Synthesis of Silane Functionalized LDH-Modified Nanopowders to Improve Compatibility and Enhance Corrosion Protection for Epoxy Coatings
Abstract
1. Introduction
2. Results
2.1. Production and Characterization of Inorganic Fillers
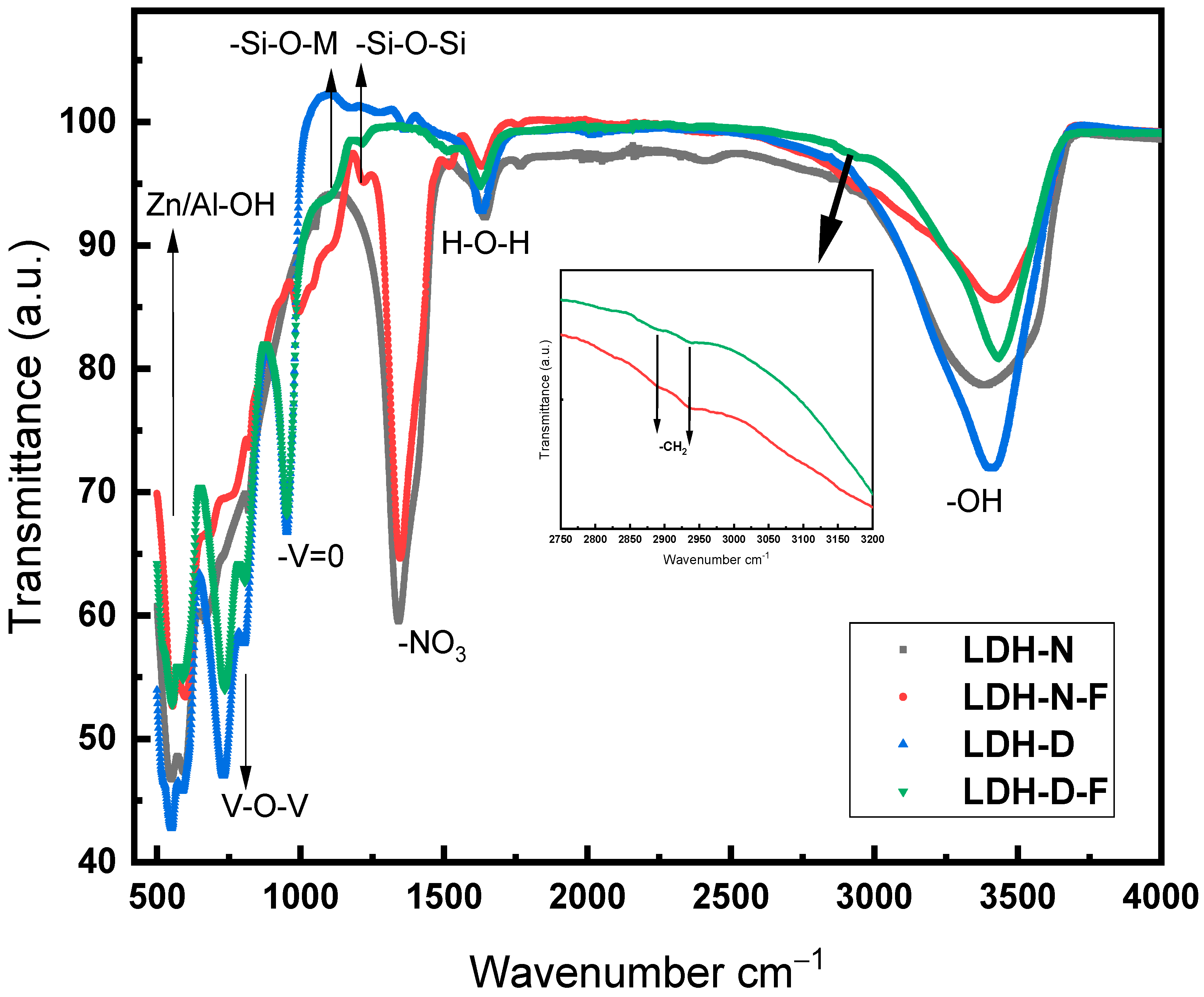
2.1.1. FT-IR Analysis of LDH-Modified Powders
2.1.2. Thermogravimetric Analysis (TGA)
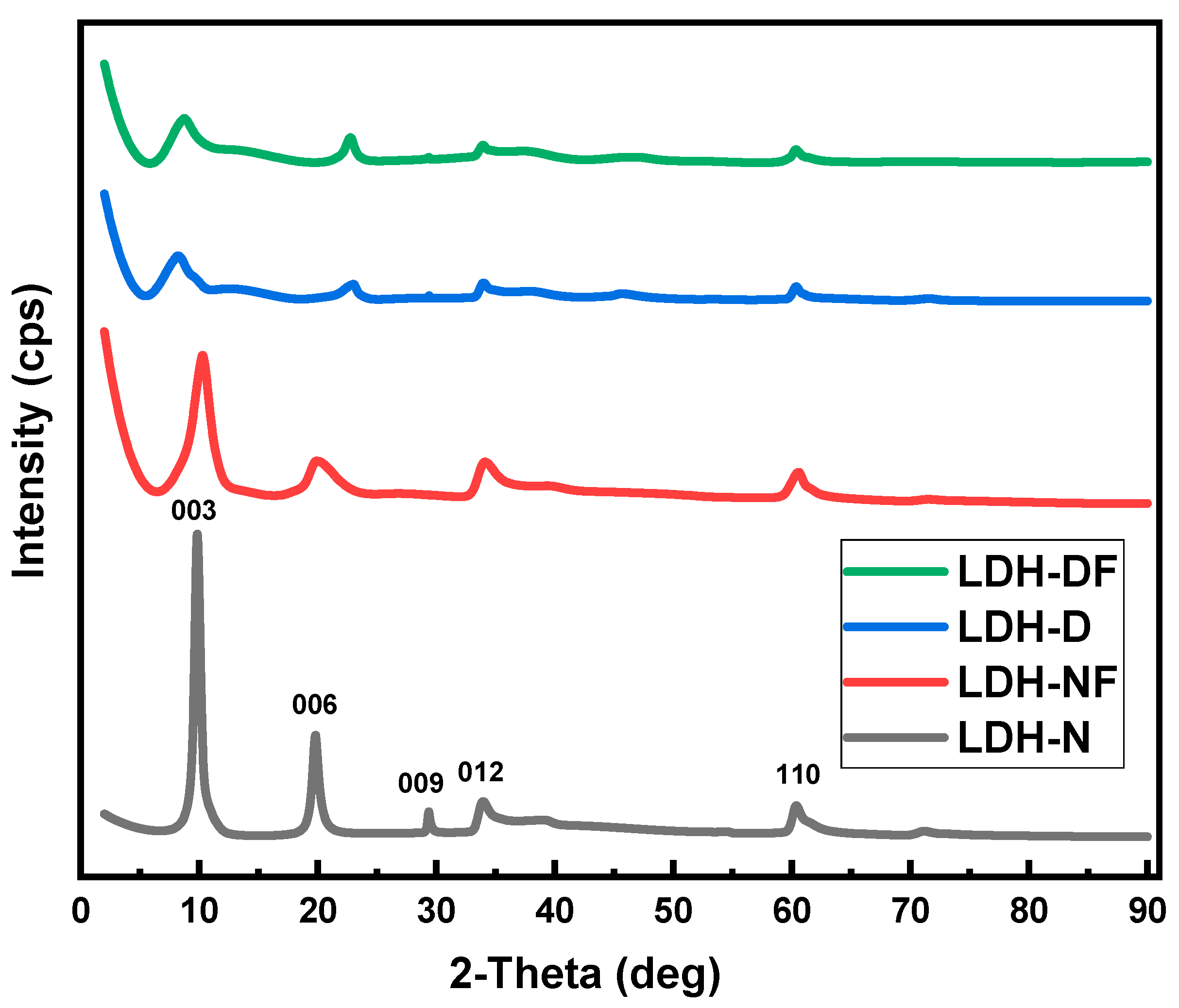
2.1.3. XRD Analysis
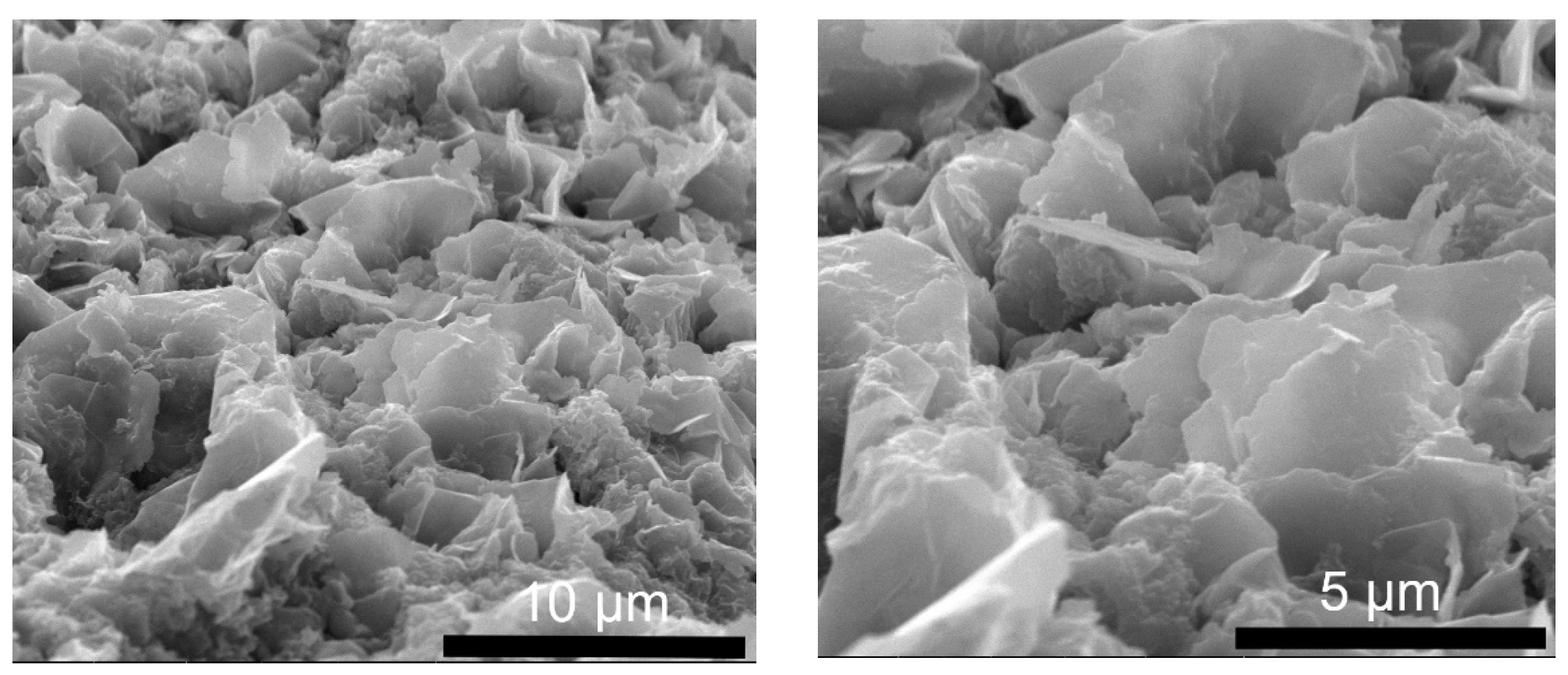
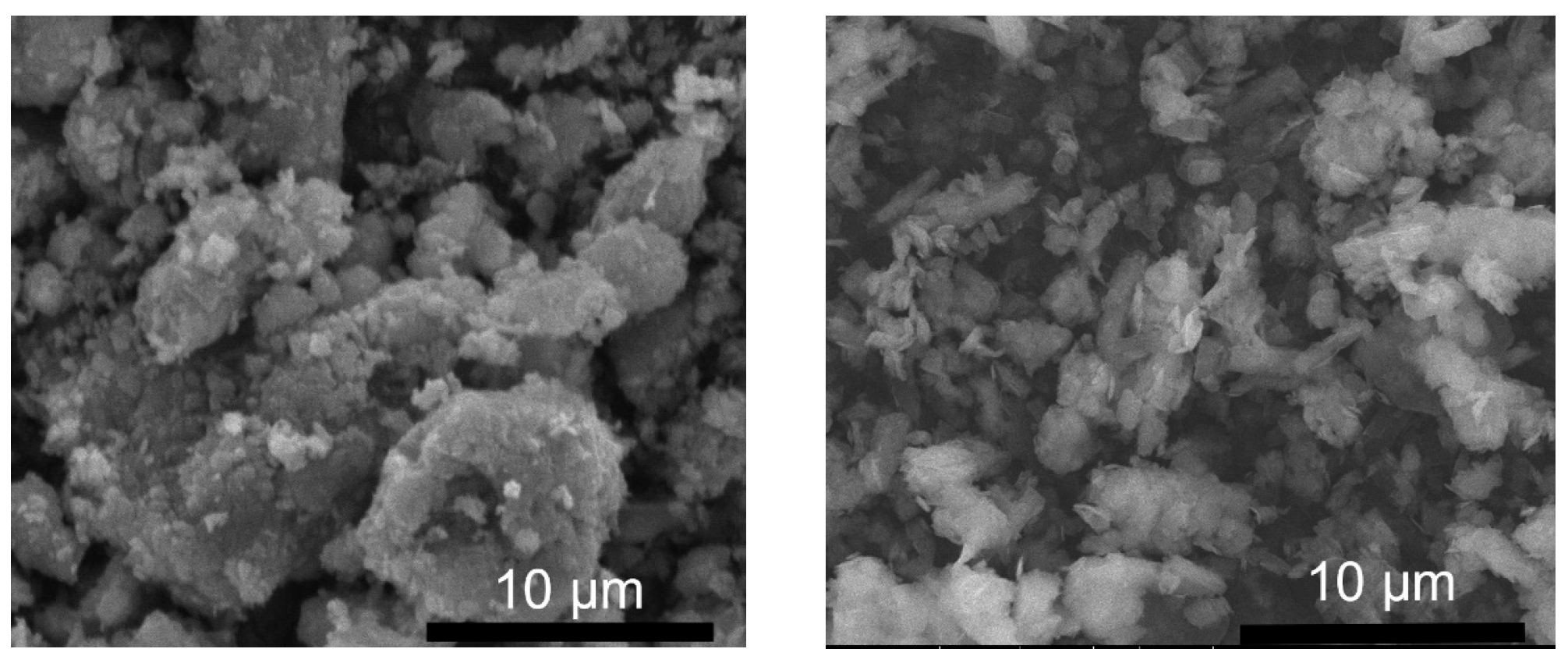
2.1.4. Morphology and Composition of Synthesized LDH Powder Nanoparticles
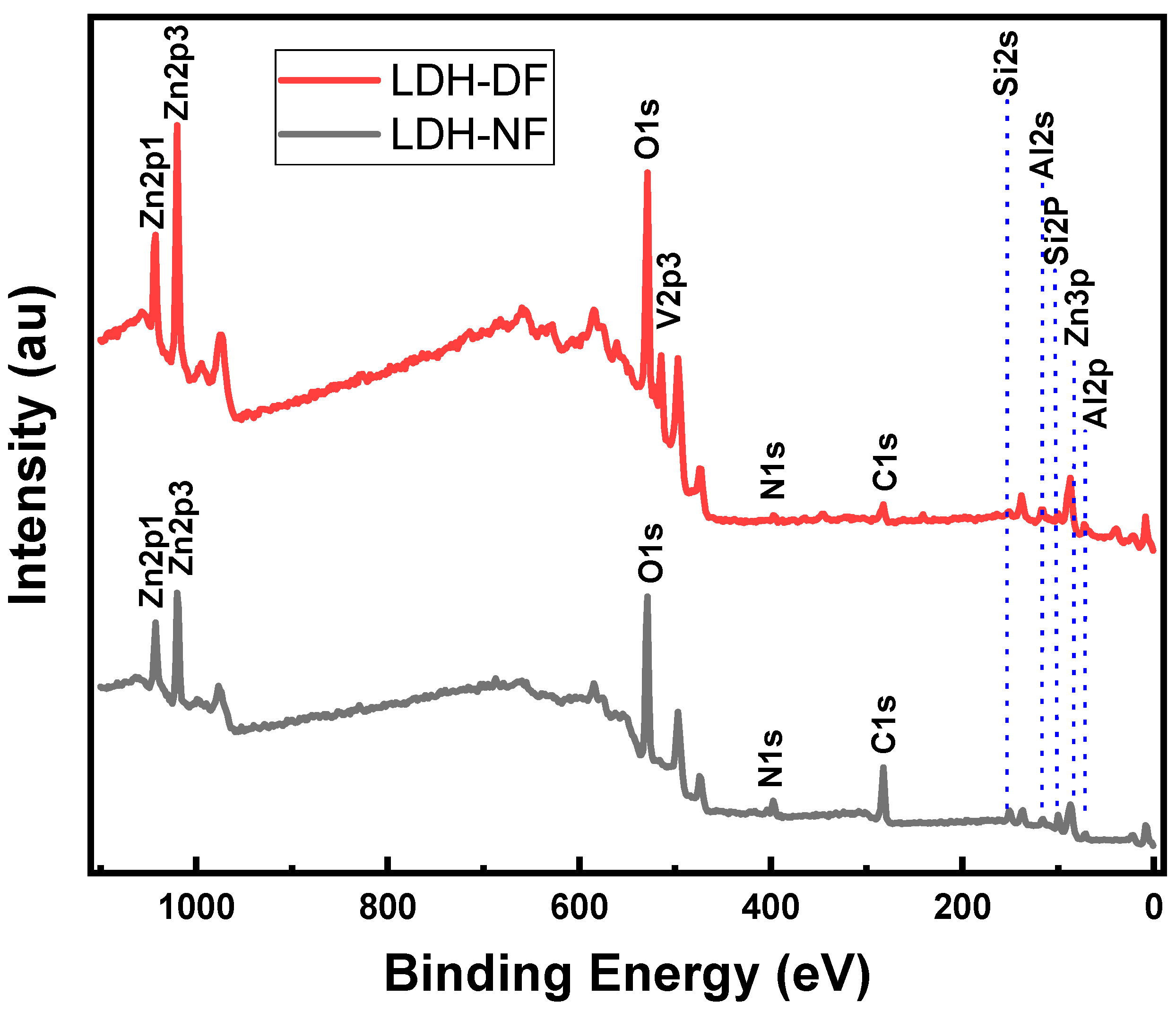
2.1.5. X-ray Photoelectron Spectroscopy of Synthesized LDH Powder Nanoparticles
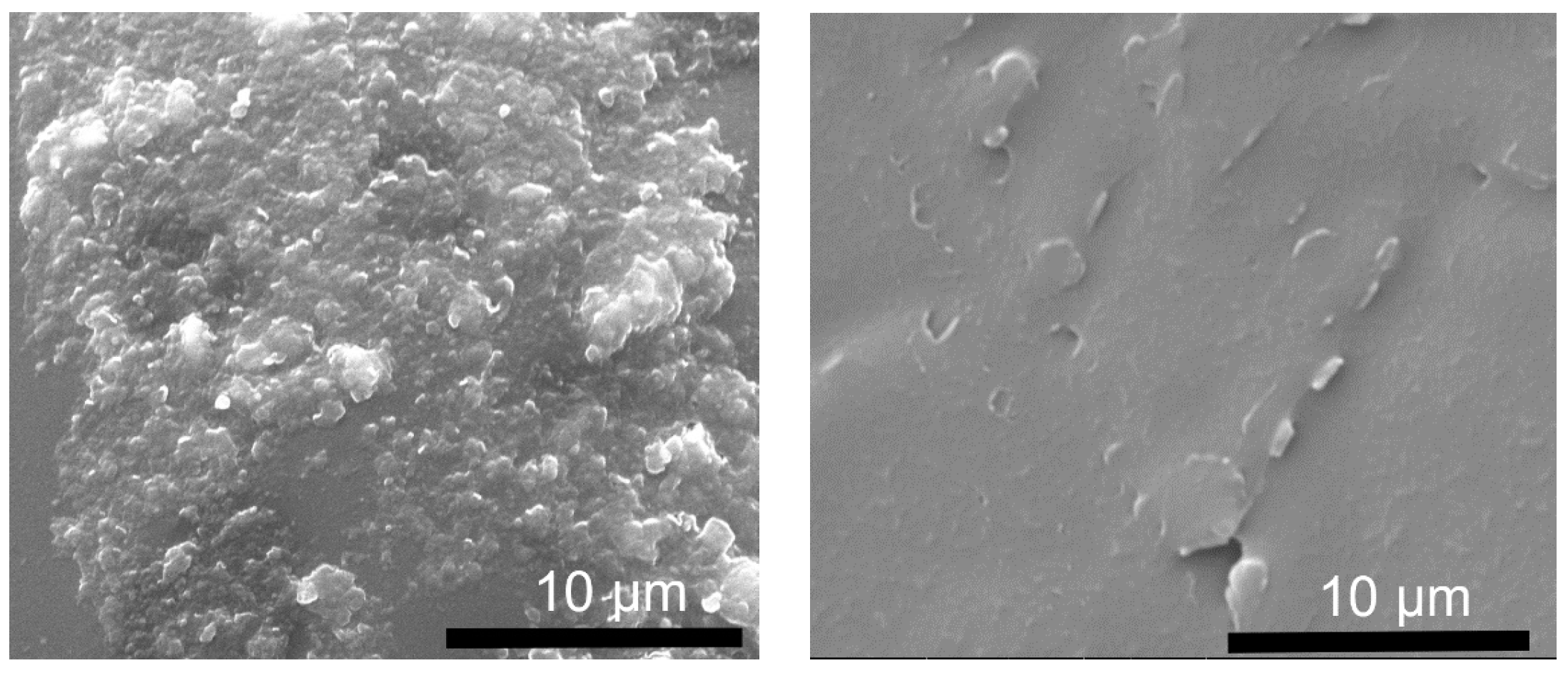
2.2. Production and Characterization of Epoxy-LDH Coatings
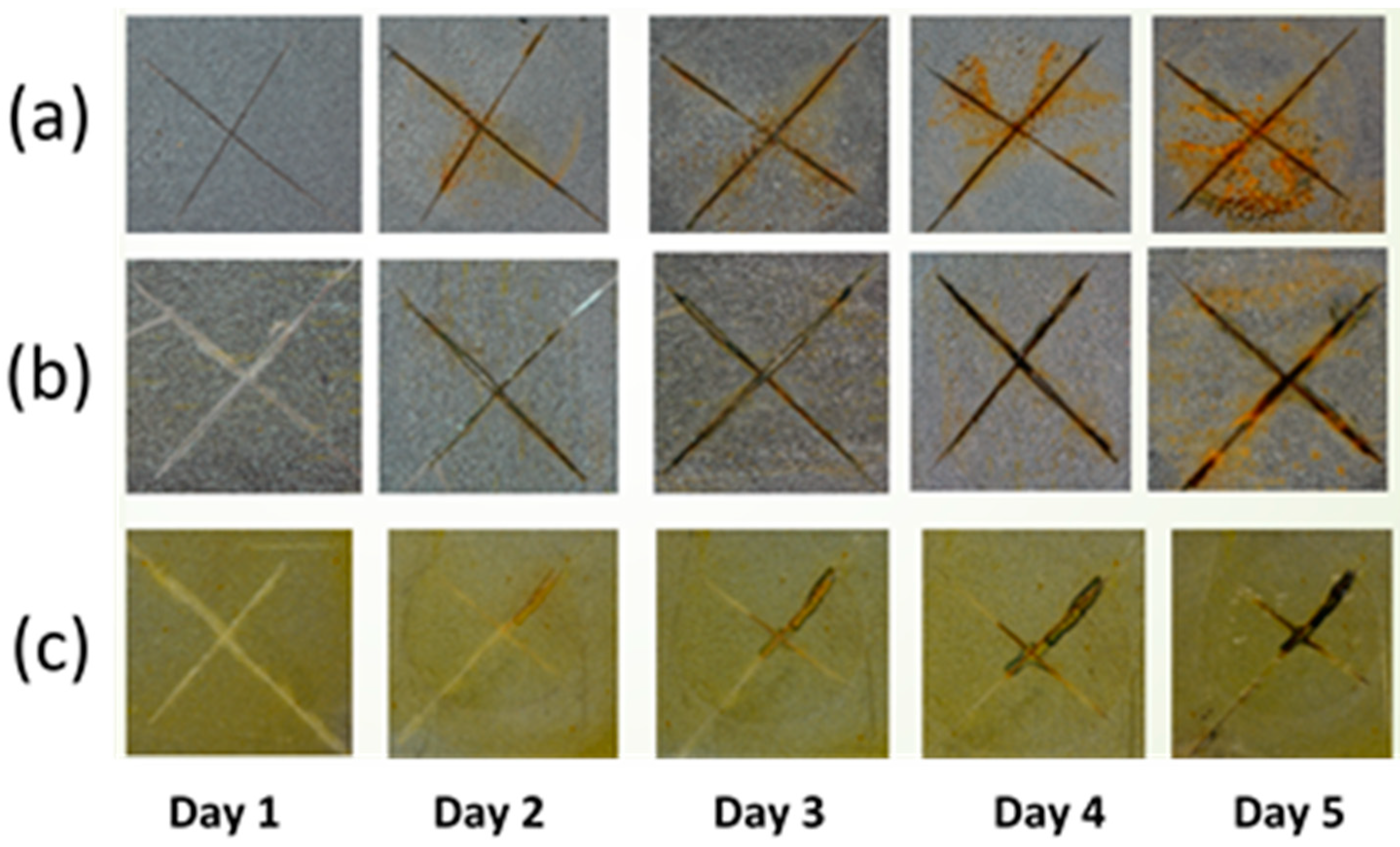
2.2.1. Corrosion Studies of Epoxy/LDH Coating: Salt Solution Immersion Test
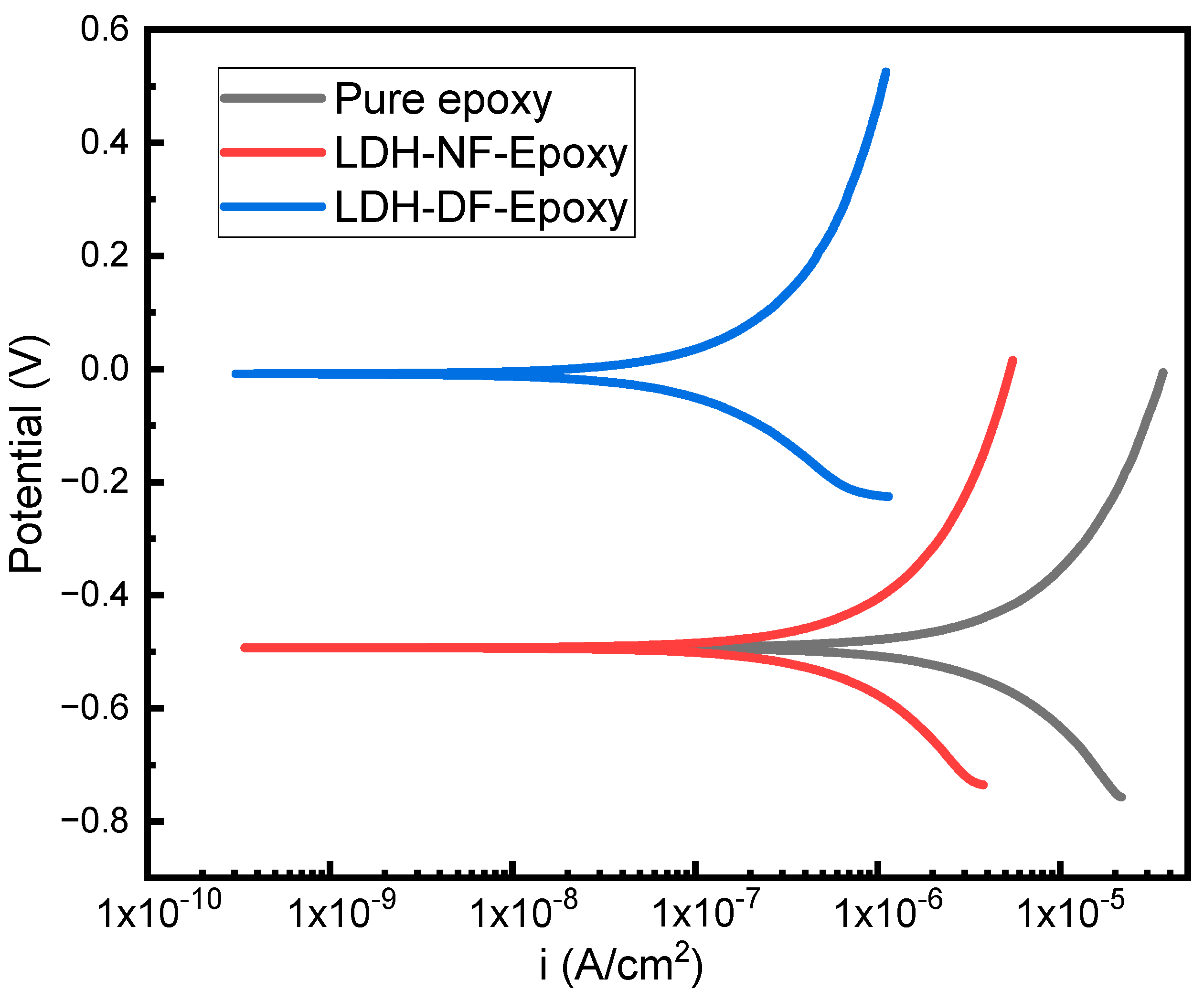
2.2.2. Corrosion Studies of Epoxy/LDH Coating: Potentiodynamic Polarization
3. Materials and Methods
3.1. Materials
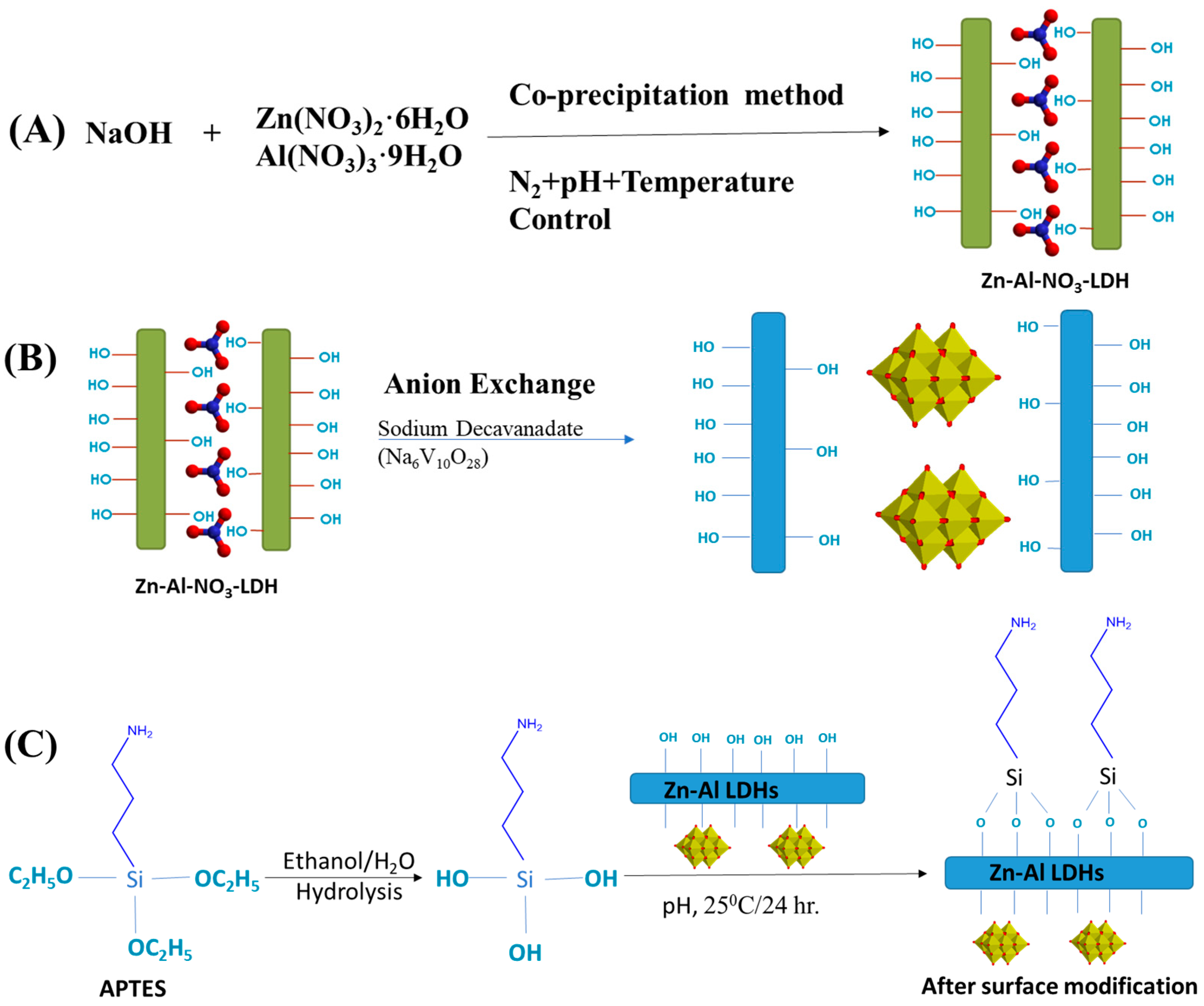
3.2. Synthesis of Nitrate and Decavanadate Intercalated LDHs
3.3. Preparation of Silane Functionalized Decavanadate and Nitrate LDHs
3.4. Preparation of Epoxy/LDH–APTES Decavanadate Composite
3.5. Characterization of Modified LDH Powders and Epoxy-Functionalized Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kania, H. Corrosion and Anticorrosion of Alloys/Metals: The Important Global Issue. Coatings 2023, 13, 216. [Google Scholar] [CrossRef]
- El-Sherik, A.M. (Ed.) Trends in Oil and Gas Corrosion Research and Technologies, Production and Transmission; Woodhead Publishing: Boston, MA, USA, 2017. [Google Scholar] [CrossRef]
- Koch, G. 1—Cost of corrosion. In Woodhead Publishing Series in Energy, Trends in Oil and Gas Corrosion Research and Technologies; El-Sherik, A.M., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–30. ISBN 9780081011058. [Google Scholar] [CrossRef]
- Motlatle, A.M.; Ray, S.S.; Scriba, M. Polyaniline-clay composite-containing epoxy coating with enhanced corrosion protection and mechanical properties. Synth. Met. 2018, 245, 102–110. [Google Scholar] [CrossRef]
- Tedim, J.; Zheludkevich, M.L.; Salak, A.N.; Lisenkov, A.; Ferreira, M.G.S. Nanostructured LDH-container layer with active protection functionality. J. Mater. Chem. 2011, 21, 15464–15470. [Google Scholar] [CrossRef]
- Ghasemi-Kahrizsangi, A.; Shariatpanahi, H.; Neshati, J.; Akbarinezhad, E. Corrosion behavior of modified nano carbon black/epoxy coating in accelerated conditions. Appl. Surf. Sci. 2015, 331, 115–126. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Ramezanzadeh, B.; Ghasemi, E. Effect of Various Spinel Ferrite Nanopigments Modified by Amino Propyl Trimethoxy Silane on the Corrosion Inhibition Properties of the Epoxy Nanocomposites. Corrosion 2016, 72, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, Y.; Guo, J.; Shen, N.Z.; Jiang, D.; Zhang, X.; Yan, X.; Zhu, J.; Wang, Q.; Shao, L.; et al. Advanced micro/nanocapsules for self-healing smart anticorrosion coatings. J. Mater. Chem. A 2014, 3, 469–480. [Google Scholar] [CrossRef]
- Chen, C. Anticorrosion Performance of Epoxy Coating Containing Reactive Poly (o-phenylenediamine) Nanoparticles. Int. J. Electrochem. Sci. 2017, 12, 3417–3431. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Deyab, M.A.; Hamdi, N.; Lachkar, M.; Bali, B.E. Clay/Phosphate/Epoxy nanocomposites for enhanced coating activity towards corrosion resistance. Prog. Org. Coat. 2018, 123, 232–237. [Google Scholar] [CrossRef]
- Jeon, H.; Park, J.; Shon, M. Corrosion protection by epoxy coating containing multi-walled carbon nanotubes. J. Ind. Eng. Chem. 2013, 19, 849–853. [Google Scholar] [CrossRef]
- Dhole, G.S.; Gunasekaran, G.; Baloji Naik, R.; Vinjamur, M. Modified Epoxy Coatings for Corrosion Detection. Corrosion 2017, 73, 326–338. [Google Scholar] [CrossRef]
- Venugopal, B.R.; Shivakumara, C.; Rajamathi, M. Effect of various factors influencing the delamination behavior of surfactant intercalated layered double hydroxides. J. Colloid Interface Sci. 2006, 294, 234–239. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Forano, C.; Costantino, U.; Prévot, V.; Taviot Gueho, C. Chapter 14.1 Layered Double Hydroxides (LDH). In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar] [CrossRef]
- Velasco, J.I.; Ardanuy, M.; Antunes, M. 4—Layered double hydroxides (LDHs) as functional fillers in polymer nanocomposites. In Advances in polymer nanocomposites; Gao, F., Ed.; Woodhead Publishing: Cambridge, UK; Elsevier: Amsterdam, The Netherlands, 2012; pp. 91–130. [Google Scholar] [CrossRef]
- Jafarbeglou, M.; Abdouss, M.; Shoushtari, A.M.; Jafarbeglou, M. Clay nanocomposites as engineered drug delivery systems. RSC Adv. 2016, 6, 52–516. [Google Scholar] [CrossRef]
- Stimpfling, T.; Leroux, F.; Hintze-Bruening, H. Organo-modified layered double hydroxide in coating formulation to protect AA2024 from corrosion. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 147–154. [Google Scholar] [CrossRef]
- Hang, T.T.X.; Truc, T.A.; Duong, N.T.; Pébère, N.; Olivier, M. Layered double hydroxides as containers of inhibitors in organic coatings for corrosion protection of carbon steel. Prog. Org. Coat. 2012, 74, 343–348. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn–Al layered double hydroxides as chloride nanotraps in active protective coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Kahl, M.; Golden, T.D. Corrosion Resistance of Electrochemically Synthesized Modified Zaccagnaite LDH-type Films on Steel Substrates. Materials 2021, 14, 7389. [Google Scholar] [CrossRef]
- Daugherty, R.E.; Zumbach, M.M.; Golden, T.D. Design Challenges in Electrodepositing Metal-anionic Clay Nanocomposites. Surf. Coat. Technol. 2018, 349, 773–782. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Urdă, A.; Rives, V.; Carrazán, S.R.G.; Martín, C.; Tichit, D.; Marcu, I. Propane oxidative dehydrogenation over V-containing mixed oxides derived from decavanadate-exchanged ZnAl–layered double hydroxides prepared by a sol–gel method. Comptes Rendus Chim. 2018, 21, 210–220. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Silane Coupling Agents, 2nd ed.; Springer Science: Berlin/Heidelberg, Germany; Plenum Press: New York, NY, USA, 1991. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, T.; Zhang, T.C.; Ouyang, L.; Yuan, S. Superhydrophobic double layered MgAl-LDH/epoxy composite coatings for enhanced anticorrosion performance of magnesium alloys. Prog. Org. Coat. 2023, 174, 107300. [Google Scholar] [CrossRef]
- Chhetri, S.; Samanta, P.; Murmu, N.C.; Kuila, T. Anticorrosion Properties of Epoxy Composite Coating Reinforced by Molybdate-Intercalated Functionalized Layered Double Hydroxide. J. Compos. Sci. 2019, 3, 11. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. A comparative study on corrosion inhibitive effect of nitrate and phosphate intercalated Zn-Al- layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their effect on cathodic delamination of epoxy topcoat. Corros. Sci. 2017, 115, 159–174. [Google Scholar] [CrossRef]
- Mahjoubi, F.Z.; Khalidi, A.; Abdennouri, M.; Barka, N. Zn–Al layered double hydroxides intercalated with carbonate, nitrate, chloride and sulphate ions: Synthesis, characterisation and dye removal properties. J. Taibah Univ. Sci. 2017, 11, 90–100. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, L.; Ling, H.; Diao, Y.; Pang, X.; Wang, Y.; Gao, K. Design and fabrication of enhanced corrosion resistance Zn-Al layered double hydroxides films based anion-exchange mechanism on magnesium alloys. Appl. Surf. Sci. 2017, 404, 246–253. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; Hussein, M.Z. Synthesis and Optimization of Electric Conductivity and Thermal Diffusivity of Zinc-Aluminum Hydroxide (Zn–Al–NO3–LDH) Prepared at Different pH Values. Mater. Today Proc. 2016, 3, 130–144. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, F.; Lu, J.; Zeng, R.; Li, S.; Song, L.; Zeng, J. A comparison of corrosion inhibition of magnesium aluminum and zinc aluminum vanadate intercalated layered double hydroxides on magnesium alloys. Front. Mater. Sci. 2018, 12, 198–206. [Google Scholar] [CrossRef]
- Karami, Z.; Jouyandeh, M.; Ali, J.A.; Ganjali, M.R.; Aghazadeh, M.; Paran, S.M.R.; Naderi, G.; Puglia, D.; Saeb, M.R. Epoxy/layered double hydroxide (LDH) nanocomposites: Synthesis, characterization, and Excellent cure feature of nitrate anion intercalated Zn-Al LDH. Prog. Org. Coat. 2019, 136, 105218. [Google Scholar] [CrossRef]
- Hu, J.; Gan, M.; Ma, L.; Li, Z.; Yan, J.; Zhang, J. Synthesis and anticorrosive properties of polymer–clay nanocomposites via chemical grafting of polyaniline onto Zn-Al layered double hydroxides. Surf. Coat. Technol. 2014, 240, 55–62. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, Y.; Niu, P.; Lu, N.; Fan, B.; Li, R. Amine-functionalized MgAl LDH nanosheets as efficient solid base catalysts for Knoevenagel condensation. Mol. Catal. 2018, 449, 31–37. [Google Scholar] [CrossRef]
- Wu, J.; Peng, D.; He, Y.; Du, X.; Zhang, Z.; Zhang, B.; Li, X.; Huang, Y. In Situ Formation of Decavanadate-Intercalated Layered Double Hydroxide Films on AA2024 and their Anti-Corrosive Properties when Combined with Hybrid Sol Gel Films. Materials 2017, 10, 426. [Google Scholar] [CrossRef]
- Layered Double Hydroxides. Handbook of Clay Science, Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Forano, C., Hibino, T., Leroux, F., Taviot-Gueho, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Darwish, A.S.; Mahmoud, S.S.; Bayaumy, F.E.A. Microwave-assisted hydrothermal fabrication of hierarchical-stacked mesoporous decavanadate-intercalated ZnAl nanolayered double hydroxide to exterminate different developmental stages of Trichinella spiralis and Schistosoma mansoni in-vitro. Heliyon 2023, 9, e18110. [Google Scholar] [CrossRef]
- Richetta, M.; Digiamberardino, L.; Mattoccia, A.; Medaglia, P.G.; Montanari, R.; Pizzoferrato, R.; Scarpellini, D.; Varone, A.; Kaciulis, S.; Mezzi, A.; et al. Surface spectroscopy and structural analysis of nanostructured multifunctional (Zn, Al) layered double hydroxides. Surf. Interface Anal. 2016, 48, 514–518. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hatami, M.; Hussain, C.M. Recent innovations in functionalized layered double hydroxides: Fabrication, characterization, and industrial applications. Adv. Colloid Interface Sci. 2020, 283, 102216. [Google Scholar] [CrossRef]
- Tabish, M.; Zhao, J.; Wang, J.; Anjum, M.J.; Qiang, Y.; Yang, Q.; Mushtaq, M.A.; Yasin, G. Improving the corrosion protection ability of epoxy coating using CaAl LDH intercalated with 2-mercaptobenzothiazole as a pigment on steel substrate. Prog. Org. Coat. 2022, 165, 106765. [Google Scholar] [CrossRef]
- Zeng, Q.; Min, X.; Luo, Z.; Dai, H.; Liao, B. In-situ preparation of superhydrophobic Zn-Al layered double hydroxide coatings for corrosion protection of aluminum alloy. Mater. Lett. 2022, 328, 133077. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, F.; Fang, L.; Guan, T.; Hu, J.; Zhang, S. A comparative study and optimization of corrosion resistance of ZnAl layered double hydroxides films intercalated with different anions on AZ31 Mg alloys. Surf. Coat. Technol. 2019, 358, 594–603. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, T.D.; Nguyen, A.S.; Paint, Y.; Gonon, M.; To, T.X.H.; Olivier, M. A comparative study of the structure and corrosion resistance of ZnAl hydrotalcite conversion layers at different Al3+/Zn2+ ratios on electrogalvanized steel. Surf. Coat. Technol. 2022, 429, 127948. [Google Scholar] [CrossRef]
- Mahajanam, S.P.V.; Buchheit, R.G. Characterization of Inhibitor Release from Zn-Al-[V10O28]6− Hydrotalcite Pigments and Corrosion Protection from Hydrotalcite-Pigmented Epoxy Coatings. Corrosion 2008, 64, 230–240. [Google Scholar] [CrossRef]
- Pope, C.G. X-ray diffraction and the Bragg equation. J. Chem. Ed. 1997, 74, 129–131. [Google Scholar] [CrossRef]
- Angst, U.; Buchler, M. On the applicability of the Stern–Geary relationship to determine instantaneous corrosion rates in macro-cell corrosion. Mater. Corros. 2015, 66, 1017–1028. [Google Scholar] [CrossRef]
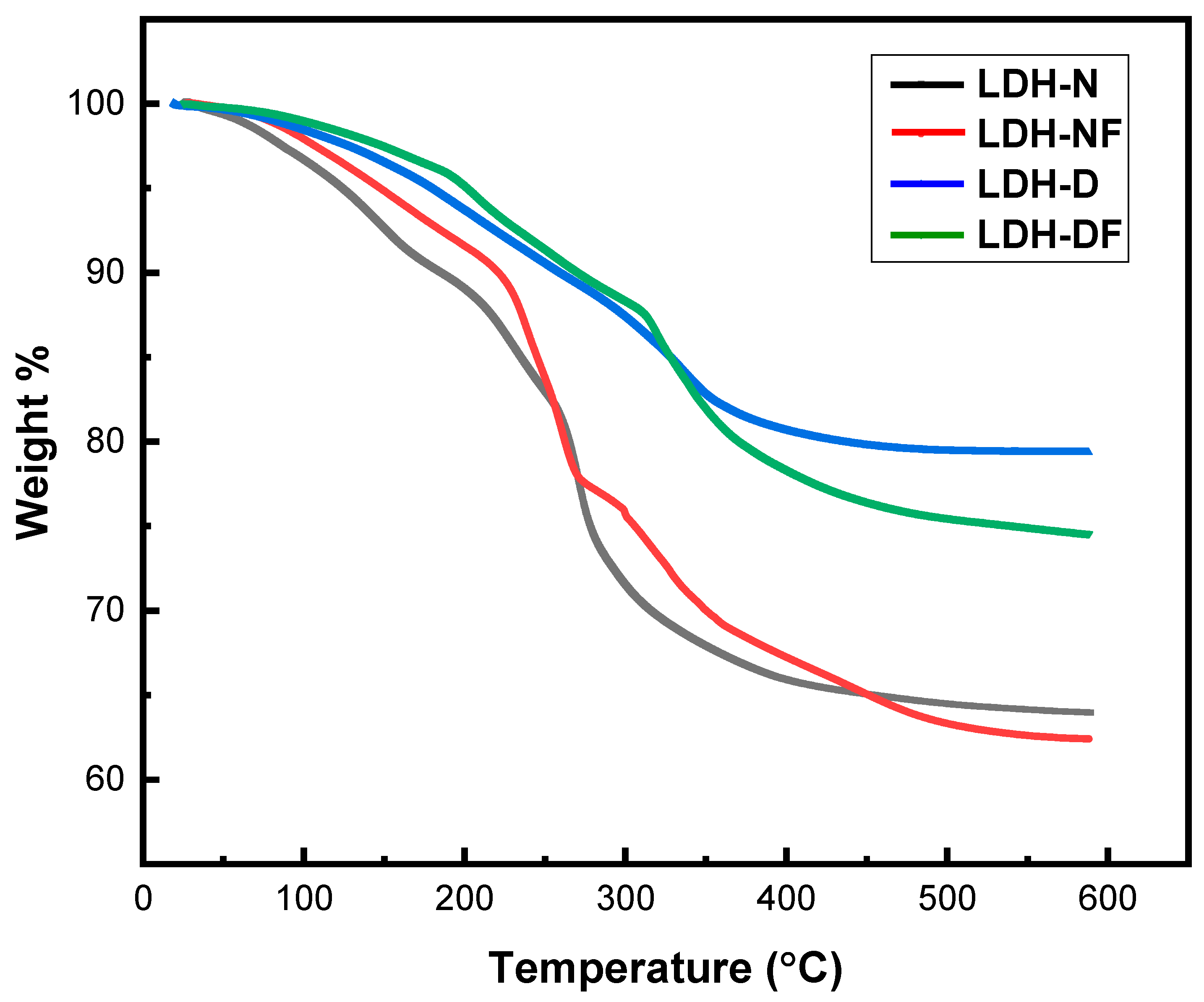



| Samples | Weight Loss% (25–150 °C) | Weight Loss% (150–300 °C) | Weight Loss% (300–600 °C) | Total Weight Loss% | T10 (°C) | Td (°C) |
|---|---|---|---|---|---|---|
| LDH-N | 7.5 | 21.0 | 7.6 | 36.1 | 184 | 262 |
| LDH-NF | 5.3 | 18.9 | 13.4 | 37.6 | 220 | 275 |
| LDH-D | 3.5 | 9.1 | 8.6 | 21.7 | 258 | 324 |
| LDH-DF | 2.6 | 9.2 | 13.7 | 25.4 | 270 | 340 |
| Sample | Element | XPS Peak | Chemical Bonding | Peak Values (eV) |
|---|---|---|---|---|
| LDH-NF | Aluminum | Al 2p | Al-O, Al-OH | 74.2 |
| Zinc | Zn 2p3/2; Zn 2p1/2 | Zn (+2) | 1021.7; 1044.8 | |
| Nitrogen | N 1s | -NO3; NH2 | 405.8; 399.9 | |
| Oxygen | O 1s | Oxides | 531.7 | |
| Silicon | Si 2p | Si-O | 101.8 | |
| Carbon | C 1s | C-C; C-N | 284.5; 285.7 | |
| LDH-DF | Aluminum | Al 2p | Al-O, Al-OH | 74.0 |
| Zinc | Zn 2p3/2; Zn 2p1/2 | Zn (+2) | 1021.8; 1045.9 | |
| Nitrogen | N 1s | -NH2 | 399.8 | |
| Oxygen | O 1s | Oxides | 530.6 | |
| Silicon | Si 2p | Si-O | 102.2 | |
| Carbon | C 1s | C-C; C-N | 284.5; 285.7 | |
| Vanadium | V 2p3/2; V 2p1/2 | V-O | 516.2; 523.6 |
| Sample | Ecorr (V) | βa (mV/dec) | βc (mV/dec) | Rp (Ω/cm2) | Icorr (A/cm2) |
|---|---|---|---|---|---|
| Pure Epoxy | −0.49 | 676 | −397 | 1.3 × 104 | 3.4 × 10−5 |
| LDH-NF Epoxy | −0.49 | 767 | −333 | 5.7 × 104 | 7.7 × 10−6 |
| LDH-DF Epoxy | −0.006 | 855 | −249 | 3.8 × 105 | 1.2 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminifazl, A.; Karunarathne, D.J.; Golden, T.D. Synthesis of Silane Functionalized LDH-Modified Nanopowders to Improve Compatibility and Enhance Corrosion Protection for Epoxy Coatings. Molecules 2024, 29, 819. https://doi.org/10.3390/molecules29040819
Aminifazl A, Karunarathne DJ, Golden TD. Synthesis of Silane Functionalized LDH-Modified Nanopowders to Improve Compatibility and Enhance Corrosion Protection for Epoxy Coatings. Molecules. 2024; 29(4):819. https://doi.org/10.3390/molecules29040819
Chicago/Turabian StyleAminifazl, Alireza, Darshan Jayasinghe Karunarathne, and Teresa D. Golden. 2024. "Synthesis of Silane Functionalized LDH-Modified Nanopowders to Improve Compatibility and Enhance Corrosion Protection for Epoxy Coatings" Molecules 29, no. 4: 819. https://doi.org/10.3390/molecules29040819
APA StyleAminifazl, A., Karunarathne, D. J., & Golden, T. D. (2024). Synthesis of Silane Functionalized LDH-Modified Nanopowders to Improve Compatibility and Enhance Corrosion Protection for Epoxy Coatings. Molecules, 29(4), 819. https://doi.org/10.3390/molecules29040819






