Abstract
Catalytic oxidation is widely recognized as a highly effective approach for eliminating highly toxic CO. The current challenge lies in designing catalysts that possess exceptional low-temperature activity and stability. In this work, we have prepared ultrafine platinum particles of ~1 nm diameter dispersed on a MgFe2O4 support and found that the addition of 3 wt.% FeOx into the 3Pt/MgFe2O4 significantly improves its activity and stability. At an ultra-low temperature of 30 °C, the CO can be totally converted to CO2 over 3FeOx-3Pt/MgFe2O4. High and stable performances of CO-catalytic oxidation can be obtained at 60 °C on 3FeOx-3Pt/MgFe2O4 over 35 min on-stream at WHSV = 30,000 mL/(g·h). Based on a series of characterizations including BET, XRD, ICP, STEM, H2-TPR, XPS, CO-DRIFT, O2-TPD and CO-TPD, it was disclosed that the relatively high activity and stability of 3FeOx-3Pt/MgFe2O4 is due to the fact that the addition of FeOx could facilitate the antioxidant capacity of Pt and oxygen mobility and increase the proportion of adsorbed oxygen species and the amounts of adsorbed CO. These results are helpful in designing Pt-based catalysts exhibiting higher activity and stability at low temperatures for the catalytic oxidation of CO.
1. Introduction
The incomplete combustion of carbon-containing compounds and some important industrial processes such as ironmaking and steelmaking, as well as vehicle exhaust, produce carbon monoxide (CO) widely [1,2]. CO is a highly toxic gas for humans, and exposure to ppm levels of CO in an enclosed environment can cause serious poisoning. In industry, the trace amounts of CO can poison the Fe-based ammonia-synthesis catalysts [3], Pt-based fuel cell [4] and hydrogenation [5] catalysts, and so on. Catalytic oxidation is recognized as a highly effective method for removing low concentrations of CO. The major challenge of CO catalytic oxidation is to design catalysts with outstanding performance at low temperatures.
The catalysts employed in CO oxidation mainly consisted of transition-metal oxides (such as CuO [6], MnO2 [7] and Co3O4 [8]) and noble metal catalysts (like supported Au [9], Pt [10], Pd [11], and Rh [12]). The attractive activity and thermal stability of noble metal-based catalysts make them highly appealing for CO oxidation compared to transition-metal oxide catalysts. Among numerous reported noble metal-based catalysts, Pt-based catalysts have gained great interest in various catalytic oxidation reactions, including formaldehyde oxidation [13], CO oxidation [14], propane oxidation [15], and so on. It is well known that ultrafine platinum particles (<2 nm) can efficiently enhance the catalytic oxidation activity [16,17], probably owing to the increase in their surface area and the presence of more edge and corner atoms. In addition, decreasing the size of platinum particles can significantly improve the Pt atom utilization efficiency, and therefore can save catalyst costs. The typical methods of preparing ultrafine metallic platinum particles are colloidal synthesis. However, the usage of organic capping agents during the colloidal synthesis process often blocks the catalytic active sites, thereby causing a decrease in catalytic activity. Therefore, using the capping agents-free synthesis method to prepare ultrafine metallic platinum particles is more advantageous. It was reported that FeOx possesses an excellent ability to disperse Pt owing to the strong interaction of Pt–FeOx species [18]. Considering the fact that the single metal oxide has a lower surface area and poorer thermal stability than spinel materials, the usages of iron-based spinel oxides such as MgFe2O4 to disperse Pt are very promising. Additionally, it has been reported that the incorporation of FeOx as an additive can boost the activities of supported Pt catalysts in the oxidation of CO [19,20,21]. Thus, we expected that FeOx modification further improves the activities of MgFe2O4-supported Pt catalysts.
In this paper, we developed FeOx-modified MgFe2O4-supported Pt catalysts with ultra-small-sized Pt particles of ~1 nm. It was found that FeOx modification significantly promotes the activity and stability of Pt/MgFe2O4 in the oxidation of CO reactions, and the CO conversion can reach 100.0% at the low temperature of 30 °C. The as-prepared catalyst structure was comprehensively characterized by using BET, ICP, XRD, STEM, H2-TPR, XPS, CO-DRIFT, O2-TPD, and CO-TPD. The results reveal that the interaction between FeOx and Pt exists, and the transfer of electrons from Pt to FeOx was determined. The incorporation of 3 wt.% FeOx into the 3Pt/MgFe2O4 improved the antioxidant capacity of Pt, increased the ratio of adsorbed oxygen species and the adsorbed amount of CO and promoted oxygen mobility, which are regarded as the main reasons responsible for the higher performance for CO oxidation on 3FeOx-3Pt/MgFe2O4.
2. Results
2.1. Catalyst Performance
Figure 1a displays the CO oxidation activities of 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 at 30–70 °C at WHSV = 30,000 mL/(g·h). The conversion of CO over 3Pt/MgFe2O4 displayed an increase from 74.0% to 100.0% as the reaction temperature was elevated from 30 to 52 °C. However, the 100.0% conversion of CO was achieved within the range of 30–70 °C over 3FeOx-3Pt/MgFe2O4. Figure 1b displays the catalytic stability of the 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 catalysts in the CO oxidation reaction at 60 °C. It is obvious that the 3FeOx-3Pt/MgFe2O4 catalyst displays a stable CO conversion of 100.0% during the total test time of 35 min, while the CO conversion over 3Pt/MgFe2O4 decreases from 100.0% to 84.2% in the last 29 min of the reaction. Based on the results provided above, it can be inferred that the incorporation of 3 wt.% FeOx into 3Pt/MgFe2O4 is beneficial in enhancing the CO-oxidation performance.
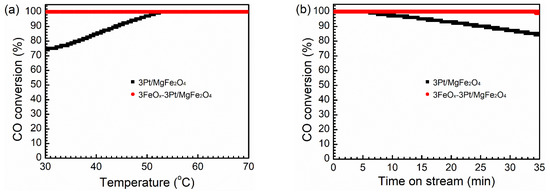
Figure 1.
(a) CO catalytic-oxidation activities and (b) catalytic stability at 60 °C of 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 at WHSV = 30,000 mL/(g·h).
2.2. Catalyst Characterization
The isotherms of nitrogen physical adsorption as well as the pore-size distribution curves for the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 samples are presented in Figure 2. Both 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 samples demonstrated type IV isotherms accompanied by hysteresis loops, which are indicative of their mesoporous structure (Figure 2a). The pore-size distributions plot also showed a mesoporous structure for both samples (Figure 2b). The corresponding properties of the 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 are listed in Table 1. Based on the nitrogen-adsorption results, the specific surface areas for the 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 samples are 93.8 and 77.9 m2/g, respectively. Additionally, the average pore sizes were determined to be 11.3 and 11.5 nm, and the pore volumes were measured to be 0.29 and 0.25 cm3/g for the 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 samples, respectively. The Pt loadings, as determined by ICP-OES, are 3.1 and 2.9 wt.% for the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4, respectively.
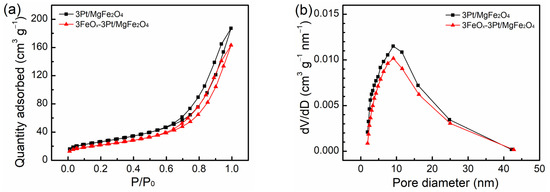
Figure 2.
(a) Isotherms of nitrogen physical adsorption and (b) pore-size distribution plots for the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4.

Table 1.
Properties of the 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4.
The XRD patterns for the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 are presented in Figure 3. The characteristic diffraction peaks for MgFe2O4 (JCPDS 73-1720) can be observed in both 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4. In addition to MgFe2O4, no diffraction peak of Pt was observed on these two catalysts, suggesting that the sizes of Pt nanoparticles are smaller than that of the XRD detection limit (<3 nm). To further confirm this result, the STEM technique was employed. As shown in Figure 4a,d, both the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 have high Pt-nanoparticle density with narrow size distributions. According to statistical data of over 200 Pt-nanoparticle sizes, the average sizes of Pt nanoparticles were determined to be 1.2 and 1.3 nm for 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4, respectively. Pt nanoparticles in 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 can be seen more clearly in Figure 4c,f.
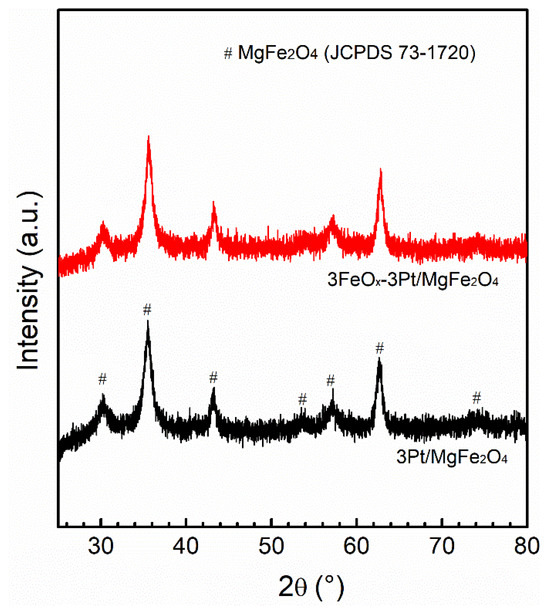
Figure 3.
XRD patterns of the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4.
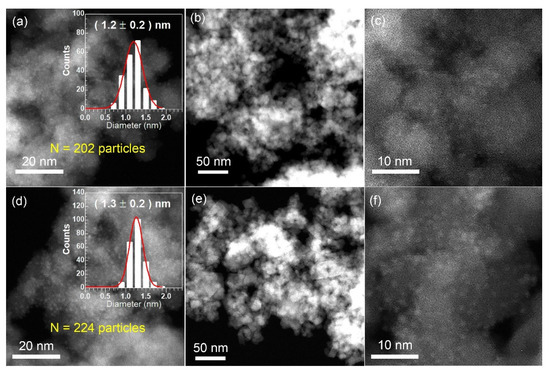
Figure 4.
STEM images and the size histogram of Pt-particle distribution for the fresh (a–c) 3Pt/MgFe2O4 and (d–f) 3FeOx-3Pt/MgFe2O4.
We conducted H2-TPR experiments to explore in detail the reducibility of 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 as well as MgFe2O4 support, and the obtained outcomes are illustrated in Figure 5. For MgFe2O4 support, the reduction peak above 250 °C belongs to the reduction of iron species because of the nonreducible of Mg2+. For 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4, a distinct reduction peak was found at 90–150 °C in addition to a reduction peak above 250 °C, which belongs to the PtOx reduction [22,23]. As shown in insert figure, the PtOx reduction peak for 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 is centered at 107 and 112 °C, respectively. The introduction of FeOx to 3Pt/MgFe2O4 raises the reduction temperature of PtOx species obviously. It suggests that the interaction that exists between FeOx and PtOx and FeOx is expected to modify the electron properties of metallic Pt nanoparticles.
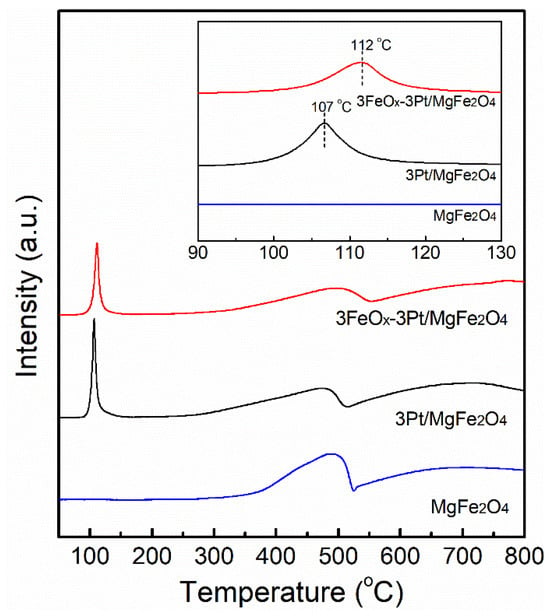
Figure 5.
H2-TPR diagrams of the calcined 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 as well as MgFe2O4 support.
The valence states of platinum and the type of surface oxygen species in 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 were explored using XPS. The XPS spectra for the Pt 4f orbitals of the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 are presented in Figure 6a. These spectra can be fitted well with six peaks. The Pt 4f spectra of the fresh 3Pt/MgFe2O4 exhibit two fitted peaks observed at binding energy values of 71.4 and 74.7 eV, which can be ascribed to Pt 4f7/2 and Pt 4f5/2 orbitals for Pt0, respectively. Two fitted peaks observed at binding energy values of 72.3 and 75.5 eV can be associated with Pt 4f7/2 and Pt 4f5/2 orbitals for Pt2+, respectively. The presence of two additional peaks observed at 74.1 and 77.7 eV can be assigned to Pt 4f7/2 and Pt 4f5/2 orbitals of the Pt4+ state, respectively [24,25,26,27]. Similar Pt species were observed in the fresh 3FeOx-3Pt/MgFe2O4 while their binding energy shifted to higher values by ~0.2 eV. This suggests that there is an interaction between Pt and FeOx where the electrons are transferred from Pt to FeOx. The existence of Pt2+ and Pt4+ in the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 samples could be induced by the oxidation process with air at room temperature. According to the calculation of the proportion of the Pt0 peak areas to the sum of the Pt0, Pt2+ and Pt4+ peak areas, the relative amounts of Pt0 in the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 are 43.1% and 49.0%, respectively (Table 1). These findings indicate that FeOx reduced the degree of reoxidation for the reduced-state Pt, which indirectly reflects the Pt–FeOx interaction in the 3FeOx-3Pt/MgFe2O4 sample. Figure 6b presents the XPS spectra for the O 1s orbitals of the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 samples. The spectra could be fitted well, with three peaks which are located at binding energy values of 529.6, 530.7 and 531.8 eV, respectively. The peak centered at the low binding energy of 529.6 eV corresponds to the lattice oxygen (Olatt), where the peaks centered at the high binding energies of 530.7 and 531.8 eV correspond to adsorbed oxygen (Oads) species [28,29,30]. According to the proportion of the Oads peak areas to the sum of the Olatt and Oads peak areas, the respective proportions of Oads in the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 are 31.7% and 37.4%, respectively. This indicates that the 3FeOx-3Pt/MgFe2O4 possesses a higher percentage of adsorbed oxygen species, indicating the higher capacity to adsorb and activate molecular oxygen, and therefore leading to enhanced catalyst performance [21,31,32]. Figure 6c,d present the Pt 4f and O 1s XPS results for the spent 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 catalysts. In the spent 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4, the binding energies of Pt 4f and O 1s remained unchanged. The relative amounts of Pt0 for the spent 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 are 24.5% and 31.7%, respectively. The higher percentage of Pt0 in the spent 3FeOx-3Pt/MgFe2O4 suggests that FeOx can improve the antioxidant performance of Pt/MgFe2O4 in the reaction atmosphere, which always leads to a high CO oxidation performance [25]. The relative amounts of Oads for the spent 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 are 32.5% and 40.3%, respectively. Compared to the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4, the spent 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 exhibits a higher content of adsorbed oxygen species. This indicates that the gaseous molecular oxygen could adsorb on the surface of the catalysts [33].
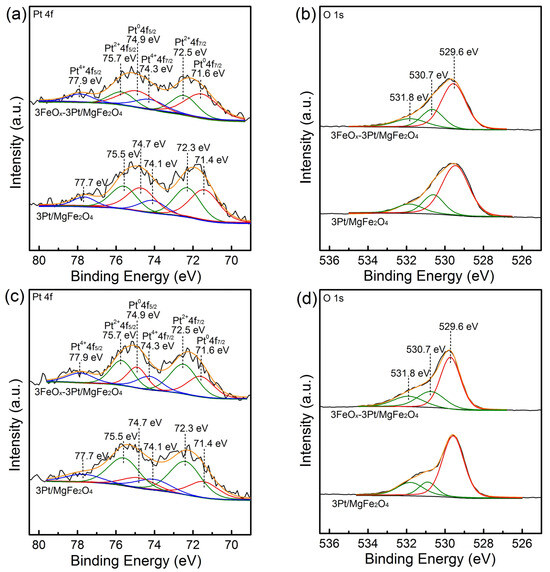
Figure 6.
(a,c) Pt 4f and (b,d) O 1s XPS spectra for the (a,b) fresh and (c,d) spent 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4.
Figure 7 shows CO-DRIFT spectra of fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4. Both 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 show bands at 2173, 2060 and 1772 cm−1, respectively. The band observed at 2173 cm−1 usually belongs to gaseous CO. The observed bands at 2060 and 1772 cm−1 are assigned to the linear and bridged CO adsorption on the surface of Pt0 [33,34,35,36,37], respectively. The findings suggest that the Pt species are primarily present in the form of a Pt0 metallic state in the pre-reduced catalysts. It is obvious that the CO bands’ intensities at 2060 and 1772 cm−1 on the 3FeOx-3Pt/MgFe2O4 catalyst are much higher than those on the 3Pt/MgFe2O4, indicating that the 3FeOx-3Pt/MgFe2O4 has a greater amount of adsorbed CO. 3FeOx-3Pt/MgFe2O4 with a high CO adsorption ability at 30 °C might result in an excellent CO-oxidation activity at low temperatures.
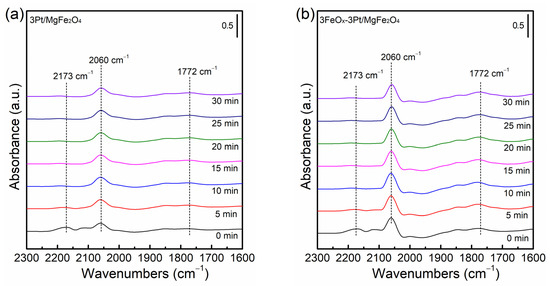
Figure 7.
CO-DRIFT spectra of fresh (a) 3Pt/MgFe2O4 and (b) 3FeOx-3Pt/MgFe2O4.
Figure 8a shows the O2-TPD profiles of fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4. The O2-TPD profiles of 3Pt/MgFe2O4 show oxygen desorption peaks at 154, 259, 555 and 776 °C, respectively. These desorption peaks shifted to lower temperatures of 136, 258, 546, and 748 °C for 3FeOx-3Pt/MgFe2O4, respectively. The lower oxygen desorption temperature in 3FeOx-3Pt/MgFe2O4 confirmed that 3FeOx-3Pt/MgFe2O4 has a higher oxygen mobility, which is advantageous to catalytic-CO oxidation. Figure 8b presents the CO-TPD profiles of fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4. Both 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4 exhibited small desorption peaks of CO and large desorption peaks of CO2. The formation of CO2 may be related to the surface reaction of the adsorbed CO species with oxygen species. It has been observed that CO2 formation in 3Pt/MgFe2O4 begins at 125 °C, while for 3FeOx-3Pt/MgFe2O4, CO2 formation occurs at 50 °C or even lower temperatures. The lower temperature at which CO2 begins to form demonstrates that the oxygen species could be easily released and could participate in CO oxidation. Therefore, CO is much easier to be oxidized over 3FeOx-3Pt/MgFe2O4.
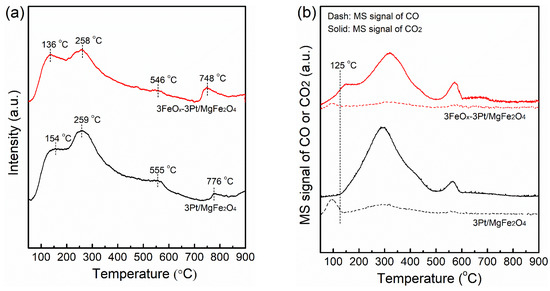
Figure 8.
(a) O2-TPD and (b) CO-TPD profiles of the fresh 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4.
3. Discussion
The introduction of FeOx (3 wt.%) into 3Pt/MgFe2O4 can greatly enhance its low-temperature catalytic activity and stability in CO oxidation. CO can be fully oxidized to CO2 at temperatures as low as 30 °C over 3FeOx-3Pt/MgFe2O4 at WHSV = 30,000 mL/(g·h) (Figure 1a). At 30 °C, the CO-turnover rate on 3FeOx-3Pt/MgFe2O4 is calculated to be 0.024 mol/(molPt-s), which is higher than the C-turnover rates reported in the literature (0.002 mol/(molPt-s)) calculated at 50 °C on Pt/TiO2 with high Pt oxidation state and a Pt size of less than 1 nm [38]. In addition, according to the literature reports, the CO-turnover rates at 30 °C on Pt/Nb2O5 modified with FeOx with a Pt size of 3.5 nm is calculated to be 0.012 mol/(molPt-s) [39], which is also lower than the CO-turnover rates on 3FeOx-3Pt/MgFe2O4. These results indicate that the FeOx-modified ultrafine Pt nanoparticles (~1 nm) prepared in our article exhibit excellent low-temperature activity for the oxidation of CO. In addition, during the 35 min duration test for stability at 60 °C, the 3FeOx-3Pt/MgFe2O4 exhibited a high and constant CO conversion of 100.0% (Figure 1b). The nitrogen physical adsorption results indicated that the FeOx modification might slightly decrease the surface area of 3Pt/MgFe2O4 (Table 1). According to the XRD and STEM results (Figure 3 and Figure 4, Table 1), the ultrafine Pt particles (~1 nm) are highly dispersed on the MgFe2O4 support for 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4, and FeOx modification has little/no effect on the size of Pt. Based on the H2-TPR- and XPS-characterization results (Figure 5 and Figure 6), there is an interaction between FeOx and Pt with an electron transfer from Pt to FeOx. Moreover, the addition of FeOx increased the ratio of the adsorbed oxygen species and decreased the oxidizing extent of the metallic Pt. The latter resulted in an increase in Pt0 percentage in an oxidizing atmosphere. As we know, the Pt catalyst is present in an oxidizing atmosphere during a CO-oxidation reaction. The reduced Pt can be oxidized in this reaction mixture. It is generally believed that metallic Pt is more active in CO-oxidation reactions than in that of oxidized Pt [40,41]. The CO-DRIFT results demonstrated that the FeOx modification increased the amount of CO adsorption on 3Pt/MgFe2O4 (Figure 7). The results of O2-TPD and CO-TPD showed that FeOx promoted the oxygen mobility of 3Pt/MgFe2O4 and CO2 formation at low temperatures (Figure 8a,b). The high oxygen mobility in 3FeOx-3Pt/MgFe2O4 makes CO more easily oxidized, resulting in an outstanding catalytic performance for CO oxidation. The above results indicated that the incorporation of FeOx into Pt/MgFe2O4 has successfully modified the electronic properties of Pt nanoparticles, while having almost no effect on the geometric structure of Pt nanoparticles. The relatively strong antioxidant capacity, the high proportion of adsorbed oxygen species, and a high amount of adsorbed CO as well as the good oxygen mobility of 3FeOx-3Pt/MgFe2O4 contributed to its high low-temperature activity and improved stability for CO oxidation.
4. Materials and Methods
4.1. Catalyst Preparation
A solid-phase method was employed to synthesize the MgFe2O4 support. In brief, 0.015 M of MgSO4 (Zesheng, Anqing, China), 0.03 M of Fe(NO3)3·9H2O (Guoyao, Shanghai, China), 0.12 M of NaOH (Guoyao, Shanghai, China) and 0.15 M of NaCl (Annaiji, Shanghai, China) were simultaneously poured into a mortar and grinded evenly for 40 min. The obtained mixture was subjected to calcination at 700 °C for 1 h with a ramp rate of 2 °C/min. Subsequently, it was washed with deionized water and dried at 120 °C for 12 h.
The Pt/MgFe2O4 with Pt loading of 3 wt.% was synthesized through the incipient wetness impregnation of MgFe2O4 support with H2PtCl6·6H2O (Damas-beta, Shanghai, China) dissolved in a certain amount of deionized water. After impregnation, the sample was subjected to drying at 60 °C under ambient air for 12 h and calcination at 500 °C in air for 5 h. After undergoing a repeated washing process with deionized water, the obtained solid was subjected to drying at 120 °C for 12 h and then reduction at 150 °C for 30 min in 5% H2/N2. Catalysts thus prepared were denoted as fresh 3Pt/MgFe2O4.
FeOx-modified Pt/MgFe2O4 with Fe loading of 3 wt.% (excluding the Fe content in MgFe2O4 support) was prepared by further incipient wetness impregnation of calcined 3Pt/MgFe2O4 with the appropriate amount of Fe(NO3)3·9H2O (Guoyao, Shanghai, China) dissolved in a certain amount of deionized water. After impregnation, the sample was treated in the same way as the 3Pt/MgFe2O4, obtaining the fresh 3FeOx-3Pt/MgFe2O4 catalyst. The catalysts after the stability test of 60 °C were denoted as the spent 3Pt/MgFe2O4 and 3FeOx-3Pt/MgFe2O4.
4.2. Catalyst Characterization
We conducted the surface-area testing of the as-prepared samples on a Micromeritics ASAP 2460 model adsorption instrument (Norcross, GA, USA). The Bruauer–Emmett–Teller (BET) method was employed with nitrogen as an adsorbate.
The content of Pt in the as-prepared samples was measured using inductively coupled plasma optical emission spectrometry (ICP-OES) with an Agilent 5110 instrument (Santa Clara, CA, USA).
We obtained X-ray diffraction (XRD) patterns of the as-prepared samples using a Rigaku SmartLab SE apparatus (Tokyo, Japan). The Cu Kα radiation with a wavelength of 0.15406 nm was utilized as the excitation source.
A JEOL JEM-2100F instrument (Tokyo, Japan) was employed to acquire the scanning transmission electron microscopy (STEM) images.
A Micromeritics AutoChem II 2920 instrument (Norcross, GA, USA) was utilized for performing the temperature-programmed reduction of H2 (H2-TPR). The sample (~0.1 g) was introduced into a quartz tube and maintained at 300 °C for 30 min under an Ar atmosphere. Subsequently, the sample was exposed to a 10% H2/Ar and the temperature was gradually elevated to 800 °C using a ramp rate of 10 °C/min.
X-ray photoelectron spectroscopy (XPS) was determined by employing an Al Kα X-ray on a Thermo Fisher ESCALAB 250 Xi apparatus (Waltham, MA, USA). A reference of the C 1s peak at 284.6 eV was employed to correct all the obtained binding energies.
A Bruker Tensor 27 spectrometer (Ettlingen, Germany) was employed to record the in situ CO-diffuse reflectance infrared Fourier transform (CO-DRIFT) spectroscopy. The sample was subjected to reduction in a H2 atmosphere at 150 °C for a duration of 30 min before CO adsorption. After cooling down to 30 °C under Ar, 20% CO/Ar of 30 mL/min was passed through the sample until the saturated adsorption of CO was reached. The 20% CO/Ar was subsequently switched to Ar and the data was collected.
The Micromeritics AutoChem II 2920 apparatus (Norcross, GA, USA) was utilized to conduct temperature-programmed desorption of O2 (O2-TPD). The sample (~0.1 g) was firstly subjected to reduction in a 10% H2/Ar atmosphere at 150 °C for a duration of 30 min. After cooling down to 60 °C under Ar, 3% O2/He was introduced for a duration of 1 h to achieve the saturated adsorption of O2. Finally, the sample was subjected to a heating process up to 900 °C in the presence of an Ar atmosphere, and the data was recorded.
The Microtrac Belcat II apparatus (Osaka, Japan) was utilized to conduct temperature-programmed desorption (TPD) of CO. A 0.1 g sample was subjected to prereduction in a 10% H2/Ar atmosphere at 150 °C for a duration of 30 min. After the temperature decreased to 50 °C in the presence of an Ar atmosphere, 10% CO/He was introduced for 1 h for the saturated adsorption of CO. Finally, the sample was subjected to heating up to 900 °C in Ar, and the mass signals corresponding to CO with m/z of 28 and CO2 with m/z of 44 were recorded.
4.3. Catalytic Performance Tests
The oxidation of CO was conducted at atmospheric pressure in a U-shaped quartz fixed-bed reactor. Prior to the catalytic testing, all samples were exposed to 5% H2/N2 of 150 °C for a duration of 30 min for reduction. For each activity and stability test, a mixture consisting of 50 mg of catalysts and 1g of quartz sand was utilized. The reactant mixture consisted of 1% CO, 1% O2 and 98% N2. The total flow rate was 25 mL/min, which corresponded to a weighted hourly space velocity (WHSV) of 30,000 mL/(g·h). An FGA 10 model infrared gas analyzer was employed to monitor online the concentrations of reactants and products. We calculated the CO conversion as (CCO, inlet − CCO, outlet)/CCO, inlet × 100%, in which CCO, inlet and CCO, outlet represent the inlet and outlet CO concentrations of the reactor, respectively.
5. Conclusions
To summarize, we successfully synthesized a 3FeOx-3Pt/MgFe2O4 catalyst with ultrafine Pt particles that had an average size of ~1 nm. The incorporation of FeOx into the Pt/MgFe2O4 catalyst significantly boosted the low-temperature catalytic activity and stability of the Pt catalyst for CO oxidation. CO conversion could reach 100.0% on 3FeOx-3Pt/MgFe2O4 at temperatures as low as 30 °C, and the elevated stability was obtained with a CO conversion of 100.0% at 60 °C for a 35 min run under a WHSV of 30,000 mL/(g·h). It was found that modifying 3Pt/MgFe2O4 with FeOx tunes the electronic properties while hardly changing the size of the Pt particles. Compared with 3Pt/MgFe2O4, the remarkably increased catalytic performance of 3FeOx-3Pt/MgFe2O4 for CO oxidation might be attributed to its relatively strong antioxidant capacity, high ratio of adsorbed oxygen species, a greater amount of adsorbed CO, and an increased oxygen mobility. Overall, a deeper understanding of the crucial structure of catalysts needed for oxidizing CO in this work will contribute significantly to the advancement of a more high-performance Pt-based catalyst for the oxidation of CO.
Author Contributions
Conceptualization, C.W., F.W. and J.S.; methodology, C.W. and F.W.; validation, C.W.; investigation, C.W. and F.W.; data curation, C.W.; writing—original draft preparation, C.W. and F.W.; writing—review and editing, F.W. and J.S.; supervision, F.W. and J.S.; project administration, F.W. and J.S.; funding acquisition, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Research Project of Colleges and Universities in Anhui Province (2023AH051207), the National Natural Science Foundation of China (22308007), the Anhui Provincial Natural Science Foundation (2108085QB49) and the Research Foundation of the Institute of Environment-friendly Materials and Occupational Health of Anhui University of Science and Technology (Wuhu) (ALW2022YF05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, K.; Tian, H.; Hua, S.; Zhu, C.; Gao, J.; Xue, Y.; Hao, J.; Wang, Y.; Zhou, J. A comprehensive emission inventory of multiple air pollutants from iron and steel industry in China: Temporal trends and spatial variation characteristics. Sci. Total Environ. 2016, 559, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zou, Y.; Li, X.; Xu, T.; Cen, W.; Li, B.; Zhu, T. Ultralow doping of Mn species into Pt catalyst enhances the CO oxidation performance in the presence of H2O and SO2. ACS Catal. 2023, 13, 14580–14597. [Google Scholar] [CrossRef]
- Waugh, K.C.; Butler, D.; Hayden, B.E.J.C.L. The mechanism of the poisoning of ammonia synthesis catalysts by oxygenates O2, CO and H2O: An in situ method for active surface determination. Catal. Lett. 1994, 24, 197–210. [Google Scholar] [CrossRef]
- Liu, K.; Wang, A.; Zhang, T. Recent advances in preferential oxidation of CO reaction over platinum group metal catalysts. ACS Catal. 2012, 2, 1165–1178. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Gao, R.; Liang, X.; Yu, Q.; Deng, Y.; Liu, J.; Peng, M.; Jiang, Z.; Li, S.; et al. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation. Nat. Nanotechnol. 2019, 14, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chi, X.; Li, L.; Yang, J.; Liu, S.; Lu, X.; Xiao, W.; Wang, L.; Luo, Z.; Yang, W.; et al. Elucidating the nature of the Cu (I) active site in CuO/TiO2 for excellent low-temperature CO oxidation. ACS Appl. Mater. Interfaces 2020, 12, 7091–7101. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zong, R.; Zhu, Y. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J. Phys. Chem. C 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, L.; Bajdich, M.; García-Melchor, M.; Li, H.; He, J.; Wilcox, J.; Wu, W.; Vojvodic, A.; Zheng, X. Enhancing catalytic CO oxidation over Co3O4 nanowires by substituting Co2+ with Cu2+. ACS Catal. 2015, 5, 4485–4491. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.M.; Henkelman, G. CO oxidation mechanism on CeO2-supported Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Allian, A.D.; Takanabe, K.; Fujdala, K.L.; Hao, X.; Truex, T.J.; Cai, J.; Buda, C.; Neurock, M.; Iglesia, E. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 2011, 133, 4498–4517. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Henkelman, G. CO oxidation on the Pd (111) surface. ACS Catal. 2014, 4, 3435–3443. [Google Scholar] [CrossRef]
- Guan, H.; Lin, J.; Qiao, B.; Yang, X.; Li, L.; Miao, S.; Liu, J.; Wang, A.; Wang, X.; Zhang, T. Catalytically active Rh sub-nanoclusters on TiO2 for CO oxidation at cryogenic temperatures. Angew. Chem. Int. Ed. 2016, 55, 2820–2824. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gueddida, S.; Badawi, M.; Lebègue, S.; Giraudon, J.-M.; Dhainaut, J.; Royer, S.; Lamonier, J.-F. Unravelling the critical role of silanol in Pt/SiO2 for room temperature HCHO oxidation: An experimental and DFT study. Appl. Catal. B Environ. 2023, 331, 122672. [Google Scholar] [CrossRef]
- Xu, L.; Pan, Y.; Li, H.; Xu, R.; Sun, Z. Highly active and water-resistant lanthanum-doped platinum-cobalt oxide catalysts for CO oxidation. Appl. Catal. B Environ. 2023, 331, 122678. [Google Scholar] [CrossRef]
- Wang, X.-F.; Xu, L.-Y.; Wen, C.-H.; Li, D.-D.; Li, B.; Lu, J.-Q.; Yang, Q.-H.; Luo, M.-F.; Chen, J. WO3 boosted water tolerance of Pt nanoparticle on SO42−-ZrO2 for propane oxidation. Appl. Catal. B Environ. 2023, 338, 123000. [Google Scholar] [CrossRef]
- Lu, J.; Stair, P.C. Low-temperature ABC-type atomic layer deposition: Synthesis of highly uniform ultrafine supported metal nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 2547–2551. [Google Scholar] [CrossRef] [PubMed]
- Vajda, S.; Pellin, M.; Greeley, J.; Marshall, C.; Curtiss, L.; Ballentine, G.; Elam, J.; Catillon-Mucherie, S.; Redfern, P.; Mehmood, F.; et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 2009, 8, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Siani, A.; Alexeev, O.S.; Captain, B.; Lafaye, G.; Marécot, P.; Adams, R.D.; Amiridis, M.D. Synthesis of cluster-derived PtFe/SiO2 catalysts for the oxidation of CO. J. Catal. 2008, 255, 162–179. [Google Scholar] [CrossRef]
- Tomita, A.; Shimizu, K.-i.; Kato, K.; Tai, Y. Pt/Fe-containing alumina catalysts prepared and treated with water under moderate conditions exhibit low-temperature CO oxidation activity. Catal. Commun. 2012, 17, 194–199. [Google Scholar] [CrossRef]
- Song, S.; Wu, Y.; Ge, S.; Wang, L.; Wang, Y.; Guo, Y.; Zhan, W.; Guo, Y. A facile way to improve Pt atom efficiency for CO oxidation at low temperature: Modification by transition metal oxides. ACS Catal. 2019, 9, 6177–6187. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, P.; Cao, H.; Bals, S.; Heeres, H.J.; Pescarmona, P.P. Pt/ZrO2 prepared by atomic trapping: An efficient catalyst for the conversion of glycerol to lactic acid with concomitant transfer hydrogenation of cyclohexene. ACS Catal. 2019, 9, 9953–9963. [Google Scholar] [CrossRef]
- Meher, S.K.; Cargnello, M.; Troiani, H.; Montini, T.; Rao, G.R.; Fornasiero, P. Alcohol induced ultra-fine dispersion of Pt on tuned morphologies of CeO2 for CO oxidation. Appl. Catal. B Environ. 2013, 130, 121–131. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, Y.; Yang, X. Promoting effect of Pt on the activity and stability of Pd/MgFe2O4 for catalytic combustion of methane. J. Energy Inst. 2023, 110, 101341. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Li, X.; Liao, W.-M.; Jia, A.-P.; Wang, Y.-J.; Luo, M.-F.; Lu, J.-Q. Highly active Pt/BN catalysts for propane combustion: The roles of support and reactant-induced evolution of active sites. ACS Catal. 2019, 9, 1472–1481. [Google Scholar] [CrossRef]
- Li, J.; Guan, Q.; Wu, H.; Liu, W.; Lin, Y.; Sun, Z.; Ye, X.; Zheng, X.; Pan, H.; Zhu, J.; et al. Highly active and stable metal single-atom catalysts achieved by strong electronic metal–support interactions. J. Am. Chem. Soc. 2019, 141, 14515–14519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.-P.; Chen, J.; Yu, H.-B.; Cen, B.-H.; Wang, W.-Y.; Luo, M.-F.; Lu, J.-Q. Insights into propane combustion over MoO3 promoted Pt/ZrO2 catalysts: The generation of Pt-MoO3 interface and its promotional role on catalytic activity. J. Catal. 2020, 391, 80–90. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Deng, J.; Yu, X.; Han, Z.; Zhang, K.; Dai, H. Alloying of gold with palladium: An effective strategy to improve catalytic stability and chlorine-tolerance of the 3DOM CeO2-supported catalysts in trichloroethylene combustion. Appl. Catal. B Environ. 2019, 257, 117879. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Chen, X.; Fan, S.; Zheng, Y. Engineering multicomponent metal-oxide units for efficient methane combustion over palladium-based catalysts. Catal. Sci. Technol. 2021, 11, 152–161. [Google Scholar] [CrossRef]
- Xie, S.; Deng, J.; Zang, S.; Yang, H.; Guo, G.; Arandiyan, H.; Dai, H. Au–Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J. Catal. 2015, 322, 38–48. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt–Pd bimetallic nanoparticles anchored on uniform mesoporous MnO2 sphere as an advanced nanocatalyst for highly efficient toluene oxidation. Green Energy Environ. 2022, 7, 1349–1360. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Tahini, H.A.; Scott, J.; Tan, X.; Dai, H.; Gale, J.D.; Rohl, A.L.; Smith, S.C.; Amal, R. The controlled disassembly of mesostructured perovskites as an avenue to fabricating high performance nanohybrid catalysts. Nat. Commun. 2017, 8, 15553. [Google Scholar] [CrossRef]
- Zhu, M.-T.; Zhang, K.-F.; Du, W.-P.; Jia, A.-P.; Luo, M.-F.; Lu, J.-Q. Highly active and water tolerant Pt/MFe2O4 (M = Co and Ni) catalysts for low temperature CO oxidation. Appl. Catal. A Gen. 2021, 619, 118142. [Google Scholar] [CrossRef]
- Peng, R.; Sun, X.; Li, S.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene. Chem. Eng. J. 2016, 306, 1234–1246. [Google Scholar] [CrossRef]
- Kim, M.H.; Ebner, J.R.; Friedman, R.M.; Vannice, M.A. Dissociative N2O adsorption on supported Pt. J. Catal. 2001, 204, 348–357. [Google Scholar] [CrossRef]
- Meunier, F.C.; Cardenas, L.; Kaper, H.; Šmíd, B.; Vorokhta, M.; Grosjean, R.; Aubert, D.; Dembélé, K.; Lunkenbein, T. Synergy between metallic and oxidized Pt sites unravelled during room temperature CO oxidation on Pt/Ceria. Angew. Chem. Int. Ed. 2021, 60, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, W.; Wang, X.; Liu, C.; Lu, J.; Luo, M.; Chen, J. The effects of MoO3 impregnation order on the catalytic activity for propane combustion over Pt/ZrO2 catalysts: The crucial roles of Pt–MoO3 interfacial sites density. New J. Chem. 2021, 45, 14695–14702. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Zhang, G.; Qiu, W.; He, H.; Chen, G. High active platinum clusters on titanium dioxide supports toward carbon monoxide oxidation. Appl. Catal. B Environ. 2020, 266, 118629. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.-F.; Wang, W.-Y.; Liu, C.-F.; He, L.-C.; Luo, M.-F.; Chen, J. Identifying the surface active sites of FeOx-modified Pt/Nb2O5 catalysts in CO and propane oxidation. Appl. Catal. A Gen. 2023, 649, 118960. [Google Scholar] [CrossRef]
- Jeong, H.; Shin, D.; Kim, B.-S.; Bae, J.; Shin, S.; Choe, C.; Han, J.W.; Lee, H. Controlling the oxidation state of Pt single atoms for maximizing catalytic activity. Angew. Chem. Int. Ed. 2020, 59, 20691–20696. [Google Scholar] [CrossRef]
- Kim, G.J.; Kwon, D.W.; Hong, S.C. Effect of Pt particle size and valence state on the performance of Pt/TiO2 catalysts for CO oxidation at room temperature. J. Phys. Chem. C 2016, 120, 17996–18004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).