Novel 4-Chromanone-Derived Compounds as Plant Immunity Inducers against CMV Disease in Passiflora spp. (Passion Fruit)
Abstract
1. Introduction
2. Results and Discussion
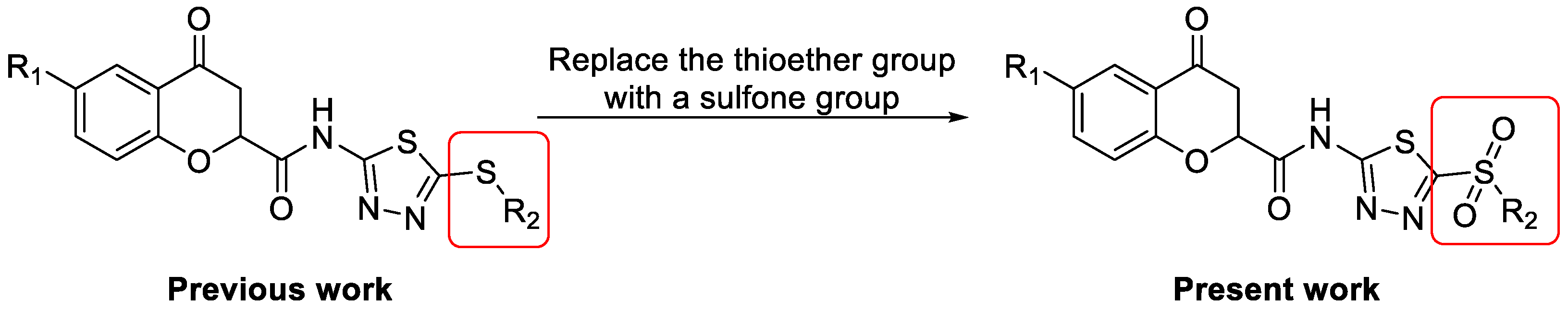
2.1. Chemistry
2.2. Results of Anti-CMV Activity and Field Trial Tests
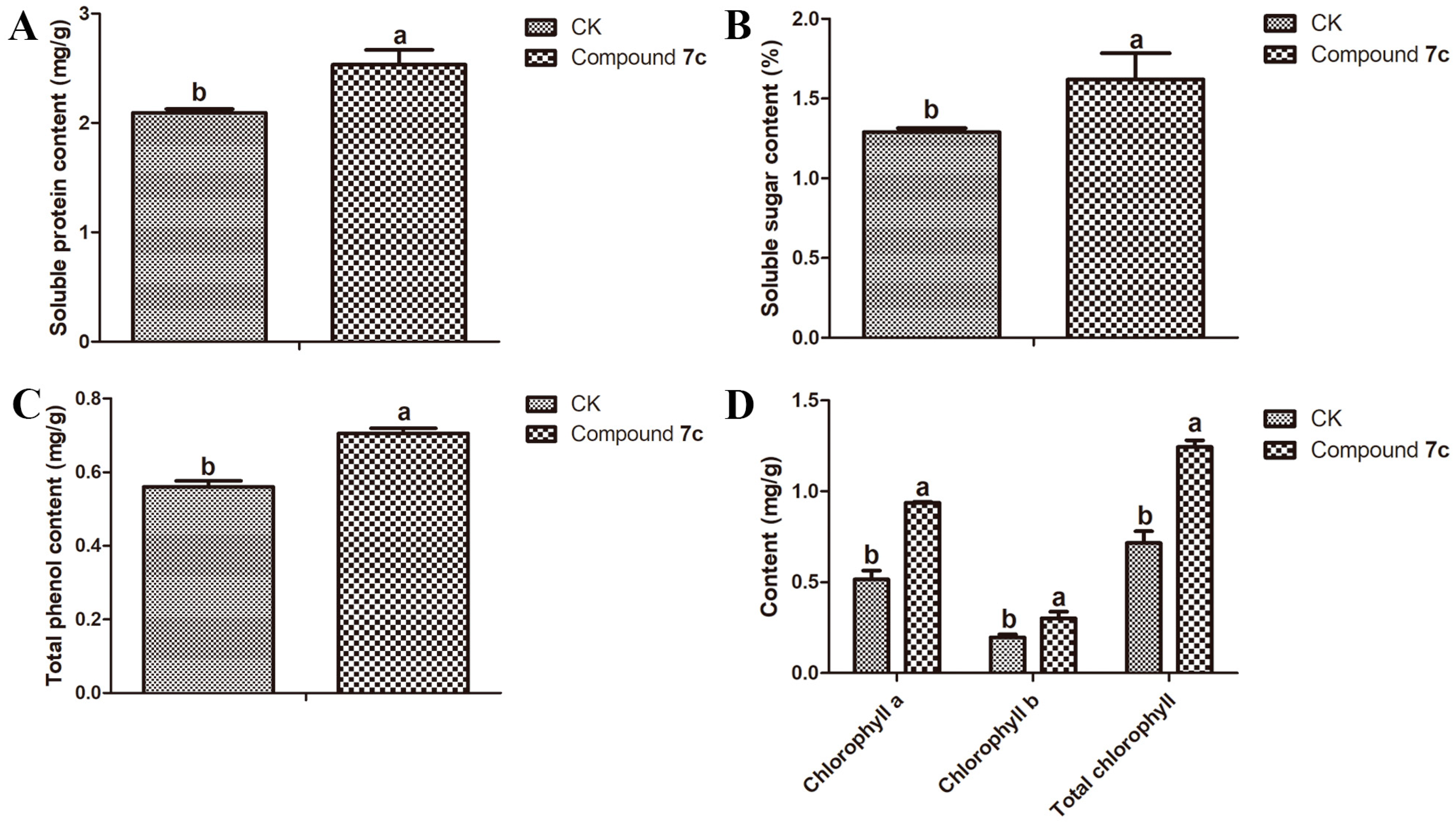
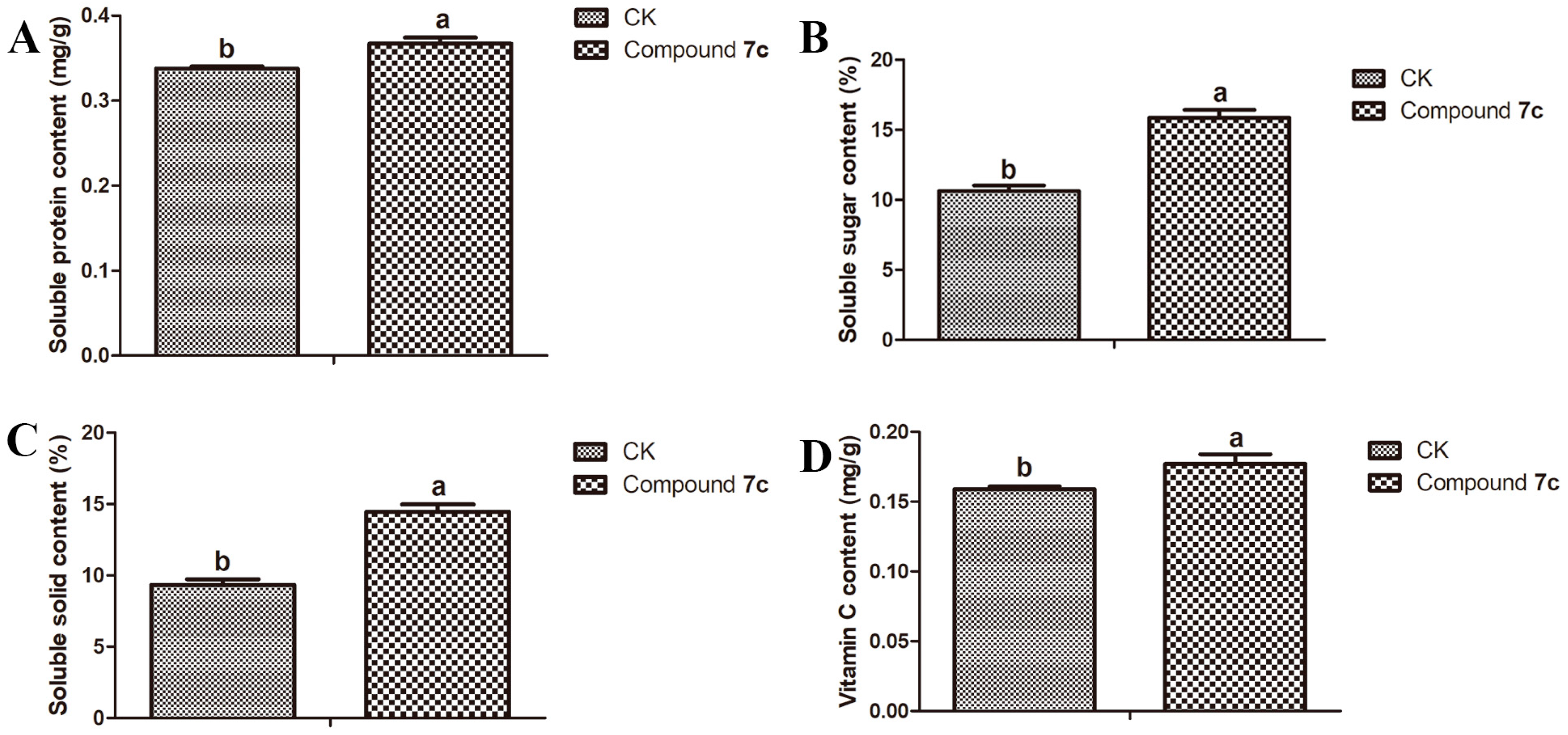
2.3. Results of Nutritional Quality of Passiflora spp. Leaves and Fruits
2.4. Quality Check of Transcriptome Sequencing Data
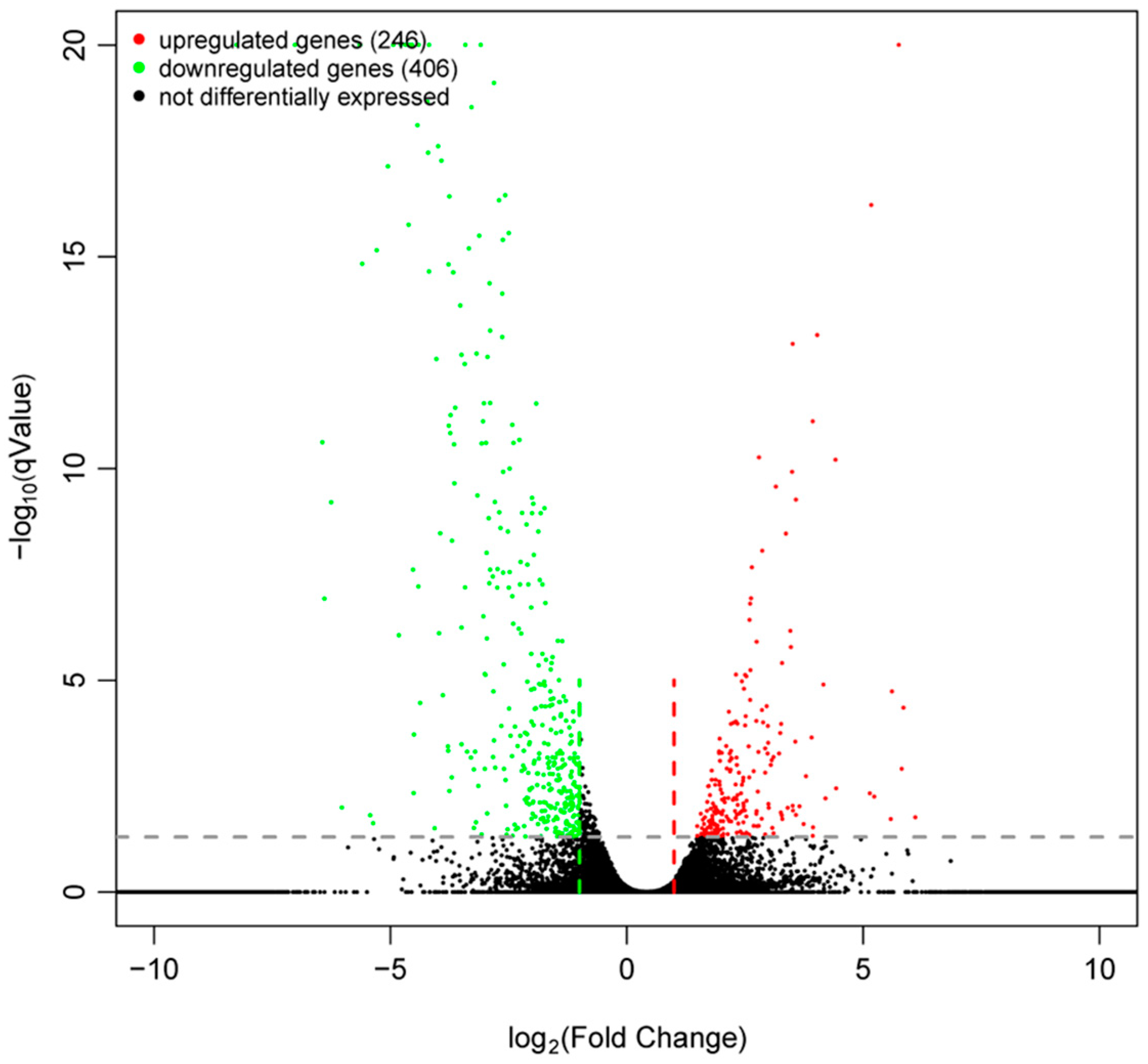
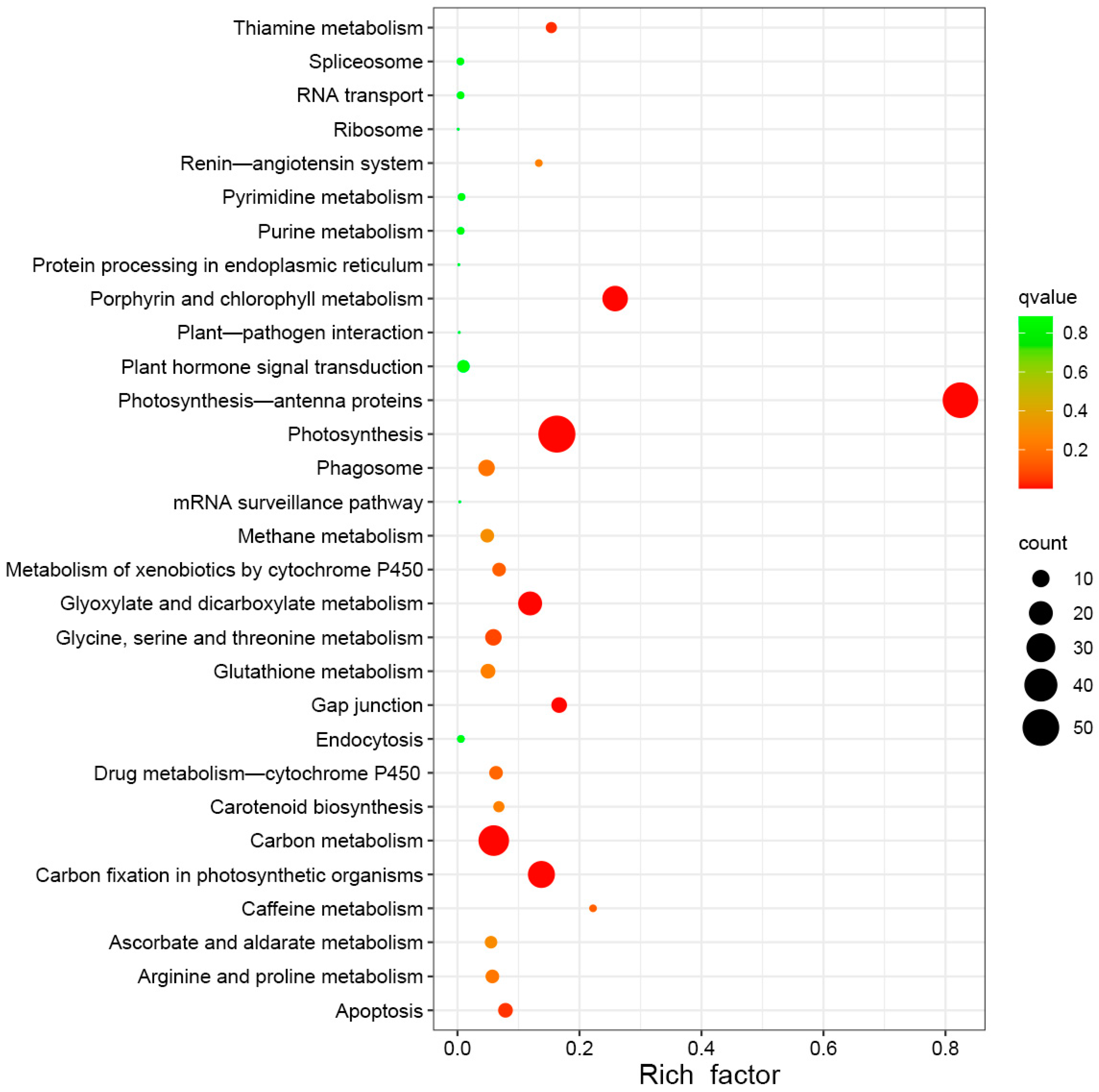
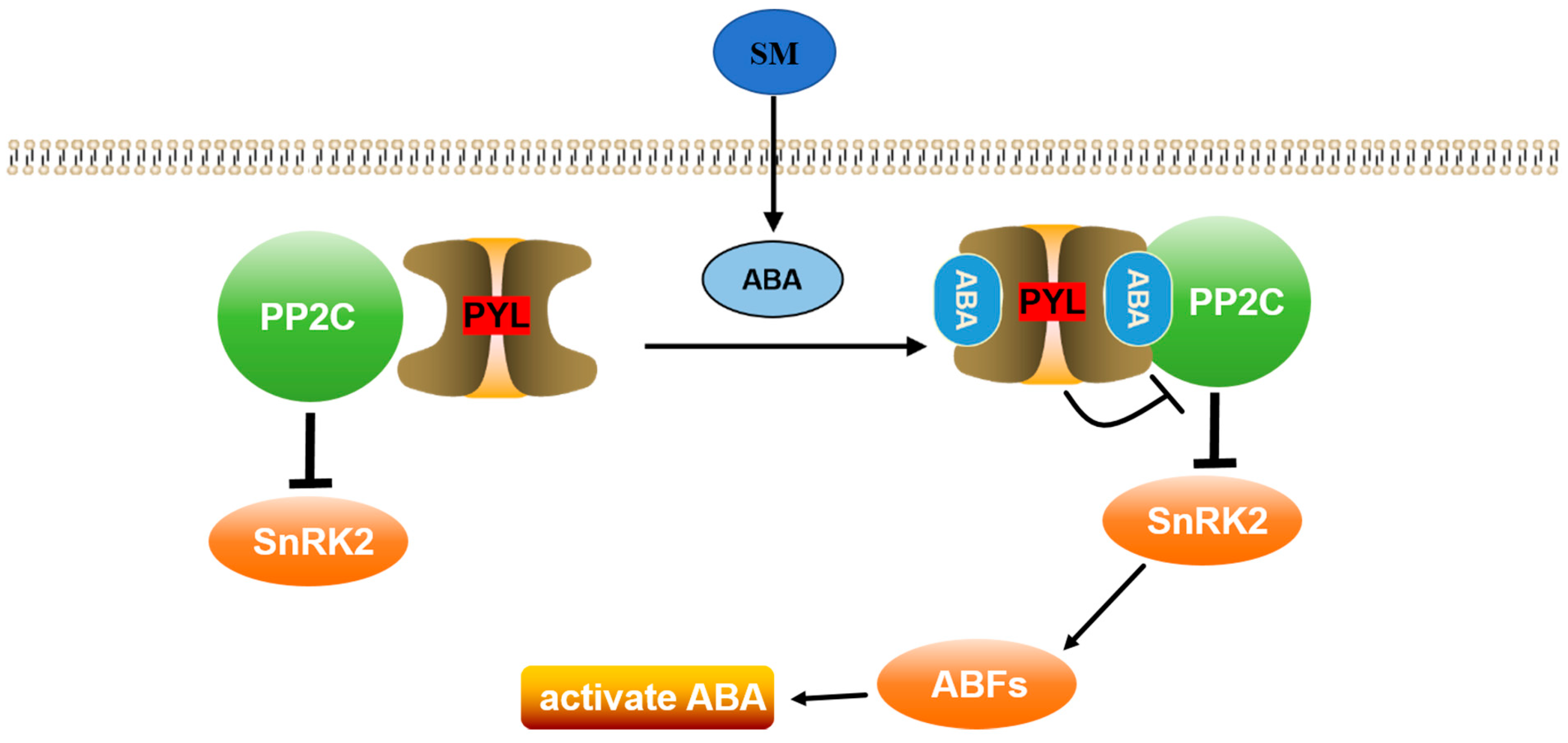
2.5. Results of DEG and Bioinformatic Analyses
3. Materials and Methods
3.1. Chemical Synthesis
3.1.1. General Protocols for the Synthesis of Intermediates 2–6
3.1.2. General Protocols for the Synthesis of Target Compounds 7a–7o
3.2. In Vivo Anti-CMV Activity Test
3.2.1. The Curative Activities of the Title Compounds against CMV In Vivo
3.2.2. The Protective Activities of the Title Compounds against CMV In Vivo
3.3. Field Trials Test against Passiflora spp. CMV Disease
3.4. Nutritional Quality Test of Passiflora spp. Leaves and Fruits
3.4.1. Soluble Protein Test of Passiflora spp. Leaves and Fruits
3.4.2. Soluble Sugar Test of Passiflora spp. Leaves and Fruits
3.4.3. Chlorophyll Test of Passiflora spp. Leaves
3.4.4. Total Phenol Test of Passiflora spp. Leaves
3.4.5. Vitamin C Test of Passiflora spp. Fruits
3.4.6. Soluble Solid Test of Passiflora spp. Fruits
3.5. Transcriptome Sequencing
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrêa, R.C.G.; Peralta, R.M.; Haminiuk, C.W.I.; Maciel, G.M.; Bracht, A.; Ferreira, I.C.F.R. The past decade findings related with nutritional composition, bioactive molecules and biotechnological applications of Passiflora spp. (Passion fruit). Trends Food Sci. Technol. 2016, 58, 79–95. [Google Scholar] [CrossRef]
- Chen, L.J.; Sun, D.L.; Zhang, X.X.; Shao, D.Q.; Lu, Y.L.; An, Y.X. Transcriptome analysis of yellow passion fruit in response to cucumber mosaic virus infection. PLoS ONE 2021, 16, e0247127. [Google Scholar] [CrossRef]
- Huang, A.J.; Gu, P.P.; Wang, Y.; Li, J.L.; Yang, Z.X.; Yi, L. Simultaneous detection and differentiation of four viruses in passion fruit plants by a multiplex RT-PCR. Trop. Plant Pathol. 2023, 48, 23–29. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Yu, L.; Zhao, Z.; Li, P.; Tan, S.M. Chitosan oligosaccharide as a plant immune inducer on the Passiflora spp. (Passion fruit) CMV disease. Front. Plant Sci. 2023, 14, 1131766. [Google Scholar] [CrossRef]
- Song, B.A.; Yang, S.; Jin, L.H.; Bhadury, P.S. Environment-Friendly Antiviral Agents for Plants; Springer Science & Business Media: Guizhou, China, 2011. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Duke, S.O. Structure simplification of natural products as a lead generation approach in agrochemical discovery. J. Agric. Food Chem. 2021, 69, 8324–8346. [Google Scholar] [CrossRef]
- Gao, B.; Yang, B.; Feng, X.D.; Li, C. Recent advances in the biosynthesis strategies of nitrogen heterocyclic natural products. Nat. Prod. Rep. 2022, 39, 139–162. [Google Scholar] [CrossRef]
- Saengchantara, S.T.; Wallace, T.W. Chromanols, chromanones, and chromones. Nat. Prod. Rep. 1986, 3, 465–475. [Google Scholar] [CrossRef]
- Abdel Ghani, S.B.; Mugisha, P.J.; Wilcox, J.C.; Gado, E.A.M.; Medu, E.O.; Lamb, A.J.; Brown, R.C.D. Convenient one-pot synthesis of chromone derivatives and their antifungal and antibacterial evaluation. Synth. Commun. 2013, 43, 1549. [Google Scholar] [CrossRef]
- Malefo, M.S.; Ramadwa, T.E.; Famuyide, I.M.; McGaw, L.J.; Eloff, J.N.; Sonopo, M.S.; Selepe, M.A. Synthesis and antifungal activity of chromones and benzoxepines from the leaves of Ptaeroxylon obliquum. J. Nat. Prod. 2020, 83, 2508. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Seo, S.H.; Na, Y.; Kwon, Y. The synthesis and anticancer activities of chiral epoxy-substituted chromone analogs. Bioorg. Chem. 2019, 84, 347. [Google Scholar] [CrossRef]
- Li, M.; Zan, N.N.; Huang, M.X.; Jiang, D.H.; Hu, D.Y.; Song, B.A. Design, synthesis and anti-TMV activities of novel chromone derivatives containing dithioacetal moiety. Bioorg. Med. Chem. Lett. 2020, 30, 126945. [Google Scholar] [CrossRef]
- Shen, S.Y.; Xiong, W.; Li, S.S.; Liu, X.S.; Li, Y.K.; Miao, D.; Li, X.M.; Wang, W.G.; Du, G.; Gong, D.P.; et al. Chromones from the tobacco derived fungus Aspergillus versicolor and their antiviral activity. Chem. Nat. Compd. 2023, 59, 462–466. [Google Scholar] [CrossRef]
- Wu, Y.P.; Zhao, G.K.; Liu, Z.Y.; Tan, T.; Li, Z.M.; Zhou, M.; Yao, H.; Li, Y.K.; Wang, W.G.; Hu, Q.F.; et al. Antiviral Chromone alkaloids from the cigar tobacco leaves derived endophytic fungus Aspergillus lentulus. Chem. Nat. Compd. 2023, 59, 721. [Google Scholar] [CrossRef]
- Jiang, D.H.; Chen, J.X.; Zan, N.N.; Li, C.Y.; Hu, D.Y.; Song, B.A. Discovery of novel chromone derivatives containing a sulfonamide moiety as anti-ToCV agents through the tomato chlorosis virus coat protein-oriented screening method. J. Agric. Food Chem. 2021, 69, 12126–12134. [Google Scholar] [CrossRef]
- Diana, E.J.; Kanchana, U.S.; Mathew, T.V. Current developments in the synthesis of 4-chromanone-derived compounds. Org. Biomol. Chem. 2021, 19, 7995–8008. [Google Scholar] [CrossRef]
- Chouchéne, N.; Toumi, A.; Boudriga, S.; Edziri, H.; Sobeh, M.; Abdelfattah, M.A.O.; Askri, M.; Knorr, M.; Strohmann, C.; Brieger, L.; et al. Antimicrobial activity and DFT studies of a novel set of spiropyrrolidines tethered with thiochroman-4-one/chroman-4-one scaffolds. Molecules 2022, 27, 582. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Alves, D.N.; de Castro, R.D.; Perez-Castillo, Y.; de Sousa, D.P. Synthesis of coumarin and homoisoflavonoid derivatives and analogs: The search for new antifungal agents. Pharmaceuticals 2022, 15, 712. [Google Scholar] [CrossRef]
- Kamboj, S.; Singh, R. Chromanone-a prerogative therapeutic scaffold: An overview. Arab. J. Sci. Eng. 2022, 47, 75–111. [Google Scholar] [CrossRef]
- Yang, J.; Lai, J.X.; Kong, W.L.; Li, S.K. Asymmetric synthesis of sakuranetin-relevant flavanones for the identification of new chiral antifungal leads. J. Agric. Food Chem. 2022, 70, 3409–3419. [Google Scholar] [CrossRef]
- Li, J.; Yu, L.; Xiao, L.L.; Yang, M.W.; Wu, T.L.; Zhang, L.Q.; Tan, S.M.; Li, P. Design, synthesis, antibacterial, and antifungal evaluation of novel 4-chromanone-derived compounds incorporating carboxamide and 1,3,4-thiadiazole thioether moieties. Phosphorus Sulfur. 2023, 198, 770–773. [Google Scholar] [CrossRef]
- Li, P.; Hu, D.Y.; Xie, D.D.; Chen, J.X.; Jin, L.H.; Song, B.A. Design, synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J. Agric. Food Chem. 2018, 66, 3093–3100. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Mangal, V.; Altaf, M.A.; Sharma, S.; Singh, B.; Kumar, M. Insight into melatonin-mediated response and signaling in the regulation of plant defense under biotic stress. Plant Mol. Biol. 2022, 109, 385–399. [Google Scholar] [CrossRef]
- Li, K.; Zhong, C.S.; Shi, Q.H.; Bi, H.G.; Gong, B. Cold plasma seed treatment improves chilling resistance of tomato plants through hydrogen peroxide and abscisic acid signaling pathway. Free Radic. Biol. Med. 2021, 172, 286–297. [Google Scholar] [CrossRef]
- Matern, U.; Kneusel, R.E. Phenolic compounds in plant disease resistance. Phytoparasitica 1988, 16, 153–170. [Google Scholar] [CrossRef]
- Yahya, M.; Saeed, N.A.; Nadeem, S.; Hamed, M.; Saleem, K. Effect of leaf rust disease on photosynthetic rate, chlorophyll contents and grain yield of wheat. Arch. Phytopathol. Plant Prot. 2020, 53, 425–439. [Google Scholar] [CrossRef]
- Huang, X.; Wang, H.K.; Luo, W.J.; Xue, S.; Hayat, F.; Gao, Z.H. Prediction of loquat soluble solids and titratable acid content using fruit mineral elements by artificial neural network and multiple linear regression. Sci. Hortic. 2021, 278, 109873. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal Behavi. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Han, Q.; Gao, H.Y.; Chen, H.J.; Fang, X.J.; Wu, W.J. Precooling and ozone treatments affects postharvest quality of black mulberry (Morus nigra) fruits. Food Chem. 2017, 221, 1947–1953. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, Y.H.; Zhang, H.Y.; Yan, X.X.; Cui, H. Methyl jasmonate treatment, aphid resistance assay, and transcriptomic analysis revealed different herbivore defensive roles between tobacco glandular and non-glandular trichomes. Plant Cell Rep. 2022, 41, 195–208. [Google Scholar] [CrossRef]
- Shen, Y.T.; Ma, Y.L.; Li, D.Y.; Kang, M.M.; Pei, Y.; Zhang, R.; Tao, W.Y.; Huang, S.X.; Song, W.J.; Li, Y.C.; et al. Biological and genomic analysis of a symbiotic nitrogen fixation defective mutant in Medicago truncatula. Front. Plant Sci. 2023, 14, 1209664. [Google Scholar] [CrossRef]
- Kusajima, M. Studies on the mechanism of agricultural chemicals focused on plant hormone signals. J. Pestic. Sci. 2019, 44, 270–274. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N. Antiviral roles of abscisic acid in plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Wang, Y.; Liu, X.; Ma, L.; Zhang, Z.; Mu, C.; Zhang, Y.; Peng, L.; Xie, S.; et al. Initiation and amplification of SnRK2 activation in abscisic acid signaling. Nat. Commun. 2021, 12, 2456. [Google Scholar] [CrossRef]
- García-Andrade, J.; González, B.; Gonzalez-Guzman, M.; Rodriguez, P.L.; Vera, P. The role of ABA in plant immunity is mediated through the PYR1 receptor. Int. J. Mol. Sci. 2020, 21, 5852. [Google Scholar] [CrossRef]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef]
- Park, S.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, S.X.; Wang, Y.; Wu, Y.K.; Yu, L.J.; Wu, J. Trifluoromethylpyridine piperazine derivatives: Synthesis and anti-plant virus activity. Pest Manag. Sci. 2023, 79, 2571–2580. [Google Scholar] [CrossRef]
- Montasser, M.S.; Tousignant, M.E.; Kaper, J.M. Viral satellite RNAs for the prevention of cucumber mosaic virus (CMV) disease in field-grown pepper and melon plants. Plant Dis. 1998, 82, 1298–1303. [Google Scholar] [CrossRef][Green Version]
- Haroon, E.T.; Zou, X.B.; Li, Z.H.; Zhu, Y.D. Comprehensive evaluation of antioxidant properties and volatile compounds of sudanese honeys. J. Food Biochem. 2015, 39, 349–359. [Google Scholar] [CrossRef]
- Vidović, M.; Morina, F.; Milić, S.; Albert, A.; Zechmann, B.; Tosti, T.; Winkler, J.B.; Jovanović, S.V. Carbon allocation from source to sink leaf tissue in relation to flavonoid biosynthesis in variegated Pelargonium zonale under UV-B radiation and high PAR intensity. Plant Physiol. Biochem. 2015, 93, 44–55. [Google Scholar] [CrossRef]
- Farhadi, H.; Babaei, K.; Farsaraei, S.; Moghaddam, M.; Pirbalouti, A.G. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crop. Prod. 2020, 152, 112570. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, J.J.; Wang, Y.Y.; Gu, J.W.; Xie, X.Z. Positive regulation of phytochrome B on chlorophyll biosynthesis and chloroplast development in rice. Rice Sci. 2013, 20, 243–248. [Google Scholar] [CrossRef]
- Maryam, A.; Anwar, R.; Malik, A.U.; Khan, S.A. Influence of macro-perforated polyethylene terephthalate and low-density polyethylene packaging films on quality and storability of strawberries. J. Food Process. Pres. 2021, 45, e15068. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; Hoyos, B.E.; Naiker, M. Phenolic profiles, phytochemical composition and vitamin C content of selected horticultural produce from Central Queensland. J. Food Meas. Charact. 2023, 17, 1096–1107. [Google Scholar] [CrossRef]
- Qu, X.L.; Zhu, K.X.; Li, Z.X.; Zhang, D.F.; Hou, L.J. The alteration of M6A-tagged transcript profiles in the retina of rats after traumatic optic neuropathy. Front. Genet. 2021, 12, 628841. [Google Scholar] [CrossRef]
- Moon, S.; Zhao, Y.T. Spatial, temporal and cell-type-specific expression profiles of genes encoding heparan sulfate biosynthesis enzymes and proteoglycan core proteins. Glycobiology 2021, 31, 1308–1318. [Google Scholar] [CrossRef]
- Zhang, H.P.; Zou, J.; Yin, Y.; Zhang, B.; Hu, Y.L.; Wang, J.J.; Mu, H.J. Bioinformatic analysis identifies potentially key differentially expressed genes in oncogenesis and progression of clear cell renal cell carcinoma. PeerJ 2019, 7, e8096. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.L.; Zeng, S.; Chen, Z.; Yang, A.M.; Shi, J. Label-free quantitative proteomics analysis of cytosinpeptidemycin responses in southern rice blackstreaked dwarf virus-infected rice. Pestic. Biochem. Phys. 2018, 147, 20–26. [Google Scholar] [CrossRef]


| Compounds | Inhibition Rate (Mean ± SD, %) * | |
|---|---|---|
| Protection Activity | Curative Activity | |
| 7a | 46.15 ± 2.28 | 30.84 ± 3.10 |
| 7b | 31.17 ± 9.32 | 19.36 ± 2.83 |
| 7c | 57.69 ± 5.06 | 51.73 ± 2.64 |
| 7d | 26.28 ± 1.14 | 28.47 ± 4.47 |
| 7e | 26.28 ± 1.14 | 30.61 ± 8.61 |
| 7f | 27.11 ± 0.83 | 35.63 ± 6.52 |
| 7g | 56.13 ± 3.59 | 52.39 ± 3.38 |
| 7h | 39.34 ± 3.32 | 33.23 ± 5.44 |
| 7i | 26.13 ± 3.36 | 22.56 ± 6.36 |
| 7j | 25.26 ± 2.88 | 21.00 ± 7.56 |
| 7k | 42.15 ± 1.56 | 39.56 ± 3.15 |
| 7l | 29.20 ± 9.98 | 21.50 ± 6.83 |
| 7m | 27.26 ± 3.16 | 32.97 ± 2.87 |
| 7n | 22.17 ± 4.33 | 28.61 ± 4.32 |
| 7o | 24.25 ± 4.31 | 16.23 ± 2.07 |
| Dufulin | 42.08 ± 6.28 | 40.78 ± 5.03 |
| Ningnanmycin | 50.13 ± 1.88 | 51.35 ± 1.95 |
| Compounds | Concentration (mg/L) | Control Efficiency (Mean ± SD, %) * | ||
|---|---|---|---|---|
| 7 Days after the First Spraying | 7 Days after the Second Spraying | 7 Days after the Third Spraying | ||
| 7c | 200 | 28.88 ± 6.63 a | 47.50 ± 8.98 a | 47.49 ± 6.22 a |
| 7g | 200 | 30.63 ± 10.77 a | 47.41 ± 9.40 a | 34.82 ± 7.60 c |
| Dufulin | 200 | 40.27 ± 17.05 a | 42.90 ± 5.43 a | 28.03 ± 1.92 c |
| Ningnanmycin | 200 | 34.77 ± 3.15 a | 40.39 ± 7.35 a | 44.17 ± 4.14 a |
| Treatment | Raw Data | Valid Data | Valid Ratio (%) | GC (%) | Q30 (%) | Q20 (%) |
|---|---|---|---|---|---|---|
| CK | 38,186,288 | 37,866,831 | 99.16 | 44.78 | 93.40 | 98.02 |
| Compound 7c reatment | 42,997,459 | 42,564,302 | 98.99 | 43.89 | 93.36 | 98.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Yu, L.; Xiao, L.; Wang, T.; Li, P.; Mu, B. Novel 4-Chromanone-Derived Compounds as Plant Immunity Inducers against CMV Disease in Passiflora spp. (Passion Fruit). Molecules 2024, 29, 1045. https://doi.org/10.3390/molecules29051045
Wu T, Yu L, Xiao L, Wang T, Li P, Mu B. Novel 4-Chromanone-Derived Compounds as Plant Immunity Inducers against CMV Disease in Passiflora spp. (Passion Fruit). Molecules. 2024; 29(5):1045. https://doi.org/10.3390/molecules29051045
Chicago/Turabian StyleWu, Tianli, Lu Yu, Lingling Xiao, Tao Wang, Pei Li, and Bo Mu. 2024. "Novel 4-Chromanone-Derived Compounds as Plant Immunity Inducers against CMV Disease in Passiflora spp. (Passion Fruit)" Molecules 29, no. 5: 1045. https://doi.org/10.3390/molecules29051045
APA StyleWu, T., Yu, L., Xiao, L., Wang, T., Li, P., & Mu, B. (2024). Novel 4-Chromanone-Derived Compounds as Plant Immunity Inducers against CMV Disease in Passiflora spp. (Passion Fruit). Molecules, 29(5), 1045. https://doi.org/10.3390/molecules29051045







