. Actuators B Chem. 2016, 231, 811–829. [Google Scholar] [CrossRef]
- Chi, S.; Li, L.; Wu, Y. Preparation of Two-Photon Fluorescent Probe and Biological Imaging Application in Cells. Chin. Opt. Lett. 2016, 14, 061603–061607. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Zhang, Y.; Zhao, N.; Zhao, H.; Wang, G.; Yu, X.; Liu, H. A Series of Carbazole Cationic Compounds with Large Two-Photon Absorption Cross Sections for Imaging Mitochondria in Living Cells with Two-Photon Fluorescence Microscopy. J. Fluoresc. 2011, 21, 497–506. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Kalytchuk, S.; Li, K.F.; Chen, R.; Adachi, C.; Chen, Z.; Rogach, A.L.; Zhu, G.; Yu, P.K.N.; et al. Self-Assembly of Electron Donor–Acceptor-Based Carbazole Derivatives: Novel Fluorescent Organic Nanoprobes for Both One- and Two-Photon Cellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 11355–11365. [Google Scholar] [CrossRef]
- Gao, F.; Cao, S.; Sun, W.; Long, S.; Fan, J.; Peng, X. Development of a Two-Photon Carbazole Derivative Probe for Fluorescent Visualization of G-Quadruplex DNA in Cells. Dye. Pigment. 2019, 171, 107749. [Google Scholar] [CrossRef]
- Gao, W.; Chao, H.; Zheng, Y.-C.; Zhang, W.-C.; Liu, J.; Jin, F.; Dong, X.-Z.; Liu, Y.-H.; Li, S.-J.; Zheng, M.-L. Ionic Carbazole-Based Water-Soluble Two-Photon Photoinitiator and the Fabrication of Biocompatible 3D Hydrogel Scaffold. ACS Appl. Mater. Interfaces 2021, 13, 27796–27805. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Purman, C.; Porter, S.I.; Nganga, V.; Saini, A.; Hayer, K.E.; Gurewitz, G.L.; Sleckman, B.P.; Bednarski, J.J.; Bassing, C.H.; et al. DNA Double-Strand Breaks Induce H2Ax Phosphorylation Domains in a Contact-Dependent Manner. Nat. Commun. 2020, 11, 3158. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Hu, Y.; Lin, G.; Wu, Y.; Huang, W.; Zhao, Q. Novel Carbazole Derivatives with Quinoline Ring: Synthesis, Electronic Transition, and Two-Photon Absorption Three-Dimensional Optical Data Storage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 139, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, L.; Zhang, Y.C.; Wu, Y.Q.; Chen, Z.M.; He, C.Y. Preparation and Photophysical Properties of Two-Photon Absorption Materials Containing Quinoline Ring as Electron Acceptors. Adv. Mater. Res. 2012, 557, 755–760. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wu, B.; Liu, X.; Liu, L.; Zhou, S.; Feng, Y. Near-Infrared Light-Excited Quinolinium-Carbazole Small Molecule as Two-Photon Fluorescence Nucleic Acid Probe. Molecules 2024, 29, 1080. https://doi.org/10.3390/molecules29051080
Sun Y, Wu B, Liu X, Liu L, Zhou S, Feng Y. Near-Infrared Light-Excited Quinolinium-Carbazole Small Molecule as Two-Photon Fluorescence Nucleic Acid Probe. Molecules. 2024; 29(5):1080. https://doi.org/10.3390/molecules29051080
Chicago/Turabian StyleSun, Yanqing, Bi Wu, Xinyu Liu, Lixin Liu, Shujing Zhou, and Yanru Feng. 2024. "Near-Infrared Light-Excited Quinolinium-Carbazole Small Molecule as Two-Photon Fluorescence Nucleic Acid Probe" Molecules 29, no. 5: 1080. https://doi.org/10.3390/molecules29051080
APA StyleSun, Y., Wu, B., Liu, X., Liu, L., Zhou, S., & Feng, Y. (2024). Near-Infrared Light-Excited Quinolinium-Carbazole Small Molecule as Two-Photon Fluorescence Nucleic Acid Probe. Molecules, 29(5), 1080. https://doi.org/10.3390/molecules29051080




