Morphology Regulation of Zeolite MWW via Classical/Nonclassical Crystallization Pathways
Abstract
:1. Introduction
2. Results and Discussion
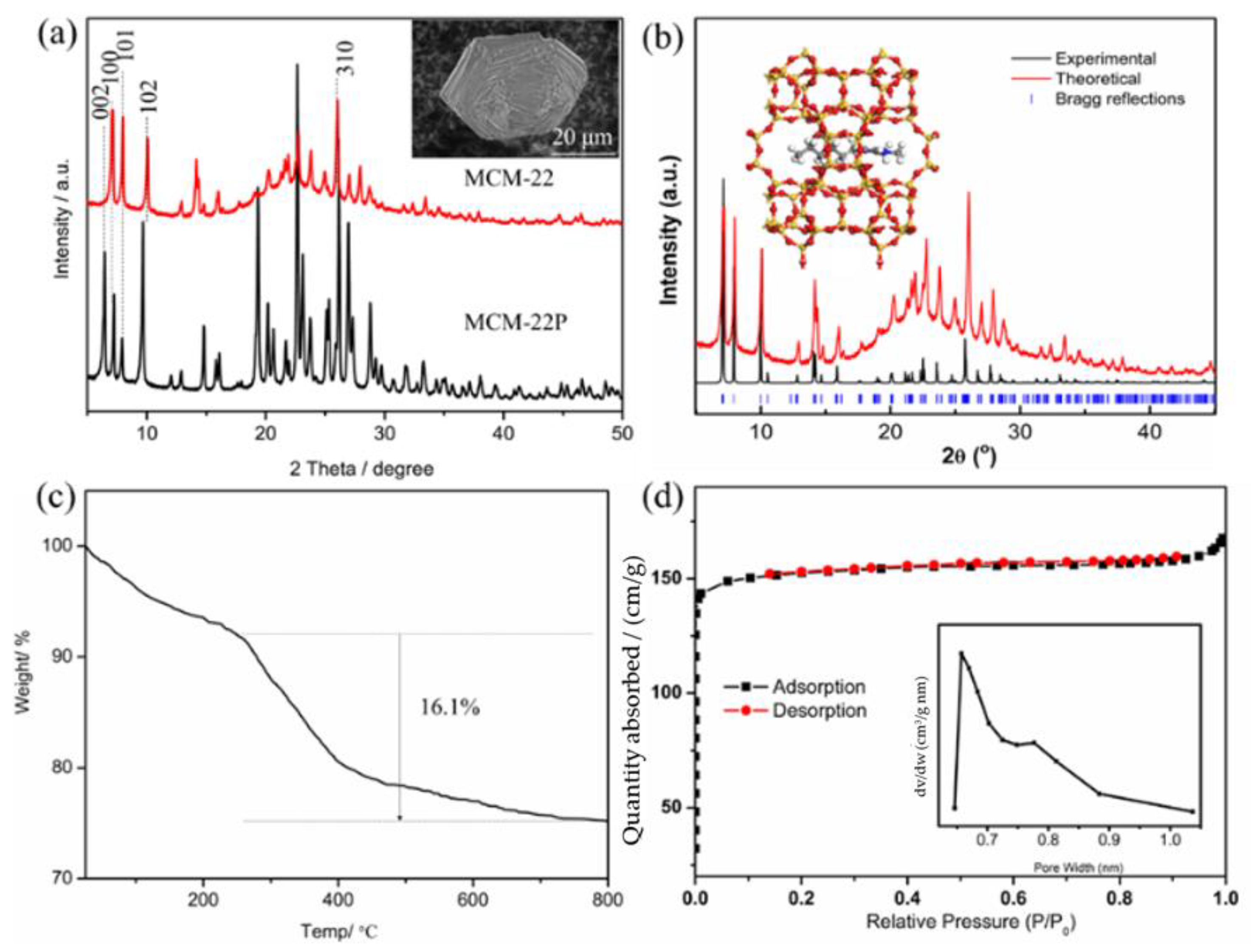
2.1. Synthesis of MWW Zeolites Only with the Organic Structure-Directing Agent
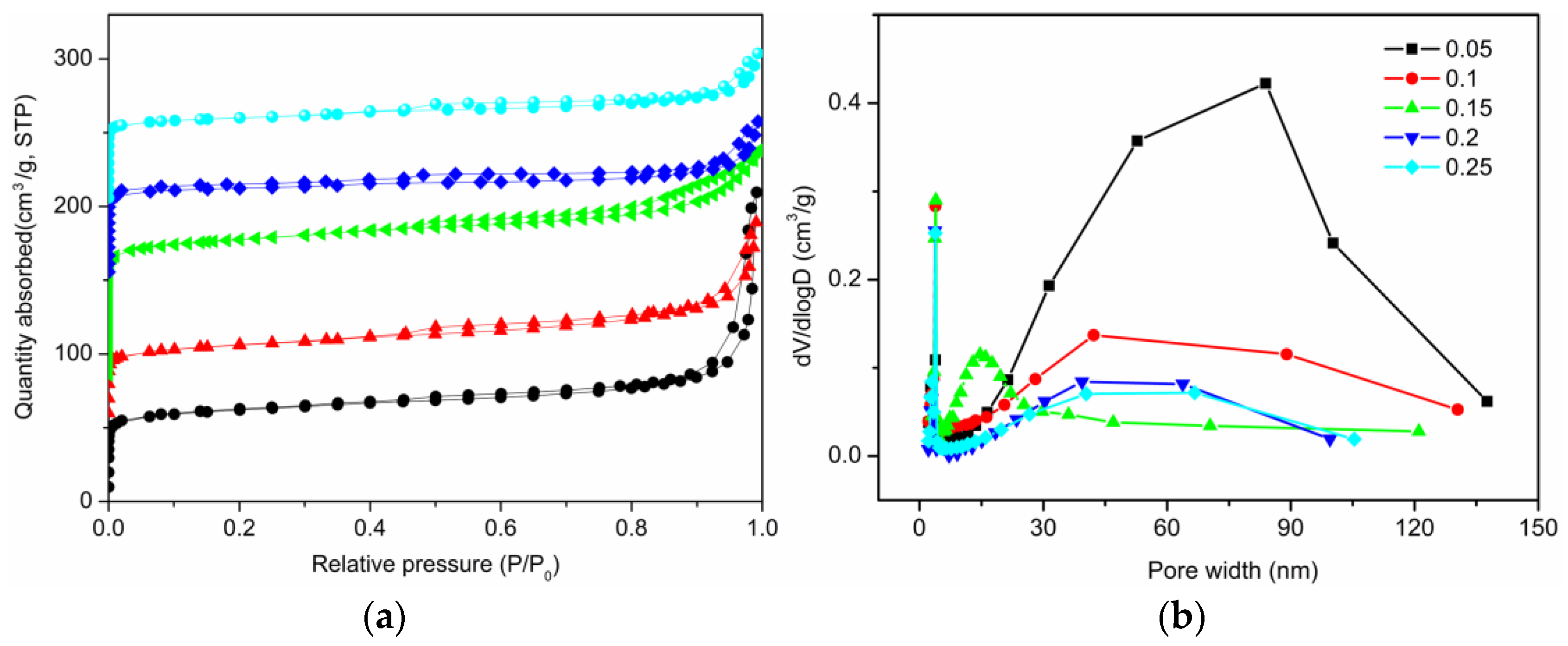
2.2. The Effect of Na Salts on the Hierarchical Structure
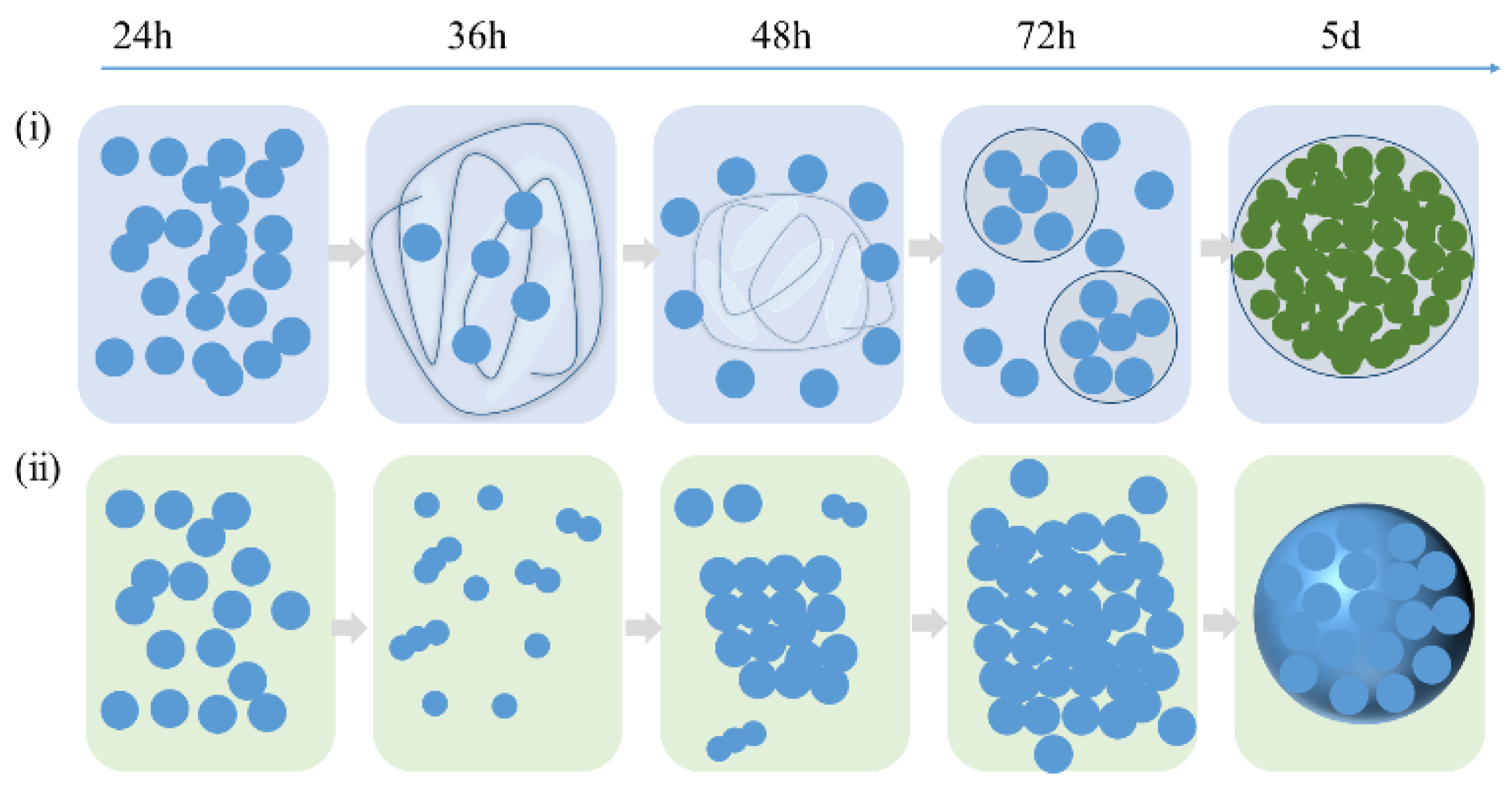
2.3. Investigation on MWW Zeolite Crystallization
2.4. The Proposed Mechanism of Crystallization of MWW Zeolite
3. Materials and Methods
3.1. Materials
3.2. Preparation of MCM-22 Zeolite
3.3. Characterization of Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.; Huang, W.; Han, H.; Zhang, J.; Wu, H.; Yan, X.; Jiang, Y.; Fang, L.; Zhang, B.; Guo, X.; et al. Facile and fast synthesis of highly active Lewis acid MWW zeolite from pure silica ITQ-1. Inorg. Chem. Front. 2022, 9, 3505–3513. [Google Scholar] [CrossRef]
- Bao, R.; Shen, Z.; Ma, C.; Li, L.; Wang, Y.; Sun, H.; Yang, W. Delaminated MWW-type zeolite and its catalytic performance in alkylation of benzene with cyclohexene. Appl. Catal. A-Gen. 2022, 643, 118741. [Google Scholar] [CrossRef]
- Barakov, R.; Shcherban, N.; Petrov, O.; Lang, J.; Shamzhy, M.; Opanasenko, M.; Čejka, J. MWW-type zeolite nanostructures for a one-pot three-component Prins–Friedel–Crafts reaction. Inorg. Chem. Front. 2022, 9, 1244–1257. [Google Scholar] [CrossRef]
- Zhou, Y.; Mu, Y.; Hsieh, M.-F.; Kabius, B.; Pacheco, C.; Bator, C.; Rioux, R.M.; Rimer, J.D. Enhanced Surface Activity of MWW Zeolite Nanosheets Prepared via a One-Step Synthesis. J. Am. Chem. Soc. 2020, 142, 8211–8222. [Google Scholar] [CrossRef] [PubMed]
- Leonowicz, M.E.; Lawton, J.A.; Lawton, S.L.; Rubin, M.K. MCM-22: A Molecular Sieve with Two Independent Multidimensional Channel Systems. Science 1994, 264, 1910–1913. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.J.; Kresge, C.T.; Vartul, J.C.; Leonowicz, M.E.; Fung, A.S.; McCullen, S.B. MCM-36: The first pillared molecular sieve with zeoliteproperties. Stud. Surf. Sci. Catal. 1995, 94, 301–308. [Google Scholar]
- Lawton, S.L.; Fung, A.S.; Kennedy, G.J.; Alemany, L.B.; Chang, C.D.; Hatzikos, G.H.; Lissy, D.N.; Rubin, M.K.; Timken, H.-K.C.; Steuernagel, S.; et al. Zeolite MCM-49: A Three-Dimensional MCM-22 Analogue Synthesized byin SituCrystallization. J. Phys. Chem. 1996, 100, 3788–3798. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Fornés, V.; Guil, J.M.; Martínez-Triguero, J.; Creyghton, E.J. Characterization and Catalytic Activity of MCM-22 and MCM-56 Compared with ITQ-2. J. Catal. 2000, 191, 218–224. [Google Scholar] [CrossRef]
- Camblor, M.A.; Corma, A.; Diaz-Cabanas, M.J.; Baerlocher, C. Synthesis and Structural Characterization of MWW Type Zeolite ITQ-1, the Pure Silica Analog of MCM-22 and SSZ-25. J. Phys. Chem. B 1998, 102, 44–51. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Guil, J.M.; Pergher, S.; Maesen, T.L.M.; Buglass, J.G. Preparation, characterisation and catalytic activity of ITQ-2, a delaminated zeolite. Microporous Mesoporous Mater. 2000, 38, 301–309. [Google Scholar] [CrossRef]
- Zones, S.I.; Hwang, S.-J.; Davis, M.E. Studies of the Synthesis of SSZ-25 Zeolite in a “Mixed-Template” System. Chemistry 2001, 7, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Ye, Z.; Kong, L.; Liu, P.; Zhang, Y.; Zhang, H.; Tang, Y. Facile Morphology and Porosity Regulation of Zeolite ZSM-5 Mesocrystals with Synergistically Enhanced Catalytic Activity and Shape Selectivity. Nanomaterials 2022, 12, 1601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, F.; Kong, L.; Ye, Z.; Pan, D.; Li, H.; Li, H.; Liu, P.; Zhang, Y.; Zhang, H.; et al. c-Axis-penetrated mesoporous MWW zeolite nanosheets: Preparation by H2O2-induced micro-explosion and their enhanced properties. Inorg. Chem. Front. 2022, 9, 4030–4040. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Wang, S.; Wang, J.; Fan, W.; Dong, M. Improvement of adsorption and catalytic properties of zeolites by precisely controlling their particle morphology. Chem. Commun. 2022, 58, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-H.; Liu, M.-N.; Luo, Q.-X.; Zhang, J.; Chen, H.; Xu, L.; Sun, M.; Ma, X.; Hao, Q.-Q. Friedel-Crafts acylation of anisole with acetic anhydride over single- to multiple-layer MWW zeolites: Catalytic behavior and kinetic mechanism. Chem. Eng. J. 2023, 466, 143098. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, B.; Zhou, L.; Yang, X.; Lu, T.; Yao, H.; Su, Y. Rational construction of hierarchical SAPO-34 with enhanced MTO performance without an additional meso/macropore template. J. Mater. Chem. A 2021, 9, 1859–1867. [Google Scholar] [CrossRef]
- Dai, W.; Kouvatas, C.; Tai, W.; Wu, G.; Guan, N.; Li, L.; Valtchev, V. Platelike MFI Crystals with Controlled Crystal Faces Aspect Ratio. J. Am. Chem. Soc. 2021, 143, 1993–2004. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.; Fan, K.; Chen, W.; Han, S.; Wu, Q.; Ma, Y.; Zheng, A.; Kunkes, E.; De Baerdemaeker, T.; et al. Design of an Organic Template for Synthesizing ITR Zeolites under Ge-Free Conditions. J. Am. Chem. Soc. 2023, 145, 17284–17291. [Google Scholar] [CrossRef]
- Ma, Z.; Deng, H.; Li, L.; Zhang, Q.; Chen, G.; Sun, C.; He, H.; Yu, J. Fluoride-free and seed-free microwave-assisted hydrothermal synthesis of nanosized high-silica Beta zeolites for effective VOCs adsorption. Chem. Sci. 2023, 14, 2131–2138. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, G.; Hu, H.; Sun, Y.; Ma, Z.; Peng, P.; Ng, E.-P.; Tian, P.; Guo, H.; Svetlana, M. SAPO-34 crystals with nanosheet morphology synthesized by pyrophosphoric acid as new phosphorus source. Microporous Mesoporous Mater. 2022, 333, 111753. [Google Scholar] [CrossRef]
- Asselman, K.; Kirschhock, C.; Breynaert, E. Illuminating the Black Box: A Perspective on Zeolite Crystallization in Inorganic Media. Acc. Chem. Res. 2023, 56, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhao, Y.; Zhang, H.; Shi, Z.; Li, H.; Yang, X.; Wang, L.; Kong, L.; Zhang, C.; Sheng, Z.; et al. Mesocrystal morphology regulation by “alkali metals ion switch”: Re-examining zeolite nonclassical crystallization in seed-induced process. J. Colloid Interface Sci. 2022, 608, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Z.; Goonetilleke, E.C.; Huang, X. Temperature-dependent kinetic pathways of heterogeneous ice nucleation competing between classical and non-classical nucleation. Nat. Commun. 2021, 12, 4954. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.I.; Andriako, E.P.; Kostyukov, I.A.; Zasukhin, D.S.; Fedosov, D.A.; Zarubin, D.N. Multinuclear MAS NMR Monitoring of the Effect of Silicate Speciation on the Mechanism of Zeolite BEA Formation: Toward Engineering of Crystal Size and Morphology. Cryst. Growth Des. 2023, 23, 5677–5689. [Google Scholar] [CrossRef]
- Jain, R.; Mallette, A.J.; Rimer, J.D. Controlling Nucleation Pathways in Zeolite Crystallization: Seeding Conceptual Methodologies for Advanced Materials Design. J. Am. Chem. Soc. 2021, 143, 21446–21460. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Liu, W.; Li, J.; Wang, Y.; Yu, Q.; Yang, Z.; Liu, X.; Xu, L.; Li, X.; Zhu, X. Synthesis and crystallization mechanism of EUO zeolite. Microporous Mesoporous Mater. 2022, 337, 111911. [Google Scholar] [CrossRef]
- Jain, R.; Niu, Z.; Choudhary, M.; Bourji, H.; Palmer, J.C.; Rimer, J.D. In Situ Imaging of Faujasite Surface Growth Reveals Unique Pathways of Zeolite Crystallization. J. Am. Chem. Soc. 2023, 145, 1155–1164. [Google Scholar] [CrossRef]
- Sheng, Z.; Li, H.; Du, K.; Gao, L.; Ju, J.; Zhang, Y.; Tang, Y. Observing a Zeolite Nucleus (Subcrystal) with a Uniform Framework Structure and Its Oriented Attachment without Single-Molecule Addition. Angew. Chem. Int. Ed. 2021, 60, 13444. [Google Scholar] [CrossRef]
- Chawla, A.; Linares, N.; Li, R.; García-Martínez, J.; Rimer, J.D. Tracking Zeolite Crystallization by Elemental Mapping. Chem. Mater. 2020, 32, 3278–3287. [Google Scholar] [CrossRef]
- Zhang, J.; Bai, R.; Zhou, Y.; Chen, Z.; Zhang, P.; Li, J.; Yu, J. Impact of a polymer modifier on directing the non-classical crystallization pathway of TS-1 zeolite: Accelerating nucleation and enriching active sites. Chem. Sci. 2022, 13, 13006–13014. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Wang, L.; Han, J.; Lou, C.; Xu, S.; Zhang, Y.; Wu, R.; Tian, P.; Liu, Z. Recognizing the Minimum Structural Units Driving the Crystallization of SAPO-34 in a Top-Down Process. Chemistry 2023, 29, e202203886. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Song, Y.; Latsch, L.; Zou, Y.; Feng, Z.; Coperet, C.; Corma, A.; Yu, J. Switching between classical/nonclassical crystallization pathways of TS-1 zeolite: Implication on titanium distribution and catalysis. Chem. Sci. 2022, 13, 10868–10877. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Jain, R.; Rimer, J.D. In situ imaging of two-dimensional surface growth reveals the prevalence and role of defects in zeolite crystallization. Proc. Natl. Acad. Sci. USA 2020, 117, 28632–28639. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Mallette, A.J.; Neeway, J.J.; Motkuri, R.K.; Rimer, J.D.; Mpourmpakis, G. Understanding formation thermodynamics of structurally diverse zeolite oligomers with first principles calculations. Dalton Trans. 2023, 52, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Ma, D.; Liu, L.; Cheng, M.; Bao, X. In situ assembly of zeolitic building blocks into high-order structures. Angew. Chem. Int. Ed. 2004, 43, 3452–3456. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, F.; Gao, Y.; Wu, P.; Xu, L. Construction of hierarchical Sn-ECNU-7 zeolite with MWW nanosheets for Baeyer-Villiger oxidation of bulky reactant. Microporous Mesoporous Mater. 2024, 364, 112846. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, M.; Huang, P.; Zheng, S.; Qiao, Y.; Liang, Y.; Chen, X.; Kita, H. Preparation and catalytic performance of the supported Ti-MWW zeolites. Microporous Mesoporous Mater. 2024, 364, 112878. [Google Scholar] [CrossRef]
- Zi, W.W.; Gao, Z.; Zhang, J.; Zhao, B.X.; Cai, X.S.; Du, H.B.; Chen, F.J. An Extra-Large-Pore Pure Silica Zeolite with 16x8x8-Membered Ring Pore Channels Synthesized using an Aromatic Organic Directing Agent. Angew. Chem. Int. Ed. 2020, 59, 3948–3951. [Google Scholar] [CrossRef]
- Zi, W.W.; Cai, X.S.; Jiao, F.; Du, H.B. Synthesis, Structure and Properties of an Extra-Large-Pore Aluminosilicate Zeolite NUD-6. Chem. Eur. J. 2020, 26, 17143–17148. [Google Scholar] [CrossRef]



| Run | NaOH/Si | Surface Area (m2/g) | Pore Volume (cm3/g) | ||||
|---|---|---|---|---|---|---|---|
| SBET [a] | Smicro [b] | Sext [c] | Vtotal | Vmicro [d] | Vmes | ||
| 1 | 0.05 | 208 | 170 | 38 | 0.12 | 0.07 | 0.05 |
| 2 | 0.1 | 228 | 194 | 34 | 0.13 | 0.08 | 0.05 |
| 3 | 0.15 | 167 | 146 | 21 | 0.09 | 0.06 | 0.03 |
| 4 | 0.2 | 191 | 175 | 16 | 0.11 | 0.07 | 0.04 |
| 5 | 0.25 | 214 | 190 | 24 | 0.11 | 0.08 | 0.03 |
| Run | Na2CO3/Si | Surface Area (m2/g) | Pore Volume (cm3/g) | ||||
|---|---|---|---|---|---|---|---|
| SBET [a] | Smicro [b] | Sext [c] | Vtotal | Vmicro [d] | Vmes | ||
| 1 | 0.05 | 218 | 177 | 41 | 0.12 | 0.07 | 0.05 |
| 2 | 0.1 | 38 | 24 | 14 | 0.03 | 0.01 | 0.02 |
| 3 | 0.15 | 118 | 102 | 16 | 0.07 | 0.04 | 0.03 |
| 4 | 0.2 | 126 | 97 | 29 | 0.08 | 0.04 | 0.04 |
| 5 | 0.25 | 26 | 7 | 19 | 0.05 | 0.003 | 0.047 |
| Run | HF/Si | Surface Area (m2/g) | Pore Volume (cm3/g) | ||||
|---|---|---|---|---|---|---|---|
| SBET [a] | Smicro [b] | Sext [c] | Vtotal | Vmicro [d] | Vmes | ||
| 1 | 0.5 | 229 | 168 | 61 | 0.32 | 0.07 | 0.25 |
| 2 | 0.65 | 211 | 148 | 63 | 0.22 | 0.06 | 0.16 |
| 3 | 0.75 | 375 | 297 | 77 | 0.24 | 0.12 | 0.12 |
| 4 | 1 | 240 | 211 | 29 | 0.17 | 0.08 | 0.09 |
| 5 | 1.25 | 229 | 190 | 39 | 0.16 | 0.08 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zi, W.; Hu, Z.; Jiang, X.; Zhang, J.; Guo, C.; Qu, K.; Tao, S.; Tan, D.; Liu, F. Morphology Regulation of Zeolite MWW via Classical/Nonclassical Crystallization Pathways. Molecules 2024, 29, 170. https://doi.org/10.3390/molecules29010170
Zi W, Hu Z, Jiang X, Zhang J, Guo C, Qu K, Tao S, Tan D, Liu F. Morphology Regulation of Zeolite MWW via Classical/Nonclassical Crystallization Pathways. Molecules. 2024; 29(1):170. https://doi.org/10.3390/molecules29010170
Chicago/Turabian StyleZi, Wenwen, Zejing Hu, Xiangyu Jiang, Junjun Zhang, Chengzhi Guo, Konggang Qu, Shuo Tao, Dengran Tan, and Fangling Liu. 2024. "Morphology Regulation of Zeolite MWW via Classical/Nonclassical Crystallization Pathways" Molecules 29, no. 1: 170. https://doi.org/10.3390/molecules29010170
APA StyleZi, W., Hu, Z., Jiang, X., Zhang, J., Guo, C., Qu, K., Tao, S., Tan, D., & Liu, F. (2024). Morphology Regulation of Zeolite MWW via Classical/Nonclassical Crystallization Pathways. Molecules, 29(1), 170. https://doi.org/10.3390/molecules29010170






