Therapeutic Potential of Pectin and Its Derivatives in Chronic Diseases
Abstract
1. Introduction
2. Materials and Method
3. Characteristics of Pectin Polymer
3.1. Chemistry of Pectin
3.1.1. Chemical Structure of Pectin
3.1.2. Chemical Structures of Pectin Derivatives
Terpyridine-Metal-Pectin Derivatives
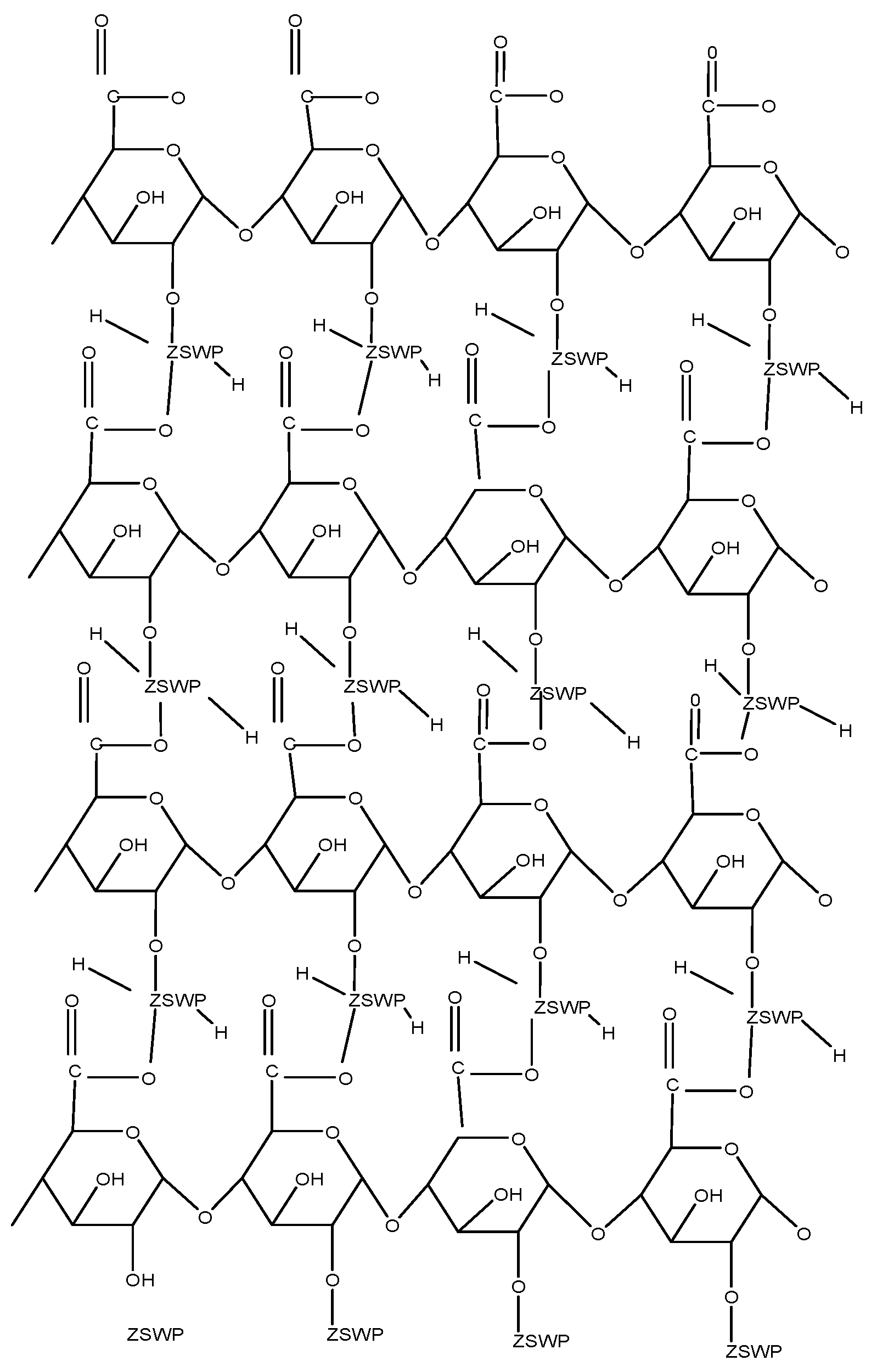
Pectin Zirconium (IV) Selenotungstophosphate
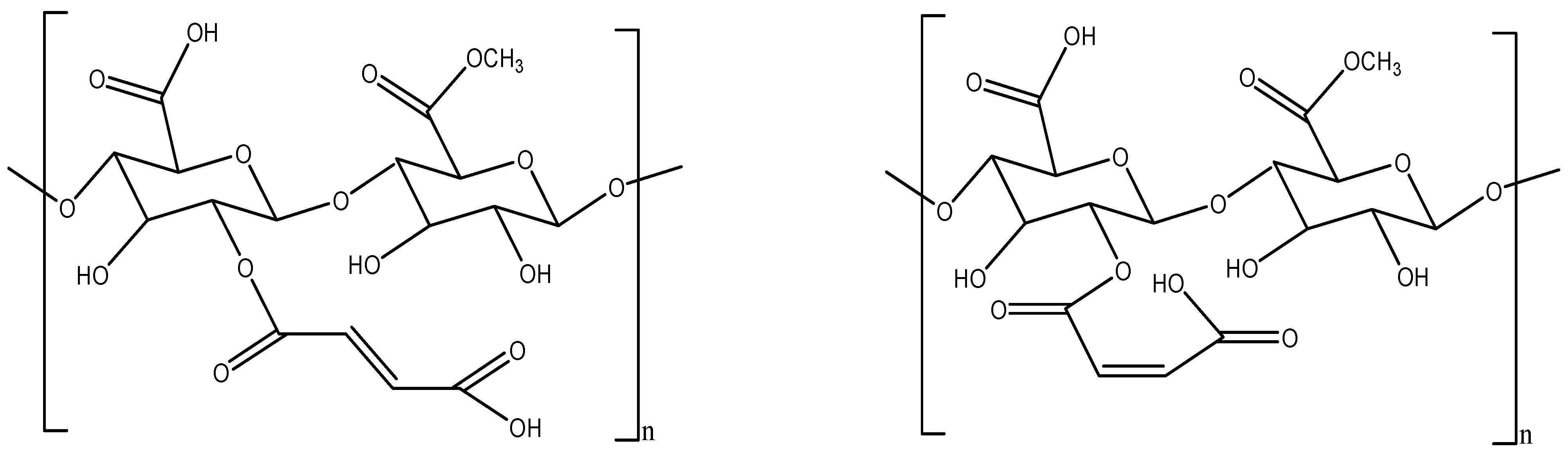
Pectin-Maleated Derivatives
Quaternary Ammonium Derivative of Pectin
4. Sources of Pectin
5. Biological Potency of Pectin
6. Extraction of Pectin
7. Biological Activities
7.1. Anticancer Activity (Colon and Breast Cancer)
7.2. Anti-Inflammatory and Immune Modulatory Activity
7.3. Antidiabetic Activity
7.4. Anti-Hypertensive Activity
7.5. Neuroprotective Activity
7.6. Anti-Hypercholesterolemic Activity
7.7. Anti-Alzheimer’s Diseases Activity
7.8. Anti-Liver Diseases Activity
7.9. Anti-Kidney Disease Activity
7.10. Clinical Trials of Pectin and Its Derivatives
7.11. Limitations of Pectin as a Therapeutic Agent
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boopathi, S.; Priya, P.S.; Haridevamuthu, B.; Nayak, S.P.R.R.; Chandrasekar, M.; Arockiaraj, J.; Jia, A.-Q. Expanding germ-organ theory: Understanding non-communicable diseases through enterobacterial translocation. Pharmacol. Res. 2023, 194, 106856. [Google Scholar] [CrossRef]
- Rao, K.D.; Mehta, A.; Kautsar, H.; Kak, M.; Karem, G.; Misra, M.; Joshi, H.; Herbst, C.H.; Perry, H.B. Improving quality of non-communicable disease services at primary care facilities in middle-income countries: A scoping review. Soc. Sci. Med. 2023, 320, 115679. [Google Scholar] [CrossRef]
- Brenyah, J.K.; Nonvignon, J.; Singh, A.; Owusu-Dabo, E. Tobacco consumption and non-communicable diseases in Ghana; Identifying accentuating factors and further evidence from 2014 Ghana demographic and health survey. Sci. Afr. 2023, 20, e01665. [Google Scholar] [CrossRef]
- Konkor, I.; Kuuire, V.; Bisung, E. Understanding perceptions of neighborhood health and non-communicable disease risk in urban contexts in Ghana. Soc. Sci. Med. 2023, 317, 115574. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Hoskins, C. Drug development: Lessons from nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, M. Pectin polymers for colon-targeted antitumor drug delivery. Int. J. Biol. Macromol. 2020, 158, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Biswas, M.M.H.; Esham, M.K.H.; Roy, P.; Khan, M.R.; Hasan, S.M.K. Jackfruit (Artocarpus heterophyllus) by-products a novel source of pectin: Studies on physicochemical characterization and its application in soup formulation as a thickener. Food Chem. Adv. 2023, 2, 100273. [Google Scholar] [CrossRef]
- Cipolla, L.; Araújo, A.C.; Bini, D.; Gabrielli, L.; Russo, L.; Shaikh, N. Discovery and design of carbohydrate-based therapeutics. Expert Opin. Drug Discov. 2010, 5, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Leivas, C.L.; Nascimento, L.F.; Barros, W.M.; Santos AR, S.; Iacomini, M.; Cordeiro, L.M.C. Substituted galacturonan from starfruit: Chemical structure and antinociceptive and anti-inflammatory effects. Int. J. Biol. Macromol. 2016, 84, 295–300. [Google Scholar] [CrossRef]
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Kumar, D.N.S. Synthesis and characterization of citrus-derived pectin nanoparticles based on their degree of esterification. J. Mater. Res. 2020, 35, 1514–1522. [Google Scholar] [CrossRef]
- Yavuz-Düzgün, M.; Zeeb, B.; Dreher, J.; Özçelik, B.; Weiss, J. The Impact of Esterification Degree and Source of Pectins on Complex Coacervation as a Tool to Mask the Bitterness of Potato Protein Isolates. Food Biophys. 2020, 15, 376–385. [Google Scholar] [CrossRef]
- Sundar Raj, A.A.; Rubila, S.; Jayabalan, R.; Ranganathan, T.V. A Review on Pectin: Chemistry due to General Properties of Pectin and its Pharmaceutical Uses. 2012, Volume 1, p. 550. Available online: https://d1wqtxts1xzle7.cloudfront.net/54511404/OPEN_ACCESS_SCIENTIFIC_REPORTS-1-libre.pdf?1506141766=&response-content-disposition=inline%3B+filename%3DA_Review_on_Pectin_Chemistry_due_to_Gene.pdf&Expires=1707462355&Signature=W7PHgckhdMtsK8E6hxFURt60QjPzihrVLPPcPCxRNNvuAa6a-gW533Rrqm~Utth8A5RQR0MTuU~lNb8wy1GajZuqWu4jhKGEzEIZE8kmakmR~cXdoLncifIrdguluqC0m6GcqB8BBXWw9PRhEZaUD-DaeIgJZ4i70v1QFScI0lAHBr5b~IbrrUO72M~Bzqro-0dUbFj2DhvDNFUSbsm6UIBpMDYWo~lBu5gnBrnNZAzOPlE-7dB5k1cmY43vVMYOctw7uUKTsIm~OK1XL1~uMy84TQ9tqLhNESF54DPWbt83IsEPNPJ3fVcwaGbCgl7dAQepSYbduZZxPLnmKqIQ~w__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 23 October 2023).
- Noreen, A.; Nazli, Z.-I.-H.; Akram, J.; Rasul, I.; Mansha, A.; Yaqoob, N.; Iqbal, R.; Tabasum, S.; Zuber, M.; Zia, K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: A review. Int. J. Biol. Macromol. 2017, 10, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.A.; Abou Elseoud, W.S.; Abo-Elfadl, M.T.; Hassan, M.L. New pectin derivatives with antimicrobial and emulsification properties via complexation with metal-terpyridines. Carbohydr. Polym. 2021, 268, 118230. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Pathania, D.; Naushad, M. Preparation, characterization and antimicrobial activity of biopolymer based nanocomposite ion exchanger pectin zirconium (IV) selenotungstophosphate: Application for removal of toxic metals. J. Ind. Eng. Chem. 2014, 20, 4482–4490. [Google Scholar] [CrossRef]
- Almeida EA, M.S.; Facchi, S.P.; Martins, A.F.; Nocchi, S.; Schuquel IT, A.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Synthesis and characterization of pectin derivative with antitumor property against Caco-2 colon cancer cells. Carbohydr. Polym. 2015, 115, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Cao, M.; Gao, S.; Wang, W.; Peng, K.; Tan, C.; Wen, F.; Tao, S.; Xie, W. Preparation and characterization of a quaternary ammonium derivative of pectin. Carbohydr. Polym. 2012, 88, 707–712. [Google Scholar] [CrossRef]
- Zimniewska, M. Hemp Fibre Properties and Processing Target Textile: A Review. Materials 2022, 15, 1901. [Google Scholar] [CrossRef]
- Niu, H.; Chen, X.; Luo, T.; Chen, H.; Fu, X. Relationships between the behavior of three different sources of pectin at the oil-water interface and the stability of the emulsion. Food Hydrocoll. 2022, 128, 107566. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Geigert, J. Indispensable Potency (Biological Activity). In The Challenge of CMC Regulatory Compliance for Biopharmaceuticals; Springer Nature: Cham, Switzerland, 2023; pp. 407–430. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Sun, D.; Chen, X.; Zhu, C. Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: A comparison of different extraction methods. Int. J. Biol. Macromol. 2020, 158, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yang, W.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.; Huang, L.; Gao, W. Comparison of characterization and antioxidant activity of different citrus peel pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef]
- Garrido, G.; Garrido-Suárez, B.B.; Mieres-Arancibia, M.; Valdes-Gonzalez, M.; Ardiles-Rivera, A. Modified pectin with anticancer activity in breast cancer: A systematic review. Int. J. Biol. Macromol. 2023, 254, 127692. [Google Scholar] [CrossRef]
- Tan, C.; Kong, Y.; Tong, Y.; Deng, H.; Wang, M.; Zhao, Y.; Wan, M.; Lin, S.; Liu, X.; Meng, X.; et al. Anti-apoptotic effects of high hydrostatic pressure treated cyanidin-3-glucoside and blueberry pectin complexes on lipopolysaccharide-induced inflammation in Caco-2 cells. J. Funct. Foods 2021, 86, 104709. [Google Scholar] [CrossRef]
- Soubhagya, A.S.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef]
- Zouambia, Y.; Youcef Ettoumi, K.; Krea, M.; Moulai-Mostefa, N. A new approach for pectin extraction: Electromagnetic induction heating. Arab. J. Chem. 2017, 10, 480–487. [Google Scholar] [CrossRef]
- Mao, Y.; Lei, R.; Ryan, J.; Arrutia Rodriguez, F.; Rastall, B.; Chatzifragkou, A.; Winkworth-Smith, C.; Harding, S.E.; Ibbett, R.; Binner, E. Understanding the influence of processing conditions on the extraction of rhamnogalacturonan-I “hairy” pectin from sugar beet pulp. Food Chem. X 2019, 2, 100026. [Google Scholar] [CrossRef] [PubMed]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Liu, J.-P.; Huang, X.; Du, L.-P.; Shi, F.-L.; Dong, R.; Huang, X.-T.; Zheng, K.; Liu, Y.; Cheong, K.-L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Thu Dao, T.A.; Webb, H.K.; Malherbe, F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwave-assisted heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar] [CrossRef]
- Deng, Z.; Pan, Y.; Chen, W.; Chen, W.; Yun, Y.; Zhong, Q.; Zhang, W.; Chen, H. Effects of cultivar and growth region on the structural, emulsifying and rheological characteristic of mango peel pectin. Food Hydrocoll. 2020, 103, 105707. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, M.; Bhatt, S.; Saini, V.; Malik, A. Cancer Causes and Treatments. Int. J. Pharm. Sci. Res. 2020, 11, 3121. [Google Scholar] [CrossRef]
- Benetou, V.; Lagiou, A.; Lagiou, P. Chemoprevention of cancer: Current evidence and future prospects. F1000Research 2015, 4, 916. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Islam, F.; Mitra, S.; Paul, S.; Nath, N.; Khan, Z.; Das, R.; Chandran, D.; Sharma, R.; Lima, C.M.G.; et al. Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecules 2022, 27, 7405. [Google Scholar] [CrossRef]
- Mohan, S.; Gupta, D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed. Pharmacother. 2018, 108, 1866–1878. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Hu, S.; Kuwabara, R.; Beukema, M.; Ferrari, M.; de Haan, B.J.; Walvoort MT, C.; de Vos, P.; Smink, A.M. Low methyl-esterified pectin protects pancreatic β-cells against diabetes-induced oxidative and inflammatory stress via galectin-3. Carbohydr. Polym. 2020, 249, 116863. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.M.; Dinneen, S.F. What is diabetes? Medicine 2019, 47, 1–4. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Feng, J.-H.; Zhang, J.-F. Efficacy and Tolerability of Insulin Degludec Versus Other Long-acting Basal Insulin Analogues in the Treatment of Type 1 and Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Clin. Ther. 2022, 44, 1520–1533. [Google Scholar] [CrossRef] [PubMed]
- Tuei, V.C.; Maiyoh, G.K.; Ha, C.E. Type 2 diabetes mellitus and obesity in sub-Saharan Africa. Diabetes Metab. Res. Rev. 2010, 26, 433–445. [Google Scholar] [CrossRef]
- Kedir, W.M.; Deresa, E.M.; Diriba, T.F. Pharmaceutical and drug delivery applications of pectin and its modified nanocomposites. Heliyon 2022, 8, e10654. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, M.; Yang, Z.; Pan, S. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2016, 89, 484–488. [Google Scholar] [CrossRef]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Whelton, P.K.; He, J. Worldwide prevalence of hypertension: A systematic review. J. Hypertens. 2004, 22, 11–19. Available online: https://journals.lww.com/jhypertension/Fulltext/2004/01000/Worldwide_prevalence_of_hypertension__a_systematic.3.aspx (accessed on 1 May 2023). [CrossRef]
- Baluja, Z. Antihypertensive properties of an apple peel-can apple a day keep a doctor away? Bull. Pharm. Med. Sci. A Peer Rev. Int. J. 2013, 1, 9–16. Available online: http://www.bopams.com (accessed on 1 July 2023).
- Agarkova, E.Y.; Kruchinin, A.G.; Glazunova, O.A.; Fedorova, T.V. Whey protein hydrolysate and pumpkin pectin as nutraceutical and prebiotic components in a functional mousse with antihypertensive and bifidogenic properties. Nutrients 2019, 11, 2930. [Google Scholar] [CrossRef]
- Na, C.-S.; Yun, D.-H.; Choi, D.-H.; Kim, J.-S.; Cao, C.-H.; Eun, J.-B. The Effect of Pear Pectin on Blood Pressure, Plasma Renin ANP and Cardiac Hypertrophy in Hypertensive Rat Induced by 2K1C. J. Korean Soc. Food Sci. Nutr. 2003, 32, 700–705. [Google Scholar]
- Das, P.; Singh, R.; Kumar, A. Current Scenario and recent advances of Neurological Disorder. J. Complement. Med. Res. 2023, 14, 110–121. [Google Scholar]
- Nuzzo, D.; Picone, P.; Giardina, C.; Scordino, M.; Mudò, G.; Pagliaro, M.; Scurria, A.; Meneguzzo, F.; Ilharco, L.M.; Fidalgo, A.; et al. New Neuroprotective Effect of Lemon IntegroPectin on Neuronal Cellular Model. Antioxidants 2021, 10, 669. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, N.N.; Wang, D.; Meng, W.H.; Chen, H.S. Modified Citrus Pectin Alleviates Cerebral Ischemia/Reperfusion Injury by Inhibiting NLRP3 Inflammasome Activation via TLR4/NF-ĸB Signaling Pathway in Microglia. J. Inflamm. Res. 2022, 15, 3369–3385. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Liu, L.; Nakano, F.; Kawakita, F.; Kanamaru, H.; Nakatsuka, Y.; Okada, T.; Suzuki, H. Modified citrus pectin prevents blood-brain barrier disruption in mouse Subarachnoid hemorrhage by inhibiting Galectin-3. Stroke 2018, 49, 2743–2751. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Stothard, P. Tissues, Metabolic Pathways and Genes of Key Importance in Lactating Dairy Cattle. Springer Sci. Rev. 2016, 4, 49–77. [Google Scholar] [CrossRef]
- Defesche, J.C.; Gidding, S.S.; Harada-Shiba, M.; Hegele, R.A.; Santos, R.D.; Wierzbicki, A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Primers 2017, 3, 17093. [Google Scholar] [CrossRef]
- Ram, L.; Singh, S. Medicinal importance of citrus products and by-products—A review. Agric. Rev. 2006, 27, 170–180. [Google Scholar]
- Wicker, L.; Kim, Y.; Kim, M.J.; Thirkield, B.; Lin, Z.; Jung, J. Pectin as a bioactive polysaccharide—Extracting tailored function from less. Food Hydrocoll. 2014, 42 Pt 2, 251–259. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Shi, S.; Gao, W.; Wu, J.; Gong, H.; Zhao, Y.; Chen, W.; Wang, H.; Wang, S. Structure characterization of pectin from the pollen of Typha angustifolia L. and the inhibition activity of lipid accumulation in oleic acid induced L02 cells. Carbohydr. Polym. 2023, 303, 120452. [Google Scholar] [CrossRef] [PubMed]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; D’Errico, S.; Ciccone, R.; De Feo, V.; Secondo, A.; Pannaccione, A. Exploring the Therapeutic Potential of Phytochemicals in Alzheimer’s Disease: Focus on Polyphenols and Monoterpenes. Front. Pharmacol. 2022, 13, 876614. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Li, P.; Zhou, L.; Ding, K. A novel pectin from Polygala tenuifolia blocks Aβ42 aggregation and production by enhancing insulin-degradation enzyme and neprilysin. Int. J. Biol. Macromol. 2020, 161, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fang, J.; Wang, P.; Du, Z.; Li, Y.; Wang, S.; Ding, K. Characterization of a pectin from Lonicera japonica Thunb. and its inhibition effect on Aβ42 aggregation and promotion of neuritogenesis. Int. J. Biol. Macromol. 2018, 107, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Houron, C.; Ciocan, D.; Trainel, N.; Mercier-Nomé, F.; Hugot, C.; Spatz, M.; Perlemuter, G.; Cassard, A.-M. Gut Microbiota Reshaped by Pectin Treatment Improves Liver Steatosis in Obese Mice. Nutrients 2021, 13, 3725. [Google Scholar] [CrossRef]
- Ciocan, D.; Spatz, M.; Trainel, N.; Hardonnière, K.; Domenichini, S.; Mercier-Nomé, F.; Desmons, A.; Humbert, L.; Durand, S.; Kroemer, G.; et al. Modulation of the Bile Acid Enterohepatic Cycle by Intestinal Microbiota Alleviates Alcohol Liver Disease. Cells 2022, 11, 968. [Google Scholar] [CrossRef]
- Ferrere, G.; Wrzosek, L.; Cailleux, F.; Turpin, W.; Puchois, V.; Spatz, M.; Ciocan, D.; Rainteau, D.; Humbert, L.; Hugot, C.; et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 2017, 66, 806–815. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Koriem KM, M.; Arbid, M.S.; Emam, K.R. Therapeutic effect of pectin on octylphenol induced kidney dysfunction, oxidative stress and apoptosis in rats. Environ. Toxicol. Pharmacol. 2014, 38, 14–23. [Google Scholar] [CrossRef]
- Bakr, E.-S.H. Utilization of Pectin and Arabic Gum to Improve Kidney Functions in Rats Inflicted with Renal Failure. J. Home Econ. 2016, 26, 97–120. Available online: http://homeEcon.menofia.edu.eg (accessed on 23 October 2023).
- Khotimchenko, M.; Piatchina, O.V.; Kolenchenko, E.A. The therapeutic and preventive effects of pectin in experimental renal failure. Patol. Fiziol. Eksperimental’naia Ter. 2009, 4, 31–33. [Google Scholar]
- Miyazawa, R.; Tomomasa, T.; Kaneko, H.; Arakawa, H.; Shimizu, N.; Morikawa, A. Effects of pectin liquid on gastroesophageal reflux disease in children with cerebral palsy. BMC Gastroenterol. 2008, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Garrett, D.O.; Longley, A.T.; Aiemjoy, K.; Yousafzai, M.T.; Hemlock, C.; Yu, A.T.; Vaidya, K.; Tamrakar, D.; Saha, S.; Bogoch, I.I.; et al. Incidence of typhoid and paratyphoid fever in Bangladesh, Nepal, and Pakistan: Results of the Surveillance for Enteric Fever in Asia Project. Lancet Glob. Health 2022, 10, e978–e988. [Google Scholar] [CrossRef] [PubMed]
- Szu, S.C.; Lin, K.F.-Y.; Hunt, S.; Chu, C.; Thinh, N.D. Phase I clinical trial of O-acetylated pectin conjugate, a plant polysaccharide based typhoid vaccine. Vaccine 2014, 32, 2618–2622. [Google Scholar] [CrossRef]
- Keizman, D.; Frenkel, M.A.; Peer, A.; Kushnir, I.; Rosenbaum, E.; Sarid, D.L.; Leibovitch, I.; Mano, R.; Yossepowitch, O.; Margel, D.; et al. 62 Poster Session P-MCP treatment in non-metastatic biochemically relapsed prostate cancer (BRPC-M0): Final long-term results of a prospective phase II study. J. Clin. Oncol. 2023, 4 (Suppl. S6), 162. [Google Scholar]
- Pascale, N.; Gu, F.; Larsen, N.; Jespersen, L.; Respondek, F. The Potential of Pectins to Modulate the Human Gut Microbiota Evaluated by In Vitro Fermentation: A Systematic Review. Nutrients 2022, 14, 3629. [Google Scholar] [CrossRef]
- Maurya, V.K.; Singh, J.; Ranjan, V.; Gothandam, K.M.; Bohn, T.; Pareek, S. Factors affecting the fate of β-carotene in the human gastrointestinal tract: A narrative review. Int. J. Vitam. Nutr. Res. 2022, 92, 385–405. [Google Scholar] [CrossRef]
- Kaleab Baye, J.-P.G.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. [Google Scholar] [CrossRef]
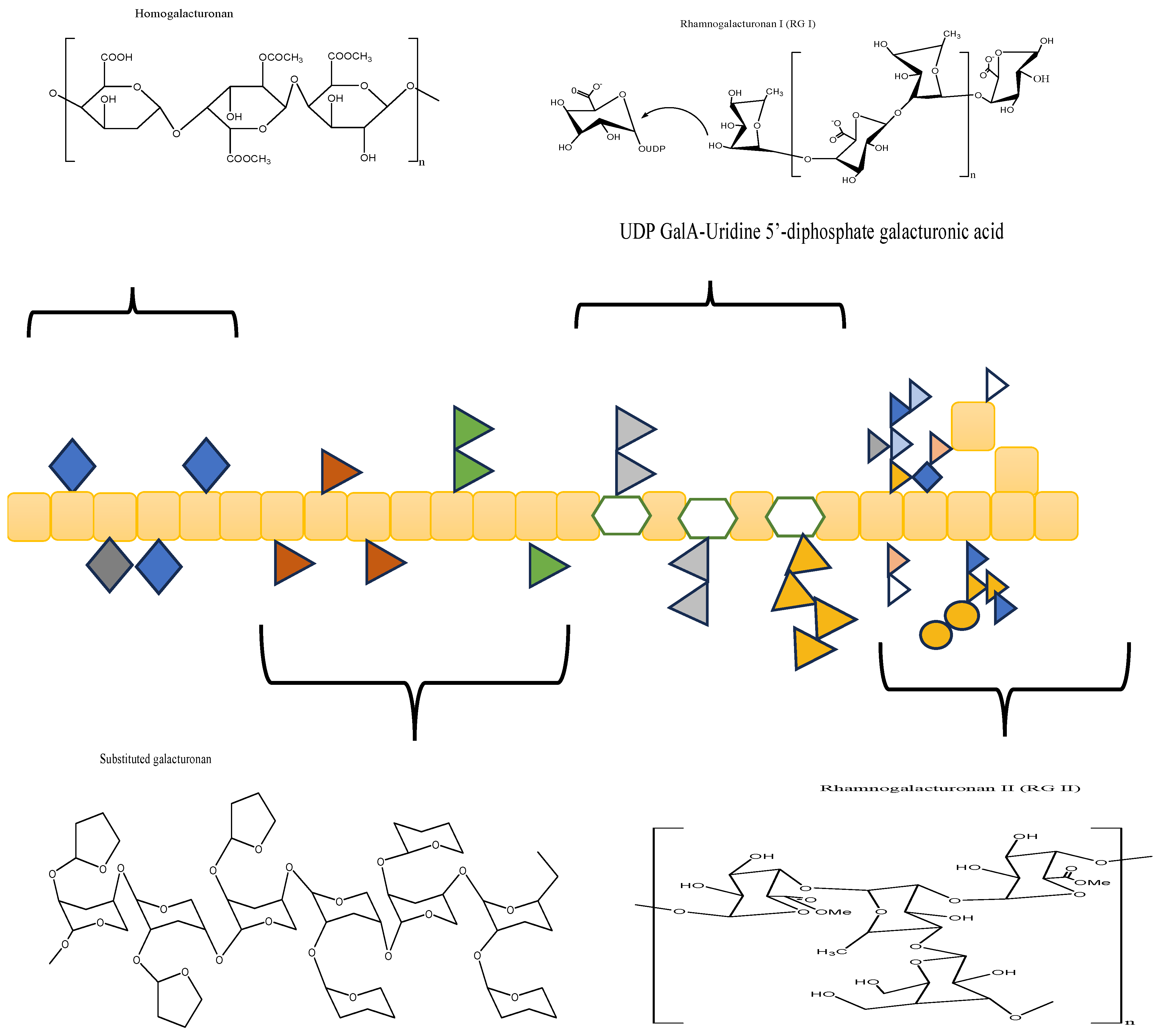
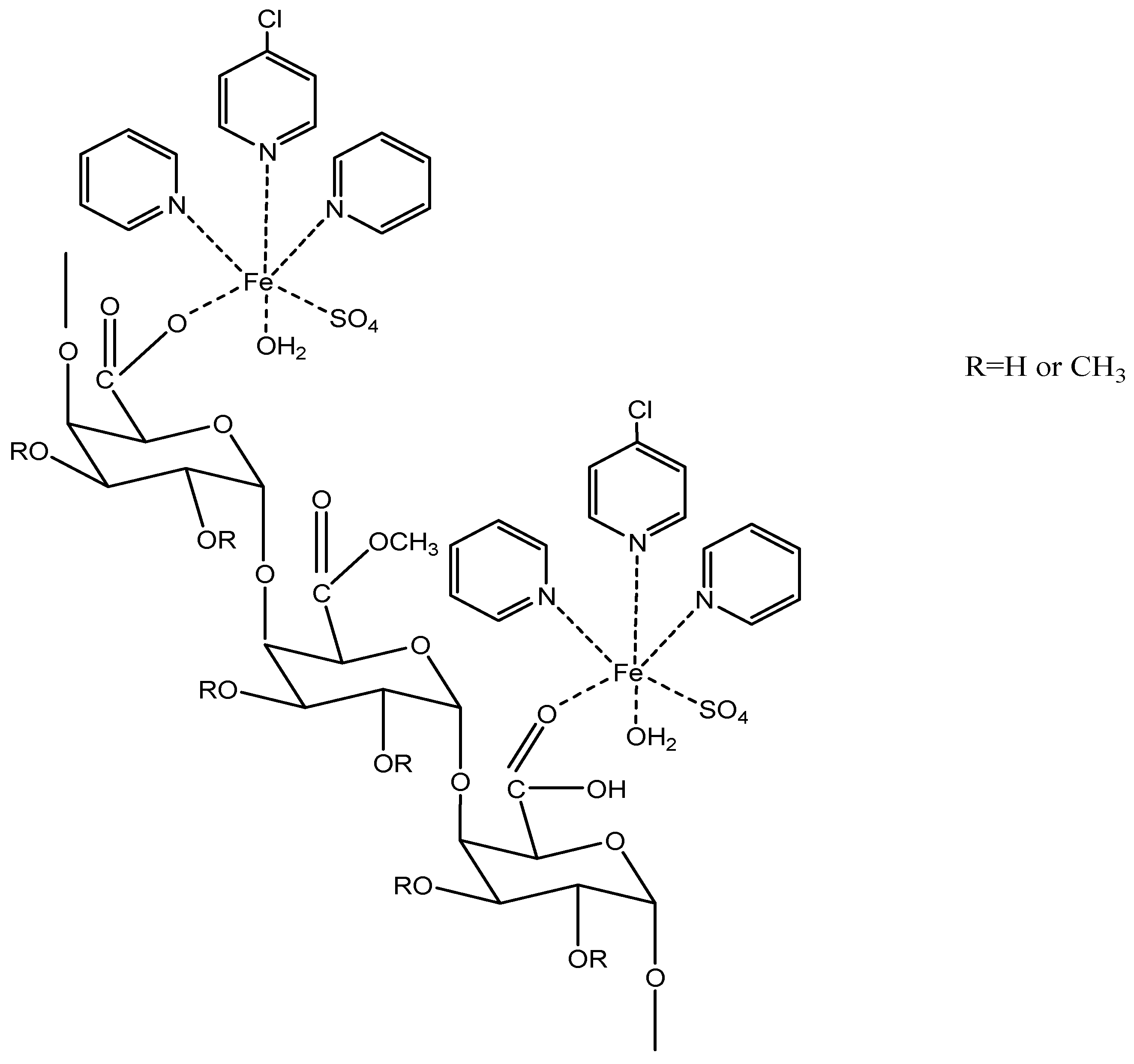
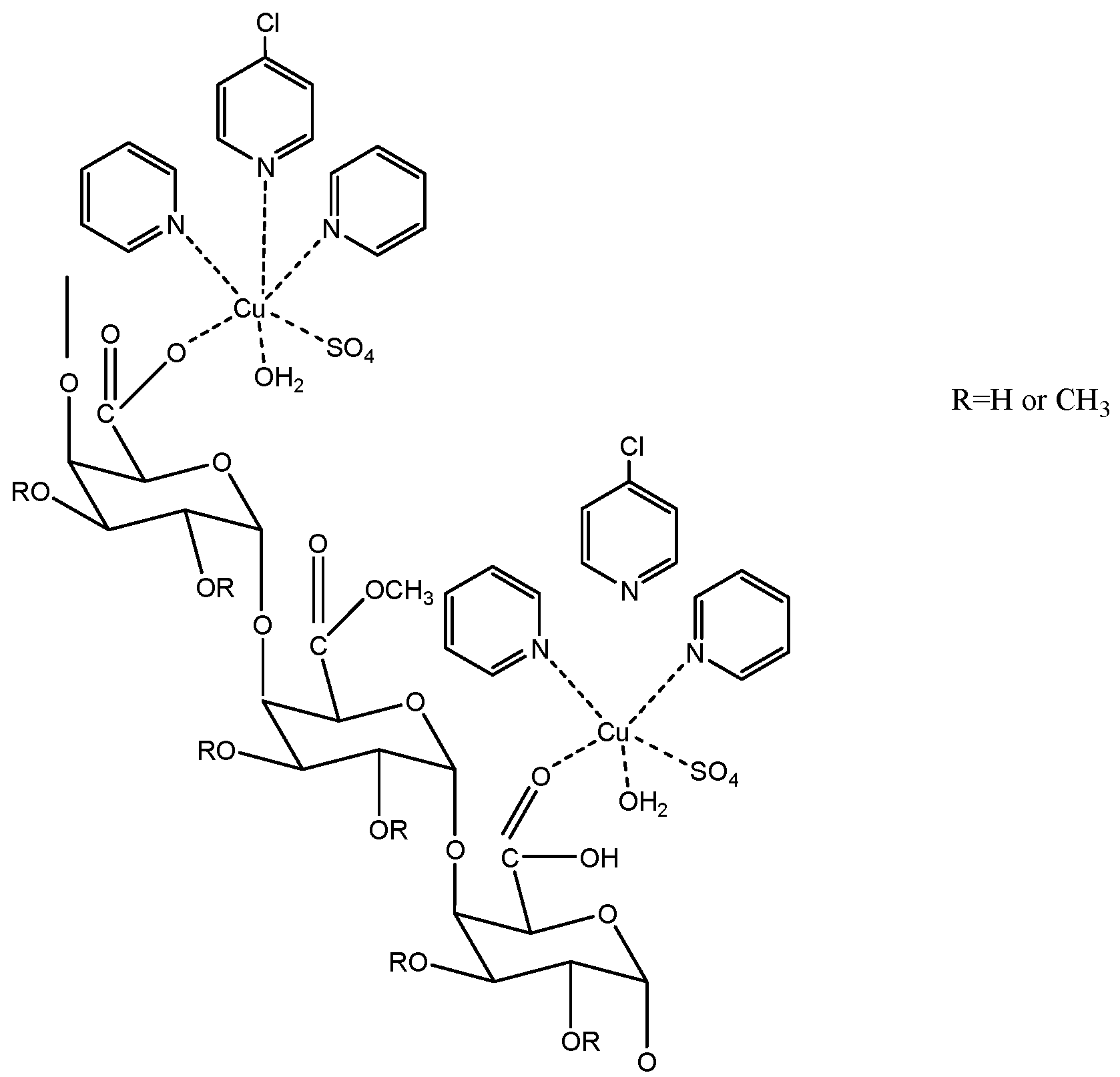

| Type of Extraction | Species | Plant Part Extracted | References |
|---|---|---|---|
| Electromagnetic induction (EMI) | Citrus sinensis × Poncirus trifoliata | Peels | [32] |
| Conventional and microwave-assisted extraction | Beta. vulgaris (sugar beet) | Sugar beet pulp | [33] |
| Ultrasonic-assisted extraction (UAE) | Opuntia ficus indica (prickly pear) | Cladodes | [34] |
| Ultrasonic microwave-assisted extraction | Artocarpus heterophyllus (Jackfruit) | Peels | [35] |
| Conventional method and microwave-assisted method | Hylocereus polyrhizus (red flesh dragon) Hylocereus undatus (white flesh dragon) Passiflora edulis (passion fruit) | Peels Peels Peels | [36] |
| Conventional extraction | Mangifera Indica (mango) | Peels | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dambuza, A.; Rungqu, P.; Oyedeji, A.O.; Miya, G.; Oriola, A.O.; Hosu, Y.S.; Oyedeji, O.O. Therapeutic Potential of Pectin and Its Derivatives in Chronic Diseases. Molecules 2024, 29, 896. https://doi.org/10.3390/molecules29040896
Dambuza A, Rungqu P, Oyedeji AO, Miya G, Oriola AO, Hosu YS, Oyedeji OO. Therapeutic Potential of Pectin and Its Derivatives in Chronic Diseases. Molecules. 2024; 29(4):896. https://doi.org/10.3390/molecules29040896
Chicago/Turabian StyleDambuza, Anathi, Pamela Rungqu, Adebola Omowunmi Oyedeji, Gugulethu Miya, Ayodeji Oluwabunmi Oriola, Yiseyon Sunday Hosu, and Opeoluwa Oyehan Oyedeji. 2024. "Therapeutic Potential of Pectin and Its Derivatives in Chronic Diseases" Molecules 29, no. 4: 896. https://doi.org/10.3390/molecules29040896
APA StyleDambuza, A., Rungqu, P., Oyedeji, A. O., Miya, G., Oriola, A. O., Hosu, Y. S., & Oyedeji, O. O. (2024). Therapeutic Potential of Pectin and Its Derivatives in Chronic Diseases. Molecules, 29(4), 896. https://doi.org/10.3390/molecules29040896







