Effects of Non-Saccharomyces Yeasts and Their Pairwise Combinations in Co-Fermentation with Saccharomyces cerevisiae on the Quality of Chunjian Citrus Wine
Abstract
1. Introduction
2. Results
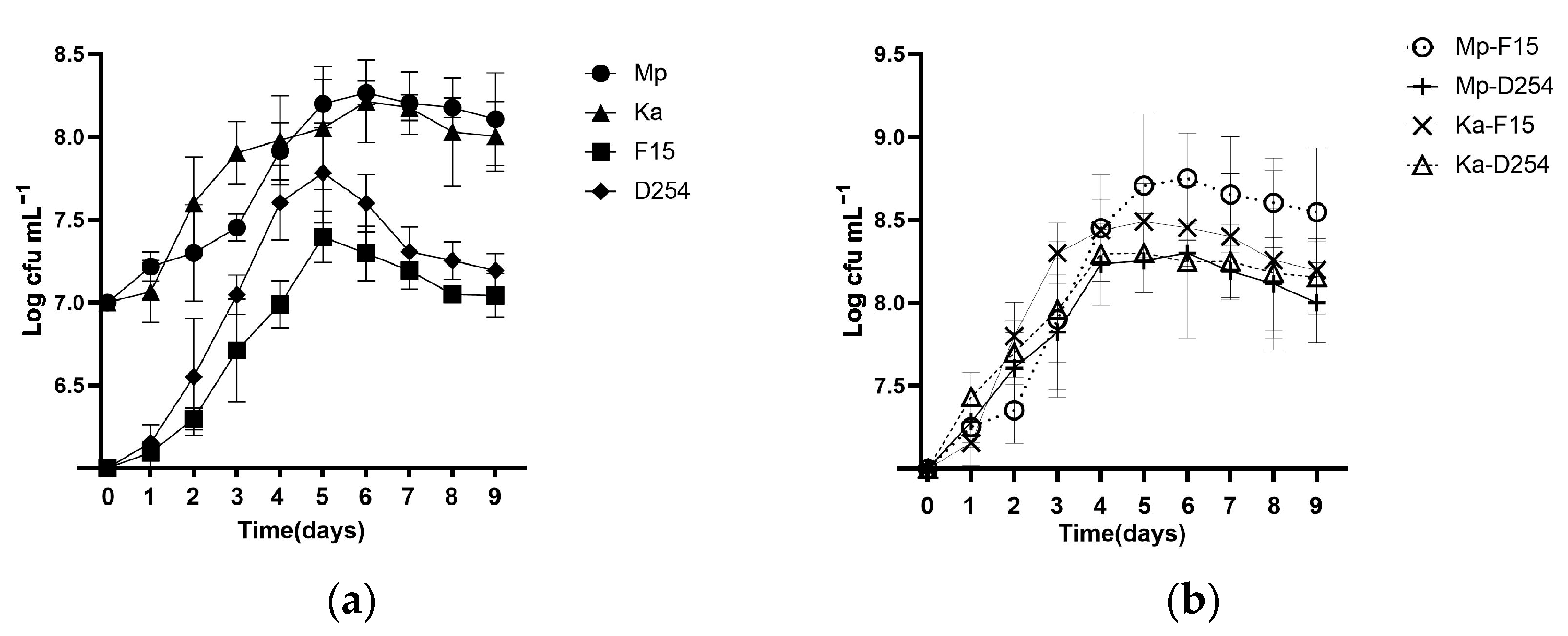
2.1. Growth and Survival of NScs and Scs in Pure and Mixed Cultures
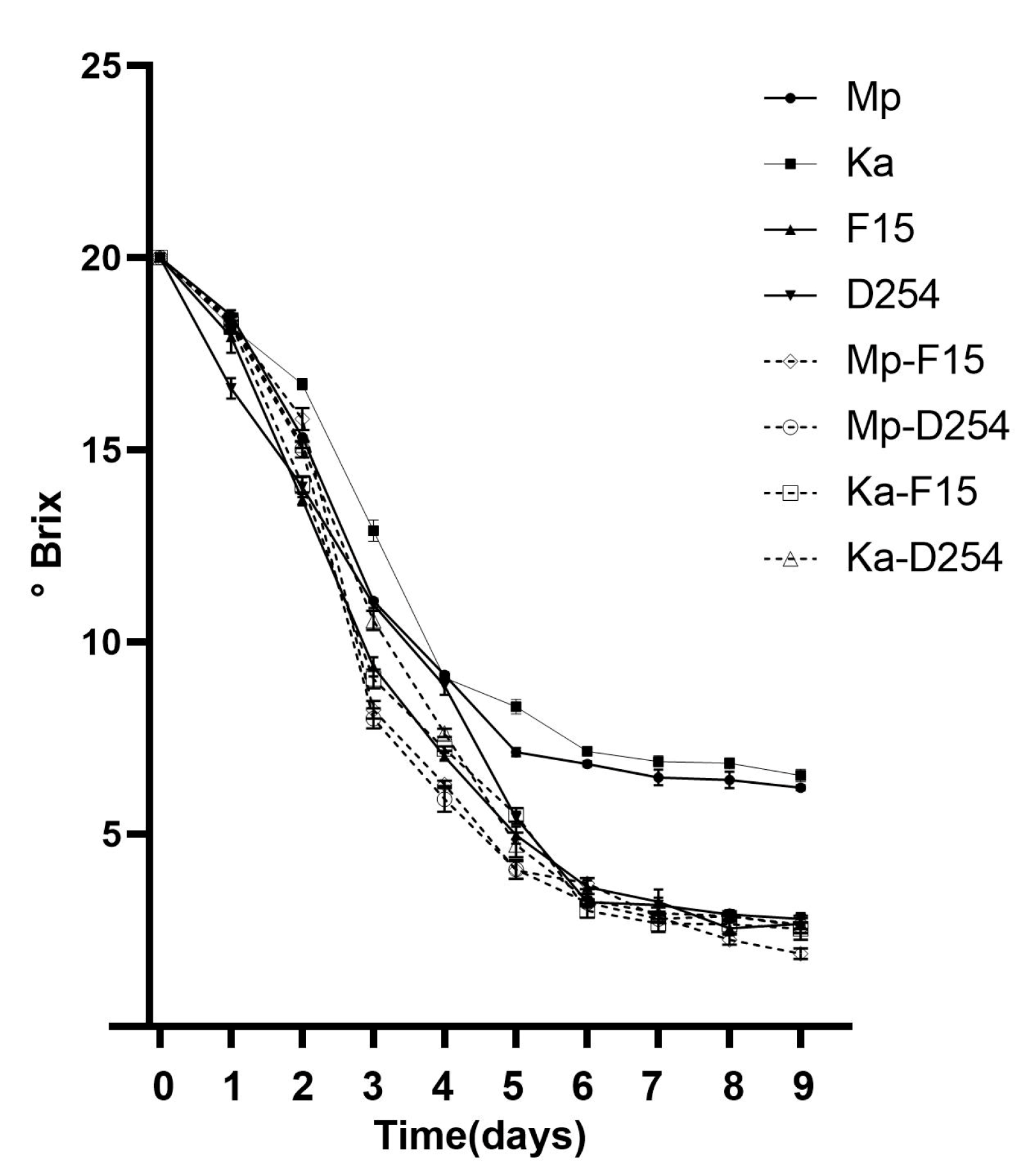
2.2. Consumption of Sugar in Mono-Culture Fermentation and Mixed Fermentation
2.3. Physical and Chemical Properties of Citrus Wine
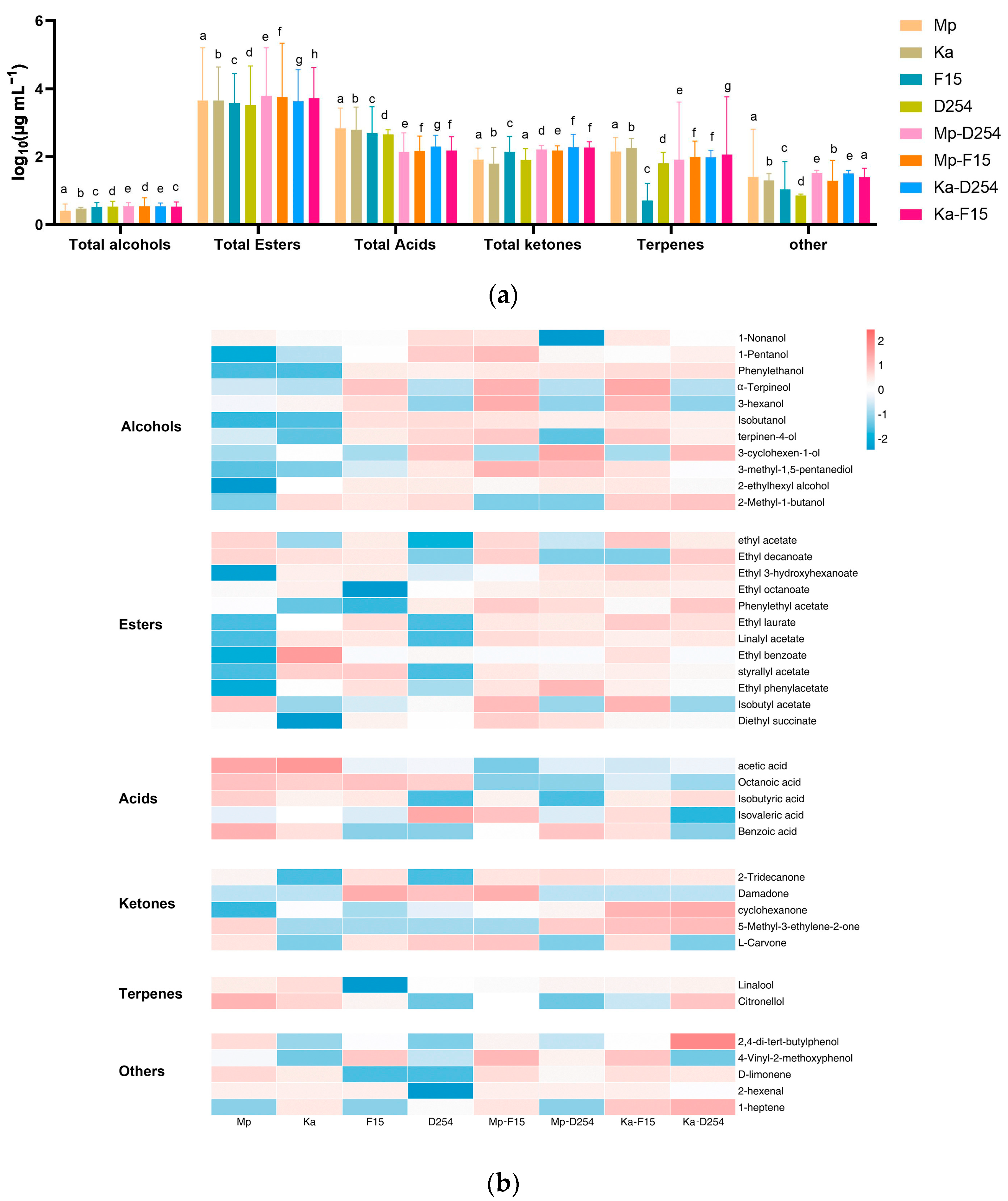
2.4. Volatile Compounds in Citrus Wine
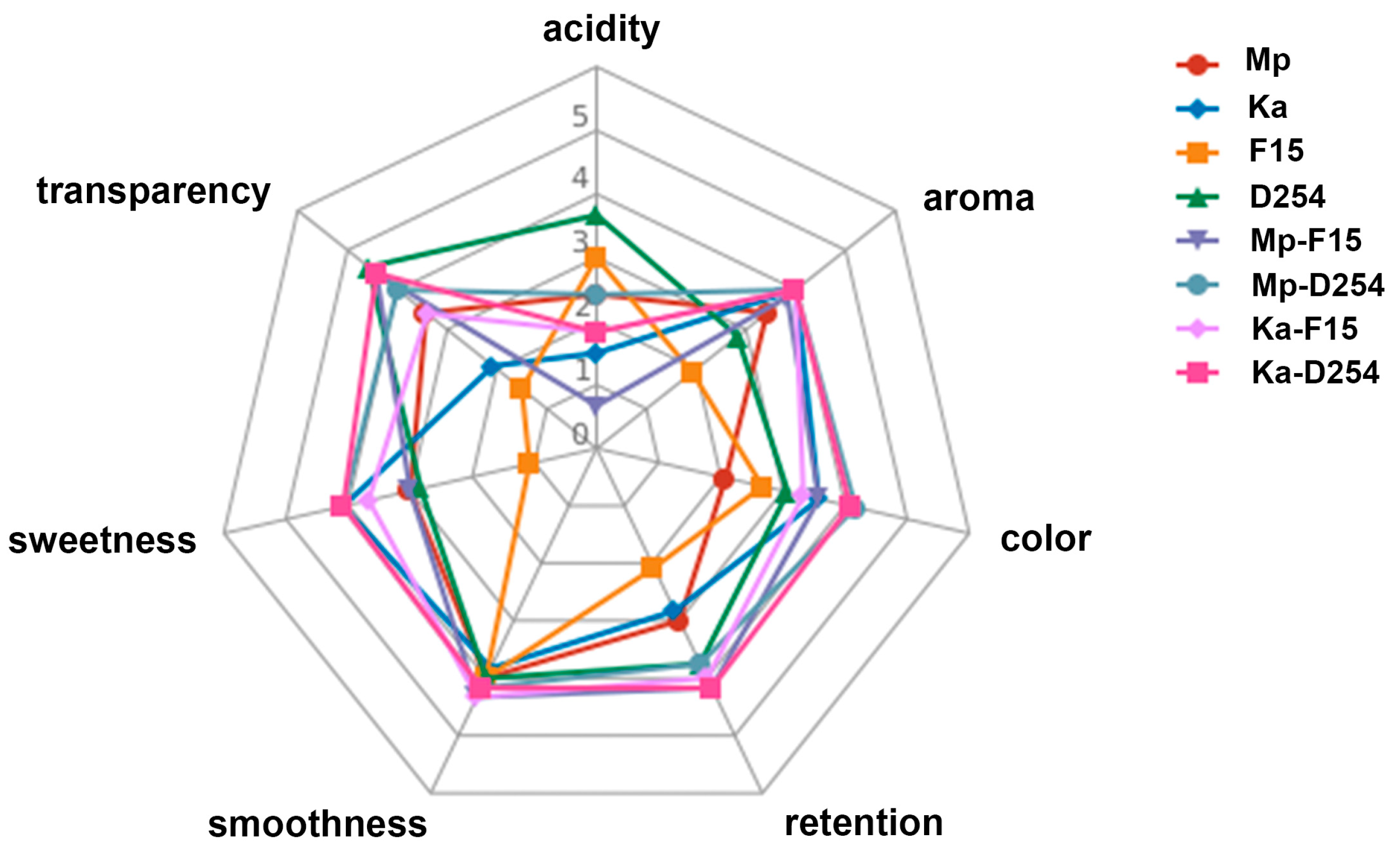
2.5. Sensory Evaluation
3. Discussion
4. Materials and Methods
4.1. Sample Processing
4.2. Yeast Preparation
4.3. Mono-Culture and Mixed Fermentation
4.4. Determination of Reduced Sugar, Alcohol Level, pH Value, and Total Acid
4.5. CI and CT Determination
4.6. Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry (SPME-GC-MS) Analysis
4.7. Calculation of ROAV
4.8. Sensory Evaluation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciani, M.; Comitini, F. Yeast ecology of wine production. In Yeasts in the Production of Wine; Springer: New York, NY, USA, 2019; pp. 1–42. [Google Scholar]
- Afonso, S.M.; Inês, A.; Vilela, A. Bio-Dealcoholization of Wines: Can Yeast Make Lighter Wines? Fermentation 2024, 10, 36. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Harlé, O.; Legrand, J.; Tesniere, C.; Pradal, M.; Mouret, J.-R.; Nidelet, T. Investigations of the mechanisms of interactions between four non-conventional species with Saccharomyces cerevisiae in oenological conditions. PLoS ONE 2020, 15, e0233285. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The sensory quality improvement of citrus wine through co-fermentations with selected non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef]
- Postigo, V.; Sanz, P.; García, M.; Arroyo, T. Impact of Non-Saccharomyces Wine Yeast Strains on Improving Healthy Characteristics and the Sensory Profile of Beer in Sequential Fermentation. Foods 2022, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, K.; Li, H.; Ma, R.; Cui, M. Effect of mixed Saccharomyces cerevisiae Y10 and Torulaspora delbrueckii Y22 on dough fermentation for steamed bread making. Int. J. Food Microbiol. 2019, 303, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rollero, S.P.; Zietsman, A.J.; Buffetto, F.; Schückel, J.; Ortiz-Julien, A.; Divol, B. Kluyveromyces marxianus secretes a pectinase in Shiraz grape must that impacts technological properties and aroma profile of wine. J. Agric. Food Chem. 2018, 66, 11739–11747. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Ma, H.; Hu, B.; Zhao, H.; Wang, J.; Rennenberg, H.; Shi, X.; Zhang, Y. Nitrogen fertilization stimulates nitrogen assimilation and modifies nitrogen partitioning in the spring shoot leaves of citrus (Citrus reticulata Blanco) trees. J. Plant Physiol. 2021, 267, 153556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, J.; Cao, J.; Wang, D.; Liu, C.; Yang, R.; Li, X.; Sun, C. Antioxidant capacity, anticancer ability and flavonoids composition of 35 citrus (Citrus reticulata Blanco) varieties. Molecules 2017, 22, 1114. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Canbas, A.; Varlet, V.; Kelebek, H.; Prost, C.; Serot, T. Characterization of the Most Odor-Active Volatiles of Orange Wine Made from a Turkish cv. Kozan (Citrus sinensis L. Osbeck). J. Agric. Food Chem. 2008, 56, 227–234. [Google Scholar] [CrossRef]
- Bi, J.; Li, H.; Wang, H. Delayed bitterness of citrus wine is removed through the selection of fining agents and fining optimization. Front. Chem. 2019, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Bapat, V.A. Wines from fruits other than grapes: Current status and future prospectus. Food Biosci. 2015, 9, 80–96. [Google Scholar] [CrossRef]
- Lee, J.-S.; Chang, C.-Y.; Yu, T.-H.; Lai, S.-T.; Lin, L.-Y. Studies on the quality and flavor of ponkan (Citrus poonensis hort.) wines fermented by different yeasts. J. Food Drug Anal. 2013, 21, 301–309. [Google Scholar] [CrossRef]
- Idise, O. Studies on wine production from orange (Citrus sinensis). Niger. J. Sci. Environ. 2007, 10, 96–100. [Google Scholar]
- Liu, S.; Lou, Y.; Li, Y.; Zhang, J.; Li, P.; Yang, B.; Gu, Q. Review of phytochemical and nutritional characteristics and food applications of Citrus L. fruits. Front. Nutr. 2022, 9, 968604. [Google Scholar] [CrossRef]
- Huang, J.; Wen, S.; Zhang, L.; Bao, R.; Wu, J.; Cai, Z. Comparison of stomatal structure among five citrus cultivars including Cara Cara Navel Orange. J. Fruit Sci. 2011, 28, 193–198. [Google Scholar]
- Saranraj, P.; Sivasakthivelan, P.; Naveen, M. Fermentation of fruit wine and its quality analysis: A review. Aust. J. Sci. Technol. 2017, 1, 85–97. [Google Scholar]
- Karabegovi, I.; Malianin, M.; Danilovi, B.; Stanojevi, J.; Stojanovi, S.S.; Nikoli, N.; Lazi, M. Potential of non-Saccharomyces yeast for improving the aroma and sensory profile of Prokupac red wine. OENO One 2021, 55, 181–195. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima Selected Strain for Ethanol Reduction in Wine: Influence of Cell Immobilization and Aeration Condition. Foods 2019, 8, 378. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Varela, C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef]
- Malićanin, M.; Danilović, B.; Stamenković Stojanović, S.; Cvetković, D.; Lazić, M.; Karabegović, I.; Savić, D. Pre-Fermentative Cold Maceration and Native Non-Saccharomyces Yeasts as a Tool to Enhance Aroma and Sensory Attributes of Chardonnay Wine. Horticulturae 2022, 8, 212. [Google Scholar] [CrossRef]
- Blanco, P.; Castrillo, D.; Graña, M.J.; Lorenzo, M.J.; Soto, E. Evaluation of autochthonous non-Saccharomyces yeasts by sequential fermentation for wine differentiation in galicia (Nw spain). Fermentation 2021, 7, 183. [Google Scholar] [CrossRef]
- Mendoza, L.M.; de Nadra, M.C.M.; Farías, M.E. Kinetics and metabolic behavior of a composite culture of Kloeckera apiculata and Saccharomyces cerevisiae wine related strains. Biotechnol. Lett. 2007, 29, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Goldner, M.C.; Zamora, M.C.; Di Leo Lira, P.; Gianninoto, H.; Bandoni, A. Effect of ethanol level in the perception of aroma attributes and the detection of volatile compounds in red wine. J. Sens. Stud. 2009, 24, 243–257. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. Levels of higher alcohols inducing aroma changes and modulating experts’ preferences in wine model solutions. Aust. J. Grape Wine Res. 2017, 23, 162–169. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of Nutrient Availability on the Fermentation and Production of Aroma Compounds Under Sequential Inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 305. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Baumes, R. Wine aroma precursors. In Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009; pp. 251–274. [Google Scholar]
- Gambetta, J.M.; Bastian, S.E.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of Chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Hernández-Orte, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.; Augustyn, O.; Pretorius, I. The Role and Use of Non-Saccharomyces Yeasts in Wine Production; South African Society for Enology and Viticulture: Stellenbosch, South Africa, 2006. [Google Scholar]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.-C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.E.; Albertin, W.; Bely, M. An innovative tool reveals interaction mechanisms among yeast populations under oenological conditions. Appl. Microbiol. Biotechnol. 2013, 97, 4105–4119. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Silva, R.D.; Chaves, S.R.; Sousa, M.J.; Côrte-Real, M. The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape musts and wines. Appl. Microbiol. Biotechnol. 2011, 89, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef]
- Chidi, B.S.; Bauer, F.; Rossouw, D. Organic Acid Metabolism and the Impact of Fermentation Practices on Wine Acidity: A Review. S. Afr. J. Enol. Vitic. 2018, 39, 1–15. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Canbas, A.; Cabaroglu, T. HPLC determination of organic acids, sugars, phenolic compositions and antioxidant capacity of orange juice and orange wine made from a Turkish cv. Kozan. Microchem. J. 2009, 91, 187–192. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Ouyang, X.; Yuan, G.; Ren, J.; Wang, L.; Wang, M.; Li, Y.; Zhang, B.; Zhu, B. Aromatic compounds and organoleptic features of fermented wolfberry wine: Effects of maceration time. Int. J. Food Prop. 2017, 20, 2234–2248. [Google Scholar] [CrossRef]
- Tufariello, M.; Capone, S.; Siciliano, P. Volatile components of Negroamaro red wines produced in Apulian Salento area. Food Chem. 2012, 132, 2155–2164. [Google Scholar] [CrossRef]
- Wedler, H.B.; Pemberton, R.P.; Tantillo, D.J. Carbocations and the complex flavor and bouquet of wine: Mechanistic aspects of terpene biosynthesis in wine grapes. Molecules 2015, 20, 10781–10792. [Google Scholar] [CrossRef]
- Perpetuini, G.; Rossetti, A.P.; Quadrani, L.; Arfelli, G.; Piva, A.; Suzzi, G.; Tofalo, R. Sequential Inoculation of Metschnikowia pulcherrima and Saccharomyces cerevisiae as a Biotechnological Tool to Increase the Terpenes Content of Pecorino White Wines. Fermentation 2023, 9, 785. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Kortesniemi, M.; Kalpio, M.; Yang, B. Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem. 2018, 266, 262–274. [Google Scholar] [CrossRef]
- Mylona, A.; Del Fresno, J.; Palomero, F.; Loira, I.; Bañuelos, M.; Morata, A.; Calderón, F.; Benito, S.; Suárez-Lepe, J.A. Use of Schizosaccharomyces strains for wine fermentation—Effect on the wine composition and food safety. Int. J. Food Microbiol. 2016, 232, 63–72. [Google Scholar] [CrossRef]
- Leonardos, G.; Kendall, D.; Barnard, N. Odor threshold determination of 53 odorant chemicals. J. Environ. Conserv. Eng. 1974, 3, 579–585. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]


| Sample | A420 nm | A520 nm | A620 nm | CI | CT |
|---|---|---|---|---|---|
| Mp | 0.31 ± 0.03 b | 0.11 ± 0.03 b | 0.05 ± 0.02 b | 0.47 ± 0.08 b | 3.00 ± 0.58 ab |
| Ka | 0.43 ± 0.15 b | 0.17 ± 0.12 b | 0.11 ± 0.07 ab | 0.70 ± 0.20 b | 3.71 ± 2.52 ab |
| F15 | 0.29 ± 0.03 b | 0.08 ± 0.03 b | 0.04 ± 0.02 b | 0.41 ± 0.07 b | 4.16 ± 1.16 a |
| D254 | 0.27 ± 0.04 b | 0.05 ± 0.01 b | 0.03 ± 0.01 b | 0.36 ± 0.04 b | 4.85 ± 0.81 a |
| Mp-D254 | 0.39 ± 0.12 b | 0.12 ± 0.35 b | 0.14 ± 0.12 ab | 0.61 ± 0.15 b | 3.24 ± 0.21 ab |
| Mp-F15 | 0.68 ± 0.11 a | 0.45 ± 0.04 a | 0.31 ± 0.33 a | 1.44 ± 0.25 a | 1.54 ± 0.36 b |
| Ka-D254 | 0.51 ± 0.25 ab | 0.22 ± 0.18 b | 0.14 ± 0.12 ab | 0.88 ± 0.56 b | 2.90 ± 1.23 ab |
| Ka-F15 | 0.42 ± 0.18 b | 0.15 ± 0.14 b | 0.10 ± 0.09 ab | 0.67 ± 0.38 b | 3.49 ± 1.46 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Gao, Y.; Yang, M.; Chen, J.; Zhu, C.; Tang, J.; Chen, L.; Cai, Z. Effects of Non-Saccharomyces Yeasts and Their Pairwise Combinations in Co-Fermentation with Saccharomyces cerevisiae on the Quality of Chunjian Citrus Wine. Molecules 2024, 29, 1028. https://doi.org/10.3390/molecules29051028
Fu Y, Gao Y, Yang M, Chen J, Zhu C, Tang J, Chen L, Cai Z. Effects of Non-Saccharomyces Yeasts and Their Pairwise Combinations in Co-Fermentation with Saccharomyces cerevisiae on the Quality of Chunjian Citrus Wine. Molecules. 2024; 29(5):1028. https://doi.org/10.3390/molecules29051028
Chicago/Turabian StyleFu, Yu, Yueyue Gao, Ming Yang, Juan Chen, Chenglin Zhu, Junni Tang, Lianhong Chen, and Zijian Cai. 2024. "Effects of Non-Saccharomyces Yeasts and Their Pairwise Combinations in Co-Fermentation with Saccharomyces cerevisiae on the Quality of Chunjian Citrus Wine" Molecules 29, no. 5: 1028. https://doi.org/10.3390/molecules29051028
APA StyleFu, Y., Gao, Y., Yang, M., Chen, J., Zhu, C., Tang, J., Chen, L., & Cai, Z. (2024). Effects of Non-Saccharomyces Yeasts and Their Pairwise Combinations in Co-Fermentation with Saccharomyces cerevisiae on the Quality of Chunjian Citrus Wine. Molecules, 29(5), 1028. https://doi.org/10.3390/molecules29051028






