Sustainable Production of Biofuels and Biochemicals via Electro-Fermentation Technology
Abstract
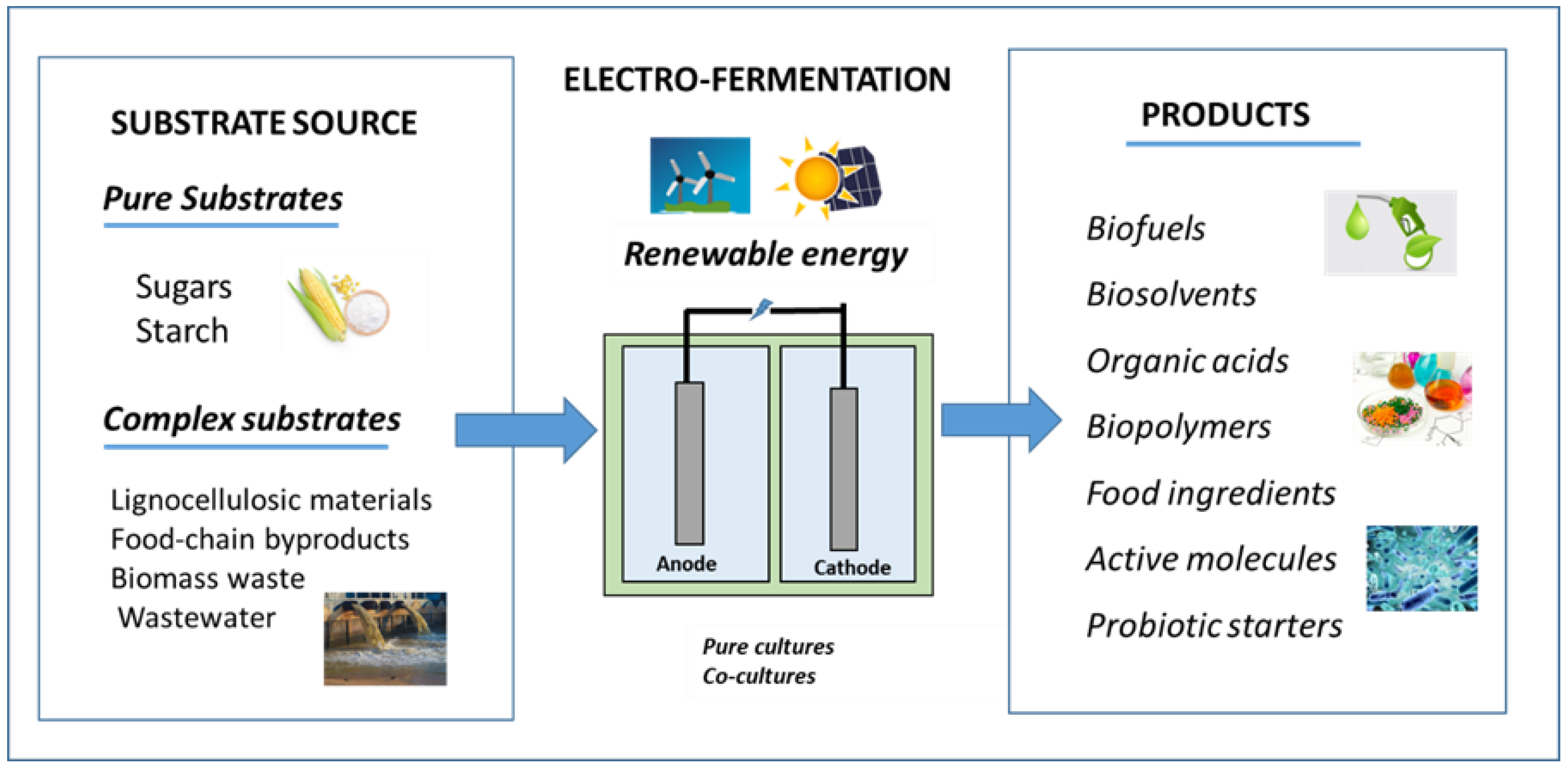
1. Electro-Fermentation as a Sustainable Platform for a Circular Bioeconomy
2. Principles of Electro-Fermentation
3. Process Design and Electro-Fermenter Configurations
3.1. Single-Chamber Electro-Fermenters
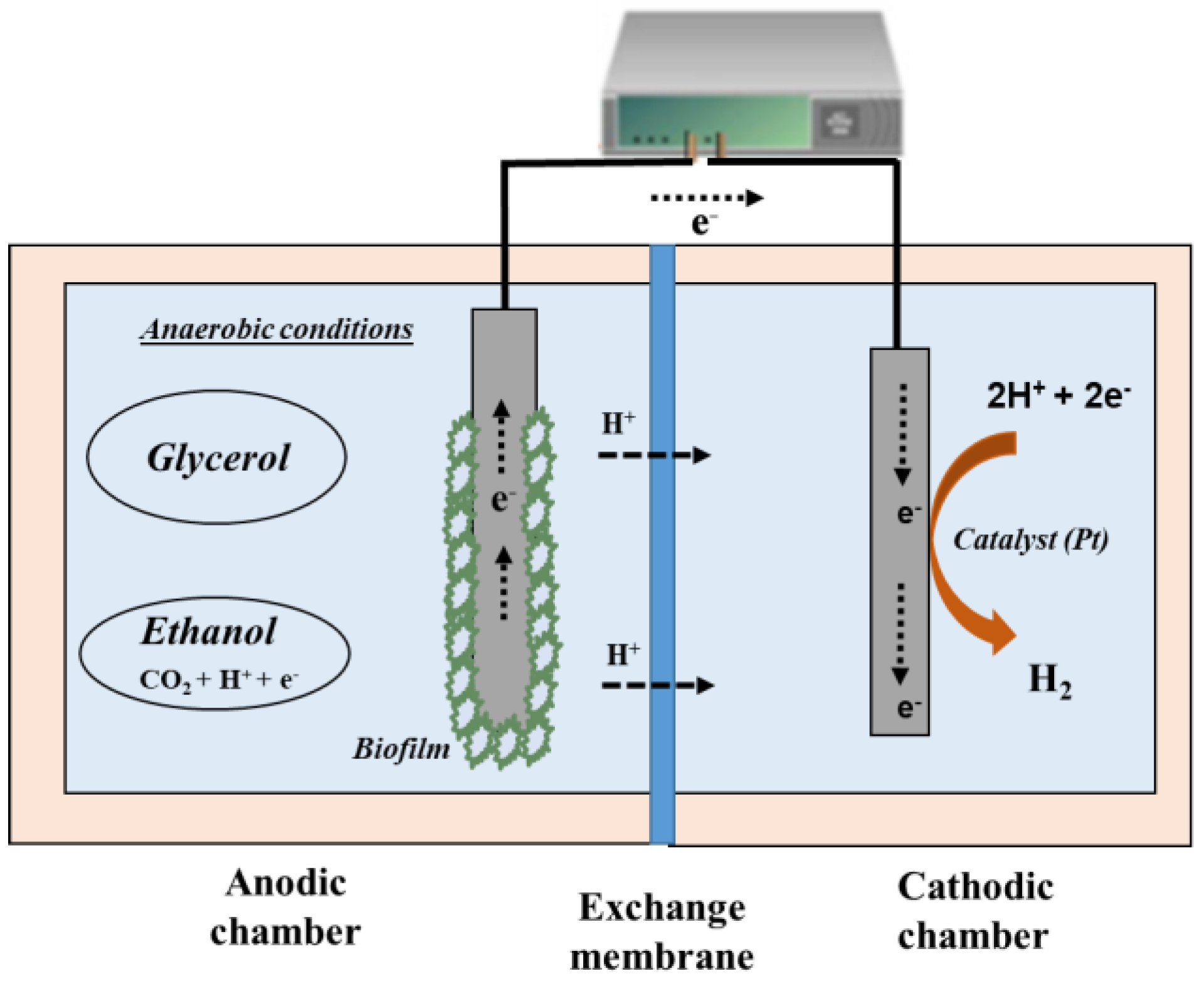
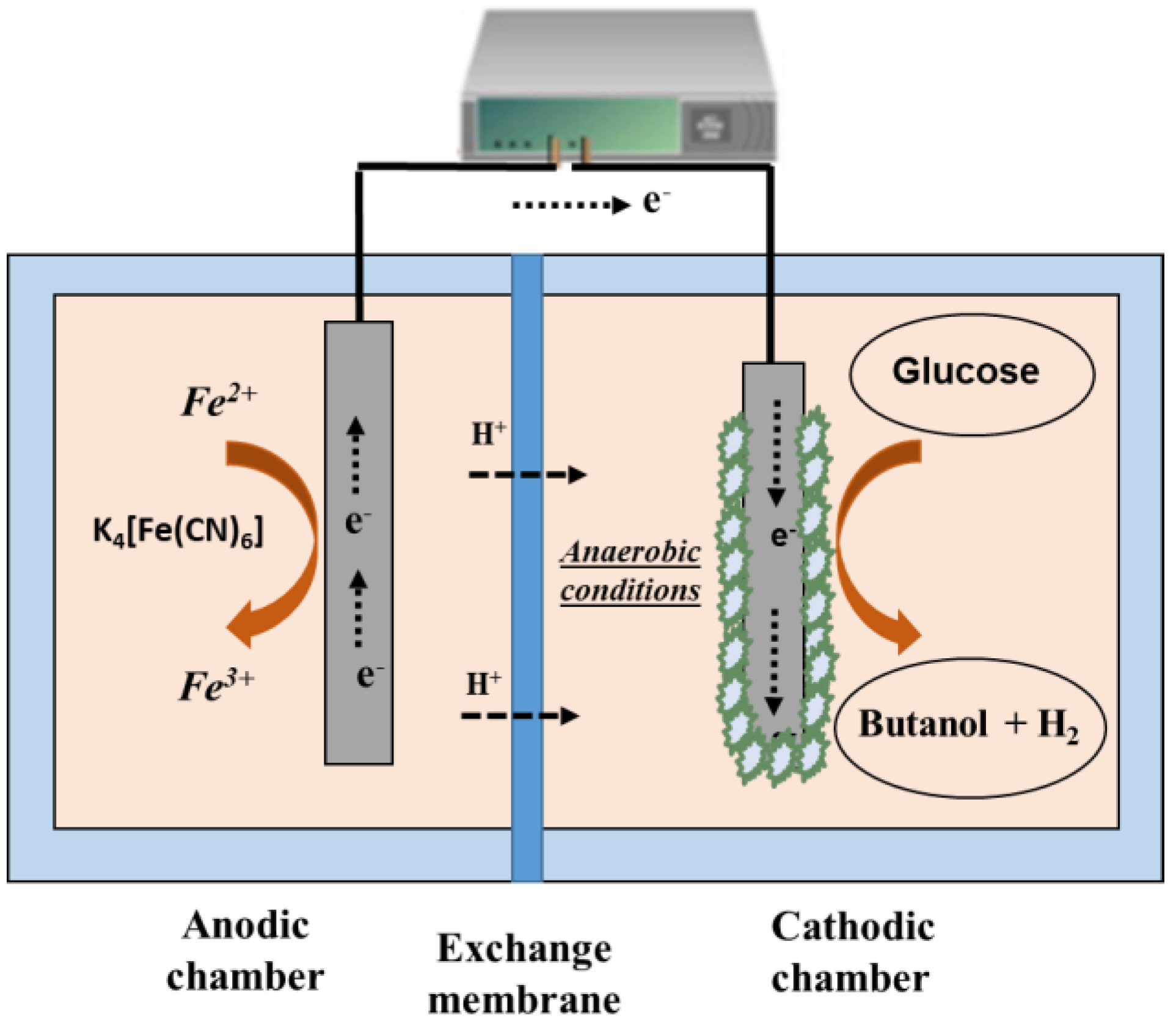
3.2. Double-Chamber Electro-Fermenters
3.3. Hybrid Configurations of Electro-Fermenters
4. Production of Platform Chemicals through Electro-Fermentation
4.1. Organic Acids
4.2. Alcohols
5. Current Challenges and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Updated Bioeconomy Strategy 2018|Knowledge for Policy. 2018. Available online: https://knowledge4policy.ec.europa.eu/publication/updated-bioeconomy-strategy-2018_en (accessed on 15 December 2023).
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus. 2021, 4, 100036. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Emerging technologies for biofuel production: A critical review on recent progress, challenges and perspectives. J. Environ. Manag. 2021, 290, 112627. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Bhagchandanii, D.D.; Babu, R.P.; Sonawane, J.M. A Comprehensive Understanding of Electro-Fermentation. Fermentation 2020, 6, 92. [Google Scholar] [CrossRef]
- Sriram, S.; Wong, J.W.C.; Pradhan, N. Recent advances in electro-fermentation technology: A novel approach towards balanced fermentation. Bioresour. Technol. 2022, 360, 127637. [Google Scholar] [CrossRef]
- Laurinavichene, T.; Tekucheva, D.; Laurinavichius, K.; Tsygankov, A. Utilization of distillery wastewater for hydrogen production in one-stage and two-stage processes involving photofermentation. Enzyme Microb. Technol. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical Effects Accompanying the Decomposition of Organic Compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276. [Google Scholar]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Muddasar, M.; Liaquat, R.; Aslam, A.; Rahman, M.Z.U.; Abdullah, A.; Khoja, A.H.; Latif, K.; Bahadar, A. Performance efficiency comparison of microbial electrolysis cells for sustainable production of biohydrogen—A comprehensive review. Int. J. Energy Res. 2022, 46, 5625–5645. [Google Scholar] [CrossRef]
- Jiang, Y.; May, H.D.; Lu, L.; Liang, P.; Huang, X.; Ren, Z.J. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019, 149, 42–55. [Google Scholar] [CrossRef]
- Virdis, B.; Hoelzle, R.D.; Marchetti, A.; Boto, S.T.; Rosenbaum, M.A.; Blasco-Gómez, R.; Puig, S.; Freguia, S.; Villano, M. Electro-fermentation: Sustainable bioproductions steered by electricity. Biotechnol. Adv. 2022, 59, 107950. [Google Scholar] [CrossRef] [PubMed]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-Fermentation: How To Drive Fermentation Using Electrochemical Systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Ragauskas, A.J. Editorial overview: Energy Biotechnology. Curr. Opin. Biotechnol. 2014, 27, v–vi. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Krömer, J.O. Identifying target processes for microbial electrosynthesis by elementary mode analysis. BMC Bioinform. 2014, 15, 410. [Google Scholar] [CrossRef]
- Mier, A.A.; Olvera-Vargas, H.; Mejía-López, M.; Longoria, A.; Verea, L.; Sebastian, P.J.; Arias, D.M. A review of recent advances in electrode materials for emerging bioelectrochemical systems: From biofilm-bearing anodes to specialized cathodes. Chemosphere 2021, 283, 131138. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, L.; Gu, J.D. Microbial electrocatalysis: Redox mediators responsible for extracellular electron transfer. Biotechnol. Adv. 2018, 36, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Yu, H.; Zhang, J.; Li, F.; Song, H. Microbial electro-fermentation for synthesis of chemicals and biofuels driven by bi-directional extracellular electron transfer. Synth. Syst. Biotechnol. 2020, 5, 304–313. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, A.N.; Kumar, G.; Kim, D.H.; Song, Y.C.; Kim, S.H. Electro-fermentation for biofuels and biochemicals production: Current status and future directions. Bioresour. Technol. 2021, 323, 124598. [Google Scholar] [CrossRef]
- Xue, G.; Lai, S.; Li, X.; Zhang, W.; You, J.; Chen, H.; Qian, Y.; Gao, P.; Liu, Z.; Liu, Y. Efficient bioconversion of organic wastes to high optical activity of L-lactic acid stimulated by cathode in mixed microbial consortium. Water Res. 2018, 131, 1–10. [Google Scholar] [CrossRef]
- Liu, C.-G.; Qin, J.-C.; Lin, Y.-H.; Liu, C.-G.; Qin, J.-C.; Lin, Y.-H. Fermentation and Redox Potential. In Fermentation Processes; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Hoelzle, R.D.; Virdis, B.; Batstone, D.J. Regulation mechanisms in mixed and pure culture microbial fermentation. Biotechnol. Bioeng. 2014, 111, 2139–2154. [Google Scholar] [CrossRef]
- Liu, L.; Bilal, M.; Luo, H.; Zhao, Y.; Iqbal, H.M.N. Metabolic Engineering and Fermentation Process Strategies for L-Tryptophan Production by Escherichia coli. Processes 2019, 7, 213. [Google Scholar] [CrossRef]
- Liu, C.G.; Xue, C.; Lin, Y.H.; Bai, F.W. Redox potential control and applications in microaerobic and anaerobic fermentations. Biotechnol. Adv. 2013, 31, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation—The foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 146906. [Google Scholar] [CrossRef] [PubMed]
- Yoruklu, H.C.; Koroglu, E.O.; Demir, A.; Ozkaya, B. The Electromotive-Induced Regulation of Anaerobic Fermentation: Electrofermentation. In Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 739–756. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Meng, J.; Sun, K.; Yan, H. A neutral red mediated electro-fermentation system of Clostridium beijerinckii for effective co-production of butanol and hydrogen. Bioresour. Technol. 2021, 332, 125097. [Google Scholar] [CrossRef] [PubMed]
- Schievano, A.; Sciarria, T.P.; Vanbroekhoven, K.; De Wever, H.; Puig, S.; Andersen, S.J.; Rabaey, K.; Pant, D. Electro-Fermentation—Merging Electrochemistry with Fermentation in Industrial Applications. Trends Biotechnol. 2016, 34, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef] [PubMed]
- Heyndrickx, M.; De Vos, P.; De Ley, J. Fermentation characteristics of Clostridium pasteurianum LMG 3285 grown on glucose and mannitol. J. Appl. Bacteriol. 1991, 70, 52–58. [Google Scholar] [CrossRef]
- Moscoviz, R.; de Fouchécour, F.; Santa-Catalina, G.; Bernet, N.; Trably, E. Cooperative growth of Geobacter sulfurreducens and Clostridium pasteurianum with subsequent metabolic shift in glycerol fermentation. Sci. Rep. 2017, 7, 44334. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Sang, B.I. Extracellular electron transfer from cathode to microbes: Application for biofuel production. Biotechnol. Biofuels. 2016, 9, 11. [Google Scholar] [CrossRef]
- Sharma, M.; Alvarez-Gallego, Y.; Achouak, W.; Pant, D.; Sarma, P.M.; Dominguez-Benetton, X. Electrode material properties for designing effective microbial electrosynthesis systems. J. Mater. Chem. A 2019, 7, 24420–24436. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Wang, L.; Quan, X. Potential for direct interspecies electron transfer in an electric-anaerobic system to increase methane production from sludge digestion. Sci. Rep. 2015, 5, 11094. [Google Scholar] [CrossRef]
- Zhen, G.; Kobayashi, T.; Lu, X.; Kumar, G.; Xu, K. Biomethane recovery from Egeria densa in a microbial electrolysis cell-assisted anaerobic system: Performance and stability assessment. Chemosphere 2016, 149, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Hu, A.; Huang, S.; Ye, J.; Tang, J.; Zhou, S. Graphite-assisted electro-fermentation methanogenesis: Spectroelectrochemical and microbial community analyses of cathode biofilms. Bioresour. Technol. 2018, 269, 74–80. [Google Scholar] [CrossRef]
- Sravan, J.S.; Butti, S.K.; Sarkar, O.; Krishna, K.V.; Mohan, S.V. Electrofermentation of food waste—Regulating acidogenesis towards enhanced volatile fatty acids production. Chem. Eng. J. 2018, 334, 1709–1718. [Google Scholar] [CrossRef]
- d’Ippolito, G.; Squadrito, G.; Tucci, M.; Esercizio, N.; Sardo, A.; Vastano, M.; Lanzilli, M.; Fontana, A.; Cristiani, P. Electrostimulation of hyperthermophile Thermotoga neapolitana cultures. Bioresour. Technol. 2021, 319, 124078. [Google Scholar] [CrossRef]
- Paiano, P.; Premier, G.; Guwy, A.; Kaur, A.; Michie, I.; Majone, M.; Villano, M. Simplified Reactor Design for Mixed Culture-Based Electrofermentation toward Butyric Acid Production. Processes 2021, 9, 417. [Google Scholar] [CrossRef]
- Mukherjee, T.; Mohan, S.V. Metabolic flux of Bacillus subtilis under poised potential in electrofermentation system: Gene expression vs product formation. Bioresour. Technol. 2021, 342, 125854. [Google Scholar] [CrossRef]
- Sasaki, D.; Sasaki, K.; Watanabe, A.; Morita, M.; Matsumoto, N.; Igarashi, Y.; Ohmura, N. Operation of a cylindrical bioelectrochemical reactor containing carbon fiber fabric for efficient methane fermentation from thickened sewage sludge. Bioresour. Technol. 2013, 129, 366–373. [Google Scholar] [CrossRef]
- Mostafazadeh, A.K.; Drogui, P.; Brar, S.K.; Tyagi, R.D.; Le Bihan, Y.; Buelna, G.; Rasolomanana, S.D. Enhancement of biobutanol production by electromicrobial glucose conversion in a dual chamber fermentation cell using C. pasteurianum. Energy Convers. Manag. 2016, 130, 165–175. [Google Scholar] [CrossRef]
- Villano, M.; Paiano, P.; Palma, E.; Miccheli, A.; Majone, M. Electrochemically Driven Fermentation of Organic Substrates with Undefined Mixed Microbial Cultures. ChemSusChem 2017, 10, 3091–3097. [Google Scholar] [CrossRef]
- Isipato, M.; Dessì, P.; Sánchez, C.; Mills, S.; Ijaz, U.Z.; Asunis, F.; Spiga, D.; De Gioannis, G.; Mascia, M.; Collins, G.; et al. Propionate Production by Bioelectrochemically-Assisted Lactate Fermentation and Simultaneous CO2 Recycling. Front. Microbiol. 2020, 11, 3184. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, L.; Wang, H.; Shen, R.; Ge, Z.; Hou, D.; Chen, X.; Liang, P.; Huang, X.; Ren, Z.J. Electrochemical Control of Redox Potential Arrests Methanogenesis and Regulates Products in Mixed Culture Electro-Fermentation. ACS Sustain. Chem. Eng. 2018, 6, 8650–8658. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Amulya, K.; Mohan, S.V. Solid phase bio-electrofermentation of food waste to harvest value-added products associated with waste remediation. Waste Manag. 2015, 45, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, G.N.; Subhash, G.V.; Yeruva, D.K.; Mohan, S.V. Synergistic yield of dual energy forms through biocatalyzed electrofermentation of waste: Stoichiometric analysis of electron and carbon distribution. Energy 2015, 88, 281–291. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Heijne, A.T.; Grootscholten, T.I.M.; Steinbusch, K.J.J.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Bioelectrochemical production of caproate and caprylate from acetate by mixed cultures. ACS Sustain. Chem. Eng. 2013, 1, 513–518. [Google Scholar] [CrossRef]
- Borole, A.P.; Tsouris, C.; Pavlostathis, S.G.; Yiacoumi, S.; Lewis, A.J.; Zeng, X.; Park, L. Efficient conversion of aqueous-waste-carbon compounds into electrons, hydrogen, and chemicals via separations and microbial electrocatalysis. Front. Energy Res. 2018, 6, 94. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S.I.; Nigam, P.S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Sravan, J.S.; Butti, S.K.; Sarkar, O.; Mohan, S.V. Electrofermentation: Chemicals and Fuels. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 723–737. [Google Scholar] [CrossRef]
- Bajracharya, S.; Vanbroekhoven, K.; Buisman, C.J.N.; Pant, D.; Strik, D.P.B.T.B. Application of gas diffusion biocathode in microbial electrosynthesis from carbon dioxide. Environ. Sci. Pollut. Res. 2016, 23, 22292–22308. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial electrosynthesis: Feeding microbes electricity to convert carbon dioxide and water to multicarbon extracellular organic compounds. mBio 2010, 1, e00103-10. [Google Scholar] [CrossRef]
- Gildemyn, S.; Verbeeck, K.; Jansen, R.; Rabaey, K. The type of ion selective membrane determines stability and production levels of microbial electrosynthesis. Bioresour. Technol. 2017, 224, 358–364. [Google Scholar] [CrossRef]
- Kracke, F.; Wong, A.B.; Maegaard, K.; Deutzmann, J.S.; Hubert, M.K.A.; Hahn, C.; Jaramillo, T.F.; Spormann, A.M. Robust and biocompatible catalysts for efficient hydrogen-driven microbial electrosynthesis. Commun. Chem. 2019, 2, 45. [Google Scholar] [CrossRef]
- Sturm-Richter, K.; Golitsch, F.; Sturm, G.; Kipf, E.; Dittrich, A.; Beblawy, S.; Kerzenmacher, S.; Gescher, J. Unbalanced fermentation of glycerol in Escherichia coli via heterologous production of an electron transport chain and electrode interaction in microbial electrochemical cells. Bioresour. Technol. 2015, 186, 89–96. [Google Scholar] [CrossRef]
- TerAvest, M.A.; Zajdel, T.J.; Ajo-Franklin, C.M. The Mtr Pathway of Shewanella oneidensis MR-1 Couples Substrate Utilization to Current Production in Escherichia coli. ChemElectroChem 2014, 1, 1874–1879. [Google Scholar] [CrossRef]
- Schuppert, B.; Schink, B.; Trösch, W. Batch and continuous production of propionic acid from whey permeate by Propionibacterium acidi-propionici in a three-electrode amperometric culture system. Appl. Microbiol. Biotechnol. 1992, 37, 549–553. [Google Scholar] [CrossRef]
- Choi, O.; Um, Y.; Sang, B.I. Butyrate production enhancement by Clostridium tyrobutyricum using electron mediators and a cathodic electron donor. Biotechnol. Bioeng. 2012, 109, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Ganigué, R.; Puig, S.; Batlle-Vilanova, P.; Balaguer, M.D.; Colprim, J. Microbial electrosynthesis of butyrate from carbon dioxide. Chem. Commun. 2015, 51, 3235–3238. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, J.; Liu, J.; Wang, Y.; Bi, C.; Zhang, X. Engineering an electroactive Escherichia coli for the microbial electrosynthesis of succinate from glucose and CO2. Microb. Cell Fact. 2019, 18, 15. [Google Scholar] [CrossRef]
- Kim, C.; Kim, M.Y.; Michie, I.; Jeon, B.H.; Premier, G.C.; Park, S.; Kim, J.R. Anodic electro-fermentation of 3-hydroxypropionic acid from glycerol by recombinant Klebsiella pneumoniae L17 in a bioelectrochemical system. Biotechnol. Biofuels. 2017, 10, 199. [Google Scholar] [CrossRef]
- Kucek, L.A.; Xu, J.; Nguyen, M.; Angenent, L.T. Waste conversion into n-caprylate and n-caproate: Resource recovery from wine lees using anaerobic reactor microbiomes and in-line extraction. Front. Microbiol. 2016, 7, 217840. [Google Scholar] [CrossRef]
- Flynn, J.M.; Ross, D.E.; Hunt, K.A.; Bond, D.R.; Gralnick, J.A. Enabling unbalanced fermentations by using engineered electrode–interfaced bacteria. mBio 2010, 1, e00190-10. [Google Scholar] [CrossRef] [PubMed]
- Awate, B.; Steidl, R.J.; Hamlischer, T.; Reguera, G. Stimulation of electro-fermentation in single-chamber microbial electrolysis cells driven by genetically engineered anode biofilms. J. Power Sources 2017, 356, 510–518. [Google Scholar] [CrossRef]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Schaap, J.D.; Kampman, C.; Buisman, C.J.N. Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures. Environ. Sci. Technol. 2010, 44, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Kim, T.; Woo, H.M.; Um, Y. Electricity-driven metabolic shift through direct electron uptake by electroactive heterotroph Clostridiumpasteurianum. Sci. Rep. 2014, 4, 6961. [Google Scholar] [CrossRef]
- Saxena, R.K.; Anand, P.; Saran, S.; Isar, J. Microbial production of 1,3-propanediol: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 895–913. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Freguia, S.; Rabaey, K.; Keller, J. Carbon and electron fluxes during the electricity driven 1,3-propanediol biosynthesis from glycerol. Environ. Sci. Technol. 2013, 47, 11199–11205. [Google Scholar] [CrossRef]
- Redwood, M.D.; Orozco, R.L.; Majewski, A.J.; Macaskie, L.E. Electro-extractive fermentation for efficient biohydrogen production. Bioresour. Technol. 2012, 107, 166–174. [Google Scholar] [CrossRef]
- Moscoviz, R.; Trably, E.; Bernet, N. Electro-fermentation triggering population selection in mixed-culture glycerol fermentation. Microb. Biotechnol. 2018, 11, 74–83. [Google Scholar] [CrossRef]
| Fermentation | Electro-Fermentation | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Potential applications |
|
|
| Operation Mode | Electrode Materials | Applied Voltage (V) | Substrate | Biocatalyst | Main Products | Ref. |
|---|---|---|---|---|---|---|
| Batch | Cathode: graphite rod Anode: graphite brush | 0.6 | Glucose | Anaerobic sludge | CH4: 2998.4 mL | [35] |
| Semi-continuous | Ti/RuO2 mesh plate | 0.4 to 1.0 | Domestic wastewater | Egeria densa | CH4: 248.2 ± 21.0 mL.L−1.d−1 | [36] |
| Batch | Graphite plate | −0.6 (vs. SCE) | Synthetic culture media | Anaerobic seed culture | CH4: 3.07 mmol | [37] |
| Fed-batch | Graphite | −0.6 (vs. Ag/AgCl) | Food waste | Sludge from sewage treatment plant | VFA: 4595 mg.L−1 H2 (26%) > CH4 (4%) | [38] |
| Fed-batch | Carbon cloth | ±1.2 (vs. Ag/AgCl) | Glucose | Thermotoga neapolitana | H2: 9.91 mM AA: 0.75 g.L−1 LA: 0.35 g.L−1 | [39] |
| Batch | Graphite | 0.6 to 1.5 | Glucose, acetate, and ethanol | Anaerobic sludge | BA: 0.38 g.L−1 | [40] |
| Batch | Graphite | 0.2, 0.4, 0.6 and 0.8 | Pyruvate | Bacillus subtilis | SA: 0.83 g.L−1 | [41] |
| Operation Mode | Membrane | Electrode Materials | Applied Voltage (V) | Substrate | Biocatalyst | Main Products | Ref. |
|---|---|---|---|---|---|---|---|
| Semi-continuous | Nafion 117 | Cathode: 8 carbon plates Anode: carbon bar | −0.8 V (vs. Ag/AgCl) | Thickened sewage sludge | Thickened sewage sludge | CH4: 3.57 L.L−1.day−1 | [42] |
| Batch | Nafion 117 | Gaphite felt | 1.32 (−540 mV vs. Ag/AgCl) | Glucose | C. pasteurianum | Butanol: 13.31 g.L−1 | [43] |
| Batch | Nafion 117 | Graphite rod | −0.7 vs. SHE | Glucose, acetate, and ethanol | Anaerobic sludge from a mesophilic anaerobic digester | H2: 1.19 mol/mol of glucose BA: 0.3 g.L−1 | [44] |
| Batch | Nafion 117 | Anode: Platinized Ti Mesh Cathode: Carbon Cloth | −1 (vs. Ag/AgCl) | Lactate | Anaerobic sludge from dairy processing plant | PA: 0.9 g.L−1 | [45] |
| Fed-batch | CMI7000 | Graphite felt | −1, −0.6, −0.2 (vs. Ag/AgCl) | Glucose | Anaerobic sludge from wastewater treatment plant | CH4: 4.6 to 6.7 mL AA: 1.3 g.L−1 PA: 0.5 g.L−1 BA: 1.3 g.L−1 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salar-García, M.J.; Ortiz-Martínez, V.M.; Sánchez-Segado, S.; Valero Sánchez, R.; Sáez López, A.; Lozano Blanco, L.J.; Godínez-Seoane, C. Sustainable Production of Biofuels and Biochemicals via Electro-Fermentation Technology. Molecules 2024, 29, 834. https://doi.org/10.3390/molecules29040834
Salar-García MJ, Ortiz-Martínez VM, Sánchez-Segado S, Valero Sánchez R, Sáez López A, Lozano Blanco LJ, Godínez-Seoane C. Sustainable Production of Biofuels and Biochemicals via Electro-Fermentation Technology. Molecules. 2024; 29(4):834. https://doi.org/10.3390/molecules29040834
Chicago/Turabian StyleSalar-García, María José, Víctor Manuel Ortiz-Martínez, Sergio Sánchez-Segado, Raúl Valero Sánchez, Antonia Sáez López, Luis Javier Lozano Blanco, and Carlos Godínez-Seoane. 2024. "Sustainable Production of Biofuels and Biochemicals via Electro-Fermentation Technology" Molecules 29, no. 4: 834. https://doi.org/10.3390/molecules29040834
APA StyleSalar-García, M. J., Ortiz-Martínez, V. M., Sánchez-Segado, S., Valero Sánchez, R., Sáez López, A., Lozano Blanco, L. J., & Godínez-Seoane, C. (2024). Sustainable Production of Biofuels and Biochemicals via Electro-Fermentation Technology. Molecules, 29(4), 834. https://doi.org/10.3390/molecules29040834






