Hydroxylation of Aryl Sulfonium Salts for Phenol Synthesis under Mild Reaction Conditions
Abstract
1. Introduction
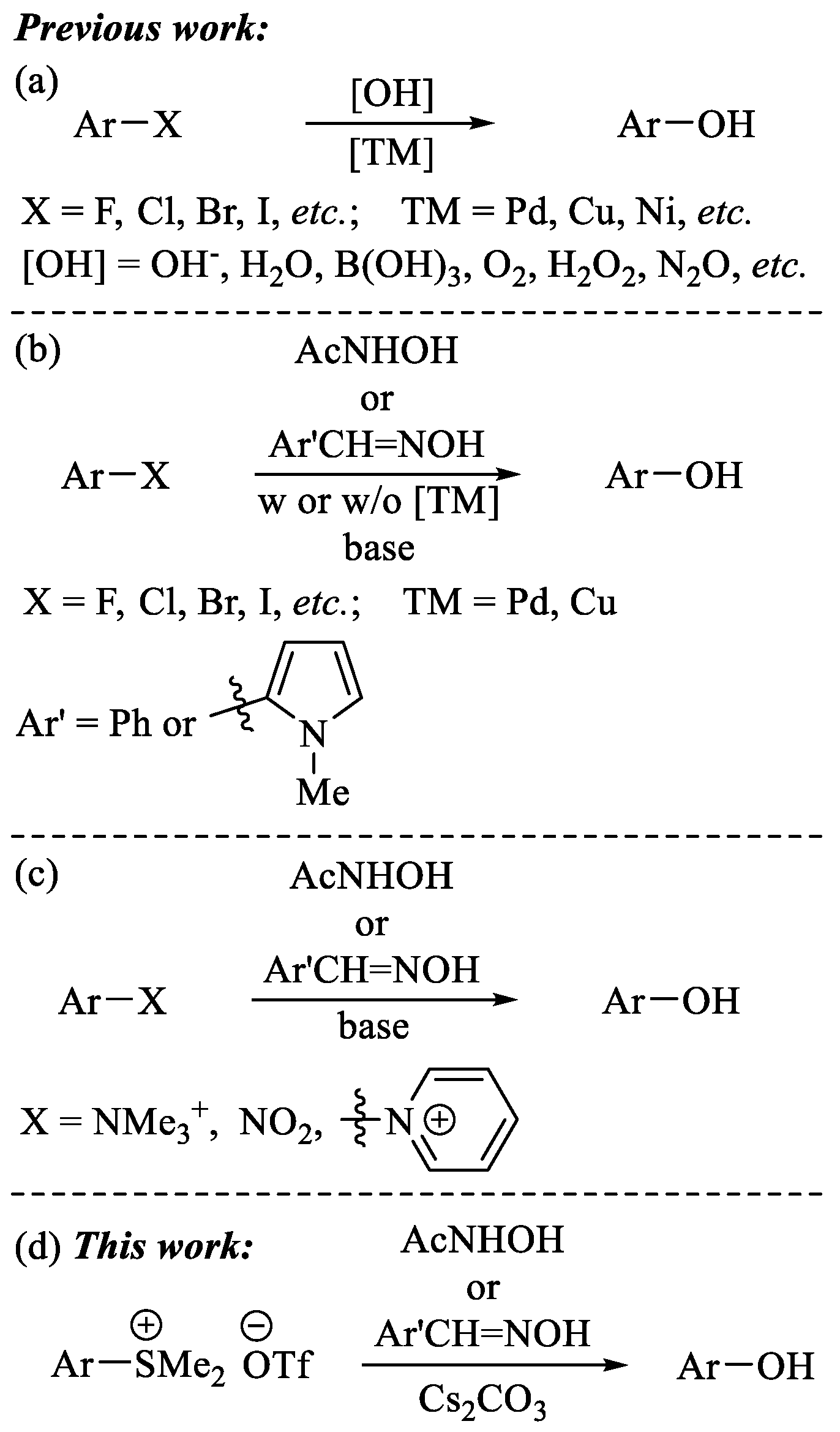
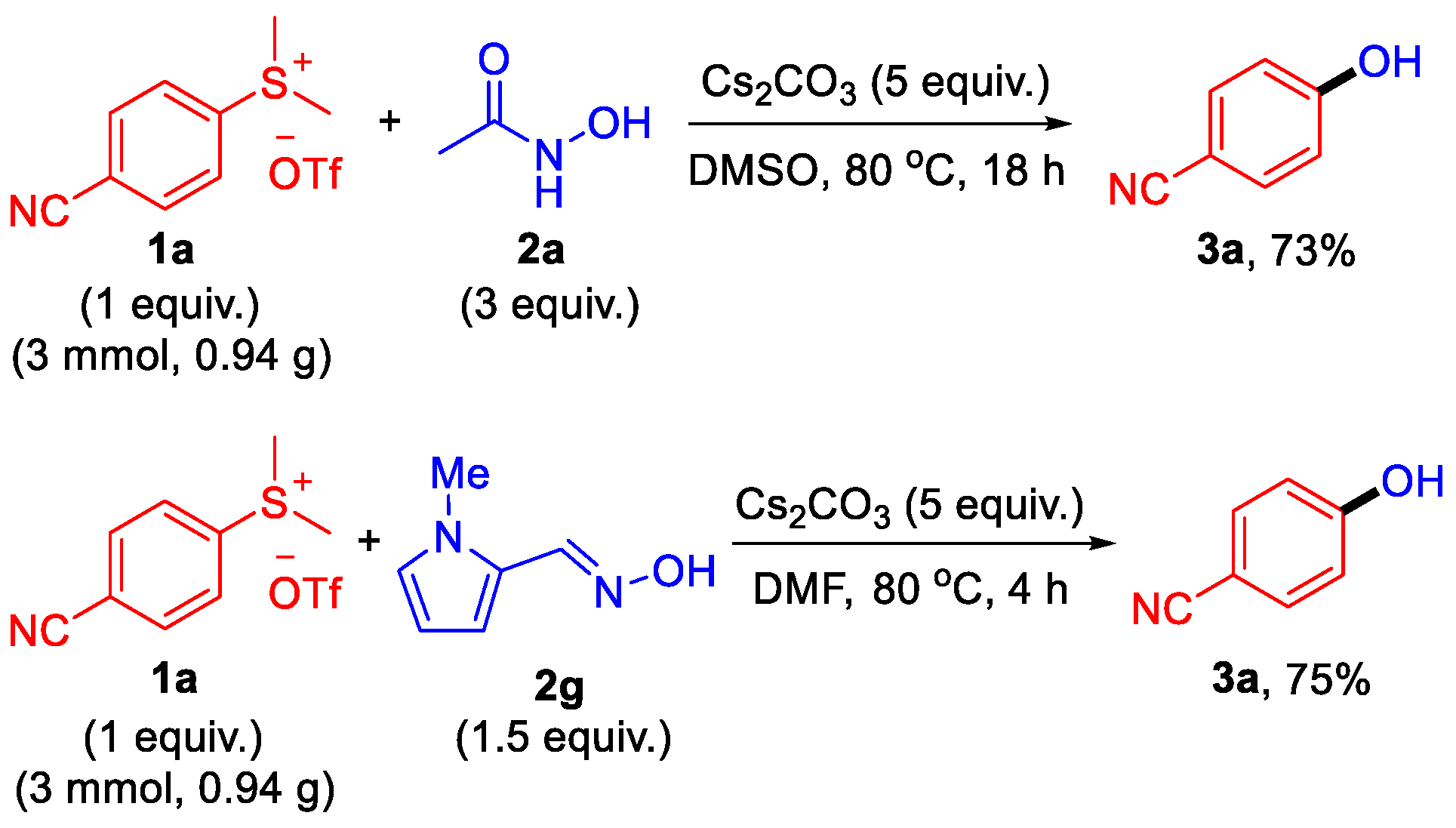
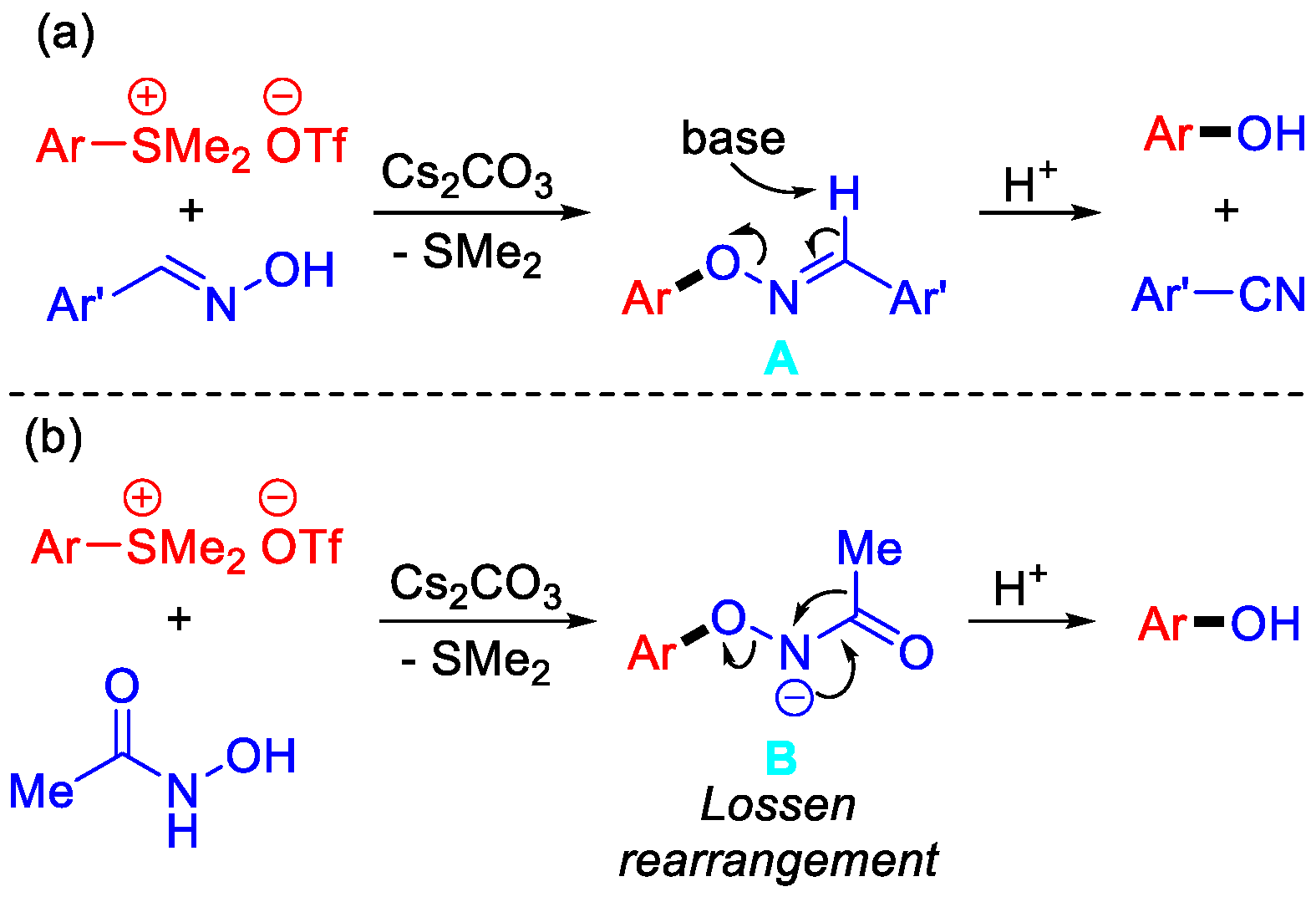
2. Results
3. Materials and Methods
3.1. General Information
3.2. Experimental
3.2.1. General Procedure for the Synthesis of Aryl Sulfonium Salts 1a–k
3.2.2. General Procedure for the Synthesis of Oximes 2b–g
3.2.3. General Procedure for the Reaction of Aryl Sulfonium Salt with Acetohydroxamic Acid
3.2.4. General Procedure for the Reaction of Aryl Sulfonium Salt with Oxime
3.2.5. Scale-Up Reaction of Sulfonium Salt 1a with Acetohydroxamic Acid 2a
3.2.6. Scale-Up Reaction of Sulfonium Salt 1a with Oxime 2g
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rappoport, Z. The Chemistry of Phenols; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Sandmeyer, T. The Substitution of the Amine Group with Chlorine Atom in Aromatic Systems. Ber. Dtsch. Chem. Ges. 1884, 17, 1633–1635. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, H.; Cai, H.; Zhang, J.; Gong, X.; Han, W. Iron-Catalyzed Arene C-H Hydroxylation. Science 2021, 374, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.; Ma, X.; Nguyen, K.T.; Zhao, Y. A Vanadyl Complex Grafted to Periodic Mesoporous Organosilica: A Green Catalyst for Selective Hydroxylation of Benzene to Phenol. Angew. Chem. Int. Ed. 2012, 51, 7756–7761. [Google Scholar] [CrossRef]
- Kamata, K.; Yamaura, T.; Mizuno, N. Chemo- and Regioselective Direct Hydroxylation of Arenes with Hydrogen Peroxide Catalyzed by a Divanadium-Substituted Phosphotungstate. Angew. Chem. Int. Ed. 2012, 51, 7275–7278. [Google Scholar] [CrossRef]
- Shoji, O.; Kunimatsu, T.; Kawakami, N.; Watanabe, Y. Highly Selective Hydroxylation of Benzene to Phenol by Wild-type Cytochrome P450BM3 Assisted by Decoy Molecules. Angew. Chem. Int. Ed. 2013, 52, 6606–6610. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Xu, Y.; Komiyama, M. A Chemistry-Based Method to Detect Individual Telomere Length at a Single Chromosome Terminus. J. Am. Chem. Soc. 2013, 135, 5368–5371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Rodrigalvarez, J.; Martin, R. C(sp2)-H Hydroxylation via Catalytic 1,4-Ni Migration with N2O. J. Am. Chem. Soc. 2023, 145, 17564–17569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, Y.; Yang, K.; Hu, S.; Lv, C.; Huang, J.; Mei, J.; Zhao, W.; Mei, L. Modification of the 4-Hydroxyphenylacetate-3-hydroxylase Substrate Pocket to Increase Activity towards Resveratrol. Molecules 2023, 28, 5602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Chen, X.; Yang, D.; Liu, C.-Y.; Zhou, X.-Y. A Facile and General Oxidative Hydroxylation of Organoboron Compounds: Citric Acid as an Efficient Catalyst in Water to Access Phenolic and Alcoholic Motifs. Molecules 2023, 28, 7915. [Google Scholar] [CrossRef]
- Willis, M.C. Palladium-Catalyzed Coupling of Ammonia and Hydroxide with Aryl Halides: The Direct Synthesis of Primary Anilines and Phenols. Angew. Chem. Int. Ed. 2007, 46, 3402–3404. [Google Scholar] [CrossRef]
- Alonso, D.A.; Nájera, C.; Pastor, I.M.; Yus, M. Transition-Metal-Catalyzed Synthesis of Hydroxylated Arenes. Chem. Eur. J. 2010, 16, 5274–5284. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S.; Company, A. Palladium-Catalysed Hydroxylation and Alkoxylation. Chem. Soc. Rev. 2011, 40, 4912–4924. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, J.; Pawar, G.G.; Kumar, S.V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C−N, C−O, C−S, and C−C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179. [Google Scholar] [CrossRef] [PubMed]
- Amal Joseph, P.J.; Priyadarshini, S. Copper-Mediated C-X Functionalization of Aryl Halides. Org. Process Res. Dev. 2017, 21, 1889–1924. [Google Scholar] [CrossRef]
- Yang, L.; Xue, D. Transition-Metal-Catalyzed Hydroxylation Reaction of Aryl Halide for the Synthesis of Phenols. Synlett 2021, 32, 1891–1896. [Google Scholar]
- Das, R.; Rohit, K.R.; Anilkumar, G. Recent Trends in Non-Noble Metal-Catalyzed Hydroxylation Reactions. J. Organomet. Chem. 2022, 977, 122456. [Google Scholar] [CrossRef]
- Xia, S.; Gan, L.; Wang, K.; Li, Z.; Ma, D. Copper-Catalyzed Hydroxylation of (Hetero)aryl Halides under Mild Conditions. J. Am. Chem. Soc. 2016, 138, 13493–13496. [Google Scholar] [CrossRef]
- Rodrigo, E.; Wiechert, R.; Walter, M.W.; Braje, W.; Geneste, H. One-Step Hydroxylation of Aryl and Heteroaryl Fluorides Using Mechanochemistry. Green Chem. 2022, 24, 1469–1473. [Google Scholar] [CrossRef]
- Tao, S.; Xü, M.; Zou, G. Co-Catalytic Borinate Enables Air-Tolerant Copper/Oxalamide Catalysis for Cost-Effective Hydroxylation of Aryl Bromides. Tetrahedron Lett. 2023, 123, 154586. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.S.; Kim, K. Complete Regioselective Formation of 2-(Arylsulfinyl)diphenyl Sulfides from 5-Arylthianthreniumyl Perchlorates. J. Heterocyclic Chem. 1999, 36, 617–622. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Z.; Li, G.; Zhang, W.; Cao, R.; Wang, C.; Xiao, J.; Xue, D. Synthesis of Phenols: Organophotoredox/Nickel Dual Catalytic Hydroxylation of Aryl Halides with Water. Angew. Chem. Int. Ed. 2018, 57, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.-K.; Lin, Y.; Li, Y.; Xu, L.; Li, K.; Shi, H. Catalytic SNAr Hydroxylation and Alkoxylation of Aryl Fluorides. Angew. Chem. Int. Ed. 2021, 60, 20391–20399. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, Y.; Cao, N.; Hao, J.; Li, G.; Zhang, W.; Cao, R.; Wang, C.; Xiao, J.; Xue, D. Ni(I)-Catalyzed Hydroxylation of Aryl Halides with Water under Thermal Catalysis. Org. Lett. 2022, 24, 9431–9435. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, H.; Liu, H.; Chen, H.; Zhang, F. Accelerated Direct Hydroxylation of Aryl Chlorides with Water to Phenols via the Proximity Effect in a Heterogeneous Metallaphotocatalyst. ACS Catal. 2022, 12, 6068–6080. [Google Scholar] [CrossRef]
- Katagiri, K.; Kuriyama, M.; Yamamoto, K.; Demizu, Y.; Onomura, O. Organocatalytic Synthesis of Phenols from Diaryliodonium Salts with Water under Metal-Free Conditions. Org. Lett. 2022, 24, 5149–5154. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Han, C.; Shi, P.; Gao, W.; Mei, W. Copper-Catalyzed Synthesis of Phenol and Diaryl Ether Derivatives via Hydroxylation of Diaryliodoniums. RSC Adv. 2019, 9, 21525–21529. [Google Scholar] [CrossRef]
- Song, Z.-Q.; Wang, D.-H. Palladium-Catalyzed Hydroxylation of Aryl Halides with Boric Acid. Org. Lett. 2020, 22, 8470–8474. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, G.; Gao, W.; Ding, J.; Huang, X.; Liu, M.; Wu, H. Synergistic Photo-Copper-Catalyzed Hydroxylation of (Hetero)aryl Halides with Molecular Oxygen. Org. Lett. 2018, 20, 708–711. [Google Scholar] [CrossRef]
- Cai, Y.-M.; Xu, Y.-T.; Zhang, X.; Gao, W.-X.; Huang, X.-B.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Photoinduced Hydroxylation of Organic Halides under Mild Conditions. Org. Lett. 2019, 21, 8479–8484. [Google Scholar] [CrossRef]
- Reitti, M.; Gurubrahamam, R.; Walther, M.; Lindstedt, E.; Olofsson, B. Synthesis of Phenols and Aryl Silyl Ethers via Arylation of Complementary Hydroxide Surrogates. Org. Lett. 2018, 20, 1785–1788. [Google Scholar] [CrossRef]
- Le Vaillant, F.; Mateos Calbet, A.; Gonzalez-Pelayo, S.; Reijerse, E.J.; Ni, S.; Busch, J.; Cornella, J. Catalytic Synthesis of Phenols with Nitrous Oxide. Nature 2022, 604, 677–683. [Google Scholar] [CrossRef]
- Ni, S.; Cornella, J. Catalytic Hydroxylation of Arylthianthrenium Salts with Nitrous Oxide. Tetrahedron 2023, 145, 133602. [Google Scholar] [CrossRef]
- Fier, P.S.; Maloney, K.M. Synthesis of Complex Phenols Enabled by a Rationally Designed Hydroxide Surrogate. Angew. Chem. Int. Ed. 2017, 56, 4478–4482. [Google Scholar] [CrossRef] [PubMed]
- Fier, P.S.; Maloney, K.M. Reagent Design and Ligand Evolution for the Development of a Mild Copper-Catalyzed Hydroxylation Reaction. Org. Lett. 2017, 19, 3033–3036. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.S.; Krabbe, S.W.; Li, C.; Sun, L.; Liu, Y.; Nett, A.J. Identification of an Oxalamide Ligand for Copper-Catalyzed C-O Couplings from a Pharmaceutical Compound Library. ChemCatChem 2019, 11, 5748–5753. [Google Scholar] [CrossRef]
- Zhou, Y. Facile and Metal-Free Synthesis of Phenols from Benzaldoxime and Diaryliodonium Salts. J. Chem. Res 2017, 41, 591–593. [Google Scholar] [CrossRef]
- Fier, P.S.; Maloney, K.M. Direct Conversion of Haloarenes to Phenols under Mild, Transition-Metal-Free Conditions. Org. Lett. 2016, 18, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Greener, A.J.; Ubysz, P.; Owens-Ward, W.; Smith, G.; Ocana, I.; Whitwood, A.C.; Chechik, V.; James, M.J. Radical–Anion Coupling through Reagent Design: Hydroxylation of Aryl Halides. Chem. Sci. 2021, 12, 14641–14646. [Google Scholar] [CrossRef] [PubMed]
- Korch, K.M.; Watson, D.A. Cross-Coupling of Heteroatomic Electrophiles. Chem. Rev. 2019, 119, 8192–8228. [Google Scholar] [CrossRef]
- Zhao, B.; Rogge, T.; Ackermann, L.; Shi, Z. Metal-Catalysed C–Het (F, O, S, N) and C–C Bond Arylation. Chem. Soc. Rev. 2021, 50, 8903–8953. [Google Scholar] [CrossRef]
- Zhao, B.; Prabagar, B.; Shi, Z. Modern Strategies for C–H Functionalization of Heteroarenes with Alternative Coupling Partners. Chem 2021, 7, 2585–2634. [Google Scholar] [CrossRef]
- Dolewski, R.D.; Hilton, M.C.; McNally, A. 4-Selective Pyridine Functionalization Reactions via Heterocyclic Phosphonium Salts. Synlett 2018, 29, 8–14. [Google Scholar]
- Garcίa-Cárceles, J.; Bahou, K.A.; Bower, J.F. Recent Methodologies that Exploit Oxidative Addition of C-N Bonds to Transition Metals. ACS Catal. 2020, 10, 12738–12759. [Google Scholar] [CrossRef]
- Xu, J.; Bercher, O.P.; Talley, M.R.; Watson, M.P. Nickel-Catalyzed, Stereospecific C-C and C-B Cross-Couplings via C-N and C-O Bond Activation. ACS Catal. 2021, 11, 1604–1612. [Google Scholar] [CrossRef]
- He, F.-S.; Ye, S.; Wu, J. Recent Advances in Pyridinium Salts as Radical Reservoirs in Organic Synthesis. ACS Catal. 2019, 9, 8943–8960. [Google Scholar] [CrossRef]
- Rössler, S.L.; Jelier, B.J.; Magnier, E.; Dagousset, G.; Carreira, E.M.; Togni, A. Pyridinium Salts as Redox-Active Functional Group Transfer Reagents. Angew. Chem. Int. Ed. 2020, 59, 9264–9280. [Google Scholar] [CrossRef] [PubMed]
- Ghiazza, C.; Wagner, L.; Fernández, S.; Leutzsch, M.; Cornella, J. Bio-Inspired Deaminative Hydroxylation of Aminoheterocycles and Electron-Deficient Anilines. Angew. Chem. Int. Ed. 2023, 62, e202212219. [Google Scholar] [CrossRef]
- Duff, L.; Meakin, H.; Richardson, A.; Greener, A.J.; Smith, G.W.A.; Ocaña, I.; Chechik, V.; James, M.J. Denitrative Hydroxylation of Unactivated Nitroarenes. Chem. Eur. J. 2023, 29, e202203807. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Yang, L.; Shen, Y.; Zhang, L.; Ma, Y.; Sun, M.; Cheng, R.; Ye, J. Synthesis of Phenols from Aryl Ammonium Salts under Mild Conditions. J. Org. Chem. 2022, 87, 12677–12687. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Hu, Y.-T.; Teng, H.-B.; Zhang, C.-P. Application of Arylsulfonium Salts as Arylation Reagents. Tetrahedron Lett. 2018, 59, 299–309. [Google Scholar] [CrossRef]
- Kaiser, D.; Klose, I.; Oost, R.; Neuhaus, J.; Maulide, N. Bond-Forming and -Breaking Reactions at Sulfur(IV): Sulfoxides, Sulfonium Salts, Sulfur Ylides, and Sulfinate Salts. Chem. Rev. 2019, 119, 8701–8780. [Google Scholar] [CrossRef]
- Kozhushkov, S.I.; Alcarazo, M. Synthetic Applications of Sulfonium Salts. Eur. J. Inorg. Chem. 2020, 2020, 2486–2500. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.-Y.; Ma, Y.; Zhang, C.-P. Alkylation Reactions with Alkylsulfonium Salts. Synthesis 2022, 54, 1478–1502. [Google Scholar]
- Meng, H.; Liu, M.-S.; Shu, W. Organothianthrenium Salts: Synthesis and Utilization. Chem. Sci. 2022, 13, 13690–13707. [Google Scholar] [CrossRef] [PubMed]
- Dektar, J.L.; Hacker, N.P. Photochemistry of Triarylsulfonium Salts. J. Am. Chem. Soc. 1990, 112, 6004–6015. [Google Scholar] [CrossRef]
- Wang, S.-M.; Song, H.-X.; Wang, X.-Y.; Liu, N.; Qin, H.-L.; Zhang, C.-P. Palladium-Catalyzed Mizoroki-Heck-Type Reactions of [Ph2SRfn][OTf] with Alkenes at Room Temperature. Chem. Commun. 2016, 52, 11893–11896. [Google Scholar] [CrossRef] [PubMed]
- Minami, H.; Otsuka, S.; Nogi, K.; Yorimitsu, H. Palladium-Catalyzed Borylation of Aryl Sulfoniums with Diborons. ACS Catal. 2018, 8, 579–583. [Google Scholar] [CrossRef]
- Minami, H.; Nogi, K.; Yorimitsu, H. Palladium-Catalyzed Alkoxycarbonylation of Arylsulfoniums. Org. Lett. 2019, 21, 2518–2522. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.-Y.; Zhang, C.-P. Ullmann-Type N-Arylation of Anilines with Alkyl(aryl)sulfonium Salts. Chem. Commun. 2019, 55, 11936–11939. [Google Scholar] [CrossRef]
- Engl, P.S.; Häring, A.P.; Berger, F.; Berger, G.; Pérez-Bitrián, A.; Ritter, T. C-N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts. J. Am. Chem. Soc. 2019, 141, 13346–13351. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Wang, S.-M.; Jia, S.-J.; Song, H.-X.; Zhang, C.-P. Sonogashira Reaction Using Arylsulfonium Salts as Cross-Coupling Partners. Org. Lett. 2017, 19, 5454–5457. [Google Scholar] [CrossRef]
- Yanagi, T.; Somerville, R.J.; Nogi, K.; Martin, R.; Yorimitsu, H. Ni-Catalyzed Carboxylation of C(sp2)-S Bonds with CO2: Evidence for the Multifaceted Role of Zn. ACS Catal. 2020, 10, 2117–2123. [Google Scholar] [CrossRef]
- Yamada, K.; Yanagi, T.; Yorimitsu, H. Generation of Organozinc Reagents from Arylsulfonium Salts Using a Nickel Catalyst and Zinc Dust. Org. Lett. 2020, 22, 9712–9718. [Google Scholar] [CrossRef]
- Ma, N.-N.; Ren, J.-A.; Liu, X.; Chu, X.-Q.; Rao, W.; Shen, Z.-L. Nickel-Catalyzed Direct Cross-Coupling of Aryl Sulfonium Salt with Aryl Bromide. Org. Lett. 2022, 24, 1953–1957. [Google Scholar] [CrossRef]
- Srogl, J.; Allred, G.D.; Liebeskind, L.S. Sulfonium Salts. Participants par Excellence in Metal-Catalyzed Carbon-Carbon Bond-Forming Reactions. J. Am. Chem. Soc. 1997, 119, 12376–12377. [Google Scholar] [CrossRef]
- Vanier, C.; Lorgé, F.; Wagner, A.; Mioskowski, C. Traceless, Solid-Phase Synthesis of Biarylmethane Structures through Pd-Catalyzed Release of Supported Benzylsulfonium Salts. Angew. Chem. Int. Ed. 2000, 39, 1679–1683. [Google Scholar] [CrossRef]
- Vasu, D.; Yorimitsu, H.; Osuka, A. Palladium-Assisted “Aromatic Metamorphosis” of Dibenzothiophenes into Triphenylenes. Angew. Chem. Int. Ed. 2015, 54, 7162–7166. [Google Scholar] [CrossRef] [PubMed]
- Cowper, P.; Jin, Y.; Turton, M.D.; Kociok-Köhn, G.; Lewis, S.E. Azulenesulfonium Salts: Accessible, Stable, and Versatile Reagents for Cross-Coupling. Angew. Chem. Int. Ed. 2016, 55, 2564–2568. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Plutschack, M.B.; Riegger, J.; Yu, W.; Speicher, S.; Ho, M.; Frank, N.; Ritter, T. Site-Selective and Versatile Aromatic C-H Functionalization by Thianthrenation. Nature 2019, 567, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Aukland, M.H.; Talbot, F.J.T.; Fernández-Salas, J.A.; Ball, M.; Pulis, A.P.; Procter, D.J. An Interrupted Pummerer/Nickel-Catalyzed Cross-Coupling Sequence. Angew. Chem. Int. Ed. 2018, 57, 9785–9789. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.-Y.; Lin, Z.-H.; Zhang, C.-P. Pd/Cu-Catalyzed C-H/C-H Cross Coupling of (Hetero)Arenes with Azoles through Arylsulfonium Intermediates. Org. Lett. 2021, 23, 4400–4405. [Google Scholar] [CrossRef]
- Xu, G.; Han, Z.; Guo, L.; Lu, H.; Gao, H. Transition-Metal-Free Cascade Approach for the Synthesis of Functionalized Biaryls by SNAr of Arylhydroxylamines with Arylsulfonium Salts. J. Org. Chem. 2022, 87, 10449–10453. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Korkis, S.E.; Su, W.; Ye, F.; Engl, P.S.; Berger, F.; Ritter, T. Site-Selective C-H Oxygenation via Aryl Sulfonium Salts. Angew. Chem. Int. Ed. 2019, 58, 16161–16166. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-D.; Guo, M.-M.; Xu, S.; Shen, C.; Zhou, X.; Chu, X.-Q.; Ma, M.; Shen, Z.-L. Nickel-Catalyzed Diastereoselective Reductive Cross-Coupling of Disubstituted Cycloalkyl Iodides with Aryl Iodides. Org. Lett. 2021, 23, 5118–5122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, N.-N.; Yu, Z.-L.; Shen, C.; Zhou, X.; Chu, X.-Q.; Rao, W.; Shen, Z.-L. Palladium-Catalyzed Direct Reductive Cross-Coupling of Aryltrimethylammonium Salts with Aryl Bromides. Org. Chem. Front. 2021, 8, 4865–4870. [Google Scholar] [CrossRef]
- Cui, Y.-Y.; Li, W.-X.; Ma, N.-N.; Shen, C.; Zhou, X.; Chu, X.-Q.; Rao, W.; Shen, Z.-L. Nickel-Catalyzed Direct Cross-Coupling of Heterocyclic Phosphonium Salts with Aryl Bromides. Org. Chem. Front. 2021, 8, 6931–6936. [Google Scholar] [CrossRef]
- Li, W.-X.; Yang, B.-W.; Ying, X.; Zhang, Z.-W.; Chu, X.-Q.; Zhou, X.; Ma, M.; Shen, Z.-L. Nickel-Catalyzed Direct Cross-Coupling of Diaryl Sulfoxide with Aryl Bromide. J. Org. Chem. 2022, 87, 11899–11908. [Google Scholar] [CrossRef]
- Ma, N.-N.; Hu, X.-B.; Wu, Y.-S.; Zheng, Y.-W.; Ma, M.; Chu, X.-Q.; Xu, H.; Luo, H.; Shen, Z.-L. Nickel-Catalyzed Direct Cross-Coupling of Aryl Thioether with Aryl Bromide. Org. Lett. 2023, 25, 1771–1775. [Google Scholar] [CrossRef]
- Ren, J.-A.; Na, J.-H.; Gui, C.; Miao, C.; Chu, X.-Q.; Ma, M.; Xu, H.; Zhou, X.; Shen, Z.-L. Nickel-Catalyzed Direct Cross-Coupling of Unactivated Aryl Fluorides with Aryl Bromides. Org. Lett. 2023, 25, 5525–5529. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-A.; Chen, X.; Gui, C.; Miao, C.; Chu, X.-Q.; Xu, H.; Zhou, X.; Ma, M.; Shen, Z.-L. Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Phosphates with Aryl Bromides. Adv. Synth. Catal. 2023, 365, 2511–2515. [Google Scholar] [CrossRef]
- Liu, X.; He, C.-Y.; Yin, H.-N.; Miao, C.; Chu, X.-Q.; Rao, W.; Xu, H.; Zhou, X.; Shen, Z.-L. Nickel-Catalyzed Cross-Electrophile Coupling of Triazine Esters with Aryl Bromides. Chin. J. Chem. 2023, 41, 3539–3546. [Google Scholar] [CrossRef]
- Xu, H.; He, C.-Y.; Huo, B.-J.; Jing, J.-W.; Miao, C.; Rao, W.; Chu, X.-Q.; Zhou, X.; Shen, Z.-L. Nickel-Catalyzed Cross-Electrophile Coupling of Aryl Thiols with Aryl Bromides via C–S Bond Activation. Org. Chem. Front. 2023, 10, 5171–5179. [Google Scholar] [CrossRef]
- Wang, Q.-D.; Liu, X.; Zheng, Y.-W.; Wu, Y.-S.; Zhou, X.; Yang, J.-M.; Shen, Z.-L. Iron-Mediated Reductive Amidation of Triazine Esters with Nitroarenes. Org. Lett. 2024, 26, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tong, M.; Yang, Y.; Zhang, B.; Liu, H.; Li, H.; Zhang, F. Visible Light-Catalytic Hydroxylation of Aryl Halides with Water to Phenols by Carbon Nitride and Nickel Complex Cooperative Catalysis. Green Chem. 2020, 22, 7417–7423. [Google Scholar] [CrossRef]
- Natarajan, P.; Chaudhary, R.; Rani, N.; Sakshi; Venugopalan, P. 3-(Ethoxycarbonyl)-1-(5-methyl-5-(nitrosooxy)hexyl)pyridin-1-ium Cation: A Green Alternative to tert-Butyl Nitrite for Synthesis of Nitro-Group-Containing Arenes and Drugs at Room Temperature. Tetrahedron Lett. 2020, 61, 151529. [Google Scholar] [CrossRef]
- Yang, X.-J.; Zheng, Y.-W.; Zheng, L.-Q.; Wu, L.-Z.; Tung, C.-H.; Chen, B. Visible Light-Catalytic Dehydrogenation of Benzylic Alcohols to Carbonyl Compounds by Using an Eosin Y and Nickel–Thiolate Complex Dual Catalyst System. Green Chem. 2019, 21, 1401–1405. [Google Scholar] [CrossRef]
- Suchand, B.; Satyanarayana, G. Palladium-Catalyzed Direct Acylation: One-Pot Relay Synthesis of Anthraquinones. Synthesis 2019, 51, 769–779. [Google Scholar]
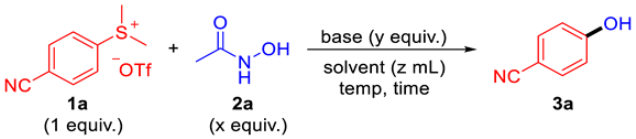 | ||||||
|---|---|---|---|---|---|---|
| Entry | 2a (x equiv.) | Base (y equiv.) | Solvent (z mL) | Temp. (°C) | Time (h) | Yield (%) b |
| 1 | 3 equiv. | DBU (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 9 |
| 2 | 3 equiv. | DABCO (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | <5 |
| 3 | 3 equiv. | CsF (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 31 |
| 4 | 3 equiv. | NaOAc (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | <5 |
| 5 | 3 equiv. | K3PO4 (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 27 |
| 6 | 3 equiv. | Na2CO3 (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 10 |
| 7 | 3 equiv. | K2CO3 (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 52 |
| 8 | 3 equiv. | Cs2CO3 (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 66 |
| 9 | 3 equiv. | KOH (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 40 |
| 10 | 3 equiv. | tBuOK (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 6 |
| 11 | 3 equiv. | LDA (5 equiv.) | DMSO (1 mL) | 80 °C | 18 h | 11 |
| 12 | 3 equiv. | Cs2CO3 (5 equiv.) | DMF (1 mL) | 80 °C | 18 h | 53 |
| 13 | 3 equiv. | Cs2CO3 (5 equiv.) | 1,4-dioxane (1 mL) | 80 °C | 18 h | 11 |
| 14 | 3 equiv. | Cs2CO3 (5 equiv.) | NMP (1 mL) | 80 °C | 18 h | 56 |
| 15 | 3 equiv. | Cs2CO3 (5 equiv.) | MeCN (1 mL) | 80 °C | 18 h | 24 |
| 16 | 3 equiv. | Cs2CO3 (5 equiv.) | toluene (1 mL) | 80 °C | 18 h | 6 |
| 17 | 3 equiv. | Cs2CO3 (5 equiv.) | THF (1 mL) | 80 °C | 18 h | 5 |
| 18 | 3 equiv. | Cs2CO3 (5 equiv.) | H2O (1 mL) | 80 °C | 18 h | <5 |
| 19 | 3 equiv. | Cs2CO3 (5 equiv.) | DMSO (1 mL) | 60 °C | 18 h | 66 |
| 20 | 3 equiv. | Cs2CO3 (5 equiv.) | DMSO (1 mL) | 100 °C | 18 h | 69 |
| 21 | 3 equiv. | Cs2CO3 (5 equiv.) | DMSO (2 mL) | 80 °C | 18 h | 81 (86) c |
| 22 | 3 equiv. | Cs2CO3 (3 equiv.) | DMSO (2 mL) | 80 °C | 18 h | 78 |
| 23 | 3 equiv. | Cs2CO3 (7 equiv.) | DMSO (2 mL) | 80 °C | 18 h | 81 |
| 24 | 3 equiv. | Cs2CO3 (5 equiv.) | DMSO (2 mL) | 80 °C | 12 h | 80 |
| 25 | 3 equiv. | Cs2CO3 (5 equiv.) | DMSO (2 mL) | 80 °C | 24 h | 82 |
| 26 | 2 equiv. | Cs2CO3 (5 equiv.) | DMSO (2 mL) | 80 °C | 18 h | 69 |
| 27 | 4 equiv. | Cs2CO3 (5 equiv.) | DMSO (2 mL) | 80 °C | 18 h | 79 |
| 28 | 3 equiv. | Cs2CO3 (5 equiv.) | DMSO (4 mL) | 80 °C | 18 h | 86 |
 | |||
|---|---|---|---|
| Entry | Product | Yield (%) b | |
| 1 | 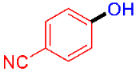 | 3a | 86 |
| 2 | 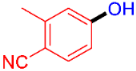 | 3b | 49 |
| 3 | 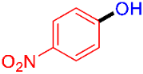 | 3c | 95 |
| 4 |  | 3d | 79 |
| 5 | 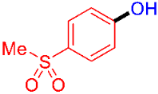 | 3e | 81 |
| 6 | 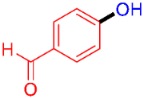 | 3f | 61 |
| 7 | 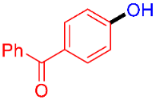 | 3g | 56 |
| 8 | 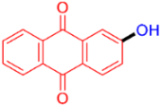 | 3h | 73 |
| 9 | 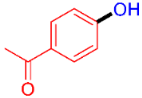 | 3i | 51 |
| 10 | 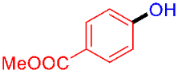 | 3j | 54 |
| 11 | 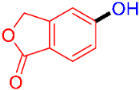 | 3k | 70 |
 | |||
|---|---|---|---|
| Entry | Base | Solvent | Yield (%) b |
| 1 | Cs2CO3 | DMSO | 58 |
| 2 | Cs2CO3 | NMP | 63 |
| 3 | Cs2CO3 | MeCN | 62 |
| 4 | Cs2CO3 | 1,4-dioxane | 54 |
| 5 | Cs2CO3 | toluene | 15 |
| 6 | Cs2CO3 | THF | 56 |
| 7 | Cs2CO3 | DMF | 67 |
| 8 | DBU | DMF | 16 |
| 9 | DABCO | DMF | <5 |
| 10 | DIPEA | DMF | <5 |
| 11 | CsF | DMF | 57 |
| 12 | NaOAc | DMF | <5 |
| 13 | K3PO4 | DMF | 56 |
| 14 | NaHCO3 | DMF | <5 |
| 15 | KHCO3 | DMF | 22 |
| 16 | Na2CO3 | DMF | <5 |
| 17 | K2CO3 | DMF | 47 |
| 18 | tBuOK | DMF | 66 |
| 19 | LDA | DMF | 58 |
| 20 | Cs2CO3 | DMF | 74 c (70) d |
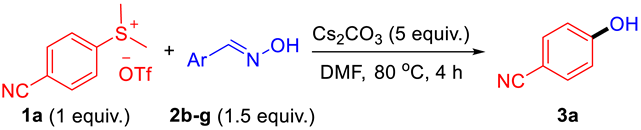 | |||
|---|---|---|---|
| Entry | Oxime | Yield (%) b | |
| 1 |  | 2b | 74 |
| 2 | 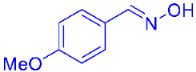 | 2c | 41 |
| 3 | 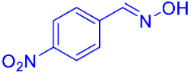 | 2d | 82 |
| 4 | 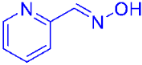 | 2e | 45 |
| 5 | 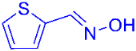 | 2f | 38 |
| 6 | 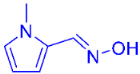 | 2g | 89 (87) c |
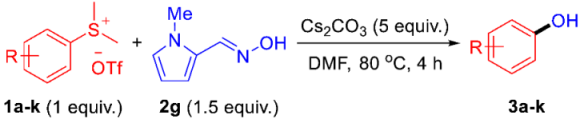 | |||
|---|---|---|---|
| Entry | Product | Yield (%) b | |
| 1 | 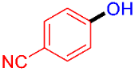 | 3a | 87 |
| 2 | 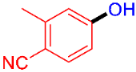 | 3b | 59 |
| 3 | 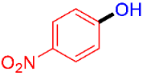 | 3c | 95 |
| 4 | 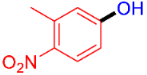 | 3d | 55 |
| 5 | 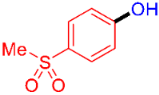 | 3e | 79 |
| 6 |  | 3f | 52 |
| 7 | 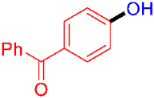 | 3g | 53 |
| 8 | 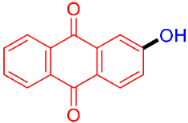 | 3h | 68 |
| 9 | 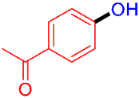 | 3i | 60 |
| 10 | 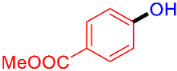 | 3j | 47 |
| 11 | 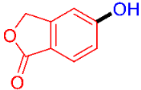 | 3k | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.-B.; Fu, Q.-Q.; Huang, X.-Y.; Chu, X.-Q.; Shen, Z.-L.; Miao, C.; Chen, W. Hydroxylation of Aryl Sulfonium Salts for Phenol Synthesis under Mild Reaction Conditions. Molecules 2024, 29, 831. https://doi.org/10.3390/molecules29040831
Hu X-B, Fu Q-Q, Huang X-Y, Chu X-Q, Shen Z-L, Miao C, Chen W. Hydroxylation of Aryl Sulfonium Salts for Phenol Synthesis under Mild Reaction Conditions. Molecules. 2024; 29(4):831. https://doi.org/10.3390/molecules29040831
Chicago/Turabian StyleHu, Xuan-Bo, Qian-Qian Fu, Xue-Ying Huang, Xue-Qiang Chu, Zhi-Liang Shen, Chengping Miao, and Weiyi Chen. 2024. "Hydroxylation of Aryl Sulfonium Salts for Phenol Synthesis under Mild Reaction Conditions" Molecules 29, no. 4: 831. https://doi.org/10.3390/molecules29040831
APA StyleHu, X.-B., Fu, Q.-Q., Huang, X.-Y., Chu, X.-Q., Shen, Z.-L., Miao, C., & Chen, W. (2024). Hydroxylation of Aryl Sulfonium Salts for Phenol Synthesis under Mild Reaction Conditions. Molecules, 29(4), 831. https://doi.org/10.3390/molecules29040831







