Abstract
Zeolites, a group of minerals with unique properties, have been known for more than 250 years. However, it was the development of methods for hydrothermal synthesis of zeolites and their large-scale industrial applications (oil processing, agriculture, production of detergents and building materials, water treatment processes, etc.) that made them one of the most important materials of the 20th century, with great practical and research significance. The orderly, homogeneous crystalline and porous structure of zeolites, their susceptibility to various modifications, and their useful physicochemical properties contribute to the continuous expansion of their practical applications in both large-volume processes (ion exchange, adsorption, separation of mixture components, catalysis) and specialized ones (sensors). The following review of the knowledge available in the literature on zeolites aims to present the most important information on the properties, synthesis methods, and selected applications of this group of aluminosilicates. Special attention is given to the use of zeolites in agriculture and environmental protection.
1. Introduction
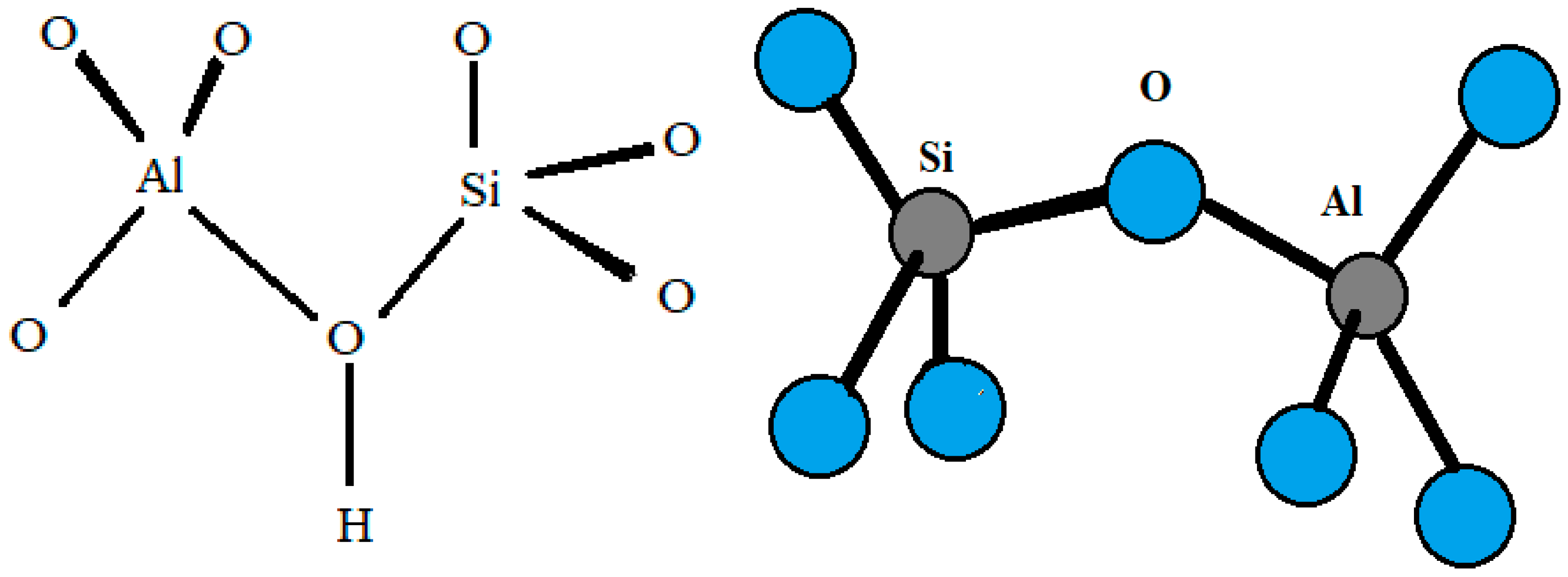
Zeolites are porous hydrated aluminosilicates with a three-dimensional structure containing cations of alkaline elements (sodium, potassium), alkaline earth (calcium, magnesium, less frequently barium, and strontium), or other monovalent or multivalent metals [1]. Due to their differentiated structure, which contains large free spaces and channels, zeolites exhibit properties characteristic of nanoporous materials and show the ability to lose and absorb water in amounts greater than 30% of their dry weight [2]. Zeolites have an ordered crystalline structure whose primary building units (PBU) are silicon [SiO4] and aluminum [AlO4] tetrahedra connected by common oxygen atoms [3], forming so-called secondary building units (SBU). According to Löwenstein’s rule, silicon–oxygen tetrahedra can be adjacent to each other (Si–O–Si), while aluminum–oxygen tetrahedra can only be connected to silicon–oxygen tetrahedra (Si–O–Al) [4]. Replacement of the Si4+ cation in the tetrahedral position by Al3+ results in an excess of electrons, i.e., a negative charge, which is usually compensated by so-called exchangeable cations (e.g., Na+, K+, NH4+, H+, Ca2+, Sr2+, or Mg2+) [5]. These off-grid cations, together with water molecules, are located in the free spaces of the aluminosilicate skeleton, moving freely inside the mineral and easily exchanging with other ions present in the environment [6]. The peculiar internal structure of zeolites is the result of a diverse distribution of tetrahedra, forming a network of structural chambers and channels of different sizes that, under normal temperature conditions, are filled with water molecules, the so-called zeolitic water [7]. By means of thermal treatment, this water can be easily removed without disturbing the crystal structure of the zeolite (Figure 1). The released pores can be filled with water molecules or other adsorbates [5,8].
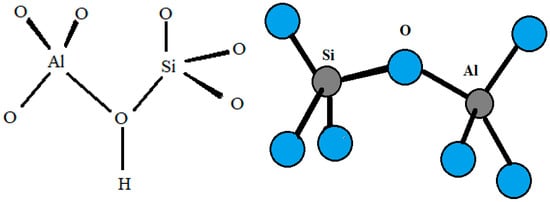
Figure 1.
Scheme of silicon and aluminum tetrahedra in the zeolite structure (own elaboration based on Khaleque et al. [9]).
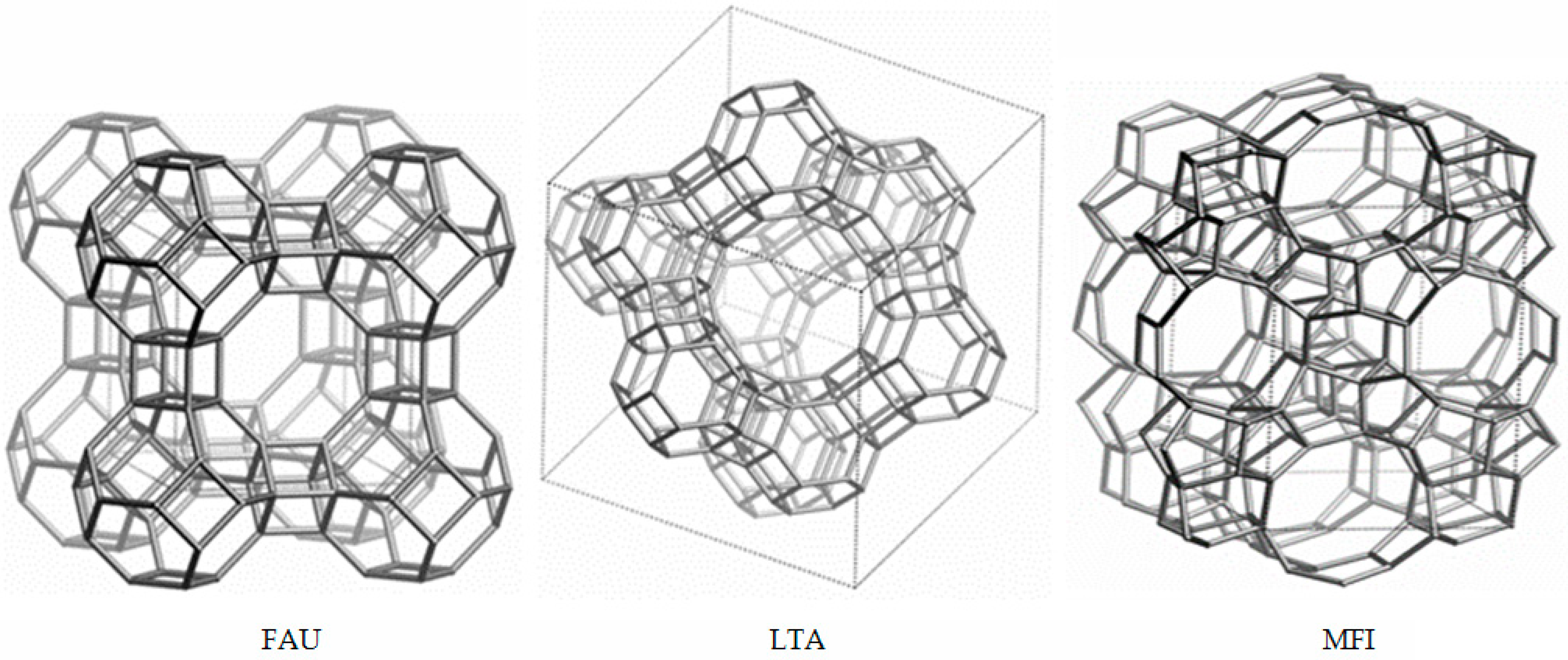
An example of an elementary cell and channel system of FAU, LTA, and MFI zeolites is shown in Figure 2.
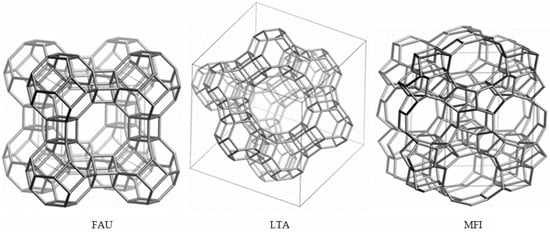
Figure 2.
Elemental cell and channel system of FAU, LTA, and MFI zeolites (from Database of Zeolite Structures, International Zeolite Association [10]).
The first zeolite (stilbite) was discovered in 1756 by the Swedish mineralogist Axel Frederic von Cronstedt [11,12]. The genesis of zeolite formation in nature is the reaction of volcanic rocks and ashes with water of high pH and high salt concentration [13]. Currently, we can distinguish about 50 natural zeolites, the most important of which are clinoptilolite, analcime, mordenite, and chabazite [14], and more than 150 synthetic zeolites [2]. Zeolites, both natural and synthetic, are used in various fields of human activity. On a larger scale, synthetic zeolites are mainly used because natural zeolites often contain various types of impurities, such as other minerals or metals [12,15]. In addition, synthetic zeolites tend to have better chemical and physical properties than natural zeolites [16,17]. The advantage of synthetic zeolites over natural zeolite applications is related to their greater stability in the reaction environment [18], as well as the pore size, which is larger in synthetic zeolites and allows adsorption of larger molecules (e.g., diesel oil) [13,18]. The kinetics of the removal of radioactive contaminants and heavy metal ions from synthetic materials are also several times higher compared to natural zeolites [9].
The aim of this paper is to present the sources in the natural environment as well as the properties and methods of synthesis of zeolites. The possibilities of using zeolites, mainly in agriculture and environmental protection, are also presented.
2. Properties and Classification of Zeolites
The presence of channels and chambers in the skeletal structure of zeolite gives it a number of desirable physicochemical properties and makes it a material with a wide range of applications. Zeolites have surface-active centers of acid–base or oxidation–reduction character. These are responsible for their exceptional adsorption and catalytic activity [19,20]. A characteristic feature of zeolites is the presence of micropores with diameters in the range of 0.3 to 1.0 nm [12] and a volume of micropores in the range of 0.10 to 0.35 cm3 g−1 [21]. The classification of zeolites based on the diameter of their pores is shown in Table 1.

Table 1.
Classification of zeolites based on the size of pores in the structure (own elaboration based on Mijailović et al. [13]; Kulprathipanja [21]).
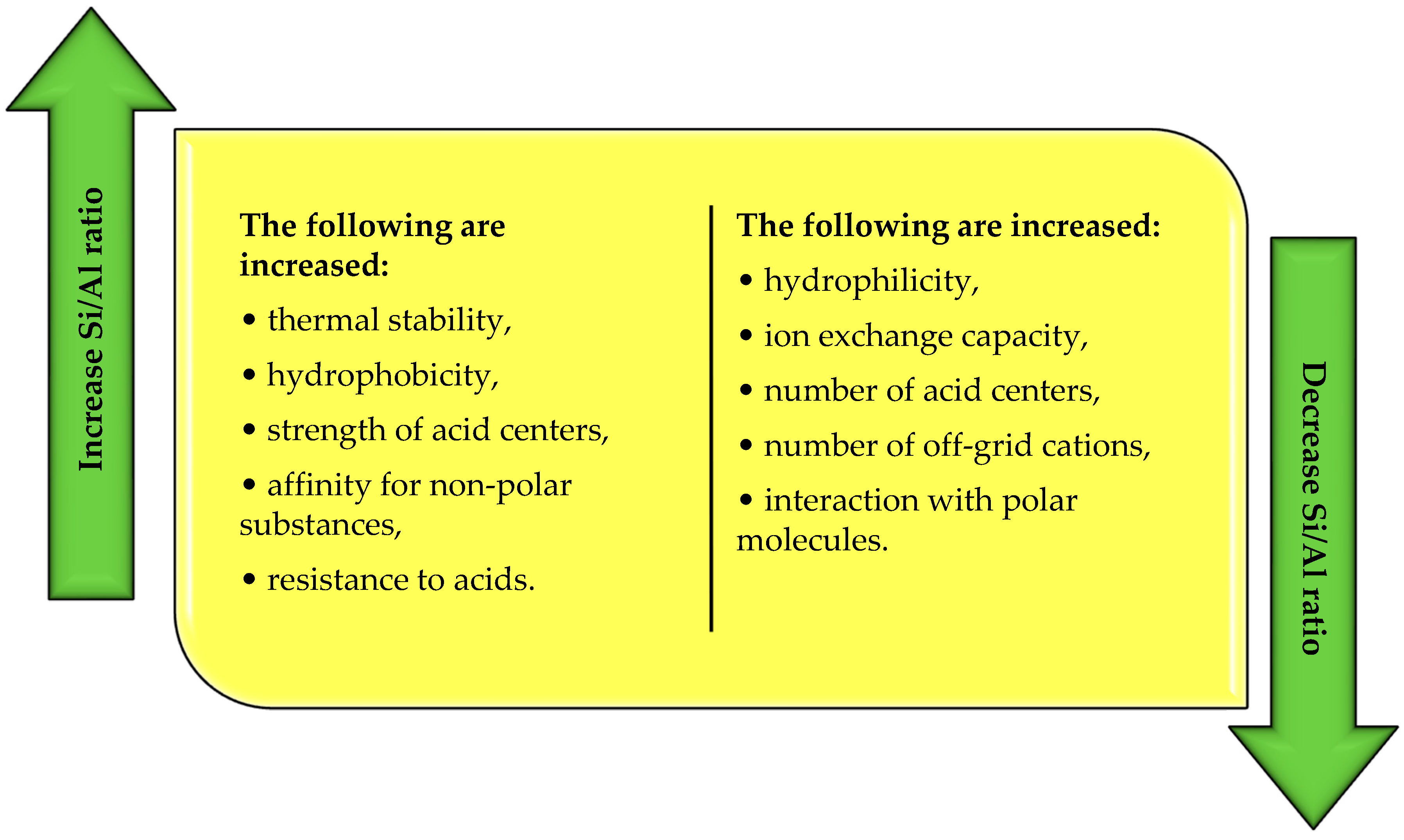
Another classification of zeolites concerns the molar ratio of Si/Al. According to Szostak [22], this ratio determines the physicochemical properties of zeolites (Figure 3). Based on the value of the Si/Al ratio, zeolites with low, medium, and high silicon content in the structure are distinguished (Table 2).
As the Si/Al ratio increases, the thermal stability of the zeolite structure, i.e., the resistance to amorphization or dealumination, increases. This relationship means that the structure of low-silicon zeolites can be affected as early as 700 °C, while the stability of high-silicon zeolites is preserved up to 1300 °C [21,23]. Low-silicon zeolites are also characterized by increased ion exchange capacity and hydrophilicity. High-silicon zeolites, on the other hand, are more hydrophobic and are characterized by an increased power of active centers, which predestines them for catalytic applications [13,18]. As the Si/Al ratio increases, the acidity also increases. On the other hand, under the same conditions, the amount of off-grid exchangeable cations in the structure and, consequently, the ion exchange capacity, which is proportional to the number of AlO4− tetrahedra present in the zeolite skeleton, decreases [24].
The specific structure of zeolites gives them a number of unique properties [18]. They are good sorbents for water and adsorbents for uncharged molecules, effective ion exchangers and molecular sieves [25], and environmentally friendly catalysts [26]. Other important properties include the large internal surface area of the zeolite framework (several hundred m2 g−1) [13] and the cation exchange capacity, which varies between 200 and 300 cmol(+) kg−1 [27]. In addition, zeolites are characterized by low crystal density (from 1.9 to 2.2 Mg m−3) and low bulk density (e.g., 0.8 to 1.5 Mg m−3) [27]. Due to their unique physicochemical properties, zeolites have found applications in many industries, including environmental protection and agriculture [28,29].

Table 2.
Classification of zeolites in terms of Si/Al ratio values (own elaboration based on Guisnet, Gilson [23]; Payra, Dutta [24]; Sharma et al. [30]).
Table 2.
Classification of zeolites in terms of Si/Al ratio values (own elaboration based on Guisnet, Gilson [23]; Payra, Dutta [24]; Sharma et al. [30]).
| Type of Zeolite | Si/Al Ratio | Example of Zeolite |
|---|---|---|
| Low silicon | 1.0–1.5 | 4A, X, UZM-4, UZM-5 |
| Medium silicon | ~2.0–5.0 | mordenite, zeolite Y, L |
| High silicon | >10 | Beta, ZSM-5, ZSM-12 |
| Silica molecular sieves | >100 | silicites |
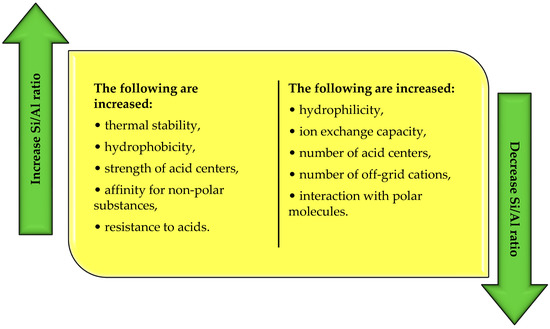
Figure 3.
Changes in the physicochemical properties of zeolites as a function of the molar ratio of silicon to aluminum (own elaboration based on Payra, Dutta [24]; Jakubowski et al. [31]).
Figure 3.
Changes in the physicochemical properties of zeolites as a function of the molar ratio of silicon to aluminum (own elaboration based on Payra, Dutta [24]; Jakubowski et al. [31]).
3. Zeolite Synthesis Methods
Interest in synthetic zeolites has increased as newer possibilities for their application in various industrial fields have been discovered. Modern synthesis techniques make it possible to obtain zeolite material with specific parameters that can be modeled and adapted for different applications. In the zeolite process, the starting materials are usually silica and clay minerals, which are sources of aluminum and silicon. The reactants can also be waste materials such as red sludge, glass pellets, or fly ash [6], which show considerable similarity to zeolites in terms of chemical composition.
The hydrothermal synthesis method is considered to be the most common technique for zeolite synthesis, where the solvent is always water [11]. In solvothermal synthesis, water can be used, but mainly organic solvents are used, including alcohols (methanol, ethanol), ethylene glycol, hydrocarbons, and pyridine [9], while in the ionothermal method, ionic liquids are used, whose main advantage is their low melting point (<100 °C) [11]. Based on the above division of zeolite synthesis methods, it can be seen that all hydrothermal and ionothermal methods are, in principle, included in solvothermal methods, while solvothermal methods are not [32].
3.1. Hydrothermal Synthesis
Conventional zeolite synthesis is a time-consuming hydrothermal process carried out in an alkaline environment in the temperature range of 90–150 °C at a pressure of 1–15 bar [6] in a closed system for 24–96 h [17,33]. This involves several steps in which the aluminosilicate hydrogel, organic molecules, and metal cations are converted into crystalline aluminosilicate [34]. Aluminosilicate hydrogels are most commonly obtained from a mixture of compounds containing aluminum (aluminate, aluminum nitrate, and aluminum sulfate) and silicon (water glass, kaolinite, and SO2 colloid). Due to the high cost of pure substrates, natural clay materials (e.g., halloysite or kaolin) [9] and waste materials (e.g., fly ash [35], rice husk [36], and paper sludge [37]) are often used as reactants. Crystal nuclei are formed throughout the crystallization process, but the highest formation rate is observed in the initial phase. Hydrothermal synthesis of zeolites at about 100 °C generally results in the formation of crystals between 0.1 and 10 μm [33]. Depending on the transformation parameters used, hydrothermal synthesis can produce different types of zeolite materials, including chabazite, Na-P1, phillipsite, faujasite, or zeolite (Y, X, A, P) [17]. As reported by Johnson and Arshad [32], several key factors must be considered in the hydrothermal synthesis of kaolin-based zeolites as follows:
- (a)
- the Si/Al molar ratio; low (Si/Al ≤ 5) gives SAPO, different types of LTA, and zeolites X, while high (Si/Al ≥ 5) gives beta and ZSM-5 zeolites and different types of zeolite Y;
- (b)
- the appropriate concentration of NaOH (optimum ≤ 3 Mol L−1); higher reduces the relative crystallinity and favors the formation of (hydroxy)sodalites as impurities;
- (c)
- the crystallization temperature, which should be between 70 °C and 200 °C; a temperature ≤ 70 °C is not sufficient for the synthesis of crystalline compounds;
- (d)
- the crystallization time (interval < 24 h < 120 h).
Novembre et al. [38] carried out an experiment to obtain Na-X zeolite by a hydrothermal method using natural substrates (naturally zeolitized alkaline volcanic rock and siliceous opaque). The process was carried out at 80 °C using a sodium hydroxide solution at a concentration of 3 Mol L−1. The authors showed that the synthesis of the Na-X zeolite started after 5 h and reached the peak of crystallization after 18 h of the process, and the zeolite obtained had a wide (temporal) stability range (500 h).
3.2. Various Techniques of Hydrothermal Synthesis
3.2.1. Alkali Fusion
In zeolite synthesis, the fusion method precedes conventional hydrothermal treatment. In this process, the raw material is fused with an alkali (e.g., solid sodium hydroxide), which acts as an activator for zeolitization [34]. The first synthesis step in the process discussed above is the thermal activation of the starting material, which is carried out at temperatures in the range of 500–650 °C [17,39]. This is followed by the aging of the reaction mixture at temperatures between 20 and 50 °C for a period of several to tens of hours [32]. The final step is the crystallization of the reaction mixture, which is typically performed at 100 °C for 24 to 48 h [39,40]. During the fusion process, sodium ions, when the introduced base is sodium hydroxide, act as stabilizers of the crystal structure of the zeolite subunit, increasing the amount of zeolite formed during chemical synthesis [34].
Among others, colloidal silica and sodium silicate are used as siliceous components in the method described above, while aluminum isopropanolate and sodium aluminate are used as zeolitic components [40,41]. The quality of the resulting synthesis product depends on the Na2O:SiO2:Al2O3:H2O molar ratio, the temperature and activation time, the aging time of the reaction mixture, and the crystallization temperature and time [42]. According to Aylele et al. [43], the advantage of alkaline fusion in the synthesis of zeolite A is the possibility of using low-quality primary kaolin without purification, whereas the conventional hydrothermal method requires high-quality raw material. The suitability of the hydrothermal method preceded by alkaline fusion in the synthesis of Na-A zeolite (Z-S1) from one of the volcanic rocks (scoria) was demonstrated by Lee et al. [44]. Based on their results, the authors concluded that control of the NaOH/precursor ratio is important to ensure high crystallinity of the zeolite product, as well as the size of the particles, which decreases with increasing alkali content in the medium. Thuadaij and Nuntiya [45] also used alkali fusion to obtain Na-X zeolites from fly ash, powdered amorphous silica, metakaolin, and their mixtures. The authors demonstrated that a mixture of metakaolin should be used to produce this type of zeolite and achieved high efficiency in converting mixtures to Na-X zeolite for a SiO2/Al2O3 = 3.25 ratio, where fly ash, amorphous silica, and metakaolin were present in a 1:3:6 ratio.
3.2.2. Alkaline Activation
Synthetic zeolites can be obtained by crystallization in a process known as alkaline activation. This process is mainly used to obtain geopolymers, which are inorganic polymers produced at low temperatures (<100 °C) and consist of chains or networks of mineral molecules linked by covalent bonds [46]. The geopolymers are produced by reacting a low-calcium aluminosilicate, such as silica fly ash, with an alkaline solution [47]. The alkaline activator in this process is a concentrated base, which can be hydroxide, silicate, carbonate, or sulfate [30]. The reactive aluminosilicates are dissolved in an aqueous alkaline solution, then the [SiO4]4− and [AlO4]5− tetrahedra join corners in a polycondensation process and form subcrystalline or amorphous aluminosilicate space structures with polymeric Si–O–Al–O bonds [48,49]. The process of alkaline activation of aluminosilicate phases contained in fly ash has been described by Garcia-Lodeiro et al. [50]. According to these authors, in materials with significant aluminosilicate content, amorphous hydrated aluminosilicates, zeolites, and gels are formed as a result of alkaline activation. The next reaction products can be zeolites:hydrosodalite, zeolite P, chabazite-Na, and faujasite-Ca. The mechanism of the geopolymerization reaction is not fully understood, and the simultaneous occurrence of stages in the process makes it even more difficult to understand. However, three main stages can be distinguished [51] as follows:
- (1)
- dissolution of silica and alumina in a strong alkaline solution (decomposition of solid aluminosilicates, whose products are a mixture of silicates, aluminosilicates, and aluminates);
- (2)
- diffusion or transport of solutes, polycondensation, and gel formation (condensation reaction of alumina and hydroxylated silica to form the inorganic gel phase of a geopolymer);
- (3)
- hardening of the gel phase—polymerization (formation of a three-dimensional aluminosilicate structure by increasing the connectivity in the geopolymer gel, crosslinking, and reorganization of the network).
Villa et al. [47] synthesized geopolymers by alkaline activation of natural zeolite. They used sodium silicate and sodium hydroxide as activators in proportions of 0.4, 1.5, 5, 10, and 15, using a 7 M sodium hydroxide solution. The time and temperature conditions used during setting and curing were variable, while the activator/precursor ratio was kept constant at 0.6. The results of this experiment showed that increasing the activator/precursor ratio, as well as the curing time, promoted the mechanical strength of the material, with the best results obtained at conditions of 90 days and 40 °C.
Alkaline activation is a polycondensation reaction and leads to the formation of new structures where the resulting negative charge is compensated by monovalent cations (Na+ or K+) from the alkaline activator (KOH or Na2SiO3) [3,52]. The combined use of alkali metal silicate with alkali metal hydroxide allows the reaction to occur to a greater extent [3]. Alkali metal cations play a fundamental role in controlling synthesis steps such as curing and crystal formation [53]. Alkali-activated materials are characterized by exceptional mechanical strength, fire and corrosion resistance, durability, rapid curing, and low thermal conductivity. Due to these advantages, the aforementioned materials are mainly used in the construction and thermal insulation industries, but also as catalysts or membranes [3,54].
3.3. Molten Salt Method
This method of zeolite synthesis was developed by Park et al. [55]. It involves the reaction of a mixture of NaOH–NaNO3 or NaOH–KNO3 with fly ash under anhydrous conditions at temperatures above 250 °C [34]. The advantages of this method are the simplicity and versatility of the implementation, the low temperatures, and the favorable cost/purity ratio of the products obtained in one phase. In addition, this method allows the synthesis of zeolites from various types of mineral wastes, has a shorter synthesis time compared to other methods, and does not generate alkaline liquid waste due to the anhydrous conditions [56]. Unfortunately, the lack of water in the environment can lead to insufficient contact between the reactants during the crystallization process, which can reduce the rate of conversion of the precursor to the product and result in an irregular morphological structure of the zeolite [34]. In their experiment, Park et al. [55] used different combinations of salt mixtures on zeolite fly ash, using NaOH, KOH, or NH4F as mineralizers and NaNO3, KNO3, or NH4NO3 as stabilizers. The reaction mixture contained 0.7 g fly ash, 0.3 g alkali, and 1 g salt, and the whole mixture was heated at 350 °C. The zeolite materials obtained by the authors consisted of sodalite and cancrinite as the main crystalline phases. The suitability of the molten salt method for the synthesis of zeolite materials from sewage sludge was demonstrated by Yoo et al. [57].
3.4. Microwave Assisted Synthesis
Zeolites can also be obtained by synthesis using microwave irradiation. This is a simple and effective technique that can reduce the synthesis time of zeolites, improve the homogeneity of their dimensions and composition, and improve the dissolution of the precursor gel [58]. As reported by Panzarella et al. [59], the efficiency of microwave-assisted synthesis is affected by the size of the vessel in which it is performed and the volume of the reaction mixture. For example, microwave heating has been used to obtain zeolite A, ZSM-5, faujazyite, analcime, AIPO4-5, and VPI-5 [60,61]. The advantages of synthesizing zeolites using microwave radiation include [61,62]:
- (a)
- much faster heating of the reaction mixture compared to conventional methods,
- (b)
- high reaction efficiency,
- (c)
- ability to control morphology, phase purity, and pore size,
- (d)
- rapid formation of crystallization nuclei,
- (e)
- uniform heating of the entire volume of the reaction mixture.
Anuwattana et al. [63] showed that microwave heating at 150 °C (frequency 2.45 GHz, maximum power up to 1200 W) increased the rate of formation of ZSM-5 zeolite from iron slag by a factor of four compared to hydrothermal heating. It also affected the formation of smaller ZSM-5 particles (0.3 μm vs. 3 μm in diameter). Serrano et al. [64] also demonstrated the usefulness of microwave heating in the synthesis of TS-2 zeolite. The authors demonstrated that it allows a shorter process time, with 100% crystalline samples being obtained after only 15 h, as opposed to the 48 h required by the conventional process.
3.5. Other Methods
An interesting method to obtain zeolites is the synthesis inside an inert mesoporous material (confined space synthesis), which is usually carbon. In this preparative technique, the zeolite is crystallized inside the pore system of an inert matrix. In this way, the crystals cannot grow larger than the surrounding pores [65]. The crystals are then separated from the matrix by pyrolysis at 550 °C. The undoubted advantages of confined space synthesis are [66,67] as follows:
- (a)
- high reproducibility,
- (b)
- control of the maximum crystal size by the size of the matrix mesopores,
- (c)
- high purity of the obtained samples,
- (d)
- the possibility of selecting the synthesis conditions to obtain highly crystalline zeolites.
With this method of synthesis, the zeolites obtained are characterized by a well-developed specific surface area and have the same number of acid sites as in the corresponding large zeolite crystals [68].
Another method of obtaining zeolites is the use of dry aluminosilicate gels, amines, and water in the vapor phase (vapor phase transport synthesis, VPT) [69,70]. According to Kim et al. [71], in the first stage of the process, water vapor condenses on the micropores of the precursor, resulting in the establishment of a liquid–vapor equilibrium after some time. The silica present in the gel then reacts with organic cations. The formation of crystal nuclei and the growth of the crystals take place on the outer surface of the precursor. The main influence on crystallization during VPT synthesis is the amount of water in the solvent mixture. The greater the amount of water, the more crystalline and structurally complex the product obtained [70]. The alkalinity of the system also has a significant effect on the crystallization process, determining the rate of this step and the particle size [72]. Liu et al. [73] synthesized MCM-22 zeolite and Niu et al. [72] beta zeolite using this method.
An increasingly popular method of obtaining zeolites is mechanochemical synthesis, in which crude precursors are subjected only to mechanical energy in an environment with little or sometimes no solvent [74]. This method reduces waste, energy consumption, and overhead costs and allows the type, density, and availability of active sites to be influenced by controlled amorphization [75]. Wu et al. [76] synthesized zeolites in the presence of NH4F by grinding anhydrous starting solids and heating at 140–240 °C. Under these conditions, they obtained zeolites with MFI, BEA, EUO, and TON structures. The process itself was characterized by a simplified procedure (compared to hydrothermal synthesis) and high efficiency. Ren et al. [77] demonstrated that grinding (10–20 min) of a mixture of chemical reactants (Na2SiO3·9H2O, NH4Cl fumed silica, and TPABr) followed by heating at 180 °C for 24–48 h leads to the formation of ZSM-5 type zeolite. The suitability of ball milling in the synthesis of ZSM-5 zeolite and mordenite has also been confirmed by Nada et al. [78]. The obtained zeolites were characterized by a high specific surface area (~300 m2 g−1), and the whole process took place in the absence of solvents, organic structure directing agents, or grafting crystals.
4. Applications of Zeolites
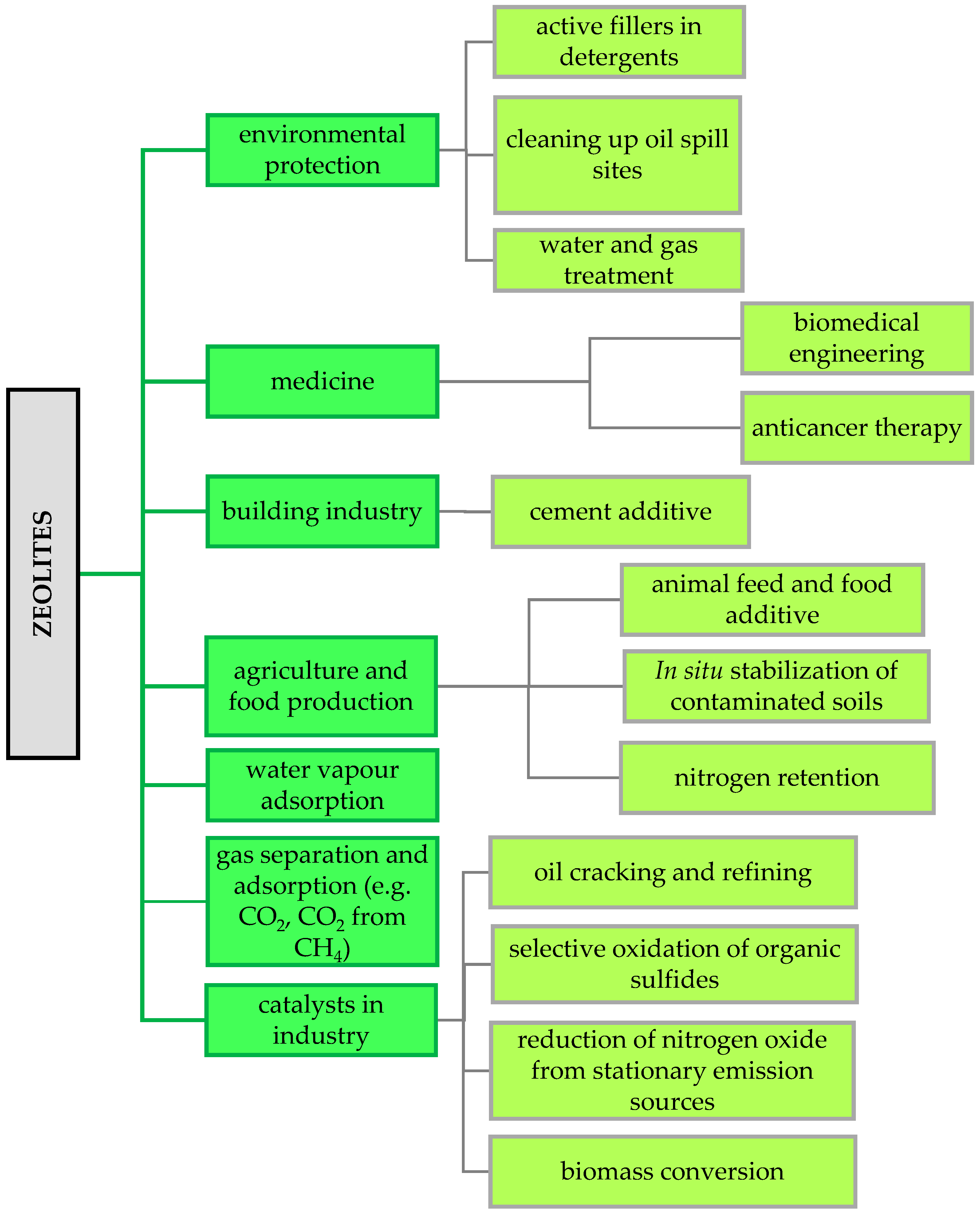
Zeolites, as microporous materials, have unique physicochemical properties and a unique structure, making them widely used in many modern scientific and industrial fields [79,80] (Figure 4).
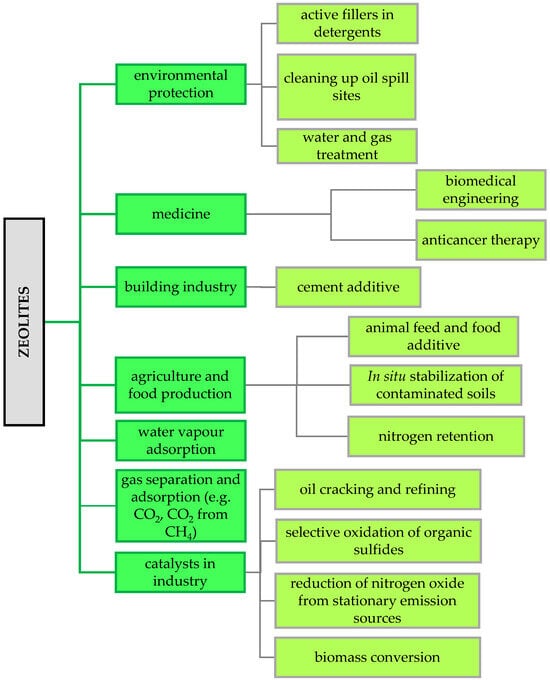
Figure 4.
Examples of zeolite applications in industry (own elaboration based on Rhodes [80]).
The magnitude of the negative charge, compensated by mobile cations, determines the sorption and ion exchange capacity and ion exchange selectivity of the zeolite. Their spatial structure allows for molecular-scale and thread processes and catalytic activity, which can be further enhanced by modifying their surfaces and pores [81]. Due to their unique properties, they are used in catalysis, ion exchange, adsorption, and separation of mixture components [25]. They are also a perspective material in the synthesis of nanostructures, inclusion chemistry, or guest–host complexes [82].
The industrial use of zeolites began in the 1960s, when the US-based Mobil Oil Corporation and Union Carbide used zeolite Y (FAU) as a cracking catalyst in oil processing [83]. A few years later, modified forms of zeolite Y, including rare earth Y (REY) and ultra-stable Y (USY), were obtained in the company’s laboratories, and in 1973, an innovative method for synthesizing high-silicon zeolite ZSM-5 using organic alkylammonium cations as crystallization guides was developed [83]. In the following years, a number of new and modified zeolites were synthesized and found wide applications in industrial processes related to adsorption, ion exchange, and catalysis [11].
Hierarchical (or mesoporous) zeolites are of increasing interest. They are obtained by top–down (desilication, dealumination, recrystallization, and irradiation) and bottom-up (template-free, soft templating, hard templating, double templating with surfactant, nanoparticle assembly, and zeolization of materials) methods [84]. However, the most efficient and cost-effective way to synthesize hierarchical zeolites is the desilication process, which involves the preferential removal of silicon from the zeolite structure by OH− ions in an alkaline medium (usually a NaOH solution) [85]. The obtained product is characterized by the presence of a secondary mesopore system within each grain (which ensures a relatively free diffusion of reactants to and from the active centers and the transport of branched molecules) while maintaining its microporous nature and high-performance acid centers [84]. The best-studied technique for removing silicon from the zeolite skeleton is treatment with NaOH solution (0.2 Mol L−1) at 65 °C for 30 min at a ratio of 1 g of zeolite per 30 mL of solution [86]. Hierarchical zeolites have been used primarily as catalysts in reactions such as cracking, hydrocracking, alkylation, or isomerization [87].
4.1. Zeolite Applications in Agriculture
Worldwide, agriculture is the main user of natural zeolites. However, in the agricultural sector, zeolites are mainly used in animal husbandry (bedding and feed additives), and about 30% of the mineral is used as a soil additive [88].
4.1.1. Soil Amendment with Multidirectional Action
Zeolites are considered to be one of the most widely used natural inorganic agents to improve the physical and chemical properties of soils [89]. The presence of large pores in zeolites allows them to retain water in their structures [29], and thus, due to their unique properties, zeolites can increase water use efficiency (WUE) by increasing the water holding capacity (WHC) of the soil [90]. Xiubin and Zhanbin [91] showed that the WHC of zeolite-treated soil increased by 0.4–1.8% under drought conditions and by 5–15% under normal conditions compared to the control soil. According to the authors, zeolite application can reduce surface runoff and protect the soil from erosion, as well as regulate crop water supply under severe drought conditions. Zeolites also improve infiltration rate and saturated hydraulic conductivity [92], cation exchange capacity (CEC) [93], water-holding capacity, and aeration [28,89]. Soils with zeolites can, therefore, better retain rain and snowmelt water and prevent it from percolating deep into the soil profile (beyond the root zone) [89]. Zeolites have a positive effect on the geometric properties of soils, including specific surface area and porosity [88]. An increase in specific surface area and a decrease in pore size can result in, among other things, reduced oxygen diffusion, mineralization of humic compounds, and loss of organic carbon stocks. This phenomenon is particularly beneficial in soils with low organic matter content and relatively poor aeration [94]. Zeolite incorporation can also improve nutrient retention [14] and help buffer soil pH, reducing the need for lime [28]. Bikkinina et al. [95] demonstrated in a field experiment that the application of zeolites to leached black soil resulted in an increase in soil pH, plant-available phosphorus and potassium, improved microbial activity in the rhizosphere, and accelerated microbial biomass growth. Due to its alkaline nature and the presence of a negative charge, phosphorus availability is increased in zeolite-enriched soils following an increase in soil pH and a decrease in the amount of exchangeable iron and aluminum ions [90,96]. The specific physicochemical properties of zeolites make them capable of releasing nutrients gradually, increasing productivity and efficiency of fertilizer use, reducing losses, and thus reducing environmental pollution [97].
By reducing the intensity of the nitrification process, the addition of zeolites reduces the risk of nitrate leaching into groundwater [98]. Zeolites have been shown to have a particularly high affinity for NH4+ [99,100]. The presence of small pores (nominal pore size 4–5 Å) in the structure of the zeolite crystal lattice, in which ammonium cations are easily adsorbed, makes them unavailable to nitrifying microorganisms and conversion to NO3+ [90]. Thus, in zeolite-treated soils, there is improved retention of this cation and slower release into the soil substrate, which increases the efficiency of its utilization and improves crop yields [101]. Ahmed et al. [102] showed that the application of inorganic fertilizers mixed with zeolites significantly increased the uptake of nitrogen, potassium, and phosphorus and their application efficiency in maize crops. Similar observations were made by Li et al. [103], who found an increase in spinach yield and plant nutrient assimilation in a greenhouse experiment after the combined application of zeolite, ammonium, and potassium.
4.1.2. Crop Protection
The ion exchange capacity and potentially high sorption capacity of zeolites can also be successfully exploited when used as carriers for pesticides and herbicides [14]. Shirvani et al. [104] conducted a study to develop slow-release formulations (SRFs) of 2,4-dichlorophenoxyacetic acid (2,4-D) using, among others, zeolite modified with cetyltrimethylammonium bromide (CTAB) as a surfactant. The authors showed that the SRF had the same herbicidal efficacy as free (technical) 2,4-D. In addition, it significantly reduced the mobility of the herbicide in the soil and reduced its desorption. After 168 h, between 62% and 64% of the adsorbed 2,4-D was released into the solution phase. According to the researchers, the SFR can be considered an effective tool for weed control in sustainable agriculture. The formulations release the active ingredients of the herbicides gradually, reducing their loss through leaching and biodegradation, thereby reducing the negative environmental impact of herbicides. Similar conclusions were reached by Bakhtiary et al. [105].
Another use of zeolites in agriculture is as plant protection products against pests and fungal diseases. Calzarano et al. [106] used a spray of crushed zeolite (15 kg L ha−1) to control gray mold and sour rot in a white grapevine variety. The researchers showed that this strategy was effective, reducing the risk of infection by more than 70% for both diseases. The antifungal effect of zeolite is based on the formation of a layer of mineral particles on the treated plant, which forms a physical barrier that inhibits the germination and development of acid rot and gray mold fungi. According to the authors, reflectance measurements performed on the leaves of the treated grapevines showed no differences compared to the control series in terms of NDVI (Normalized Difference Vegetation Index) and GNDVI (Green Normalized Difference Vegetation Index), whose values correlate with the amount of biomass and chlorophyll content. A similar study was carried out by Prisa [107], which confirmed the usefulness of micronized zeolite as a fungicide in viticulture. Its foliar application effectively reduced the development of diseases caused by Botrytis cinerea, Oidium tuckeri, and powdery mildew compared to the application of copper and sulfur. At the same time, zeolite had a positive effect on the vegetative and root growth of Vitis vinifera and showed no phytotoxicity. In his opinion, zeolite is an ecological and cost-effective tool for increasing plant productivity in sustainable agriculture.
4.1.3. Heat Stress and Photosynthesis Enhancement on Crops
Another advantage of the foliar application of zeolites is the increase in carbon dioxide near the stomata and the reduction in leaf temperature by reflecting infrared radiation [108]. Zeolites are able to adsorb carbon dioxide molecules and release them gradually into the ecosystem [109], which in turn can increase the photosynthetic rate of C3 plants [108], such as grapevines, tomatoes, apple, and orange trees [14]. This results in increased vegetative growth [110], an increased leaf area production rate, and a reduced transpiration rate [111].
4.1.4. Aquaculture
In aquaculture, zeolites are used to reduce the amount of algae in water reservoirs or farm ponds, assist in the elimination of ammonia from water [112], and are also used to aerate aquatic organisms with oxygen produced by air separation [14]. The addition of zeolites to fish ponds reduces turbidity, with positive effects on water quality, fish health, and growth performance [113].
4.2. Zeolites in Environmental Protection
The use of zeolites in environmental protection is mainly based on their ion exchange properties [114]. Other properties that determine their suitability in this field are their significant adsorption capacity, long-term mechanical and physical stability, and strong selectivity and molecular sorption capacity [11,115].
4.2.1. Sorption of Radionuclides
Zeolites are widely used to sequester cationic contaminants such as the trace elements lead, cadmium, zinc, nickel, manganese, chromium, copper, and iron [90]. They are also used to extinguish chemical fires and to deactivate nuclear and other hazardous industrial wastes [114]. A study by Osmanlioglu [116] evaluated the usefulness of clinoptilolite in the removal of radionuclides (137Cs, 60Co, 90Sr, and 110Ag) from liquid radioactive waste. It was found to be an effective sorbent of radionuclides under dynamic processing conditions and can be used as a cheaper alternative to chemicals in the chemical precipitation process. A limitation in the use of clinoptilolite is the high content of inactive salts in the radioactive waste, which reduces the ion exchange capacity of zeolite towards 90Sr and 60Co.
Lihareva et al. [117] also demonstrated the usefulness of clinoptilolite for the removal of Cs+ and Sr+ from aqueous solutions, reporting a maximum adsorption capacity of 122.7 and 21.50 mg g−1, respectively. Borai et al. [118] evaluated four different zeolite minerals (natural clinoptilolite, chabazite, mordenite, and synthetic mordenite) for their utility in removing certain radionuclides from low-level radioactive liquid waste (LLRLW). They demonstrated that of the materials tested, natural chabazite had the highest decay rates and Cs ion exchange capacity. Promising results for the removal of radium isotopes from mine water using zeolite NaP1 were obtained by Chałupnik et al. [119]. The water purification efficiency exceeded 98% for the radium isotopes 226Ra and 228Ra. In addition, they confirmed the possibility of removing radium from very saline waters (TSD > 100 g L−1) using the zeolite material.
4.2.2. Immobilization of Trace Elements in the Soil
Zeolite remediation of contaminated soils reduces the amount of phytoavailable forms of trace elements, leading to the restoration of soil homeostasis [120,121]. When mixed with Portland cement, zeolites are an effective stabilizing agent and, in the case of trace elements, an immobilizing agent [122]. The mechanism of trace element adsorption using zeolites includes the following phenomena: (1) ion exchange; (2) electrostatic attraction; (3) intrapore adsorption; (4) surface complexation; and (5) surface precipitation [123]. The pH value of the solution has an important influence on the above-mentioned processes, as it affects the surface charge of the zeolite and, consequently, the adsorption of trace elements [124]. The presence of free cations in the zeolite skeleton allows ion exchange with cations present in solution (mainly off-grid sodium participates in the exchange process) [125]. The effect of zeolites as contaminant sorbents is to bind harmful trace elements into insoluble compounds or organic–mineral complexes [126], which are less available to plants and immobilized in the soil in a safe form for a long time [127]. This is confirmed by our study [128], in which the application of a molecular sieve (crystalline aluminosilicate with a micropore size of 0.3 nm) reduced the concentration of iron (by 5%), nickel (by 8%), cadmium (by 18%), chromium (by 22%), zinc (by 22%), copper (by 13%), and manganese (by 44%) in the aerial parts of sunflowers grown in copper-contaminated soil compared to the control. In contrast, in the roots of sunflowers, zeolite application to soil contributed to a decrease in chromium and zinc content by 15% and 4%, respectively. The beneficial effect of zeolite application on the immobilization of trace elements in contaminated soil was also demonstrated by Cadar et al. [79]. They showed that a 10% addition of this material to the soil reduced the bioaccumulation of Co, Cr, Cu, Mn, Ni, Pb, and Zn in the roots and shoots of spinach, parsley, and lettuce, with the exception of Cd in the spinach roots. Zeolite had an analogous effect on the content of nickel and copper in oat roots [129]. Li et al. [130] also confirmed the usefulness of zeolite in the remediation of lead-contaminated garden soil. The addition of natural zeolite (20 g kg−1) increased soil pH, exchange capacity, and organic matter content and facilitated the formation of soil aggregates. It also reduced the bioavailability of lead and its uptake by canola. At the highest level of soil contamination with lead (2000 mg kg−1), the content of the analyzed element in plant roots decreased by 49% and in shoots by 30% compared to the control series. In a study by Wyszkowski and Brodowska [131], zeolite reduced the content of zinc, manganese, and cobalt, and in the experiment by Kosiorek and Wyszkowski [132], it reduced the content of copper and nickel in maize. The application of zeolite to soil contaminated with trace elements also affects the structure and microbial activity by increasing the activity of the enzyme dehydrogenase, thus improving soil condition and fertility [133].
4.2.3. Gas Adsorption and Catalysis
All natural and synthetic zeolites can be used for the selective adsorption of components of gas mixtures, their drying and purification, and odor control due to the variation in pore size and the presence of cations in the structure [14]. They are used in intensive livestock farming to reduce odors caused by H2S and NH3 [134,135]. They also reduce the humidity in such areas [14]. The ammonium adsorption capacity of zeolites ranges from 8.149 mg N g−1 to 15.169 mg N g−1 [136]. In addition, zeolite can be widely used in combination with other additives to reduce gas emissions, salinity, and nutrient loss during the composting process [137,138]. Wang et al. [139] evaluated the effect of adding zeolite, wood vinegar, and biocarbon on the composting process of pig manure. After 50 days, the authors reported a reduction in methane (by 50.39–61.15%), carbon dioxide (by 33.90–46.98%), and nitrous oxide (by 79.51–81.10%), and a reduction in ammonia loss (by 64.45–74.32%) compared to the control (no additives). The positive effect of zeolite addition on the composting process of dewatered fresh sludge has also been demonstrated by Awasthi et al. [137]. The best results were observed in the series with a 30% zeolite and 1% lime addition. This treatment significantly reduced ammonia, methane, nitrous oxide emissions, and nitrogen losses (by 50%) compared to the control series. The addition of zeolite increased the initial pH, had an activating effect on the total aerobic bacterial population, and increased the porosity of the feedstock and the composting rate. Under these conditions, compost maturity was achieved in 37% less time (35 days versus 56 days) than in the control sample.
The specific size of the channels inside the structure of zeolites allows them to act as molecular sieves and selectively adsorb components of gaseous mixtures. In addition, physicochemical modification of zeolites makes it possible to obtain materials with desired properties. Akyalcin et al. [140] conducted an experiment to develop a method for obtaining hydrogen (H2) storage materials. The authors used clinoptilolite, which they treated with various chemical compounds (HCl, C2H2O4, and HNO3). The authors evaluated the effects of the applied solution concentration (0.1–1.0 Mol L−1), temperature (60–80 °C), and treatment time (2–4 h) on the hydrogen adsorption capacity of the zeolite. The best results were observed after treatment with 0.5 Mol L−1 HNO3 at 80 °C for 2 h. Clinoptilolite modified under these conditions exhibited a 7.3-fold higher H2 adsorption capacity relative to the crude material. The better H2 adsorption capacity of the modified clinoptilolite was associated with an increase in the zeolite’s specific surface area, volume, and size of micropores, as well as an increase in the strength of the acid centers.
The catalytic properties of zeolites are related to their unique properties with respect to the specific surface area, pore size (shape-selective catalysis), crystallinity, and thermal stability. In addition, the presence of proton donor (bridged -OH; Brønsted acid centers), electron acceptor (Al cross-linked tri-correlated; Lewis acid centers), and electrodonor (O2− and AlO42−; Lewis base (alkali) centers) groups enables zeolites to catalyze many reactions on an industrial scale [25]. Clinoptilolite and mordenite are used as adsorber catalysts for the removal of SO2 from gas and flue gas streams from factory stacks [141]. Zeolites are also used in environmental catalysis as adsorbents and catalysts for the reduction of nitrogen oxides (NOx) and volatile organic compounds (VOCs) [142]. The aforementioned compounds are major air pollutants, and their source is the combustion of fossil fuels, both stationary (power plants) and mobile (automobiles) [143], as well as metallurgy [142] and nitric acid plants [144]. Most techniques to reduce the formed nitrogen oxides involve the introduction of a reductant (e.g., ammonia or urea) into the waste gas and its reduction to molecular nitrogen and water [145,146]. Due to its high efficiency and wide temperature window (150–450 °C), the most widely used NOx abatement technology is selective catalytic reduction using ammonia as the reducing agent (NH3-SCR) [147]. A commonly used commercial catalyst in the NH3-SCR process is vanadium oxide supported on titanium oxide (V2O5-TiO2) [148]. However, due to its unsatisfactory efficiency and the toxicity of vanadium compounds to the environment [144], alternative catalysts are being sought for the NH3-SCR process. Zeolite systems doped with transition metal ions (mainly copper and iron) are promising [149].
The characteristics of zeolites that support their usefulness in the deNOx (NOx destruction) process are mainly high catalytic activity, favorable temperature window, high thermostability, chemical resistance [150,151], and hydrothermal stability [152]. Zeolites of great interest for application in the deNOx process include zeolite ZSM-5 [153,154], SAPO-34 [155], Fe-Beta [156], or iron-modified clinoptilolite (after its application, the NOx conversion rate exceeded 90%) [144]. Iron-enriched zeolite catalysts are mainly active at medium, and high temperatures [150]. An example is the Fe-ZSM-5 zeolite, which shows high activity in the temperature range of 300 °C to 450 °C [157]. However, at such high temperatures, carbon deposition can occur and slow down the process [158]. Consequently, copper-based zeolite catalysts, which are highly active at lower temperatures (<300 °C) despite their lower hydrothermal stability, are of greater interest [150,159]. The deposition of copper in the zeolite support (in the amount of 2–4 wt%) increases the deNOx efficiency up to 95% [158]. The Cu-SSZ-13 catalyst has found commercial application in diesel engines to reduce nitrogen oxides in the exhaust [160]. Paolucci et al. [161] presented the mechanism of the deNOx process on Cu-SSZ-34, paying particular attention to the redox cycle of copper forms that change their degree of oxidation: CuII ↔ CuI. The desired N2 product is formed within two half-cycles (reduction and oxidation), which is a new insight into the mechanism of the SCR reaction. Ammonia as a reductant in the SCR process can be replaced by methane, which has an inert chemical character and lower cost [162]. Cobalt-ion-supported zeolite-based systems catalyze the selective NO reduction reaction with methane in the presence of water [163,164]. The addition of a second metal, such as palladium or platinum, favorably affects their catalytic properties and improves the efficiency of the reduction process [165,166]. Cobalt-enriched zeolites alone exhibit high NOx reduction activity and nearly 100% reduction to nitrogen at temperatures above 400 °C [167,168]. In contrast, the ZSM-5 zeolite based on cobalt, platinum, and nickel shows high activity in the catalytic oxidation of VOCs such as benzene and toluene to CO2 and H2O [169].
In terms of energy and heating solutions, zeolites can serve as components of heat exchangers [170]. They are used to store heat from solar radiation, off-peak electricity, or waste heat and then use this energy in air temperature control or water heating processes [171].
4.2.4. Wastewater Treatment
In wastewater treatment technologies, zeolites are mainly used for the removal of biogenic compounds (nitrogen and phosphorus), radioactive elements, and trace elements [172]. Natural zeolites allow the removal of ammonium nitrogen from wastewater in amounts ranging from 0.4 to 25.5 mg g−1 adsorbent [173,174]. According to Huang et al. [175], the equilibrium state of ammonium ion sorption on zeolites is reached after about 60–120 min. The following parameters influence the efficiency of the process: the contact time of the wastewater with the zeolites, the pH of the solution, the dose and type of zeolites, and the presence of other ions in the solution [172]. Modification of zeolite surfaces with alkalis and strong acids improves the sorption capacity of the mineral for cations [90]. Liang and Ni [176] carried out a modification of a natural zeolite (55% was clinoptilolite) to increase the absorption of ammonium ions. The modification included pretreatment (grinding and sieving), treatment with 1.5 Mol L−1 sodium chloride (NaCl), and calcination. After these processes, the specific surface area, total pore volume, and average pore size of the initial zeolite increased. The authors noted the most favorable effects after the combined use of NaCl modification and calcination. Such treatment increased the temperature resistance of the zeolite (from 150 °C to 400 °C) and also increased the ammonium ion uptake rate (AIU) by 4.3 times compared to the raw zeolite. The effect of clinoptilolite modification with ultrasound-assisted chemicals (NaOH, HCl, and FeCl3) on the efficiency of ammonium ion removal from water was evaluated by Jahani et al. [177]. The highest ammonium removal efficiency (≥99%) was observed after the acid-modified zeolite. In comparison, the original zeolite removed the contaminant with an efficiency of 51.66%. Importantly, the authors used the modified zeolite five times in the ammonium removal process, and it showed stability in terms of its structural and adsorption properties.
Clinoptilolite shows high selectivity towards heavy metal cations in the following order: Pb2+ > Cd2+ > Cu2+ > Co2+ > Cr3+ > Zn2+ > Ni2+ > Hg2+ [178]. For this reason, Galletti et al. [179] conducted an experiment to evaluate the suitability of clinoptilolite as a low-cost adsorbent for the removal of Zn2+ and Cd2+ ions from wastewater in a batch system. Complete adsorption for both analyzed ions was achieved by the authors at a solution pH of 4.5 and a sorbent concentration of 10 mg L−1. Interestingly, in the presence of both ions, clinoptilolite showed a higher affinity towards Zn2+ than towards Cd2+, i.e., the opposite of the single system. This was probably due to a higher partition coefficient for zinc than for cadmium and a stronger binding of the zeolite to zinc. In contrast, Senila et al. [180] showed that clinoptilolite, in addition to being an adsorbent of contaminants, can also act as a carrier for biofilm formation and microbial growth involved in biological wastewater treatment, which has a beneficial effect on the overall process. The usefulness of silver-modified clinoptilolite in immobilizing Cr(VI) ions from model wastewater was demonstrated by Panayotova [181]. In this study, the effect of the reaction (pH 4, 6, and 8) and initial Cr(VI) ion concentration (10 and 20 mg L−1) on the removal efficiency of the analyzed pollutant was evaluated. The studies were conducted at a constant ratio of v:m = 100, i.e., 100 mL of wastewater per 1 g of zeolite. It was shown that Cr(VI) immobilization increased with increasing pH values. The best results were obtained after 45 min at pH 8 and an initial Cr(VI) concentration of 20 mg L−1. Under these conditions, 82.4% of the chromium ions were removed from the model solution. In the case of industrial wastewater, the analyzed zeolite allowed a reduction of Cr(VI), Cu(II), and Zn(II) contents of more than 80%, 75%, and 70%, respectively, within 30 min. Zeolites can also be used to remove phosphorus compounds from wastewater, but only at an acidic pH. Under these conditions, sites on the zeolite surface become protonated, acquiring a positive electrical charge and attracting phosphate ions. A chemical interaction then takes place between the zeolite surface and the phosphate molecules, which are immobilized [6]. This is confirmed by the studies of Zhang et al. [182] and Goscianska et al. [183].
Table 3 summarizes the directions of application of selected natural and synthetic zeolites.

Table 3.
Examples of practical applications of selected zeolites in various industries.
The application of a particular type of zeolite in a particular field is determined by the properties it exhibits. These include the chemical nature of the surface, the strength of the active sites, the porous structure, the number, type, and distribution of pores, or the molar ratio of Si/Al. For example, Garshasbi et al. [187] used synthetic zeolite 13X in their study of H2S separation from a butane gas mixture because of its high operating efficiency at low partial pressures of the recovered components and high selectivity at temperatures up to 100 °C. In addition, it tends to adsorb smaller molecules due to its uniform pore entrance diameter. In contrast, Hashemi et al. [189] used a synthetic faujasite or zeolite Y to remove organic pollutants from wastewater, mainly because of its structural stability and large available pore volume, as well as its Si/Al ratio of ≥1.5, which increases its affinity for non-polar substances.
Clinoptilolite is the most abundant natural zeolite, forming extensive and abundant deposits throughout the world. It has a two-dimensional structure of 8-ring and 10-ring channels [116], and the Si/Al ratio is greater than 4, which makes it characterized by the high thermal stability of the structure [140]. It is also characterized by a high ion exchange capacity and a particular affinity for heavy metal cations. It can adsorb elements such as 137Cs, 90Sr, and other radioactive isotopes from solution and hold them in its three-dimensional crystal structure [117]. The low cost of clinoptilolite makes its use in the treatment of radioactive waste very attractive [116]. In addition, synthetic zeolites are produced by hydrothermal methods using fly ash as a raw material, which may contain elevated levels of radionuclides. This, in turn, requires monitoring of the natural radioactivity of synthetic zeolites during their synthesis [119]. The aforementioned properties of clinoptilolite support its superiority in the application of radionuclide sorption processes, as confirmed in their studies by Osmanlioglu [116] or Lihareva et al. [117].
Clinoptilolite contains the exchangeable cations Na+, K+, Ca2+, and Mg2+ [140], which allow ion exchange and are the basis of many processes, including drinking water treatment. These properties of zeolite, as well as economic considerations, determined the use of this natural zeolite in a study by Erdem et al. [188] to remove Co2+, Cu2+, Zn2+, and Mn2+ ions from industrial wastewater.
In an experiment carried out by Goscianska et al. [183], zeolites Na-P1 and Na-A were used to remove phosphorus compounds from wastewater, since synthetic zeolites obtained from fly ash have a high cation exchange capacity and are able to capture phosphates from solution in oxyanionic forms.
4.3. Other Applications of Zeolites
As mentioned earlier in this paper, zeolites are mainly used in traditional industries, such as catalysts in the petroleum industry, molecular sieves, adsorbents in environmental protection, or soil additives in agriculture. Their innovative and future applications seem to be in medicine and biotechnology. According to Pavelić and Hadžija [196], natural and synthetic zeolites have great potential for biomedical applications and can, therefore, contribute to significant advances in the pharmaceutical industry and biology.
Zeolites have found medical applications in modern drug delivery systems [197], wound healing [198], hemodialysis [199], and tooth root canal filling [200]. The adsorption and ion exchange properties of natural clinoptilolite have been exploited in the development of an anti-diarrheal drug [201] and a gastric acid neutralizer [202]. The aforementioned chewable tablets proved effective in the treatment of people suffering from hyperacidity due to gastric dyspepsia and gastric and duodenal ulcers. They had no side effects, did not alter the structure of pepsin, and their physical and chemical properties remained stable after three years of storage at room temperature [202].
4.3.1. Adsorption of Harmful Substances
Due to their detoxifying properties, zeolites are used as materials to remove harmful substances such as pesticides, mycotoxins, or heavy metals. The neuroprotective potential of clinoptilolite in mice exposed to Pb2+ was demonstrated by Basha et al. [203]. The experiment consisted of intraperitoneal administration of lead acetate (100 mg kg bw−1 day−1) to three-week-old mice for 21 days, followed by combined treatment with EDTA and clinoptilolite (100 mg kg bw−1) for 2 weeks. The authors observed that this contributed to a reduction in lipid peroxidation and the induction of antioxidant mechanisms, as manifested by an increase in catalase, superoxide dismutase, glutathione peroxidase, and glutathione activity.
4.3.2. Tissue Engineering
Zeolites are also used in tissue engineering [204], in the formation of implant coatings, and in the preparation of fungicidal dressings and antibacterial agents [194]. Their porous nature and high biocompatibility make them ideal materials for bone tissue cell adhesion and proliferation [205]. The high osteogenic potential of zeolite materials and the ability to customize pore shape and size allow the creation of diverse scaffolds with a wide range of biomedical applications. As implant coatings, zeolites increase bone conductivity, aid in local elastic modeling, and reduce local inflammation [204]. As reported by Banu et al. [206], zeolites may also help prevent postmenopausal bone loss. In their experiment, Bedi et al. [195] prepared biocompatible zeolite coatings for use in biomedical implants. Synthesized on commercially pure titanium and Ti6Al4V alloys, the MFI zeolite coating had higher corrosion resistance than the titanium alloy and reduced the release of cytotoxic Al and V ions into the surrounding tissue. In addition, it had excellent adhesion to the substrate, which could potentially prevent implant loosening. The tested coating also reduced the mismatch between the module and the bone tissue and had a positive effect on implant osteointegration. The aforementioned benefits of MFI zeolite coating indicate its potential in dental and orthopedic applications to facilitate patient recovery after surgery.
4.3.3. Carriers of Bioactive Compounds
Zeolites, due to their high specific surface area, stability, and susceptibility to modification, provide encapsulation of biologically active substances and their controlled release, making them an excellent biomaterial for drug delivery systems [207]. Linares et al. [208] conducted a study to use zeolite cancrinite as a carrier for acetylsalicylic acid and to determine the hydrolytic stability of the drug. They demonstrated that it is possible to administer zeolite as an anti-acid drug and an acetylsalicylic acid carrier at the same time, since no loss of individual pharmaceutical effect was observed for either substance analyzed. In an experiment carried out by Arruebo et al. [193], nanocomposites of magnetite and commercial Na-Y zeolite were formed by mechanical activation during high-energy milling at room temperature. They were characterized by a high specific surface area (442.9 m2 g−1) and cationic capacity, which allowed the adsorption, storage, and release of significant amounts of doxorubicin, an antibiotic widely used in cancer chemotherapy. Such a drug carrier can be directed directly to the tumor cells and released upon application of an external or internal magnetic field. In this way, the therapeutic dose used to date can be reduced, and the side effects associated with drug application can be reduced [209].
Zeolites can also be used as carriers for substances with antimicrobial properties. Neidrauer et al. [194] evaluated the usefulness of an antimicrobial ointment for the treatment of acute and chronic wounds, in which the active ingredient was nitric oxide embedded in zeolite A. The researchers observed that the zeolite, when subjected to ion exchange with zinc ions and loaded with nitric oxide, gradually released it over 3 h after contact with water in the skin. The minimum microbicidal concentrations (MMC) of the tested ointment against bacterial organisms (5 × 107 c.f.u.) ranged from 50 to 100 mg, while against the yeast C. albicans (5 × 104 c.f.u.), the MMC was 50 mg. After 8 h of exposure to zeolite ointment, a reduction in bacterial (by 5–8 log cycles) and fungal (by 3 log cycles) cell viability was observed compared to the control series. In addition to its therapeutic effect, zeolite also promotes the healing process of bacterially infected wounds. The mechanism of antimicrobial action of zeolites is not fully understood but is probably based on physical adsorption, ion exchange, and indirect catalysis [210]. Due to their physical adsorption capacity, zeolites immobilize microbial cells on their surface, leading to their death [211]. In contrast, the chemical interaction of zeolites with microorganisms involves the release of positively charged metal ions (e.g., copper and silver) [212] or reactive oxygen species (e.g., hydrogen peroxide) from their structures into the microenvironment [213], resulting in damage to microbial cell walls and membranes, loss of membrane potential, and ultimately cell destruction and death.
Zeolites, therefore, have many applications. Applications related to the reduction in agriculture and environmental pollution appear to be particularly interesting.
5. Conclusions
The popularity of zeolites has been growing steadily since their first industrial applications (second half of the 20th century) until the present day, and research into their properties, synthesis methods, and directions of use has not lost its relevance. This is evidenced by the ever-increasing number of known zeolite structures described in the IZA (International Zeolite Association) database. This environmentally and economically friendly material remains a challenge for science, contributing to the further development of highly active catalysts, adsorbents, and ion exchangers with high selectivity.
The development of methods for the chemical synthesis of zeolites and the modification of their surface has led to materials with new porous structures and physical properties, contributing to almost unlimited possibilities for their commercial and environmental applications.
Zeolites remain a material of the future. Increasing industrialization, climate change, and many years of anthropopressure are expected to be the main factors influencing the development of the market for the aforementioned materials due to the potential for the use of zeolites in environmental protection and agriculture, as well as the growing demand for innovative materials in biomedical processes, active substance delivery systems, and other applications.
Author Contributions
Conceptualization, M.W. and N.K.; methodology, N.K.; analysis, N.K.; writing—review and editing, N.K. and M.W.; supervision, M.W.; and funding acquisition, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was written as part of a comprehensive study financed by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry, Department of Agricultural and Environmental Chemistry (grant No. 30.610.004-110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the manuscripts and from the authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The use of zeolites as an addition to fertilisers—A review. CATENA 2022, 213, 106125. [Google Scholar] [CrossRef]
- Ahmadi, B.; Shekarchi, M. Use of natural zeolite as a supplementary cementitious material. Cem. Concr. Compos. 2010, 32, 134–141. [Google Scholar] [CrossRef]
- Hrachovcová, K.; Tišler, Z.; Svobodová, E.; Šafář, J. Modified alkali activated zeolite foams with improved textural and mechanical properties. Minerals 2020, 10, 483. [Google Scholar] [CrossRef]
- Larin, V.A. The Loewenstein rule: The increase in electron kinetic energy as the reason for instability of Al-O-Al linkage in aluminosilicate zeolites. Phys. Chem. Miner. 2013, 40, 771–780. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C. Zeolite application in wastewater treatment. Adsorp. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Bandura, L.; Białoszewska, M.; Leiviskä, T.; Franus, M. The Role of zeolite structure in its β-cyclodextrin modification and tetracycline adsorption from aqueous solution: Characteristics and sorption mechanism. Materials 2022, 15, 6317. [Google Scholar] [CrossRef]
- Kuldeyev, E.; Seitzhanova, M.; Tanirbergenova, S.; Tazhu, K.; Doszhanov, E.; Mansurov, Z.; Azat, S.; Nurlybaev, R.; Berndtsson, R. Modifying natural zeolites to improve heavy metal adsorption. Water 2023, 15, 2215. [Google Scholar] [CrossRef]
- Khaleque, A.; Alam, M.M.; Hoque, M.; Mondal, S.; Haider, J.B.; Xu, B.; Johir, M.A.H.; Karmakar, A.K.; Zhou, J.L.; Ahmed, M.B.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Database of Zeolite Structures, International Zeolite Association. Available online: http://www.iza-structure.org/databases (accessed on 5 February 2024).
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in adsorption processes: State of the art and future prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.C.; Bieseki, L.; Melguizo, P.V.; Pergher, S.B.C. Environmentally Friendly Zeolites—Synthesis and Source Materials; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Mijailović, N.R.; Nedić Vasiljević, B.; Ranković, M.; Milanović, V.; Uskoković-Marković, S. Environmental and pharmacokinetic aspects of zeolite/pharmaceuticals systems—Two facets of adsorption ability. Catalysts 2022, 12, 837. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Massini, C.M.; Mattii, G.B. Application of zeolites in agriculture and other potential uses: A review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- De Souza, V.C.; Villarroel-Rocha, J.; De Araújo, M.J.G.; Sapag, K.; Pergher, S.B.C. Basic treatment in natural clinoptilolite for improvement of physicochemical properties. Minerals 2018, 8, 595. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, J.; Ma, H.; Ma, X.; Yuan, J. Synthesis of pure NaA zeolites from coal fly ashes for ammonium removal from aqueous solutions. Clean Technol. Environ. Policy 2016, 18, 629–637. [Google Scholar] [CrossRef]
- Längauer, D.; Čablík, V.; Hredzák, S.; Zubrik, A.; Matik, M.; Danková, Z. Preparation of synthetic zeolites from coal fly ash by hydrothermal synthesis. Materials 2021, 14, 1267. [Google Scholar] [CrossRef]
- Król, M. Natural vs. synthetic zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Geerlings, P.; Pidko, E.A.; van Santen, R.A. The framework basicity of zeolites. J. Mater. Chem. 2012, 22, 18705–18717. [Google Scholar] [CrossRef]
- Walkowiak, A.; Wolska, J.; Wojtaszek-Gurdak, A.; Sobczak, I.; Wolski, L.; Ziolek, M. Modification of gold zeolitic supports for catalytic oxidation of glucose to gluconic acid. Materials 2021, 14, 5250. [Google Scholar] [CrossRef]
- Kulprathipanja, S. Zeolites in Industrial Separation and Catalysis; Wiley VCH: Weinheim, Germany, 2010. [Google Scholar]
- Szostak, R. Molecular Sieves. Principles of Synthesis and Identififaction; Springer: New York, NY, USA, 1989. [Google Scholar]
- Guisnet, M.; Gilson, J.P. Zeolites for Cleaner Technologies; Catalytic Science Series; Imperial College Press: London, UK, 2002. [Google Scholar] [CrossRef]
- Payra, P.; Dutta, P. Zeolites: A Primer. In Handbook of Zeolites Science and Technology; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Moshoeshoe, M.; Nadiye-Tabbiruka, M.S.; Obuseng, V. A Review of the chemistry, structure, properties and applications of zeolites. Am. J. Mater. Sci. 2017, 7, 196–221. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Ming, D.W.; Allen, E.R. Use of natural zeolites in agronomy, horticulture and environmental soil remediation. Rev. Mineral. Geochem. 2001, 45, 619–654. [Google Scholar] [CrossRef]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. 2004, 12, 183–189. [Google Scholar]
- Ramesh, K.; Reddy, D.D. Zeolites and their potential uses in agriculture. Adv. Agron. 2011, 113, 219–240. [Google Scholar] [CrossRef]
- Sharma, V.; Javed, B.; Byrne, H.; Curtin, J.; Tian, F. Zeolites as carriers of nano-fertilizers: From structures and principles to prospects and challenges. Appl. Nano 2022, 3, 163–186. [Google Scholar] [CrossRef]
- Jakubowski, M.; Voelkel, A.; Sandomierski, M. Crystalline zeolite layers on the surface of titanium alloys in biomedical applications: Current knowledge and possible directions of development. Crystals 2022, 12, 1520. [Google Scholar] [CrossRef]
- Johnson, E.B.G.; Arshad, S.E. Hydrothermally synthesized zeolites based on kaolinite: A review. Appl. Clay Sci. 2014, 97–98, 215–221. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.A.; Len, T.; de Oliveira, A.d.N.; Costa, A.A.F.d.; Souza, A.R.d.S.; Costa, C.E.F.d.; Luque, R.; Rocha Filho, G.N.d.; Noronha, R.C.R.; Nascimento, L.A.S.d. Zeolites: A theoretical and practical approach with uses in (bio)chemical processes. Appl. Sci. 2023, 13, 1897. [Google Scholar] [CrossRef]
- Grela, A.; Hebda, M.; Łach, M.; Mikuła, J. Thermal behavior and physical characteristic of synthetic zeolite from CFB-coal fly ash. Microporous Mesoporous Mater. 2016, 220, 155–162. [Google Scholar] [CrossRef]
- Khabuanchalad, S.; Khemthong, P.; Prayoonpokarach, S.; Wittayakun, J. Transformation of zeolite NaY synthesized from rice husk silica o NaP during hydrothermal synthesis. Suranaree J. Sci. Technol. 2008, 15, 225–231. [Google Scholar]
- Wajima, T.; Haga, M.; Kuzawa, K.; Ishimoto, H.; Tamada, O.; Ito, K.; Nishiyama Downs, R.T.; Rakovan, J.F. Zeolite synthesis from paper sludge ash at low temperature (90 °C) with addition of diatomite. J. Hazard. Mater. 2006, 132, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Novembre, D.; Sabatino, B.D.; Gimeno, D.; Garcia-Vallès, M.; Martínez-Manent, S. Synthesis of Na-X zeolites from tripolaceous deposits (Crotone, Italy) and volcanic zeolitised rocks (Vico volcano, Italy). Microporous Mesoporous Mater. 2004, 75, 1–11. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Q.; Wu, H. Synthesis of zeolite-like material by hydrothermal and fusion methods using municipal solid waste fly ash. Procedia Environ. Sci. 2016, 13, 662–667. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, D.; Zhao, J.; Li, X. Synthesis of NaX zeolite at room temperature and its characterization. Mater. Lett. 2013, 104, 80–83. [Google Scholar] [CrossRef]
- Kustova, M.Y.; Kustov, A.L.; Christensen, C.H. Aluminum-rich mesoporous MFI-type zeolite single crystals. Stud. Surf. Sci. Catal. 2005, 158, 255–262. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Dong, D.; Li, D.; Hill, M.R.; Hill, A.J.; Wang, H. Synthesis of hierarchical porous zeolite NaY particles with controllable particle size. Microporous Mesoporous Mater. 2010, 127, 167–175. [Google Scholar] [CrossRef]
- Aylele, L.; Pérez-Pariente, J.; Chebude, Y.; Díaz, I. Conventional versus alkali fusion synthesis of zeolite A from low grade kaolin. Appl. Clay Sci. 2016, 132–133, 485–490. [Google Scholar] [CrossRef]
- Lee, M.-G.; Park, J.-W.; Kam, S.-K.; Lee, C.-H. Synthesis of Na-A zeolite from Jeju Island Scoria using fusion/hydrothermal method. Chemosphere 2018, 207, 203–208. [Google Scholar] [CrossRef]
- Thuadaij, P.; Nuntiya, A. Effect of the SiO2/Al2O3 ratio on the synthesis of Na-X zeolite from Mae Moh fly ash. Sci. Asia 2012, 38, 295–300. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-like inorganic polymers. J. Ceram. Sci. Technol. 2017, 08, 335–350. [Google Scholar] [CrossRef]
- Villa, C.; Pecina, E.T.; Torres, R.; Gómez, L. Geopolymer synthesis using alkaline activation of natural zeolite. Constr. Build. Mater. 2010, 24, 2084–2090. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Jwaida, Z.; Dulaimi, A.; Mashaan, N.; Othuman Mydin, M.A. Geopolymers: The green alternative to traditional materials for engineering applications. Infrastructures 2023, 8, 98. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, J.; Maltceva, O.; Palomo, A.; Fernandes-Jiménez, A. Hybrid alkaline cements: Part I. Fundamentals. Rom. J. Mater. 2012, 42, 330–335. [Google Scholar]
- Cong, P.; Chen, Y. Advances in geopolymer materials: A comprehensive review. J. Traffic Transp. Eng. (Engl. Ed.) 2021, 8, 283–314. [Google Scholar] [CrossRef]
- Król, M.; Rożek, P.; Chlebda, D.; Mozgawa, W. ATR/FT-IR studies of zeolite formation during alkali-activation of metakaolin. Solid State Sci. 2019, 94, 114–119. [Google Scholar] [CrossRef]
- van Jaarsveld, J.G.S.; van Deventer, J.S.J. Effect of the alkali metal activator on the properties of fly ash-based geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Vegere, K.; Vitola, L.; Argalis, P.P.; Bajare, D.; Krauklis, A.E. Alkali-activated metakaolin as a zeolite-like binder for the production of adsorbents. Inorganics 2019, 7, 141. [Google Scholar] [CrossRef]
- Park, M.; Choi, J. Molten-salt method for the synthesis of zeolitic materials I. Zeolite formation in alkaline molten-salt system. Microporous Mesoporous Mater. 2000, 37, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Zheng, S.; Di, Y.; Sun, Z. A Review of the synthesis and application of zeolites from coal-based solid wastes. Int. J. Miner. Metall. Mater. 2022, 29, 1–21. [Google Scholar] [CrossRef]
- Yoo, Y.-S.; Cheon, K.-H.; Lee, J.-I.; Kim, B.-S.; Shin, W.-S.; Seo, G.-T. Zeolite synthesis using sewage sludge by molten-salt method. Mater. Sci. Forum 2008, 569, 329–332. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, X.; Song, H.; Xia, G.; Shen, Z.-Y.; Yu, R.; Moskovits, M. Microwave synthesis of zeolites and their related applications. Microporous Mesoporous Mater. 2021, 323, 111262. [Google Scholar] [CrossRef]
- Panzarella, B.; Tompsett, G.A.; Ynqvesson, K.S.; Conner, W.C. Microwave synthesis of zeolites. 2. Effect of vessel size, precursor volume, and irradiation method. J. Phys. Chem. B 2007, 111, 12657–12667. [Google Scholar] [CrossRef] [PubMed]
- Tompsett, G.A.; Conner, W.C.; Yngvesson, K.S. Microwave synthesis of nanoporous materials. ChemPhysChem 2006, 7, 296–319. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W. Microwave synthesis of zeolite membranes: A review. J. Membr. Sci. 2008, 316, 3–17. [Google Scholar] [CrossRef]
- Conner, W.C.; Tompsett, G.; Lee, K.-H.; Yngvesson, K.S. Microwave synthesis of zeolites: 1. Reactor engineering. J. Phys. Chem. B 2004, 108, 13913–13920. [Google Scholar] [CrossRef]
- Anuwattana, R.; Balkus, K.J., Jr.; Asavapisit, S.; Khummongkol, P. Conventional and microwave hydrothermal synthesis of zeolite ZSM-5 from the cupola slag. Microporous Mesoporous Mater. 2008, 111, 260–266. [Google Scholar] [CrossRef]
- Serrano, D.P.; Uguina, M.A.; Sanz, R.; Castillo, E.; Rodríguez, A.; Sánchez, P. Synthesis and crystallization mechanism of zeolite TS-2 by microwave and conventional heating. Microporous Mesoporous Mater. 2004, 69, 197–208. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Madsen, C.; Janssens, T.V.W.; Jakobsen, H.; Skibsted, J. Zeolites by confined space synthesis—Characterization of the acid sites in nanosized ZSM-5 by ammonia desorption and 27Al/29Si-MAS NMR spectroscopy. Microporous Mesoporous Mater. 2000, 39, 393–401. [Google Scholar] [CrossRef]
- Madsen, C.; Jacobsen, C.J.H. Nanosized zeolite crystals—Convenient control of crystal size distribution by confined space synthesis. Chem. Commun. 1999, 8, 673–674. [Google Scholar] [CrossRef]
- Schmidt, I.; Madsen, C.; Jacobsen, C.J.H. Confined space synthesis. A novel route to nanosized zeolites. Inorg. Chem. 2000, 39, 2279–2283. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Madsen, C.; Houzvicka, J.; Schmidt, I.; Carlsson, A. Mesoporous zeolite single crystals. J. Am. Chem. Soc. 2000, 122, 7116–7117. [Google Scholar] [CrossRef]
- Matsukata, M.; Nishiyama, N.; Ueyama, K. Crystallization of FER and MFI zeolites by a vapor-phase transport method. Micropor. Mater. 1996, 7, 109–117. [Google Scholar] [CrossRef]
- Thoma, S.G.; Nenoff, T.M. Vapor phase transport synthesis of zeolites from sol—Gel precursors. Microporous Mesoporous Mater. 2000, 41, 295–305. [Google Scholar] [CrossRef]
- Kim, M.-H.; Li, H.-X.; Davis, M.E. Synthesis of zeolites by water-organic vapor-phase transport. Micropor. Mater. 1993, 1, 191–200. [Google Scholar] [CrossRef]
- Niu, T.; Li, Y.; Li, J.; Chen, B. Synthesis of zeolite beta by the vapour-phase transport method using tetraethylammonium bromide as the organic template. Chin. J. Catal. 2009, 30, 191–195. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Chen, B.; Wang, Y. Cleaner process for synthesis of zeolite MCM-22 by vapor-phase transport method. Asia-Pac. J. Chem. Eng. 2009, 4, 607–611. [Google Scholar] [CrossRef]
- Rainer, D.N.; Morris, R.E. New avenues for mechanochemistry in zeolite science. Dalton Trans. 2021, 50, 8995–9009. [Google Scholar] [CrossRef] [PubMed]
- Majano, G.; Borchardt, L.; Mitchell, S.; Valtchev, V.; Pérez-Ramírez, J. Rediscovering zeolite mechanochemistry—A pathway beyond current synthesis and modification boundaries. Microporous Mesoporous Mater. 2014, 194, 106–114. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, X.; Zhu, L.; Ding, L.; Gao, P.; Wang, X.; Pan, S.; Bian, C.; Meng, X.; Xu, J.; et al. Solvent-free synthesis of zeolites from anhydrous starting raw solids. J. Am. Chem. Soc. 2015, 137, 1052–1055. [Google Scholar] [CrossRef]
- Ren, L.; Wu, Q.; Yang, C.; Zhu, L.; Li, C.; Zhang, P.; Zhang, H.; Meng, X.; Xiao, F.-S. Solvent-free synthesis of zeolites from solid raw materials. J. Am. Chem. Soc. 2012, 134, 15173–15176. [Google Scholar] [CrossRef] [PubMed]
- Nada, M.H.; Larsen, S.C.; Gillan, E.G. Mechanochemically-assisted solvent-free and template-free synthesis of zeolites ZSM-5 and mordenite. Nanoscale Adv. 2019, 1, 3918–3928. [Google Scholar] [CrossRef] [PubMed]
- Cadar, O.; Stupar, Z.; Senila, M.; Levei, L.; Moldovan, A.; Becze, A.; Ozunu, A.; Levei, E.A. Zeolites reduce the transfer of potentially toxic elements from soil to leafy vegetables. Materials 2022, 15, 5657. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J. Properties and applications of zeolites. Sci. Prog. 2010, 93, 223–284. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.; Barrington, S.; Zhao, X.; El-Husseini, B. Effect of particle size on physio-chemical properties of clinoptilolite as feed additive. Microporous Mesoporous Mater. 2006, 95, 48–56. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host-guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Bai, P.; Etim, U.J.; Yan, Z.; Mintova, S.; Zhang, Z.; Zhong, Z.; Gao, X. Fluid catalytic cracking technology: Current status and recent discoveries on catalyst contamination. Cat. Rev. 2019, 61, 333–405. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Lima, R.B.; Pergher, S.B.C.; Caldeira, V.P.S. Hierarchical zeolite synthesis by alkaline treatment: Advantages and applications. Catalysts 2023, 13, 316. [Google Scholar] [CrossRef]
- Jung, J.; Jo, C.; Mota, F.M.; Cho, J.; Ryoo, R. Acid Catalytic Function of Mesopore Walls Generated by MFI zeolite desilication in comparison with external surfaces of MFI zeolite nanosheet. Appl. Catal. A Gen. 2015, 492, 68–75. [Google Scholar] [CrossRef]
- Khan, W.; Jia, X.; Wu, Z.; Choi, J.; Yip, A.C.K. Incorporating hierarchy into conventional zeolites for catalytic biomass conversions: A review. Catalysts 2019, 9, 127. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A. Hierarchical Zeolites: Synthesis and Catalytic Properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Szatanik-Kloc, A.; Szerement, J.; Adamczuk, A.; Józefaciuk, G. Effect of low zeolite doses on plants and soil physicochemical properties. Materials 2021, 14, 2617. [Google Scholar] [CrossRef]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of zeolites for sustainable agriculture: A review on water and nutrient retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Xiubin, H.; Zhanbin, H. Zeolite application for enhancing water infiltration and retention in loess soil. Resour. Conserv. Recycl. 2001, 34, 45–52. [Google Scholar] [CrossRef]
- Gholizadeh-Sarabi, S.; Sepaskhah, A.R. Effect of zeolite and saline water application on saturated hydraulic conductivity and infiltration in different soil textures. Arch. Agron. Soil Sci. 2013, 59, 753–764. [Google Scholar] [CrossRef]
- Chmielewska, E. Zeolitic adsorption in course of pollutants mitigation and environmental control. J. Radioanal. Nucl. Chem. 2014, 299, 255–260. [Google Scholar] [CrossRef]
- Belviso, C.; Satriani, A.; Lovelli, S.; Comegna, A.; Coppola, A.; Dragonetti, G.; Cavalcante, F.; Rivelli, A.R. Impact of zeolite from coal fly ash on soil hydrophysical properties and plant growth. Agriculture 2022, 12, 356. [Google Scholar] [CrossRef]
- Bikkinina, L.M.-H.; Ezhkov, V.O.; Faizrakhmanov, R.N.; Gazizov, R.R.; Ezhkova, A.M. Effect of zeolites on soil modification and productivity. BIO Web Conf. 2020, 17, 00117. [Google Scholar] [CrossRef]
- Allen, E.R.; Hossner, L.R.; Ming, D.W.; Henninger, D.L. Solubility and cation exchange in phosphate rock and saturated clinoptilolite mixtures. Soil Sci. Soc. Am. J. 1993, 57, 1368–1374. [Google Scholar] [CrossRef]
- Campisi, T.; Abbondanzi, F.; Faccini, B.; Di Giuseppe, D.; Malferrari, D.; Coltorti, M.; Laurora, A.; Passaglia, E. Ammonium-charged zeolitite effects on crop growth and nutrient leaching: Greenhouse experiments on maize (Zea mays). Catena 2016, 140, 66–76. [Google Scholar] [CrossRef]
- Torma, S.; Vilcek, J.; Adamisin, P.; Huttmanova, E.; Hronec, O. Influence of natural zeolite on nitrogen dynamics in soil. Turk. J. Agric. For. 2014, 38, 739–744. [Google Scholar] [CrossRef]
- Aiyuk, S.; Xu, H.; van Haandel, A.; Verstraete, W. Removal of ammonium nitrogen from pretreated domestic sewage using a natural ion exchanger. Environ. Technol. 2004, 25, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Englert, A.H.; Rubio, J. Characterization and environmental application of a Chilean natural zeolite. Int. J. Miner. Process. 2005, 75, 21–29. [Google Scholar] [CrossRef]
- Kavoosi, M. Effects of zeolite application on rice yield, nitrogen recovery, and nitrogen use efficiency. Commun. Soil Sci. Plant Anal. 2007, 38, 69–76. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Sumalatha, G.; Muhamad, A.N. Use of zeolite in maize (Zea mays) cultivation on nitrogen, potassium and phosphorus uptake and use efficiency. Int. J. Phys. Sci. 2010, 5, 2393–2401. [Google Scholar]
- Li, Z.; Zhang, Y.; Li, Y. Zeolite as slow release fertilizer on spinach yields and quality in a greenhouse test. J. Plant Nutr. 2013, 36, 1496–1505. [Google Scholar] [CrossRef]
- Shirvani, M.; Farajollahi, E.; Bakhtiari, S.; Ogunseitan, O.A. Mobility and efficacy of 2,4-D herbicide from slow-release delivery systems based on organo-zeolite and organo-bentonite complexes. J. Environ. Health Part B 2014, 49, 255–262. [Google Scholar] [CrossRef]
- Bakhtiary, S.; Shirvani, M.; Shariatmadari, H. Adsorption-desorption behavior of 2,4-D on NCP-modified bentonite and zeolite: Implications for slow-release herbicide formulations. Chemosphere 2013, 90, 699–705. [Google Scholar] [CrossRef]
- Calzarano, F.; Seghetti, L.; Pagnani, G.; Di Marco, S. Italian zeolitites in the control of grey mould and sour rot and their effect on leaf reflectance, grape and wine. Agriculture 2020, 10, 580. [Google Scholar] [CrossRef]
- Prisa, D. Chabazitic zeolite in the cultivation and spray protection of Vitis vinifera. Int. J. Sci. Res. Arch. 2023, 09, 630–638. [Google Scholar] [CrossRef]
- De Smedt, C.; Steppe, K.; Spanoghe, P. Beneficial effects of zeolites on plant photosynthesis. Adv. Mater. Sci. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Montanari, T.; Busca, G. On the mechanism of adsorption and separation of CO2 on LTA zeolites: An IR investigation. Vib. Spectrosc. 2008, 46, 45–51. [Google Scholar] [CrossRef]
- Bhargava, S.; Mitra, S. Elevated atmospheric CO2 and the future of crop plants. Plant Breed. 2021, 140, 1–11. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Asgharimoghadam, A.; Gharedaashi, E.; Montajami, S.; Nekoubin, H.; Salamroudi, M.; Jafariyan, H. Effect of clinoptilolite zeolite to prevent mortality of beluga (Huso huso) by total ammonia concentration. Glob. Vet. 2012, 9, 80–84. [Google Scholar]
- Ghasemi, Z.; Sourinejad, I.; Kazemian, H.; Rohani, S. Application of zeolites in aquaculture industry: A review. Aquaculture 2018, 10, 75–95. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Osmanlioglu, A.E. Treatment of radioactive liquid waste by sorption on natural zeolite in Turkey. J. Hazard. Mater. 2006, 137, 332–335. [Google Scholar] [CrossRef]
- Lihareva, N.; Petrov, O.; Dimowa, L.; Tzvetanova, Y.; Piroeva, I.; Ublekov, F.; Nikolov, A. Ion exchange of Cs+ and Sr2+ by natural clinoptilolite from bi-cationic solutions and XRD control of their structural positioning. J. Radioanal. Nucl. Chem. 2020, 323, 1093–1102. [Google Scholar] [CrossRef]
- Borai, E.H.; Harjula, R.; Malinen, L.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Chałupnik, S.; Franus, W.; Wysocka, M.; Gzyl, G. Application of zeolites for radium removal from mine water. Environ. Sci. Pollut. Res. 2013, 20, 7900–7906. [Google Scholar] [CrossRef] [PubMed]
- Inazumi, S.; Shishido, K.I.; Nontananandh, S.; Moriiwa, K. Remediation of heavy metals polluted soil using metal insolubilizing materials. J. Environ. Prot. 2018, 9, 770–789. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Siebielec, G.; Siebielec, S.; Pecio, M. The effect of soil amendments on trace elements’ bioavailability and toxicity to earthworms in contaminated soils. Appl. Sci. 2022, 12, 6280. [Google Scholar] [CrossRef]
- Rahimi, M.; Mahmoudi, J. Heavy metals removal from aqueous solution by modified natural zeolites using central composite design. Period. Polytech. Chem. Eng. 2019, 64, 106–115. [Google Scholar] [CrossRef]
- Valarde, L.; Nabavi, M.S.; Escalera, E.; Anti, M.-L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Lihareva, N.; Dimova, L.; Petrov, O.; Tzvetanova, Y. Ag+ sorption on natural and Na-exchanged clinoptilolite from Eastern Rhodopes, Bulgaria. Microporous Mesoporous Mater. 2010, 130, 32–37. [Google Scholar] [CrossRef]
- Belviso, C. Zeolite for Potential Toxic Metal Uptake from Contaminated Soil: A Brief Review. Processes 2020, 8, 820. [Google Scholar] [CrossRef]
- Kumpiene, J. Trace elements immobilization in soil using amendments. In Trace Elements in Soil; Hooda, P.S., Ed.; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Żołnowski, A.C.; Wyszkowski, M. Mineral neutralizers as a tool for improving the properties of soil contaminated with copper. Minerals 2022, 12, 895. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Wyszkowska, J.; Kordala, N.; Zaborowska, M. Molecular sieve, halloysite, sepiolite and expanded clay as a tool in reducing the content of trace elements in Helianthus annuus L. on copper-contaminated soil. Materials 2023, 16, 1827. [Google Scholar] [CrossRef]
- Sivitskaya, V.; Wyszkowski, M. Effect of heating oil and neutralizing substances on the content of some trace elements in maize (Zea mays L.). Ecol. Chem. Eng. A 2013, 20, 323–331. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.-Y.; Shao, H.-B.; Shao, M.-A. The remediation of the lead-polluted garden soil by natural zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, M.; Brodowska, M.S. Phytoextraction with maize of soil contaminated with copper after application of mineral and organic amendments. Agronomy 2020, 10, 1597. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Remediation of cobalt-polluted soil after application of selected substances and using oat (Avena sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 16762–16780. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Santona, L.; Deiana, P.; Melis, P. Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotophic microbial populations and enzyme activities in a contaminated soil. Geoderma 2007, 142, 47–57. [Google Scholar] [CrossRef]
- Schneider, A.F.; Zimmermann, O.F.; Gewehr, C.E. Zeolites in poultry and swine production. Ciência Rural 2017, 47, 1–8. [Google Scholar] [CrossRef]
- Fuss, V.L.B.; Bruj, G.; Dordai, L.; Roman, M.; Cadar, O.; Becze, A. Evaluation of the impact of different natural zeolite treatments on the capacity of eliminating/reducing odors and toxic compounds. Materials 2021, 14, 3724. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Lopez-Real, J.M. Natural zeolites and sepiolite as ammonium and ammonia adsorbent materials. Bioresour. Technol. 1993, 43, 27–33. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, Q.; Ren, X.; Zhao, J.; Huang, H.; Awasthi, S.K.; Lahori, A.H.; Li, R.; Zhou, L.; Zhang, Z. Role of biochar amendment in mitigation of nitrogen loss and greenhouse gas emission during sewage sludge composting. Bioresour. Technol. 2016, 219, 270–280. [Google Scholar] [CrossRef]
- Chan, M.T.; Selvam, A.; Wong, J.W. Reducing nitrogen loss and salinity during ‘struvite’ food waste composting by zeolite amendment. Bioresour. Technol. 2016, 200, 838–844. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Wang, M.; Chen, H.; Zhang, Z. Combining biochar, zeolite and wood vinegar for composting of pig manure: The effect on greenhouse gas emission and nitrogen conservation. Waste Manag. 2018, 74, 221–230. [Google Scholar] [CrossRef]
- Akyalcin, S.; Akyalcin, L.; Ertugrul, E. Modification of natural clinoptilolite zeolite to enhance its hydrogen adsorption capacity. Res. Chem. Intermed. 2024, 1–19. [Google Scholar] [CrossRef]
- Allen, S.J.; Ivanova, E.; Koumanova, B. Adsorption of sulfur dioxide on chemically modified natural clinoptilolite. Acid modification. Chem. Eng. J. 2009, 152, 389–395. [Google Scholar] [CrossRef]
- Cheng, H.; Ren, X.; Yao, Y.; Tang, X.; Yi, H.; Gao, F.; Zhou, Y.; Yu, Q. Application of unconventional external-field treatments in air pollutants removal over zeolite-based adsorbents/catalysts. Catalysts 2023, 13, 1461. [Google Scholar] [CrossRef]
- Li, J.; Meng, X.; Xiao, F.-S. Zeolites for control of NOx emissions: Opportunities and challenges. ChemCatalysis 2022, 2, 253–261. [Google Scholar] [CrossRef]
- Saramok, M.; Inger, M.; Antoniak-Jurak, K.; Szymaszek-Wawryca, A.; Samojeden, B.; Motak, M. Physicochemical features and NH3-SCR catalytic performance of natural zeolite modified with iron—The effect of Fe loading. Catalysts 2022, 12, 731. [Google Scholar] [CrossRef]
- Święs, A.; Kowalczyk, A.; Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Ferrierite and its delaminated and silica-intercalated forms modified with copper as effective catalysts for NH3-SCR process. Catalysts 2020, 10, 734. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Ferella, F. A review on management and recycling of spent selective catalytic reduction catalysts. J. Clean. Prod. 2020, 246, 118990. [Google Scholar] [CrossRef]
- Tran, T.; Yu, J.; Gan, L.; Guo, F.; Phan, D.; Xu, G. Upgrading V2O5-WO3/TiO2 deNOx catalyst with TiO2-SiO2 support prepared from Ti-bearing blast furnace slag. Catalysts 2016, 6, 56. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhao, Z.; Liao, J.; Chen, C.; Li, Q. Recent progress of metal-exchanged zeolites for selective catalytic reduction of NOx with NH3 in diesel exhaust. Fuel 2021, 305, 121482. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Wang, H.; Huang, B.; Yu, C.; Lu, M.; Huang, H.; Zhou, J. Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2019, 88, 117207. [Google Scholar] [CrossRef]
- Du, J.; Shi, X.; Shan, Y.; Zhang, W.; Yu, Y.; Shan, W.; He, H. Investigation of suitable templates for one-pot-synthesized CuSAPO-34 in NOx abatement from diesel vehicle exhaust. Environ. Sci. Technol. 2020, 54, 7870–7878. [Google Scholar] [CrossRef]
- Shi, X.; Liu, F.; Xie, L.; Shan, W.; He, H. NH3-SCR performance of fresh and hydrothermally aged Fe-ZSM-5 in standard and fast selective catalytic reduction reactions. Environ. Sci. Technol. 2013, 47, 3293–3298. [Google Scholar] [CrossRef]
- Zhong, C.; Wu, C.; Zuo, H.; Gu, Z. Theoretical analyses of NH3-SCR reaction-mass transfer over Cu-ZSM-5. Can. J. Chem. Eng. 2022, 100, 3263–3269. [Google Scholar] [CrossRef]
- Liu, X.; Sui, Z.; Chen, Z.; Chen, Y.; Liu, H.; Jiang, P.; Shen, Z.; Linghu, W.; Wu, X. Structures and catalytic performances of Me/SAPO-34 (Me = Mn, Ni, Co) catalysts for low-tem perature SCR of NOx by ammonia. J. Environ. Sci. 2021, 104, 137–149. [Google Scholar] [CrossRef]
- Zhu, N.; Lian, Z.; Zhang, Y.; Shan, W.; He, H. Improvement of low-temperature catalytic activity over hierarchical Fe-Beta catalysts for selective catalytic reduction of NOx with NH3. Chin. Chem. Lett. 2019, 30, 867–870. [Google Scholar] [CrossRef]
- Hui, K.; Yuan, Y.; Xi, B.; Tan, W. A review of the factors affecting the emission of the ozone chemical precursors VOCs and NOx from the soil. Environ. Int. 2023, 172, 107799. [Google Scholar] [CrossRef]
- Kumar, M.S.; Alphin, M.S.; Manigandan, S.; Vignesh, S.; Vigneshwaran, S.; Subash, T. A review of comparison between the traditional catalyst and zeolite catalyst for ammonia-selective catalytic reduction of NOx. Fuel 2023, 344, 128125. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic Materials for DeNOx Selective Catalytic Reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Li, P.; Xin, Y.; Zhang, H.; Yang, F.; Tang, A.; Han, D.; Jia, J.; Wang, J.; Li, Z.; Zhang, Z. Recent progress in performance optimization of Cu-SSZ-13 catalyst for selective catalytic reduction of NOx. Front. Chem. 2022, 10, 1033255. [Google Scholar] [CrossRef]
- Paolucci, C.; Verma, A.A.; Bates, S.A.; Kispersky, V.F.; Miller, J.T.; Gounder, R.; Delgass, W.N.; Ribeiro, F.H.; Schneider, W.F. Isolation of the Copper Redox Steps in the Standard Selective Catalytic Reduction on Cu-SSZ-13. Angew. Chem. 2014, 53, 11828–11833. [Google Scholar] [CrossRef]
- Song, J.; Wang, Z.; Cheng, X.; Wang, X. State-of-Art Review of NO Reduction Technologies by CO, CH4 and H2. Processes 2021, 9, 563. [Google Scholar] [CrossRef]
- Resini, C.; Montanari, T.; Nappi, L.; Bagnasco, G.; Turco, M.; Busea, G.; Bregani, F.; Notaro, M.; Rocchini, G. Selective catalytic reduction of NOx by methane over Co-H-MFI and Co-H-FER zeolite catalysts: Characterization and catalytic activity. J. Catal. 2003, 214, 179–190. [Google Scholar] [CrossRef]
- Ferriera, A.P.; Henriques, C.; Ribeiro, M.F. SCR of NO with methane over Co-HBEA and PdCo-HBEA catalysts: The promoting effect of steaming over bimetallic catalysts. Catal. Today 2005, 107, 181–192. [Google Scholar] [CrossRef]
- Ferriera, A.P.; Capela, S.; Da Costa, P.; Henriques, C.; Ribeiro, M.F.; Ramoa, R. CH4-SCR of NO over Co and Pd ferrierite catalysts: Effect of preoaration on catalytic performance. Catal. Today 2007, 119, 156–165. [Google Scholar] [CrossRef]
- Lee, T.J.; Nam, I.S.; Ham, S.W.; Baek, Y.S.; Shin, K.H. Effect on Pd of the wather tolerance of Co-ferrierite catalysts for NO reduction by CH4. Appl. Catal. B 2003, 4, 115–129. [Google Scholar] [CrossRef]
- De Lucas, A.; Valverde, J.L.; Dorado, F.; Romero, A.; Asencio, I. Influence of the ion exchanged metal (Cu, Co, Ni and Mn) on the selective catalytic reduction of NOx over mordenite and ZSM-5. J. Mol. Catal. A Chem. 2005, 225, 47–58. [Google Scholar] [CrossRef]
- Ogura, M.; Kage, S.; Shimojo, T.; Oba, J.; Hayashi, M.; Matsukata, M. Co Cation Effects on Activity and Stability of Isolated Pd(II) Cations in Zeolite Matrices for Selective Catalytic Reduction of Nitric Oxide with Methane. J. Catal. 2002, 211, 75–84. [Google Scholar] [CrossRef]
- Gao, W.; Tang, X.; Yi, H.; Jiang, S.; Yu, Q.; Xie, X.; Zhuang, R. Mesoporous molecular sieve-based materials for catalytic oxidation of VOC: A review. J. Environ. Sci. 2023, 125, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, A.M.; Jansen, J.C.; Maschmeyer, T. Regarding pressure in the adsorber of an adsorption heat pump with thin synthesized zeolite layers on heat exchangers. Microporous Mesoporous Mater. 2001, 3, 313–317. [Google Scholar] [CrossRef]
- Fukai, J.; Wijayanta, A.T. Potential ability of zeolite to generate high-temperature vapor using waste heat. AIP Conf. Proc. 2018, 1931, 020001. [Google Scholar] [CrossRef]
- Tasić, Ž.Z.; Bogdanović, G.D.; Antonijević, M.M. Application of natural zeolite in wastewater treatment—A review. J. Min. Metall. 2019, 55A, 67–79. [Google Scholar] [CrossRef]
- El-Shafey, O.; Fathy, N.A.; El-Nabarawy, T. Sorption of ammonium ions onto natural and modified Egyptian kaolinites: Kintic and equilibrium studies. Adv. Phys. Chem. 2014, 2014, 935854. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sadegh, H.; Yari, M.; Shahryari Ghoshekandi, R.; Maazinejad, B.; Chahardori, M. Removal of ammonium ions from wastewater a short review in development of efficient methods. Glob. J. Environ. Sci. Manag. 2015, 1, 149–158. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, X.; Yan, B.; Yang, L. Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J. Hazard. Mater. 2010, 175, 247–252. [Google Scholar] [CrossRef]
- Liang, Z.; Ni, J. Improving the ammonium ion uptake onto natural zeolite by using an integrated modification process. J. Hazard. Mater. 2009, 166, 52–60. [Google Scholar] [CrossRef]
- Jahani, F.; Sadeghi, R.; Shakeri, M. Ultrasonic-assisted chemical modification of a natural clinoptilolite zeolite: Enhanced ammonium adsorption rate and resistance to disturbing ions. J. Environ. Chem. Eng. 2023, 11, 110354. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, B97, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Galletti, C.; Dosa, M.; Russo, N.; Fino, D. Zn2+ and Cd2+ removal from wastewater using clinoptilolite as adsorbent. Environ. Sci. Pollut. Res. 2021, 28, 24355–24361. [Google Scholar] [CrossRef] [PubMed]
- Senila, L.; Hoaghia, A.; Moldovan, A.; Török, I.A.; Kovacs, D.; Simedru, D.; Tomoiag, C.H.; Senila, M. The potential application of natural clinoptilolite-rich zeolite as support for bacterial community formation for wastewater treatment. Materials 2022, 15, 3685. [Google Scholar] [CrossRef]
- Panayotova, M. Removal of Cr(VI) from wastewater by silver-loaded natural clinoptilolite. E3S Web Conf. 2021, 280, 06008. [Google Scholar] [CrossRef]
- Zhang, Y.; Kou, X.; Lu, H.; Lv, X. The feasibility of adopting zeolite in phosphorus removal from aqueous solutions. Desalin. Water Treat. 2013, 52, 4298–4304. [Google Scholar] [CrossRef]
- Goscianska, J.; Ptaszkowska-Koniarz, M.; Frankowski, M.; Franus, M.; Panek, R.; Franus, W. Removal of phosphate from water by lanthanum-modified zeolites obtained from fly ash. J. Colloid Interface Sci. 2018, 513, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Koohsaryan, E.; Anbia, M.; Heydar, K.T. Mo-modified hierarchical fau zeolite: A catalyst-adsorbent for oxidative desulfurization of fuel oil. J. Solid State Chem. 2022, 312, 123218. [Google Scholar] [CrossRef]
- Ouyang, W.; Zheng, S.; Wu, C.; Hu, X.; Chen, R.; Zhuo, L.; Wang, Z. Dynamic Ammonia Adsorption by FAU Zeolites to below 0.1 Ppm for Hydrogen Energy Applications. Int. J. Hydrogen Energy 2021, 46, 32559–32569. [Google Scholar] [CrossRef]
- Mofarahi, M.; Gholipour, F. Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 2014, 200, 1–10. [Google Scholar] [CrossRef]
- Garshasbi, V.; Jahangiri, M.; Anbia, M. Separation of Hydrogen Sulfide from Butane Gas Mixture by Zeolite 13X. Iran. J. Chem. Chem. Eng. 2022, 41, 3786–3797. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Dona, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Hashemi, M.S.H.; Eslami, F.; Karimzadeh, R. Organic contaminants removal from industrial wastewater by CTAB treated synthetic zeolite Y. J. Environ. Manag. 2019, 233, 785–792. [Google Scholar] [CrossRef]
- Fu, J.; Ding, C. Study on alkylation of benzene with propylene over MCM-22 zeolite catalyst by in situ IR. Catal. Commun. 2005, 6, 770–776. [Google Scholar] [CrossRef]
- Gerasimov, D.; Kashin, E.V.; Pigoleva, I.V.; Maslov, I.A.; Fadeev, V.V.; Zaglyadova, S.V. Effect of Zeolite Properties on Dewaxing by Isomerization of Different Hydrocarbon Feedstocks. Energy Fuels 2019, 33, 3492–3503. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, Y.; Dutt, P.K. Controlled release of paraquat from surface-modified zeolite Y. Microporous Mesoporous Mater. 2006, 88, 312–318. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Irusta, S.; Arbiol, J.; Ibarra, M.R.; Santamaría, J. Sustained release of doxorubicin from zeolite-magnetite nanocomposites prepared by mechanical activation. Nanotechnology 2006, 17, 4057–4064. [Google Scholar] [CrossRef] [PubMed]
- Neidrauer, M.; Ercan, U.K.; Bhattacharyya, A.; Samuels, J.; Sedlak, J.; Trikha, R.; Barbee, K.A.; Weingarten, M.S.; Joshi, S.G. Antimicrobial efficacy and wound-healing property of a topical ointment containing nitric-oxide-loaded zeolites. J. Med. Microbiol. 2014, 63, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Bedi, R.S.; Beving, D.E.; Zanello, L.P.; Yan, Y. Biocompatibility of corrosion-resistant zeolite coatings for titanium alloy biomedical implants. Acta Biomater. 2009, 5, 3265–3271. [Google Scholar] [CrossRef]
- Pavelić, K.; Hadžija, M. Medical Application of Zeolites. In Handbook of Zeolites Science and Technology; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.-J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug Discov. Today 2020, 4, 642–656. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Cerri, G.; Gennaro, M.; Juliano, C.; Caramella, C. Zn2+-exchanged clinoptilolite-rich rock as active carrier for antibiotics in anti-acne topical therapy: In-vitro characterization and preliminary formulation studies. Appl. Clay Sci. 2007, 36, 95–102. [Google Scholar] [CrossRef]
- Suresh, S.; Ragula, U.B.R. A regenerative adsorption technique for removal of uremic toxins: An alternative to conventional haemodialysis. Mater. Today Proc. 2020, 24, 714–723. [Google Scholar] [CrossRef]
- Deshpande, S.; Kheur, S.; Kheur, M.; Eyüboğlu, T.F.; Özcan, M. A Review on Zeolites and Their Applications in Dentistry. Curr. Oral Health Rep. 2023, 10, 36–42. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, G.; Barrios, M.A.; Cedré, B.; Perdomo, I. Enterex: Anti-diarrheic drug based on purified natural clinoptilolite. Zeolites 1997, 19, 441–448. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, G.; Denis, A.R.; Barrios Álvarez, M.A.; Colarte, A.I. Antacid drug based on purified natural clinoptilolite. Microporous Mesoporous Mater. 2006, 94, 200–207. [Google Scholar] [CrossRef]
- Basha, M.P.; Begum, S.; Mir, B.A. Neuroprotective actions of clinoptilolite and ethylenediaminetetraacetic acid against lead-introduced toxicity in mice Mus musculus. Toxicol. Int. 2013, 20, 201–207. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Chen, T.; Bao, X. Zeolites: A series of promising biomaterials in bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1066552. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Mahmodi, G.; Manouchehri, S.; Mashhadzadeh, A.H.; Khodadadi, M.; Servatan, M.; Ganjali, M.R.; Azambre, B.; Kim, S.-J.; Ramsey, J.D.; et al. Zeolite in tissue engineering: Opportunities and challenges. MedComm 2020, 1, 5–34. [Google Scholar] [CrossRef]
- Banu, J.; Varela, E.; Guerra, J.M.; Halade, G.; Williams, P.J.; Bahadur, A.N.; Hanaoka, K.; Fernandes, G. Dietary coral calcium and zeolite protects bone in a mouse model for postmenopausal bone loss. Nutr. Res. 2012, 32, 965–975. [Google Scholar] [CrossRef]
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Cadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C 2020, 116, 111225. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.F.; Solano, S.; Infante, G. The influence of hydrotalcite and cancrinite-type zeolite in acidic aspirin solutions. Microporous Mesoporous Mater. 2004, 74, 105–110. [Google Scholar] [CrossRef]
- Shan, W.; Yu, T.; Wang, B.; Hu, J.; Zhang, Y.; Wang, X.; Tang, Y. Magnetically separable nanozeolites: Promising candidates for bio-applications. Chem. Mater. 2006, 18, 3169–3172. [Google Scholar] [CrossRef]
- Matusiak, J.; Przekora, A.; Franus, W. Zeolites and zeolite imidazolate frameworks on a quest to obtain the ideal biomaterial for biomedical applications: A review. Mater. Today 2023, 67, 495–517. [Google Scholar] [CrossRef]
- Kubota, M.; Nakabayashi, T.; Matsumoto, Y.; Shiomi, T.; Yamada, Y.; Ino, K.; Yamanokuchi, H.; Matsui, M.; Tsunoda, T.; Mizukami, F.; et al. Selective adsorption of bacterial cells onto zeolites. Colloids Surf. B Biointerfaces 2008, 64, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Popovich, J.; Zhang, W.; Ganser, C.; Haydel, S.E.; Seo, D.-K. Superior ion release properties and antibacterial efficacy of nanostructured zeolites ion-exchanged with zinc, copper, and iron. RSC Adv. 2018, 8, 37949. [Google Scholar] [CrossRef]
- Inoue, Y.; Hoshino, M.; Takahashi, H.; Noguchi, T.; Murata, T.; Kanzaki, Y.; Hamashima, H.; Sasatsu, M. Bactericidal activity of Ag-zeolite mediated by reactive oxygen species under aerated conditions. J. Inorg. Biochem. 2002, 92, 37–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).