Synthesis of Taxifolin-Loaded Polydopamine for Chemo-Photothermal-Synergistic Therapy of Ovarian Cancer
Abstract
1. Introduction
2. Results and Discussion
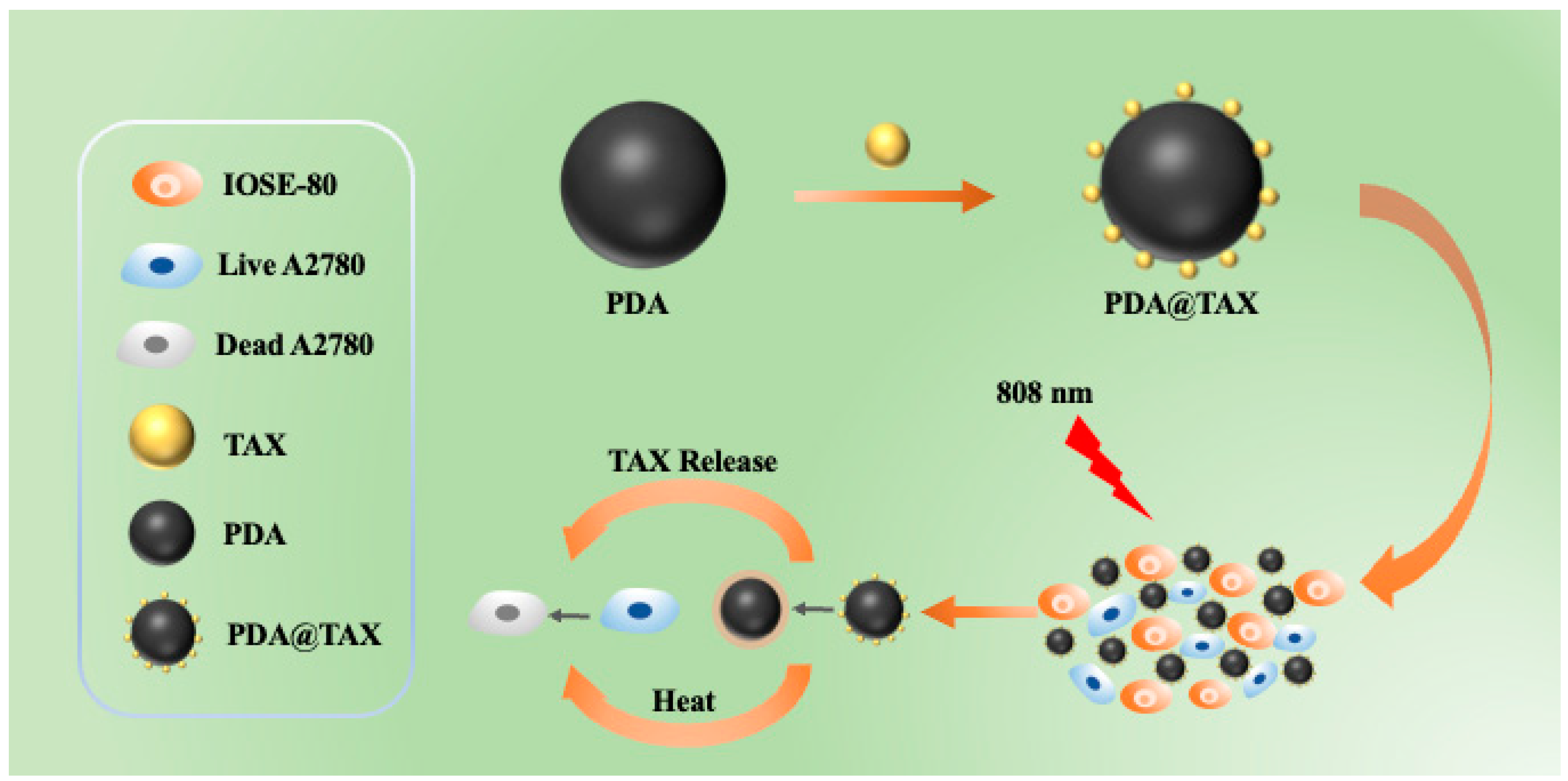
2.1. Preparation and Characterization
2.2. TAX Loading and Releasing
2.3. In Vitro Studies of Photothermal Therapy
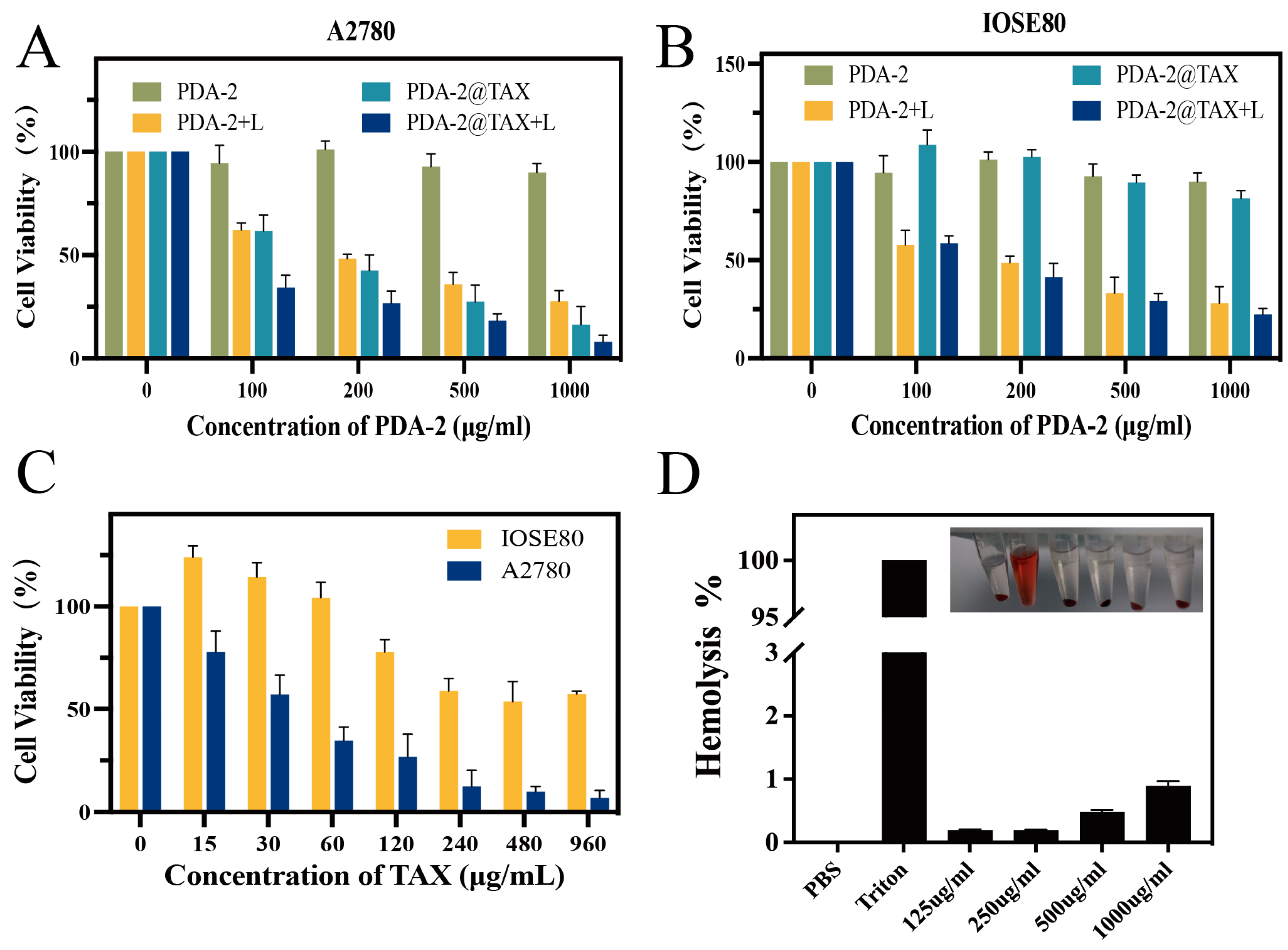
2.4. In Vitro Cytotoxicity Assay
2.5. In Vitro Hemolysis Test
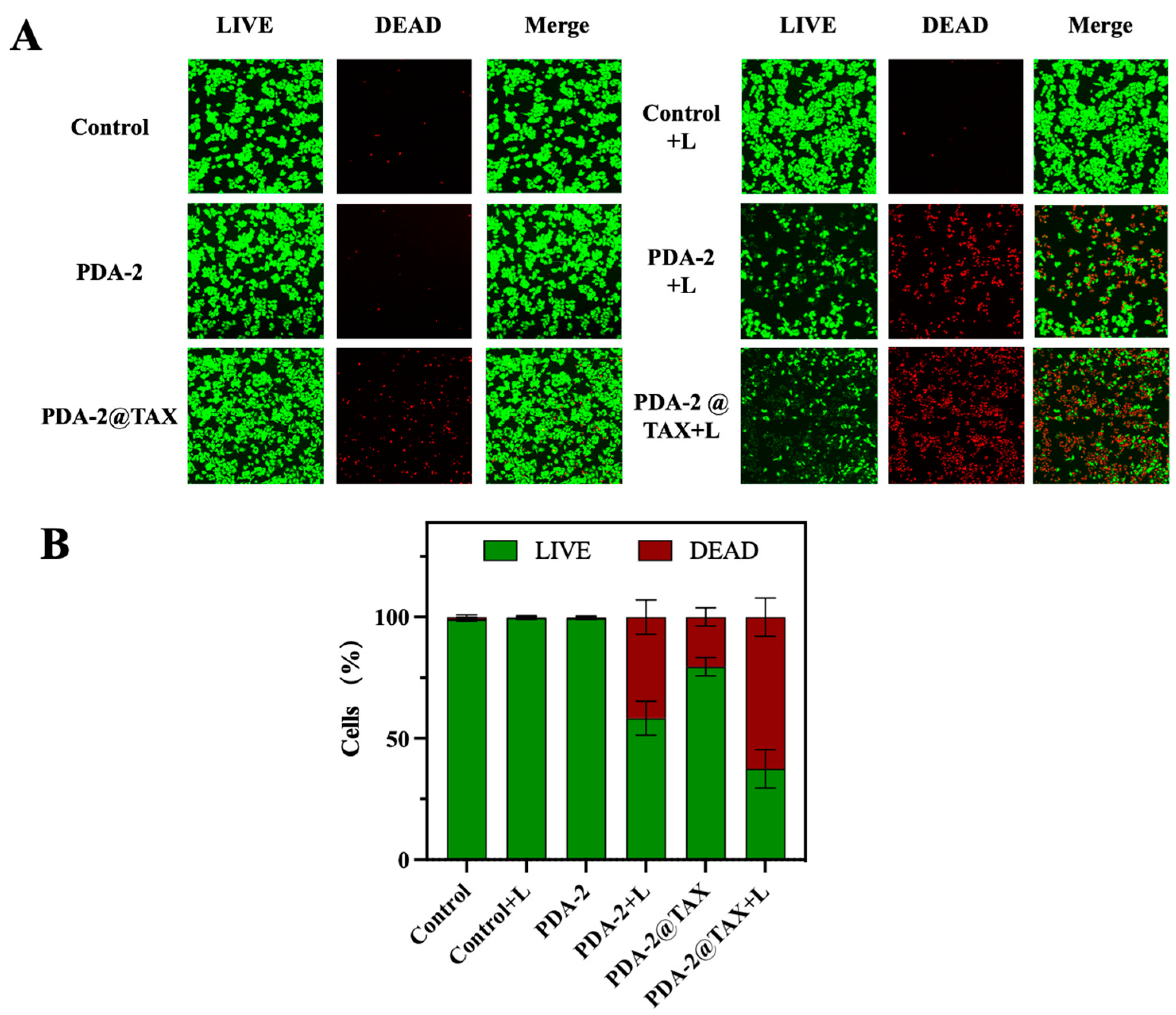
2.6. The LIVE/DEAD Assay
3. Experimental Section
3.1. Chemicals and Materials
3.2. Synthesis of Polydopamine with Different Particle Size
3.3. TAX Loading and Releasing
3.4. Photothermal Effect of the NPs
3.5. In Vitro Studies on Photothermal Therapy
3.6. The LIVE/DEAD Trial
3.7. Hemolysis Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khokhlova, S.V. The role of bevacizumab in the treatment of patients with ovarian and cervical cancer. Mod. Oncol. 2017, 19, 34–41. [Google Scholar] [CrossRef]
- Patch, A.M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; Bowtell, D.D.L. Whole–genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef]
- Robova, H.; Rob, L.; Pluta, M.; Halaska, M., Jr.; Chmel, R. Treatment of recurrent ovarian cancer. Ceska Gynekol. 2009, 74, 464–468. [Google Scholar]
- Kim, S.I.; Kim, J.W. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open 2021, 6, 100149. [Google Scholar] [CrossRef]
- Jacobson, G.; Galvan-Turner, V. Rethinking the Role of Radiation Therapy in the Management of Epithelial Ovarian Cancer. Diagnostics 2020, 10, 211. [Google Scholar] [CrossRef]
- Lim, C.K.; Kim, D.Y.; Cho, A.; Choi, J.-Y.; Park, J.-Y.; Kim, Y.-M. Role of minimally invasive surgery in early ovarian cancer. Gland Surg. 2021, 10, 1252–1259. [Google Scholar] [CrossRef]
- Flont, M.; Jastrzebska, E.; Brzozka, Z. Synergistic effect of the combination therapy on ovarian cancer cells under microfluidic conditions. Anal. Chim. Acta 2020, 1100, 138–148. [Google Scholar] [CrossRef]
- Kinoshita, T.; Goto, T. Links between Inflammation and Postoperative Cancer Recurrence. J. Clin. Med. 2021, 10, 228. [Google Scholar] [CrossRef]
- Liang, L.; Peng, S.; Yuan, Z.; Wei, C.; He, Y.; Zheng, J.; Gu, Y.; Chen, H. Biocompatible tumor-targeting nanocomposites based on CuS for tumor imaging and photothermal therapy. RSC Adv. 2018, 8, 6013–6026. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.; Uthaman, S.; Park, I.-K. External and Internal Stimuli-Responsive Metallic Nanotherapeutics for Enhanced Anticancer Therapy. Front. Mol. Biosci. 2021, 7, 597634. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tang, D.; Liu, C.; Zhang, Q.; Tang, L.; Lu, Y.; Xiao, H. Biodegradable Polymer with Effective Near-Infrared-II Absorption as a Photothermal Agent for Deep Tumor Therapy. Adv. Mater. 2022, 34, 2105976. [Google Scholar] [CrossRef]
- Lakshmi, B.A.; Kim, S. Recent insights into the development of nucleic acid-based nanoparticles for tumor-targeted drug delivery. Colloids Surf. B-Biointerfaces 2018, 172, 315–322. [Google Scholar] [CrossRef]
- Lee, C.G.; Kwon, T.-H. Controlling Morphologies of Redox-Responsive Polymeric Nanocarriers for a Smart Drug Delivery System. Chem.—Eur. J. 2023, 29, e202300594. [Google Scholar] [CrossRef]
- Kato, T.; Wakiyama, H.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy; A Review of Targets for Cancer Therapy. Cancers 2021, 13, 2535. [Google Scholar] [CrossRef]
- Wang, H.; Chang, J.; Shi, M.; Pan, W.; Li, N.; Tang, B. A Dual-Targeted Organic Photothermal Agent for Enhanced Photothermal Therapy. Angew. Chem.-Int. Ed. 2019, 58, 1057–1061. [Google Scholar] [CrossRef]
- Gheewala, T.; Skwor, T.; Munirathinam, G. Photodynamic therapy using pheophorbide and 670 nm LEDs exhibits anticancer effects in-vitro in androgen dependent prostate cancer. Photodiagn. Photodyn. Ther. 2018, 21, 130–137. [Google Scholar] [CrossRef]
- Han, R.; Xiao, Y.; Yang, Q.; Pan, M.; Hao, Y.; He, X.; Peng, J.; Qian, Z. Ag2S nanoparticle-mediated multiple ablations reinvigorates the immune response for enhanced cancer photo-immunotherapy. Biomaterials 2021, 264, 120451. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Xia, Y.; Li, T.; Wang, L.; Liu, Y.; Tan, W. Molecular Self-Assembly of Bioorthogonal Aptamer-Prodrug Conjugate Micelles for Hydrogen Peroxide and pH-Independent Cancer Chemodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jiang, B.; Yang, Y.; Zhao, M.; Sun, N.; Xia, J.; Gao, X.; Li, J. Cell membrane covered polydopamine nanoparticles with two-photon absorption for precise photothermal therapy of cancer. J. Colloid Interface Sci. 2021, 604, 596–603. [Google Scholar] [CrossRef]
- Zhu, Z.; Su, M. Polydopamine Nanoparticles for Combined Chemo- and Photothermal Cancer Therapy. Nanomaterials 2017, 7, 160. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, J.; Zhang, B.; Qu, J. Synthesis of Ag@Fe3O4 Nanoparticles for Photothermal Treatment of Ovarian Cancer. J. Nanomater. 2019, 2019, 6457968. [Google Scholar] [CrossRef]
- Tao, W.; Zeng, X.; Wu, J.; Xi, Z.; Yu, X.; Zhang, X.; Zhang, J.; Gan, L.; Lin, M. Polydopamine-Based Surface Modification of Novel Nanoparticle-Aptamer Bioconjugates for In Vivo Breast Cancer Targeting and Enhanced Therapeutic Effects. Theranostics 2016, 6, 470–484. [Google Scholar] [CrossRef]
- Kwon, I.S.; Bettinger, C.J. Polydopamine nanostructures as biomaterials for medical applications. J. Mater. Chem. B 2018, 6, 6895–6903. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, Z.; Zhang, Y.; Wang, Q.; Liang, Z.; Zeng, X. Mussel-Inspired Polydopamine: The Bridge for Targeting Drug Delivery System and Synergistic Cancer Treatment. Macromol. Biosci. 2020, 20, 2000222. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, H.; Hai, L.; Wang, T.; Hou, M.; He, D.; He, X.; Wang, K. A photosensitizer-loaded zinc oxide-polydopamine core-shell nanotherapeutic agent for photodynamic and photothermal synergistic therapy of cancer cells. Chin. Chem. Lett. 2020, 31, 189–192. [Google Scholar] [CrossRef]
- Chena, B.; Mei, L.; Fan, R.; Chuan, D.; Ren, Y.; Mua, M.; Chen, H.; Zou, B.; Guo, G. Polydopamine-coated i-motif DNA/Gold nanoplatforms for synergistic photothermal- chemotherapy. Asian J. Pharm. Sci. 2023, 18, 100781. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; An, H.J.; Park, D.H.; Yi, Y.S.; Lee, Y.H. Taxifolin Derivative with Superior Antioxidant Effect and Cosmetic Composition Containing the Same. U.S. Patent 09822104, 21 November 2017. [Google Scholar]
- Zhang, J.; Chen, K.; Ding, C.; Sun, S.; Zheng, Y.; Ding, Q.; Hong, B.; Liu, W. Fabrication of chitosan/PVP/dihydroquercetin nanocomposite film for in vitro and in vivo evaluation of wound healing. Int. J. Biol. Macromol. 2022, 206, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, X.; Wang, Q.; Li, X.; Wang, E.; Zhao, Q.; Wang, Q.; Cao, H. The anti-tumor effect of taxifolin on lung cancer via suppressing stemness and epithelial-mesenchymal transition in vitro and oncogenesis in nude mice. Ann. Transl. Med. 2020, 8, 590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shan, L.-P.; Zhao, Q.; Liu, L.; OuYang, X.; Hu, Y.; Fei, C.-J.; Chen, J. Taxifolin Inhibits WSSV Infection and Transmission by Increasing the Innate Immune Response in Litopenaeus vannamei. Viruses 2022, 14, 2731. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Saito, S.; Nakaoku, Y.; Ogata, S.; Hattori, M.; Nakatsuji, M.; Nishimura, K.; Ihara, M. Taxifolin for Cognitive Preservation in Patients with Mild Cognitive Impairment or Mild Dementia. J. Alzheimer’s Dis. 2023, 93, 743–754. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, G.; Cai, J.; Yang, J.; Zheng, Y.; Yu, D.; Liu, Q.; Gong, Y.; Zhang, Z. Taxifolin alleviates apoptotic injury induced by DEHP exposure through cytochrome P450 homeostasis in chicken cardiomyocytes. Ecotoxicol. Environ. Saf. 2019, 183, 109582. [Google Scholar] [CrossRef]
- Ding, Q.; Liu, W.; Liu, X.; Ding, C.; Zhao, Y.; Dong, L.; Chen, H.; Sun, S.; Zhang, Y.; Zhang, J.; et al. Polyvinylpyrrolidone-Modified Taxifolin Liposomes Promote Liver Repair by Modulating Autophagy to Inhibit Activation of the TLR4/NF-?B Signaling Pathway. Front. Bioeng. Biotechnol. 2022, 10, 860515. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Khabatova, V.V.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Cytoprotective Properties of a New Nanocomplex of Selenium with Taxifolin in the Cells of the Cerebral Cortex Exposed to Ischemia/Reoxygenation. Pharmaceutics 2022, 14, 2477. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Wu, W.; Zhao, X.; Li, Y.; Wang, W.; Zhong, C.; Zhang, Y.; Zhao, X. Enhancement of solubility, antioxidant ability and bioavailability of taxifolin nanoparticles by liquid antisolvent precipitation technique. Int. J. Pharm. 2014, 471, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Sundraraman, G.; Jayakumari, L.S. Meticulous Taxifolin Releasing Performance by the Zinc Oxide Nanoparticles: As a Short Road to Drug delivery System for Cancer Therapeutics. J. Clust. Sci. 2020, 31, 241–255. [Google Scholar] [CrossRef]
- Ma, L.; Lei, Z.; Liu, F.; Wang, Z. Cy5 labeled single-stranded DNA-polydopamine nanoparticle conjugate-based FRET assay for reactive oxygen species detection. Sens. Bio-Sens. Res. 2015, 3, 92–97. [Google Scholar] [CrossRef][Green Version]
- Ding, Q.; Chen, K.; Liu, X.; Ding, C.; Zhao, Y.; Sun, S.; Zhang, Y.; Zhang, J.; Liu, S.; Liu, W. Modification of taxifolin particles with an enteric coating material promotes repair of acute liver injury in mice through modulation of inflammation and autophagy signaling pathway. Biomed. Pharmacother. 2022, 152, 113242. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Zhao, H.; Zhan, G.; Zhao, Y.; Yang, X. Injectable in Situ Forming Hydrogels of Thermosensitive Polypyrrole Nanoplatforms for Precisely Synergistic Photothermo-Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 7995–8005. [Google Scholar] [CrossRef]
- Li, K.-C.; Chu, H.-C.; Lin, Y.; Tuan, H.-Y.; Hu, Y.-C. PEGylated Copper Nanowires as a Novel Photothermal Therapy Agent. Acs Appl. Mater. Interfaces 2016, 8, 12082–12090. [Google Scholar] [CrossRef]
- Alzaharna, M.; Alqouqa, I.; Cheung, H.-Y. Taxifolin synergizes Andrographolide-induced cell death by attenuation of autophagy and augmentation of caspase dependent and independent cell death in HeLa cells. PLoS ONE 2017, 12, e0171325. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Nam, Y.J.; Lee, C.S. Taxifolin reduces the cholesterol oxidation product-induced neuronal apoptosis by suppressing the Akt and NF-κB activation-mediated cell death. Brain Res. Bull. 2017, 134, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.; Xia, T.; Mao, Q.; Zhong, L. Pretreatment with dihydroquercetin, a dietary flavonoid, protected against concanavalin A-induced immunological hepatic injury in mice and TNF-α/ActD-induced apoptosis in HepG2 cells. Food Funct. 2018, 9, 2341–2352. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhao, X.; Ji, N.; Shao, C.; Fu, B.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Inhibitory effect of taxifolin on mast cell activation and mast cell-mediated allergic inflammatory response. Int. Immunopharmacol. 2019, 71, 205–214. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-Melanin Colloidal Nanospheres: An Efficient Near-Infrared Photothermal Therapeutic Agent for In Vivo Cancer Therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
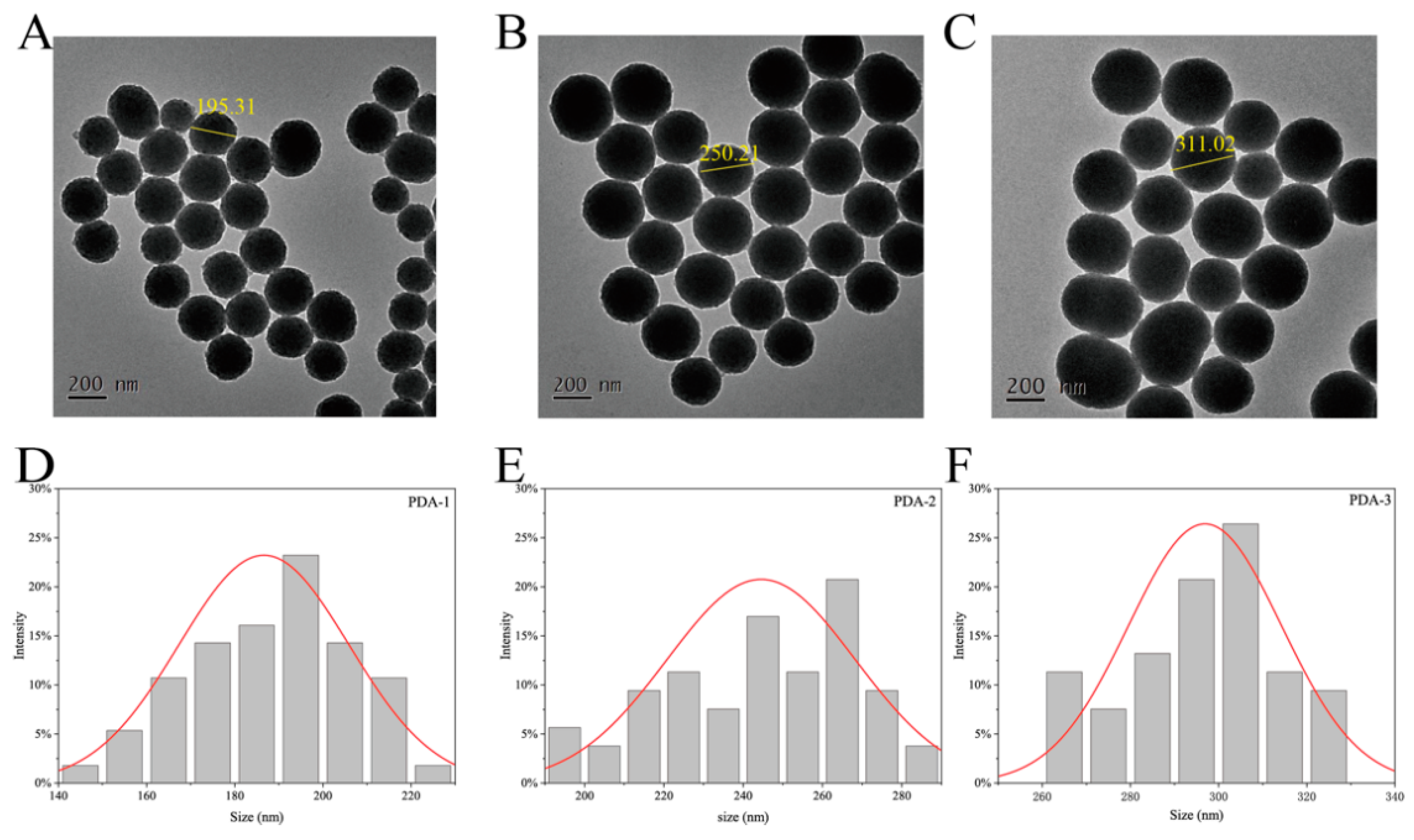



| Sample | Hydrated Particle Size Distributions (nm) | Zeta Potentials |
|---|---|---|
| PDA-1 | 200.22 ± 1.29 | 33.34 ± 6.27 |
| PDA-2 | 280.83 ± 2.07 | 38.84 ± 0.80 |
| PDA-3 | 328.78 ± 2.19 | 43.06 ± 0.65 |
| PDA-2@TAX | 300.78 ± 2.19 | 48.62 ± 0.34 |
| Sample | LC (%) | EE (%) |
|---|---|---|
| PDA-1 | 20.9 ± 1.29 | 6.09 ± 1.06 |
| PDA-2 | 36.17 ± 2.07 | 12.05 ± 0.79 |
| PDA-3 | 44.79 ± 2.19 | 14.93 ± 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Liu, X.; Zhao, T.; Ding, C.; Ding, Q.; Wang, N.; Ma, S.; Ma, L.; Liu, W. Synthesis of Taxifolin-Loaded Polydopamine for Chemo-Photothermal-Synergistic Therapy of Ovarian Cancer. Molecules 2024, 29, 1042. https://doi.org/10.3390/molecules29051042
Lu Y, Liu X, Zhao T, Ding C, Ding Q, Wang N, Ma S, Ma L, Liu W. Synthesis of Taxifolin-Loaded Polydopamine for Chemo-Photothermal-Synergistic Therapy of Ovarian Cancer. Molecules. 2024; 29(5):1042. https://doi.org/10.3390/molecules29051042
Chicago/Turabian StyleLu, Yang, Xinglong Liu, Ting Zhao, Chuanbo Ding, Qiteng Ding, Ning Wang, Shuang Ma, Lina Ma, and Wencong Liu. 2024. "Synthesis of Taxifolin-Loaded Polydopamine for Chemo-Photothermal-Synergistic Therapy of Ovarian Cancer" Molecules 29, no. 5: 1042. https://doi.org/10.3390/molecules29051042
APA StyleLu, Y., Liu, X., Zhao, T., Ding, C., Ding, Q., Wang, N., Ma, S., Ma, L., & Liu, W. (2024). Synthesis of Taxifolin-Loaded Polydopamine for Chemo-Photothermal-Synergistic Therapy of Ovarian Cancer. Molecules, 29(5), 1042. https://doi.org/10.3390/molecules29051042





