Visible Light-Mediated Monofluoromethylation/Acylation of Olefins by Dual Organo-Catalysis
Abstract
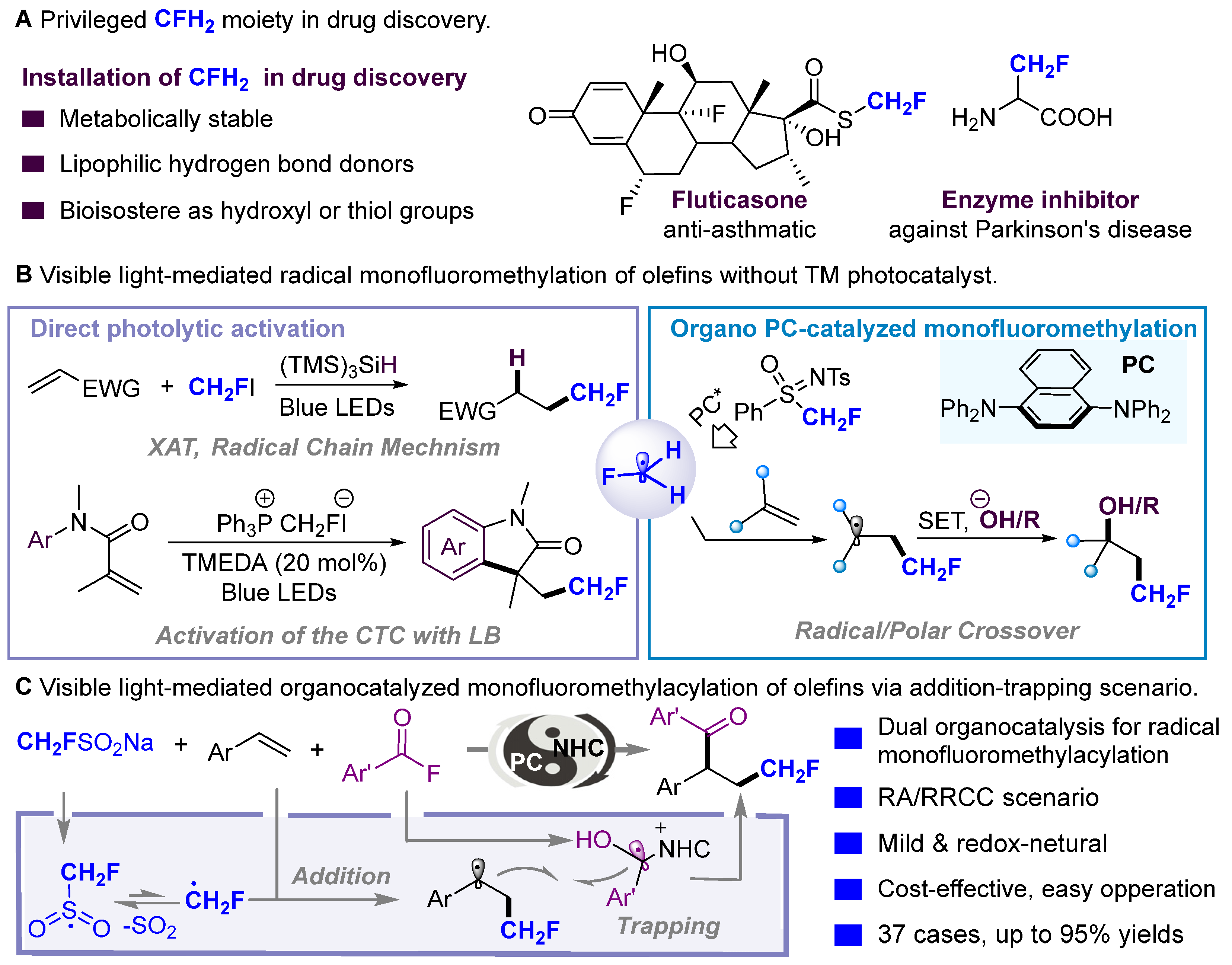
1. Introduction
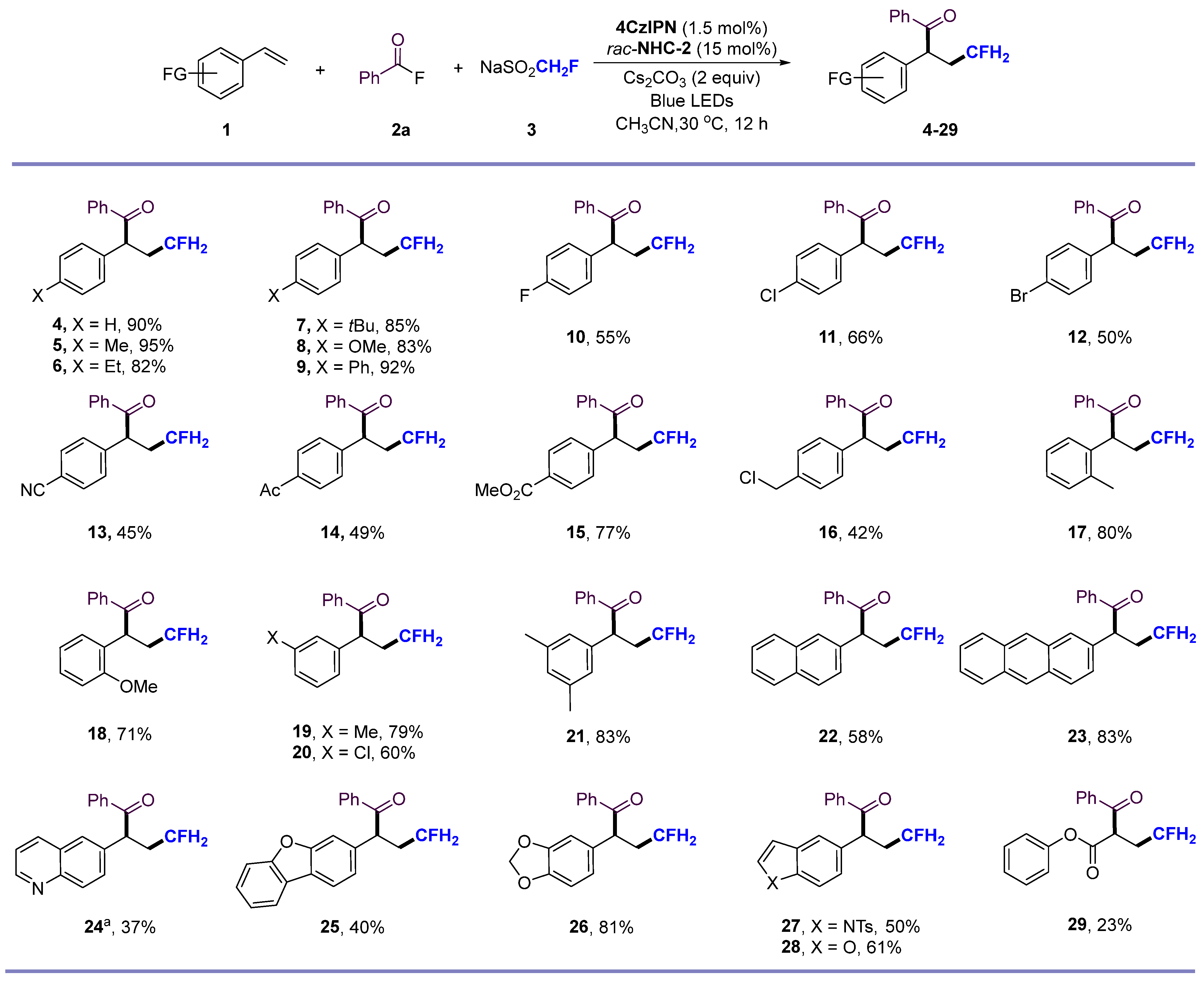
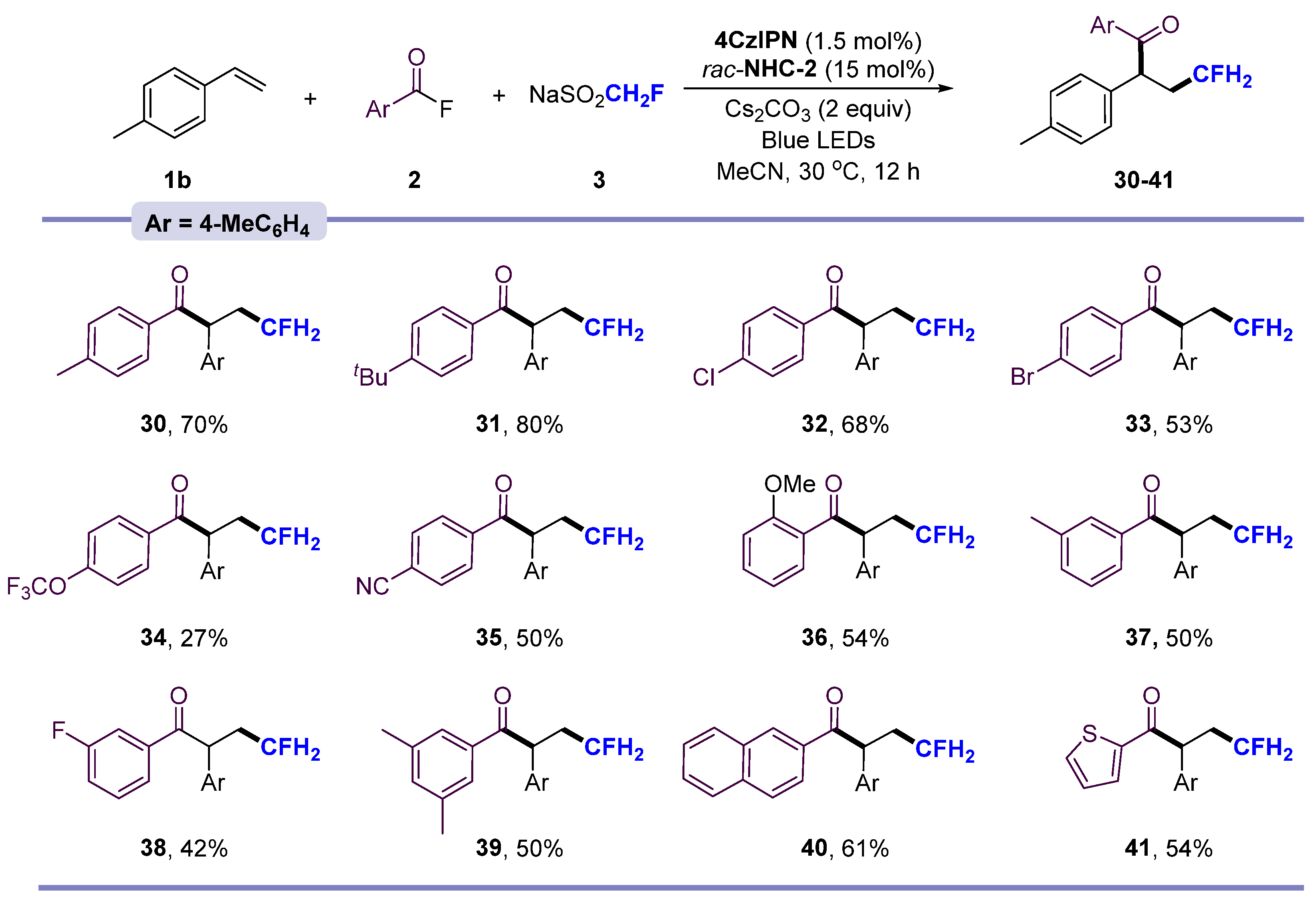
2. Results and Discussion
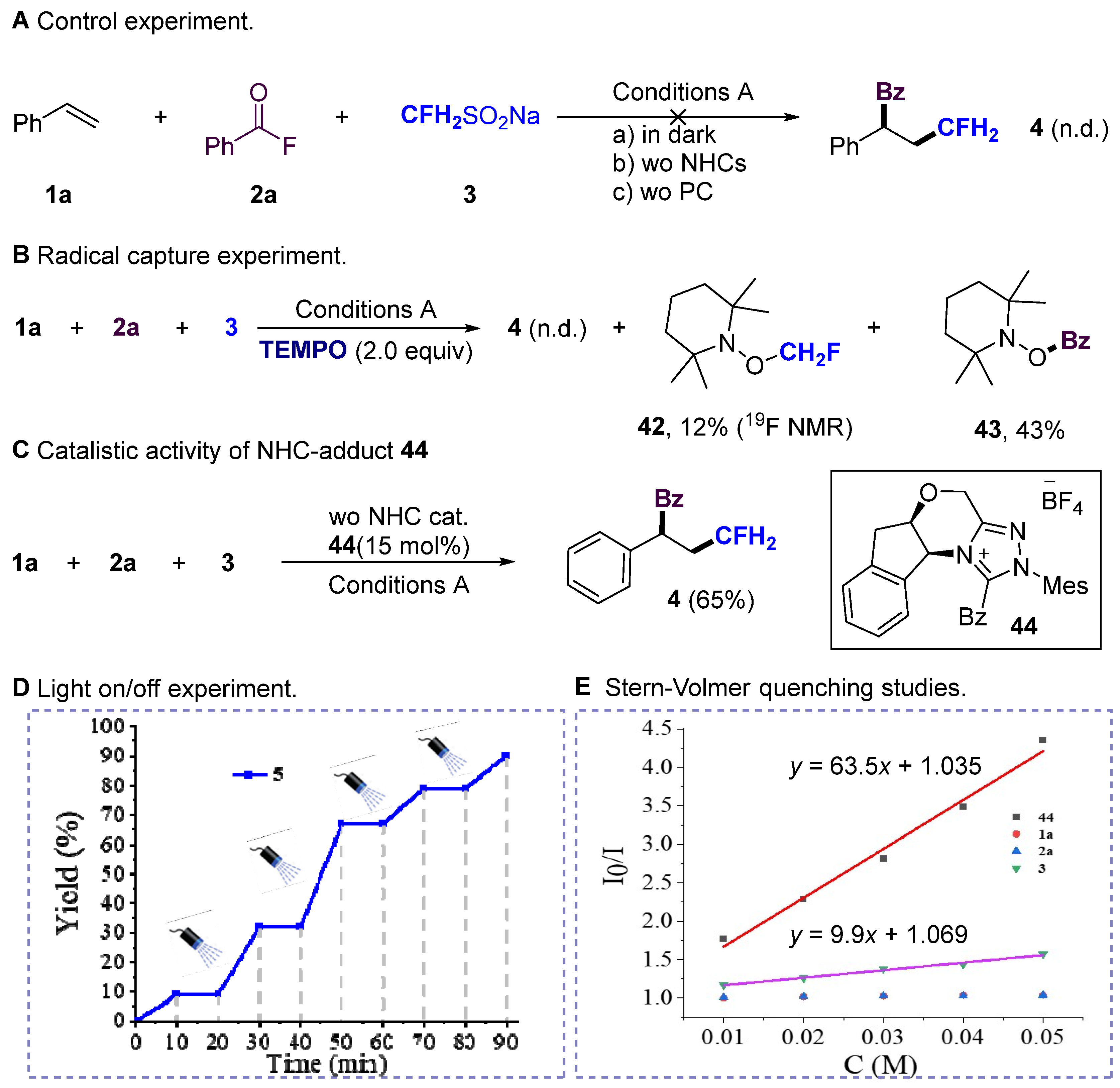
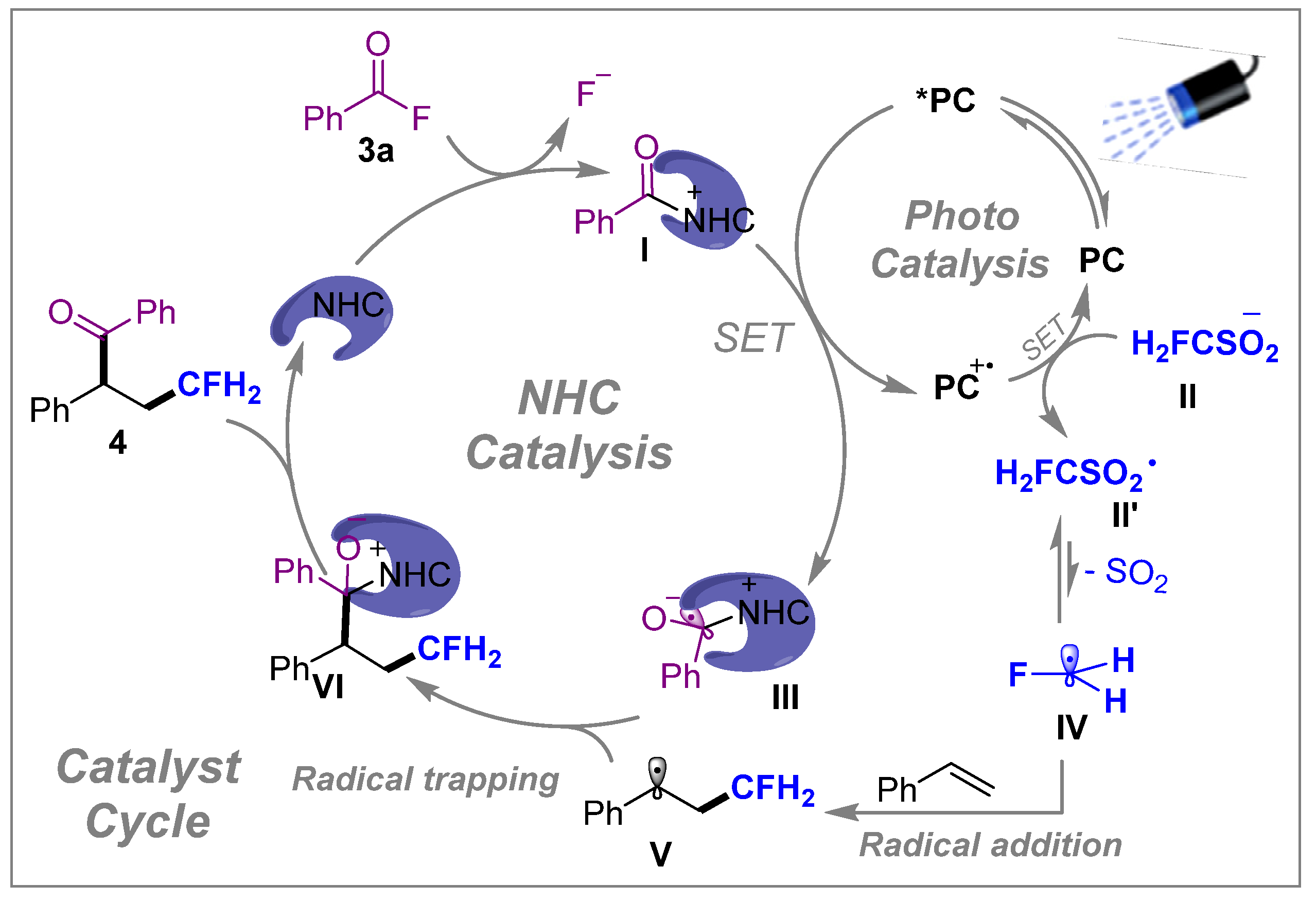
3. Mechanistic Investigation
4. Materials and Methods
4.1. Materials and Instruments
4.2. General Procedure for the Synthesis of 4
- 4-fluoro-1,2-diphenylbutan-1-one (4) Purification by column chromatography on silica- gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 22.3 mg, 92%). 1H NMR (500 MHz, Chloroform-d) δ 7.99–7.92 (m, 2H), 7.51–7.46 (m, 1H), 7.39 (d, J = 7.9 Hz, 2H), 7.34–7.29 (m, 4H), 7.26–7.18 (m, 1H), 4.84 (t, J = 7.4 Hz, 1H), 4.57–4.29 (m, 2H), 2.74–2.46 (m, 1H), 2.34–2.08 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.07, 138.49, 136.46, 133.01, 129.14, 128.79, 128.54, 128.35, 127.35, 81.76 (d, J = 164.1 Hz), 49.02 (d, J = 3.1 Hz), 34.41 (d, J = 19.2 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.37 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C16H15FNaO ([M + Na]+), 265.0999; found, 265.1003.
- 4-fluoro-1-phenyl-2-(p-tolyl)butan-1-one (5) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 24.4 mg, 92%). 1H NMR (500 MHz, Chloroform-d) δ 8.13–7.84 (m, 2H), 7.49–7.44 (m, 1H), 7.37 (t, J = 7.7 Hz, 2H), 7.22–7.14 (m, 2H), 7.10 (d, J = 7.9 Hz, 2H), 4.80 (t, J = 7.4 Hz, 1H), 4.55–4.27 (m, 2H), 2.62–2.50 (m, 1H), 2.28 (s, 3H), 2.24–2.08 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.20, 137.03, 136.50, 135.42, 132.94, 129.84, 128.78, 128.51, 128.21, 81.81 (d, J = 163.7 Hz), 48.62 (d, J = 3.1 Hz), 34.37 (d, J = 19.5 Hz), 21.01. 19F NMR (565 MHz, Chloroform-d) δ -221.34 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H17FNaO ([M + Na]+), 279.1156; found, 279.1162.
- 2-(4-ethylphenyl)-4-fluoro-1-phenylbutan-1-one (6) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 21.8 mg, 82%). 1H NMR (500 MHz, Chloroform-d) δ 7.97 (dd, J = 8.1, 1.4 Hz, 2H), 7.52–7.44 (m, 1H), 7.38 (t, J = 7.7 Hz, 2H), 7.22 (d, J = 7.9 Hz, 2H), 7.13 (d, J = 7.8 Hz, 2H), 4.82 (t, J = 7.4 Hz, 1H), 4.54–4.31 (m, 2H), 2.58 (q, J = 7.6 Hz, 3H), 2.30–2.10 (m, 1H), 1.19 (t, J = 7.7 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 199.22, 143.31, 136.51, 135.58, 132.95, 128.81, 128.62, 128.52, 128.25, 81.85 (d, J = 163.8 Hz), 48.59 (d, J = 3.2 Hz), 34.42 (d, J = 19.2 Hz), 28.39, 15.31. 19F NMR (565 MHz, Chloroform-d) δ −221.37 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H19FNaO ([M + Na]+), 293.1312; found, 293.1307.
- 2-(4-(tert-butyl)phenyl)-4-fluoro-1-phenylbutan-1-one (7) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 25.2 mg, 85%). 1H NMR (500 MHz, Chloroform-d) δ 8.02–7.96 (m, 2H), 7.51–7.46 (m, 1H), 7.43–7.35 (m, 2H), 7.33–7.28 (m, 2H), 7.24–7.21 (m, 2H), 4.82 (t, J = 7.4 Hz, 1H), 4.52–4.29 (m, 2H), 2.63–2.48 (m, 1H), 2.24–2.09 (m, 1H), 1.26 (s, 9H). 13C NMR (151 MHz, Chloroform-d) δ 199.25, 150.19, 136.57, 135.22, 132.95, 128.83, 128.52, 127.93, 126.02, 81.88 (d, J = 163.7 Hz), 48.40 (d, J = 3.5 Hz), 34.52, 34.41 (d, J = 7.0 Hz), 31.27. 19F NMR (565 MHz, Chloroform-d) δ -221.32 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C20H23FNaO ([M + Na]+), 321.1625; found, 321.1625.
- 4-fluoro-2-(4-methoxyphenyl)-1-phenylbutan-1-one (8) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 22.6 mg, 83%). 1H NMR (500 MHz, Chloroform-d) δ 8.03–7.89 (m, 2H), 7.51–7.46 (m, 1H), 7.39 (t, J = 7.7 Hz, 2H), 7.34–7.29 (m, 4H), 4.86 (t, J = 7.3 Hz, 1H), 4.52 (s, 3H), 4.51–4.26 (m, 2H), 2.65–2.49 (m, 1H), 2.26–2.08 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.86, 138.73, 136.60, 136.32, 133.17, 129.36, 128.77, 128.72, 128.62, 81.65 (d, J = 164.1 Hz), 48.60 (d, J = 3.3 Hz), 45.73, 34.39 (d, J = 19.5 Hz). 19F NMR (565 MHz, Chloroform-d) δ -221.42 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H17FNaO2 ([M + Na]+), 295.1105; found, 295.1107.
- 2-([1,1’-biphenyl]-4-yl)-4-fluoro-1-phenylbutan-1-one (9) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 28.6 mg, 90%). 1H NMR (500 MHz, Chloroform-d) δ 8.27–8.12 (m, 2H), 8.06–7.94 (m, 2H), 7.73–7.59 (m, 1H), 7.54–7.46 (m, 3H), 7.39 (dt, J = 21.2, 7.8 Hz, 3H), 7.27–7.19 (m, 2H), 7.12 (dd, J = 7.9, 2.3 Hz, 1H), 4.90 (t, J = 7.3 Hz, 1H), 4.60–4.25 (m, 2H), 2.67–2.40 (m, 1H), 2.36–2.13 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.78, 164.92, 151.46, 140.12, 136.31, 133.66, 133.24, 130.16, 130.11, 129.40, 128.80, 128.66, 128.59, 125.86, 121.55, 120.85, 81.62 (d, J = 164.2 Hz), 48.65 (d, J = 3.3 Hz), 34.48 (d, J = 19.2 Hz). 19F NMR (565 MHz, Chloroform-d) δ -221.44 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C22H19FNaO ([M + Na]+), 341.1312; found, 341.1309.
- 7. 4-fluoro-2-(4-fluorophenyl)-1-phenylbutan-1-one (10) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 14.3 mg, 55%). 1H NMR (500 MHz, Chloroform-d) δ 8.07–7.88 (m, 2H), 7.53–7.47 (m, 1H), 7.39 (dd, J = 8.5, 7.1 Hz, 2H), 7.31–7.24 (m, 2H), 7.03–6.94 (m, 2H), 4.84 (t, J = 7.4 Hz, 1H), 4.63–4.20 (m, 2H), 2.65–2.48 (m, 1H), 2.30–2.06 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.04, 162.04 (d, J = 246.3 Hz), 161.22, 136.28, 134.14 (d, J = 3.5 Hz), 133.18, 129.91 (d, J = 8.1 Hz), 128.74, 128.62, 116.06 (d, J = 21.4 Hz), 81.65 (d, J = 164.2 Hz), 48.08 (d, J = 3.1 Hz), 34.42 (d, J = 19.3 Hz).19F NMR (565 MHz, Chloroform-d) δ −114.93–−115.04 (m, 1F), −221.57 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C16H14F2NaO ([M + Na]+), 283.0904; found, 283.0900.
- 2-(4-chlorophenyl)-4-fluoro-1-phenylbutan-1-one (11) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 18.2 mg, 66%). 1H NMR (500 MHz, Chloroform-d) δ 7.95–7.91 (m, 2H), 7.54–7.48 (m, 1H), 7.39 (t, J = 7.7 Hz, 2H), 7.31–7.20 (m, 4H), 4.83 (t, J = 7.4 Hz, 1H), 4.63–4.22 (m, 2H), 2.64–2.49 (m, 1H), 2.30–2.06 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.78, 136.93, 136.19, 133.35, 133.26, 129.70, 129.34, 128.74, 128.65, 81.55 (d, J = 164.2 Hz), 48.24 (d, J = 3.2 Hz), 34.31 (d, J = 19.3 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.55 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C16H14ClFNaO ([M + Na]+), 299.0609; found, 299.0613.
- 9. 2-(4-bromophenyl)-4-fluoro-1-phenylbutan-1-one (12) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 16.1 mg, 50%). 1H NMR (500 MHz, Chloroform-d) δ 7.98–7.88 (m, 2H), 7.50 (t, J = 7.5 Hz, 1H), 7.45–7.37 (m, 4H), 7.19 (d, J = 8.4 Hz, 2H), 4.82 (t, J = 7.4 Hz, 1H), 4.54–4.28 (m, 2H), 2.65–2.42 (m, 1H), 2.23–2.03 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.68, 137.46, 136.16, 133.29, 132.30, 130.06, 128.74, 128.67, 121.46, 81.54 (d, J = 164.2 Hz), 48.31 (d, J = 3.2 Hz), 34.27 (d, J = 19.3 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.54 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C16H15BrFO ([M + H]+), 321.0285; found, 321.0283.
- 4-(4-fluoro-1-oxo-1-phenylbutan-2-yl)benzonitrile (13) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 12.0 mg, 45%). 1H NMR (500 MHz, Chloroform-d) δ 7.96–7.89 (m, 2H), 7.60 (d, J = 8.4 Hz, 2H), 7.55–7.50 (m, 1H), 7.48–7.37 (m, 4H), 4.93 (t, J = 7.3 Hz, 1H), 4.58–4.16 (m, 2H), 2.68–2.52 (m, 1H), 2.24–2.08 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.09, 143.85, 135.97, 133.61, 132.89, 129.19, 128.81, 128.71, 118.42, 111.51, 81.76 (d, J = 164.2 Hz), 48.84 (d, J = 3.2 Hz), 34.32 (d, J = 19.3 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.48 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H14FNNaO ([M + Na]+), 290.0952; found, 290.0961.
- 2-(4-acetylphenyl)-4-fluoro-1-phenylbutan-1-one (14) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 13.9 mg, 49%). 1H NMR (500 MHz, Chloroform-d) δ 7.99–7.94 (m, 2H), 7.92 (d, J = 1.9 Hz, 1H), 7.81 (dt, J = 7.8, 1.4 Hz, 1H), 7.56–7.48 (m, 2H), 7.40 (td, J = 7.6, 5.6 Hz, 3H), 4.94 (t, J = 7.4 Hz, 1H), 4.58–4.17 (m, 2H), 2.68–2.59 (m, 1H), 2.57 (s, 3H), 2.26–2.10 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.75, 197.74, 139.19, 137.92, 136.18, 133.32, 132.90, 129.43, 128.76, 128.70, 128.11, 127.55, 81.56 (d, J = 164.2 Hz), 48.71 (d, J = 3.4 Hz), 34.46 (d, J = 19.3 Hz), 26.69. 19F NMR (565 MHz, Chloroform-d) δ −221.30 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H17FNaO2 ([M + Na]+), 307.1104; found, 307.1112.
- methyl-4-(4-fluoro-1-oxo-1-phenylbutan-2-yl)benzoate (15) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 23.1 mg, 77%). 1H NMR (500 MHz, Chloroform-d) δ 7.97 (d, J = 8.2 Hz, 2H), 7.94 (dd, J = 7.0, 5.5 Hz, 2H), 7.52–7.46 (m, 1H), 7.38 (dd, J = 8.2, 6.5 Hz, 4H), 4.92 (t, J = 7.3 Hz, 1H), 4.61–4.27 (m, 2H), 3.87 (s, 3H), 2.63–2.57 (m, 1H), 2.24–2.10 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.99, 169.26, 149.89, 136.33, 135.87, 133.18, 129.34, 128.77, 128.62, 122.19, 81.65 (d, J = 164.0 Hz), 48.20 (d, J = 3.0 Hz), 34.49 (d, J = 19.5 Hz), 21.11. 19F NMR (565 MHz, Chloroform-d) δ −221.35 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H17FNaO3 ([M + Na]+), 323.1054; found, 323.1053.
- 2-(4-(chloromethyl)phenyl)-4-fluoro-1-phenylbutan-1-one (16) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 12.2 mg, 42%). 1H NMR (500 MHz, Chloroform-d) δ 7.99–7.90 (m, 2H), 7.54–7.47 (m, 1H), 7.39 (t, J = 7.7 Hz, 2H), 7.35–7.28 (m, 4H), 4.86 (t, J = 7.3 Hz, 1H), 4.52 (s, 2H), 4.51–4.28 (m, 2H), 2.66–2.49 (m, 1H), 2.27–2.10 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.86, 138.73, 136.60, 136.32, 133.17, 129.35, 128.77, 128.71, 128.61, 81.65 (d, J = 164.2 Hz), 48.61 (d, J = 3.1 Hz), 45.73, 34.38 (d, J = 19.2 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.43 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H16ClFNaO ([M + Na]+), 313.0765; found, 313.0763.
- 4-fluoro-1-phenyl-2-(o-tolyl)butan-1-one (17) Purification by column chromatography on silica- gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 20.5 mg, 80%). 1H NMR (500 MHz, Chloroform-d) δ 7.85–7.82 (m, 2H), 7.46 (t, J = 7.4 Hz, 1H), 7.36 (t, J = 7.7 Hz, 2H), 7.21 (d, J = 7.3 Hz, 1H), 7.14–7.04 (m, 3H), 4.97 (dd, J = 8.1, 6.1 Hz, 1H), 4.63–4.30 (m, 2H), 2.67–2.56 (m, 1H), 2.54 (s, 3H), 2.23–1.97 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.84, 137.38, 136.82, 135.46, 132.91, 131.22, 128.55, 128.45, 127.29 (d, J = 3.5 Hz), 126.82, 81.87 (d, J = 164.5 Hz), 45.32 (d, J = 2.9 Hz), 34.12(d, J = 19.6 Hz), 19.59. 19F NMR (565 MHz, Chloroform-d) δ −219.78 (tt, J = 45.20, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H17FNaO ([M + Na]+), 279.1155; found, 279.1159.
- 4-fluoro-2-(2-methoxyphenyl)-1-phenylbutan-1-one (18) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 19.3 mg, 71%). 1H NMR (500 MHz, Chloroform-d) δ 8.06–7.89 (m, 2H), 7.44 (t, J = 7.4 Hz, 1H), 7.34 (t, J = 7.8 Hz, 2H), 7.18 (ddd, J = 8.2, 7.4, 1.7 Hz, 1H), 7.11 (dd, J = 7.6, 1.7 Hz, 1H), 6.94–6.82 (m, 2H), 5.25 (t, J = 7.2 Hz, 1H), 4.54–4.34 (m, 2H), 3.90 (s, 3H), 2.72–2.47 (m, 1H), 2.19–2.03 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.80, 156.22, 136.46, 132.80, 128.57, 128.51 128.49, 128.35, 127.41, 111.09, 82.22 (d, J = 164.1 Hz), 55.62, 41.48 (d, J = 4.5 Hz), 33.40 (d, J = 19.9 Hz). 19F NMR (565 MHz, Chloroform-d) δ −219.92 (tt, J = 45.20, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H17FNaO2 ([M + Na]+), 295.1104; found, 295.1104.
- 4-fluoro-1-phenyl-2-(m-tolyl)butan-1-one (19) Purification by column chromatography on silica- gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 20.2 mg, 79%). 1H NMR (500 MHz, Chloroform-d) δ 8.02–7.93 (m, 2H), 7.54–7.43 (m, 1H), 7.38 (dd, J = 8.4, 7.0 Hz, 2H), 7.19 (td, J = 7.4, 1.2 Hz, 1H), 7.11 (d, J = 7.7 Hz, 2H), 7.02 (d, J = 7.6 Hz, 1H), 4.80 (t, J = 7.3 Hz, 1H), 4.55–4.28 (m, 2H), 2.64–2.48 (m, 1H), 2.30 (s, 3H), 2.23–2.09 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.17, 138.86, 138.40, 136.52, 133.00, 128.99, 128.84, 128.82, 128.54, 128.16, 125.53, 81.69 (d, J = 165.1 Hz), 48.96 (d, J = 3.1 Hz), 34.45 (d, J = 19.3 Hz), 21.42. 19F NMR (565 MHz, Chloroform-d) δ −221.30 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H17FNaO ([M + Na]+), 279.1155; found, 279.1147.
- 2-(3-chlorophenyl)-4-fluoro-1-phenylbutan-1-one (20). Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 16.6 mg, 60%). 1H NMR (500 MHz, Chloroform-d) δ 7.96 (d, J = 6.9 Hz, 2H), 7.56–7.49 (m, 1H), 7.41 (t, J = 7.7 Hz, 2H), 7.32 (d, J = 1.8 Hz, 1H), 7.28–7.18 (m, 3H), 4.84 (t, J = 7.4 Hz, 1H), 4.61–4.22 (m, 2H), 2.69–2.49 (m, 1H), 2.34–2.07 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.52, 140.45, 136.18, 134.94, 133.31, 130.36, 128.76, 128.69, 128.39, 127.68, 126.60, 81.56 (d, J = 164.3 Hz), 80.97, 48.50 (d, J = 3.0 Hz), 34.39 (d, J = 19.5 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.47 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C16H14ClFNaO ([M + Na]+), 299.0609; found, 299.0602.
- 2-(3,5-dimethylphenyl)-4-fluoro-1-phenylbutan-1-1 (21) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 22.4 mg, 83%).1H NMR (500 MHz, Chloroform-d) δ 7.97 (dd, J = 8.4, 1.4 Hz, 2H), 7.52–7.46 (m, 1H), 7.38 (dd, J = 8.4, 7.1 Hz, 2H), 6.91 (s, 2H), 6.84 (s, 1H), 4.76 (t, J = 7.3 Hz, 1H), 4.57–4.30 (m, 2H), 2.62–2.46 (m, 1H), 2.28–2.22 (m, 6H), 2.20–2.09 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.20, 138.65, 138.32, 136.57, 132.94, 129.05, 128.81, 128.51, 126.03, 81.76 (d, J = 165.3 Hz), 48.89 (d, J = 3.4 Hz), 34.49 (d, J = 19.2 Hz), 21.28. 19F NMR (565 MHz, Chloroform-d) δ −221.23 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H19FNaO ([M + Na]+), 271.1492; found, 271.1495.
- 4-fluoro-2-(naphthalen-2-yl)-1-phenylbutan-1-one (22) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 17.0 mg, 58%). 1H NMR (500 MHz, Chloroform-d) δ 8.00 (d, J = 7.7 Hz, 2H), 7.82–7.75 (m, 4H), 7.45 (dt, J = 8.1, 2.3 Hz, 4H), 7.37 (d, J = 7.7 Hz, 2H), 5.01 (t, J = 7.3 Hz, 1H), 4.64–4.14 (m, 2H), 2.77–2.51 (m, 1H), 2.45–2.17 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.03, 136.43, 135.95, 133.64, 133.06, 132.56, 129.04, 128.83, 128.57, 127.78, 127.65, 127.38, 126.34, 126.11, 126.06, 81.72 (d, J = 164.9 Hz), 49.14 (d, J = 3.2 Hz), 34.42 (d, J = 19.3 Hz). 19F NMR (565 MHz, Chloroform-d) δ -221.32 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C20H17FNaO ([M + Na]+), 315.1155; found, 315.1159.
- 2-(anthracen-2-yl)-4-fluoro-1-phenylbutan-1-one (23) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 28.4 mg, 83%). 1H NMR (500 MHz, Chloroform-d) δ 8.18 (d, J = 7.0 Hz, 2H), 7.99 (d, J = 7.7 Hz, 2H), 7.63 (d, J = 7.4 Hz, 1H), 7.56–7.49 (m, 3H), 7.40 (dt, J = 20.8, 7.8 Hz, 3H), 7.28–7.20 (m, 2H), 7.12 (dd, J = 8.1, 2.3 Hz, 1H), 4.90 (t, J = 7.4 Hz, 1H), 4.63–4.19 (m, 2H), 2.68–2.50 (m, 1H), 2.28–2.07 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.79, 164.93, 151.46, 140.13, 136.31, 133.67, 133.24, 130.17, 130.12, 129.40, 128.81, 128.66, 128.59, 125.87, 121.55, 120.86, 82.17, 81.09, 48.65 (d, J = 3.3 Hz), 34.48 (d, J = 19.4 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.44 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C24H19FNaO ([M + Na]+), 365.1312; found, 365.1315.
- 4-fluoro-1-phenyl-2-(quinolin-6-yl)butan-1-one (24) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 10.8 mg, 37%). 1H NMR (500 MHz, Chloroform-d) δ 8.92–8.83 (m, 1H), 8.08 (dd, J = 10.6, 8.4 Hz, 2H), 8.00 (s, 2H), 7.79–7.69 (m, 2H), 7.47 (t, J = 7.4 Hz, 1H), 7.40–7.35 (m, 3H), 5.06 (t, J = 7.3 Hz, 1H), 4.60–4.28 (m, 2H), 2.74–2.58 (m, 1H), 2.33–2.20 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.81, 150.57, 147.51, 136.88, 136.28, 135.92, 133.26, 130.53, 129.88, 128.79, 128.66, 121.48, 81.61 (d, J = 164.4 Hz), 48.83 (d, J = 3.2 Hz), 34.50 (d, J = 19.5 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.36 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C19H16FNNaO ([M + Na]+), 316.1108; found, 316.1113.
- 2-(5a,9a-dihydrodibenzo[b,d]furan-3-yl)-4-fluoro-1-phenylbutan-1-one (25) Purification- by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 13.3 mg, 40%). 1H NMR (500 MHz, Chloroform-d) δ 8.50 (d, J = 1.7 Hz, 1H), 8.01 (dd, J = 8.7, 1.8 Hz, 1H), 7.90 (d, J = 8.1 Hz, 1H), 7.81 (dd, J = 8.5, 3.5 Hz, 2H), 7.58–7.53 (m, 2H), 7.53–7.48 (m, 2H), 7.30 (t, J = 7.6 Hz, 2H), 7.20 (t, J = 7.4 Hz, 1H), 5.01 (t, J = 7.3 Hz, 1H), 4.60–4.31 (m, 2H), 2.73–2.56 (m, 1H), 2.31–2.15 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.05, 138.60, 135.49, 133.79, 132.44, 130.60, 129.67, 129.17, 128.51, 128.39, 128.35, 127.66, 127.36, 126.69, 124.43, 81.83 (d, J = 163.7 Hz), 49.07 (d, J = 3.3 Hz), 34.46 (d, J = 19.2 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.34 (tdd, J = 50.85, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C22H17FNaO2 ([M + Na]+), 355.1104; found, 355.1104.
- 2-(benzo[d][1,3]dioxol-5-yl)-4-fluoro-1-phenylbutan-1-one (26) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 23.2 mg, 81%). 1H NMR (500 MHz, Chloroform-d) δ 8.04–7.84 (m, 2H), 7.52–7.46 (m, 1H), 7.40 (d, J = 7.7 Hz, 2H), 6.77 (d, J = 7.4 Hz, 2H), 6.73 (d, J = 8.0 Hz, 1H), 5.92–5.87 (m, 2H), 4.75 (t, J = 7.4 Hz, 1H), 4.56–4.28 (m, 2H), 2.60–2.45 (m, 1H), 2.19–2.01 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.02, 148.23, 146.89, 136.39, 133.04, 132.04, 128.76, 128.56, 121.87, 108.79, 108.45, 101.14, 81.73 (d, J = 165.1 Hz), 48.51 (d, J = 3.1 Hz), 34.34 (d, J = 19.2 Hz), 29.71. 19F NMR (565 MHz, Chloroform-d) δ −221.48 (tdd, J = 50.85, 33.90, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H15FNaO3 ([M + Na]+), 309.0897; found, 309.0896.
- 4-fluoro-1-phenyl-2-(1-tosyl-1H-indol-5-yl)butan-1-one (27) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 21.7 mg, 50%). 1H NMR (500 MHz, Chloroform-d) δ 7.95 (dd, J = 8.4, 1.4 Hz, 2H), 7.90 (d, J = 8.6 Hz, 1H), 7.80–7.72 (m, 2H), 7.52 (d, J = 3.7 Hz, 1H), 7.49–7.41 (m, 2H), 7.36 (t, J = 7.7 Hz, 2H), 7.29–7.22 (m, 1H), 7.21 (d, J = 8.1 Hz, 2H), 6.57 (d, J = 3.7 Hz, 1H), 4.91 (t, J = 7.4 Hz, 1H), 4.54–4.23 (m, 2H), 2.65–2.50 (m, 1H), 2.32 (s, 3H), 2.23–2.07 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 199.17, 145.02, 136.41, 135.32, 133.98, 133.41, 133.04, 131.34, 129.94, 128.81, 128.55, 126.86, 126.85, 124.94, 121.04, 114.05, 108.68, 81.59 (d, J = 164.1 Hz), 48.61 (d, J = 3.3 Hz), 34.72 (d, J = 19.3 Hz), 21.56. 19F NMR (565 MHz, Chloroform-d) δ −221.38 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C25H22FNNaO3S ([M + Na]+), 458.1196; found, 458.1198.
- 2-(benzofuran-5-yl)-4-fluoro-1-phenylbutan-1-one (28) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 51.9 mg, 62%). 1H NMR (500 MHz, Chloroform-d) δ 7.99 (dd, J = 8.4, 1.4 Hz, 2H), 7.70 (dd, J = 7.5, 1.6 Hz, 1H), 7.54 (s, 1H), 7.53–7.48 (m, 1H), 7.45 (dd, J = 7.6, 1.2 Hz, 1H), 7.39 (t, J = 7.6 Hz, 2H), 7.33–7.25 (m, 2H), 5.08 (t, J = 7.3 Hz, 1H), 4.68–4.31 (m, 2H), 2.72–2.55 (m, 1H), 2.42–2.24 (m, 1H).13C NMR (151 MHz, Chloroform-d) δ 198.49, 155.52, 142.98, 136.06, 133.33, 128.67, 128.58, 126.48, 124.75, 122.95, 119.93, 117.81, 111.73, 81.69 (d, J = 164.2 Hz), 38.70 (d, J = 3.3 Hz), 32.95 (d, J = 19.4 Hz). 19F NMR (565 MHz, Chloroform-d) δ −221.32 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H15FNaO2 ([M + Na]+), 305.0948; found, 305.0956.
- phenyl-2-benzoyl-4-fluorobutanoate (29) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 6.5 mg, 23%). 1H NMR (500 MHz, Chloroform-d) δ 8.12–8.04 (m, 1H), 7.64 (td, J = 7.2, 1.3 Hz, 0H), 7.53 (t, J = 7.8 Hz, 1H), 7.38–7.29 (m, 1H), 7.24–7.17 (m, 1H), 6.98 (dd, J = 8.5, 1.3 Hz, 1H), 4.85 (dd, J = 7.8, 6.2 Hz, 0H), 4.75–4.54 (m, 1H), 2.60–2.41 (m, 1H).13C NMR (151 MHz, Chloroform-d) δ 193.34, 167.18, 149.34, 134.64, 132.98, 128.42, 127.94, 127.79, 125.15, 120.16, 80.58 (d, J = 165.6 Hz), 48.89 (d, J = 2.9 Hz), 28.86 (d, J = 19.6 Hz).19F NMR (565 MHz, Chloroform-d) δ −220.48 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H15FNaO3 ([M + Na]+), 309.0897; found, 309.0891.
- 4-fluoro-1,2-di-p-tolylbutan-1-one (30) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 18.9 mg, 70%). 1H NMR (500 MHz, Chloroform-d) δ 7.86 (d, J = 8.4 Hz, 2H), 7.21–7.12 (m, 4H), 7.09 (d, J = 7.9 Hz, 2H), 4.78 (t, J = 7.4 Hz, 1H), 4.53–4.28 (m, 2H), 2.62–2.46 (m, 1H), 2.33 (s, 3H), 2.27 (s, 3H), 2.23–2.06 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.80, 143.77, 136.92, 135.67, 133.95, 129.79, 129.21, 128.92, 128.17, 81.89 (d, J = 163.8 Hz), 48.44 (d, J = 3.2 Hz), 34.36 (d, J = 19.3 Hz), 21.58, 21.01. 19F NMR (565 MHz, Chloroform-d) δ −221.31 (tdd, J = 50.85, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H19FNaO ([M + Na]+), 293.1312; found, 293.1315.
- 1-(4-(tert-butyl)phenyl)-4-fluoro-2-(p-tolyl)butan-1-one (31) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 24.9 mg, 80%). 1H NMR (500 MHz, Chloroform-d) δ 8.09–7.79 (m, 2H), 7.39 (d, J = 8.5 Hz, 2H), 7.20 (d, J = 8.0 Hz, 2H), 7.11 (d, J = 8.0 Hz, 2H), 4.80 (t, J = 7.4 Hz, 1H), 4.57–4.25 (m, 2H), 2.60–2.48 (m, 1H), 2.28 (s, 3H), 2.23–2.06 (m, 1H), 1.28 (s, 9H). 13C NMR (151 MHz, Chloroform-d) δ 198.72, 156.68, 136.93, 135.69, 133.86, 129.80, 128.77, 128.20, 125.50, 81.89 (d, J = 163.8 Hz), 48.44 (d, J = 3.3 Hz), 35.06, 34.47 (d, J = 19.3 Hz), 31.02, 21.02. 19F NMR (565 MHz, Chloroform-d) δ −221.28 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C21H25FNaO ([M + Na]+), 335.1781; found, 335.1786.
- 1-(4-chlorophenyl)-4-fluoro-2-(p-tolyl)butan-1-one (32) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 15.4 mg, 68%). 1H NMR (500 MHz, Chloroform-d) δ 7.88 (d, J = 8.7 Hz, 2H), 7.34 (d, J = 8.6 Hz, 2H), 7.15 (d, J = 8.1 Hz, 2H), 7.11 (d, J = 7.9 Hz, 2H), 4.73 (t, J = 7.3 Hz, 1H), 4.62–4.22 (m, 2H), 2.62–2.38 (m, 1H), 2.28 (s, 3H), 2.23–2.04 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 197.96, 139.38, 137.25, 135.09, 134.73, 130.20, 129.96, 128.84, 128.15, 81.69 (d, J = 163.9 Hz), 48.74 (d, J = 3.3 Hz), 34.23 (d, J = 19.2 Hz), 21.03. 19F NMR (565 MHz, Chloroform-d) δ −221.51 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H16ClFNaO ([M + Na]+), 313.0765; found, 313.0765.
- 1-(4-bromophenyl)-4-fluoro-2-(p-tolyl)butan-1-one (33) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 17.7 mg, 53%). 1H NMR (500 MHz, Chloroform-d) δ 7.80 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.3 Hz, 2H), 7.14 (d, J = 7.8 Hz, 2H), 7.10 (d, J = 7.9 Hz, 2H), 4.72 (t, J = 7.3 Hz, 1H), 4.53–4.24 (m, 2H), 2.62–2.46 (m, 1H), 2.28 (s, 3H), 2.20–2.04 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.15, 137.26, 135.15, 135.06, 131.83, 130.30, 129.96, 128.14, 81.67 (d, J = 163.7 Hz), 48.74 (d, J = 3.2 Hz), 34.21 (d, J = 19.5 Hz), 21.01. 19F NMR (565 MHz, Chloroform-d) δ −221.52 (tdd, J = 50.85, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H16BrFNaO ([M + Na]+), 357.0260; found, 357.0265.
- 4-fluoro-2-(p-tolyl)-1-(4-(trifluoromethoxy)phenyl)butan-1-one (34) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 9.2 mg, 27%). 1H NMR (500 MHz, Chloroform-d) δ 7.80 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.3 Hz, 2H), 7.14 (d, J = 7.8 Hz, 2H), 7.10 (d, J = 7.9 Hz, 2H), 4.72 (t, J = 7.3 Hz, 1H), 4.53–4.24 (m, 2H), 2.62–2.46 (m, 1H), 2.28 (s, 3H), 2.20–2.04 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.80, 137.05, 136.18, 133.34, 129.73, 128.75, 128.70, 121.54, 120.04 (d, J = 259.2 Hz), 81.52 (d, J = 164.3 Hz), 48.07 (d, J = 2.9 Hz), 34.47 (d, J = 19.2 Hz), 21.02. 19F NMR (565 MHz, Chloroform-d) δ −57.88 (s, 3F), −221.36 (tdd, J = 50.85, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H16F4NaO2 ([M + Na]+), 363.0978; found, 363.0971.
- 4-(4-fluoro-2-(p-tolyl)butanoyl)benzonitrile (35) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 14.0 mg, 50%). 1H NMR (500 MHz, Chloroform-d) δ 7.73 (d, J = 8.2 Hz, 2H), 7.65 (d, J = 8.2 Hz, 2H), 7.14 (d, J = 7.9 Hz, 2H), 7.10 (d, J = 8.0 Hz, 2H), 4.71 (t, J = 7.3 Hz, 1H), 4.54–4.29 (m, 2H), 2.62–2.44 (m, 1H), 2.28 (s, 3H), 2.21–2.03 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.44, 137.84, 137.26, 135.67, 135.04, 130.19, 129.97, 128.15, 100.98, 81.68 (d, J = 163.7 Hz), 48.67 (d, J = 3.0 Hz), 34.18 (d, J = 19.4 Hz), 21.03. 19F NMR (565 MHz, Chloroform-d) δ −221.51 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H16FNNaO ([M + Na]+), 304.1108; found, 304.1105.
- 4-fluoro-1-(2-methoxyphenyl)-2-(p-tolyl)butan-1-one (36) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 15.4 mg, 54%). 1H NMR (500 MHz, Chloroform-d) δ 7.55 (dd, J = 7.8, 1.4 Hz, 1H), 7.51–7.46 (m, 1H), 7.27 (t, J = 8.1 Hz, 1H), 7.18 (d, J = 7.8 Hz, 2H), 7.10 (d, J = 7.9 Hz, 2H), 7.02 (dd, J = 8.2, 2.7 Hz, 1H), 4.78 (t, J = 7.4 Hz, 1H), 4.54–4.27 (m, 2H), 3.79 (s, 3H), 2.66–2.46 (m, 1H), 2.28 (s, 3H), 2.23–2.06 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.99, 159.71, 137.81, 137.05, 135.43, 129.86, 129.48, 128.18, 121.43, 119.53, 113.04, 81.81 (d, J = 163.9 Hz), 55.36, 48.75 (d, J = 3.2 Hz), 34.38 (d, J = 19.3 Hz), 21.03. 19F NMR (565 MHz, Chloroform-d) δ −221.34 (tdd, J = 58.85, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H19FNaO2 ([M + Na]+), 309.1261; found, 309.1261.
- 4-fluoro-1-(m-tolyl)-2-(p-tolyl)butan-1-one (37) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 14.6 mg, 50%). 1H NMR (500 MHz, Chloroform-d) δ 7.57 (d, J = 7.7 Hz, 1H), 7.28 (d, J = 7.4 Hz, 1H), 7.20–7.04 (m, 6H), 4.64 (t, J = 7.4 Hz, 1H), 4.56–4.30 (m, 2H), 2.67–2.51 (m, 1H), 2.31 (s, 3H), 2.27 (s, 3H), 2.21–2.06 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 203.29, 138.39, 138.09, 137.03, 134.59, 131.61, 130.94, 129.69, 128.32, 128.04, 125.46, 81.97 (d, J = 164.0 Hz), 51.55 (d, J = 3.5 Hz), 33.79 (d, J = 19.1 Hz), 21.04, 20.78. 19F NMR (565 MHz, Chloroform-d) δ −221.33 (tdd, J = 45.20, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C18H19FNaO ([M + Na]+), 293.1312; found, 293.1312.
- 4-fluoro-1-(3-fluorophenyl)-2-(p-tolyl)butan-1-one (38) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 11.5 mg, 42%). 1H NMR (500 MHz, Chloroform-d) δ 7.73 (dd, J = 7.8, 1.5 Hz, 1H), 7.62 (dt, J = 9.6, 2.1 Hz, 1H), 7.34 (td, J = 8.0, 5.5 Hz, 1H), 7.20–7.13 (m, 3H), 7.11 (d, J = 8.0 Hz, 2H), 4.73 (t, J = 7.3 Hz, 1H), 4.74–4.43 (m, 2H), 2.59–2.047 (m, 1H), 2.28 (s, 3H), 2.23–2.06 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 197.94 (d, J = 2.2 Hz), 163.57, 161.93, 138.57 (d, J = 6.2 Hz), 137.28, 134.93, 130.14 (d, J = 7.5 Hz), 129.97, 128.17, 124.51 (d, J = 2.9 Hz), 119.96 (d, J = 21.5 Hz), 115.52 (d, J = 22.5 Hz), 81.66 (d, J = 164.1 Hz), 48.94 (d, J = 3.3 Hz), 34.28 (d, J = 19.5 Hz), 21.03. 19F NMR (565 MHz, Chloroform-d) δ -111.88 (d, J = 14.0 Hz, 1F), −221.41 (tdd, J = 50.85, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C17H16F2NaO ([M + Na]+), 297.1061; found, 297.1066.
- 1-(3,5-dimethylphenyl)-4-fluoro-2-(p-tolyl)butan-1-one (39) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 14.2 mg, 50%).1H NMR (500 MHz, Chloroform-d) δ 7.56 (s, 2H), 7.18 (d, J = 7.8 Hz, 2H), 7.10 (d, J = 8.1 Hz, 3H), 4.79 (t, J = 7.4 Hz, 1H), 4.53–4.28 (m, 2H), 2.61–2.46 (m, 1H), 2.30 (s, 6H), 2.27 (s, 3H), 2.19–2.07 (m, 1H).13C NMR (151 MHz, Chloroform-d) δ 199.62, 138.09, 136.90, 136.60, 135.54, 134.67, 129.77, 128.16, 126.56, 81.92 (d, J = 163.6 Hz), 48.44 (d, J = 3.1 Hz), 34.43 (d, J = 19.3 Hz), 21.25, 21.02. 19F NMR (565 MHz, Chloroform-d) δ −221.29 (tdd, J = 50.85, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C19H21FNaO ([M + Na]+), 307.1468; found, 307.1473.
- 4-fluoro-1-(naphthalen-2-yl)-2-(p-tolyl)butan-1-one (40) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 18.7 mg, 61%). 1H NMR (500 MHz, Chloroform-d) δ 8.36 (d, J = 8.5 Hz, 1H), 7.90 (d, J = 8.3 Hz, 1H), 7.85 (dd, J = 7.2, 1.2 Hz, 1H), 7.80 (dd, J = 8.0, 1.5 Hz, 1H), 7.55–7.43 (m, 3H), 7.42 (t, J = 7.7 Hz, 1H), 7.19 (d, J = 7.9 Hz, 2H), 7.06 (d, J = 7.8 Hz, 2H), 4.83 (t, J = 7.4 Hz, 1H), 4.63–4.29 (m, 2H), 2.80–2.64 (m, 1H), 2.30–2.17 (m, 4H). 13C NMR (151 MHz, Chloroform-d) δ 203.07, 137.06, 136.38, 134.74, 133.86, 132.37, 130.54, 129.75, 128.29, 128.26, 127.76, 127.27, 126.35, 125.60, 124.28, 82.00 (d, J = 164.0 Hz), 52.06 (d, J = 3.4 Hz), 34.12 (d, J = 19.2 Hz), 21.01. 19F NMR (565 MHz, Chloroform-d) δ −221.21 (tdd, J = 50.85, 33.90, 28.25 Hz, 1F). HRMS (ESI) (m/z): calcd for C21H20NaO ([M + H]+), 307.1492; found, 307.1485.
- 4-fluoro-1-(thiophen-2-yl)-2-(p-tolyl)butan-1-one (41) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 100:1, v/v) affords the title compound as a yellow oil (yield 14.1 mg, 54%). 1H NMR (500 MHz, Chloroform-d) δ 7.71 (dd, J = 3.9, 1.1 Hz, 1H), 7.56 (dd, J = 5.0, 1.1 Hz, 1H), 7.22 (d, J = 7.9 Hz, 2H), 7.12 (d, J = 7.8 Hz, 2H), 7.04 (dd, J = 4.9, 3.8 Hz, 1H), 4.62 (t, J = 7.4 Hz, 1H), 4.56–4.27 (m, 2H), 2.61–2.45 (m, 1H), 2.28 (s, 3H), 2.18–2.03 (m, 1H). 13C NMR (151 MHz, Chloroform-d) δ 198.44, 137.84, 137.26, 135.67, 135.04, 130.19, 129.97, 128.15, 100.98, 81.68 (d, J = 163.7 Hz), 48.67 (d, J = 3.0 Hz), 34.18 (d, J = 19.4 Hz), 21.03. 19F NMR (565 MHz, Chloroform-d) δ −221.51 (tdd, J = 45.20, 28.25, 22.60 Hz, 1F). HRMS (ESI) (m/z): calcd for C15H15FNaOS ([M + Na]+), 285.0719; found, 285.0724.
- 2,2,6,6-tetramethylpiperidin-1-yl benzoate (43) Purification by column chromatography on silica gel (petroleum ether/ethyl acetate = 3:1, v/v) affords the title compound as a red solid (yield 11.2 mg, 43%). 1H NMR (500 MHz, Chloroform-d) δ 8.08 (d, J = 7.0 Hz, 2H), 7.58 (t, J = 7.5 Hz, 1H), 7.46 (t, J = 7.5 Hz, 2H), 1.83–1.74 (m, 2H), 1.75–1.66 (m, 1H), 1.61–1.57 (m, 2H), 1.50–1.43 (m, 1H), 1.28 (s, 6H), 1.12 (s, 6H). 13C NMR (151 MHz, Chloroform-d) δ 166.42, 132.88, 129.79, 129.60, 128.49, 60.44, 39.11, 32.01, 20.89, 17.05. HRMS (ESI) (m/z): calcd for C16H23NNaO2, ([M + Na]+), 284.1621, found 284.1614.
- (5aR,10bS)-1-benzoyl-2-mesityl-2,5a,6,10b-tetrahydro-4H-indeno [2,1-b][1,2,4]tri-zolo [4,3d][1,4]oxazin-11-ium tetrafluoroborate (44) The white precipitate was filtered off and washed with diethyl ether. Drying under vacuum afforded the corresponding product as a white solid. 1H NMR (500 MHz, Chloroform-d) δ 8.24 (d, J = 7.8 Hz, 2H), 7.78 (t, J = 7.4 Hz, 1H), 7.70 (t, J = 7.6 Hz, 2H), 7.35 (d, J = 4.4 Hz, 2H), 7.28–7.23 (m, 2H), 7.11 (d, J = 7.7 Hz, 1H), 7.01 (s, 1H), 6.93 (s, 1H), 5.72 (d, J = 3.3 Hz, 1H), 5.51 (d, J = 16.3 Hz, 1H), 5.49–5.46 (m, 1H), 5.12 (d, J = 16.3 Hz, 1H), 3.30 (dd, J = 17.1, 4.0 Hz, 1H), 3.20 (d, J = 17.1 Hz, 1H), 2.32 (s, 3H), 2.17 (d, J = 13.6 Hz, 6H). 13C NMR (151 MHz, Chloroform-d) δ 179.11, 150.88, 147.75, 142.66, 140.83, 138.21, 135.66, 135.31, 132.10, 131.04, 130.72, 130.25, 130.13, 129.86, 129.40, 127.82, 126.34, 123.02, 63.80, 60.82, 37.25, 21.20, 17.58, 17.47. 19F NMR (565 MHz, Chloroform-d) δ −151.49. HRMS (ESI) (m/z): calcd for C28H26N3O2+, 437.2098; found, 437.2090.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zafrani, Y.; Yeffet, D.; Moriah, G.; Berliner, A.; Amir, D.; Marciano, D.; Gershonov, E.; Saphier, S. The Difluoromethyl Bioisoster: Examining the “Lipophilic Hydrogen Bond Donor” Concept. J. Med. Chem. 2017, 60, 797–804. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2020. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2021, 26, 627. [Google Scholar] [CrossRef]
- Inoue, M.; Sumii, Y.; Shibat, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.; Gouverneur, V.; Lin, J.H.; Meanwell, M.; Ni, C.; Pupo, G.; Xiao, J.C.; Hu, J. Contemporary Synthetic Strategies in Organofluorine Chemistry. Nat. Rev. Methods Primers 2021, 1, 47. [Google Scholar] [CrossRef]
- Studer, A. “Renaissance” in Radical Trifluoromethylation. Angew. Chem. Int. Ed. 2012, 51, 8950–8958. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Akita, M. Fine Design of Photoredox Systems for Catalytic Fluoromethylation of Carbon–Carbon Multiple Bonds. Acc. Chem. Res. 2016, 49, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Xia, H.; Wu, J. Recent Advances in Photoinduced Trifluoromethylation and Difluoroalkylation. Org. Chem. Front. 2016, 3, 1163–1185. [Google Scholar] [CrossRef]
- Koike, T. Frontiers in Radical Fluoromethylation by Visible-Light Organic Photocatalysis. Asian J. Org. Chem. 2020, 9, 529–537. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, X.; Fang, H.; Zhu, L.; Li, C. Radical Trifluoromethylation. Chem. Soc. Rev. 2021, 50, 6308–6319. [Google Scholar] [CrossRef]
- Yerien, D.E.; Vallejo, S.; Postigo, A. Difluoromethylation Reactions of Organic Compounds. Chem. Eur. J. 2017, 23, 14676–14701. [Google Scholar] [CrossRef]
- Koike, T.; Akita, M. Recent Progress in Photochemical Radical Di-and Mono-Fluoromethylation. Org. Biomol. Chem. 2019, 17, 5413–5419. [Google Scholar] [CrossRef]
- Feng, J.; Jia, X.; Zhang, S.; Lu, K.; Cahard, D. State of Knowledge in Photoredox-Catalysed Direct Difluoromethylation. Org. Chem. Front. 2022, 9, 3598–3623. [Google Scholar] [CrossRef]
- Zafrani, Y.; Moriah, G.; Yeffet, D.; Berliner, A.; Amir, D.; Marciano, D.; Elias, S.; Katalan, S.; Shkenazi, N.; Madmon, M.; et al. CF2H, a Functional Group-Dependent Hydrogen-Bond Donor: Is It a More or Less Lipophilic Bioisostere of OH, SH, and CH3. J. Med. Chem. 2019, 62, 5628–5637. [Google Scholar] [CrossRef]
- Sessler, C.D.; Rahm, M.; Becker, S.; Goldberg, J.M.; Wang, F.; Lippard, S.J. CF2H, a Hydrogen Bond Donor. J. Am. Chem. Soc. 2017, 139, 9325–9332. [Google Scholar] [CrossRef]
- Cernak, T.A.; Gleason, J.L. Reagents for Selective Fluoromethylation: A Challenge in Organofluorine Chemistry. Angew. Chem. Int. Ed. 2020, 59, 12268–12281. [Google Scholar]
- Liu, Y.; Li, C.; Meng, J.; Song, D.; Liu, B.; Xu, Y. Recent Progress in Monofluoromethylation. Chin. J. Org. Chem. 2020, 40, 2322. [Google Scholar] [CrossRef]
- Colella, M.; Musci, P.; Andresini, M.; Spennacchio, M.; Degennaro, L.; Luisi, R. The synthetic versatility of fluoroiodomethane: Recent applications as monofluoromethylation platform. Org. Biomol. Chem. 2022, 20, 4669–4680. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Dixon, J.A.; Hara, A.; Funder, E.D.; Dixon, D.D.; Rodriguez, R.A.; Baxter, R.D.; Baran, P.S. Practical and innate carbon–hydrogen functionalization of heterocycle. Nature 2012, 492, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.; Colella, M.; Monticelli, S.; Romanazzi, G.; Holzer, W.; Langer, T.; Degennaro, L.; Pace, V.; Luisi, R. Exploiting a “Beast” in Carbenoid Chemistry: Development of a Straightforward Direct Radical Fluoromethylation Strategy. J. Am. Chem. Soc. 2017, 139, 13648–13651. [Google Scholar] [CrossRef]
- Tang, W.; Feng, Y.; Xu, Z.; Cheng, Z.; Dai, J.; Xu, H. Visible-Light-Enabled Decarboxylative Mono- and Difluoromethylation of Cinnamic Acids under Metal-Free Conditions. Org. Lett. 2017, 19, 5501–5504. [Google Scholar] [CrossRef]
- Su, Y.; Feng, G.; Wang, Z.; Lan, Q.; Wang, X. Nickel-Catalyzed Monofluoromethylation of Aryl Boronic Acids. Angew. Chem. Int. Ed. 2015, 127, 6101–6105. [Google Scholar] [CrossRef]
- Ruan, Z.; Zhang, S.K.; Zhu, C.; Ruth, P.N.; Stalke, D.; Ackermann, L. Ruthenium(II)-Catalyzed meta C−H Mono- and Difluoromethylations by Phosphine/Carboxylate Cooperation. Angew. Chem. Int. Ed. 2017, 56, 2045–2049. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Li, Y.; Ni, H.; Bi, Y.; Sheng, J.; Wang, X. Nickel-catalyzed hydromonofluoromethylation of unactivated alkenes for expedient construction of primary alkyl fluorides. Chin. Chem. Lett. 2023, 34, 107614. [Google Scholar] [CrossRef]
- He, Z.; Tan, P.; Ni, C.; Hu, J. Fluoroalkylative Aryl Migration of Conjugated N-Arylsulfonylated Amides Using Easily Accessible Sodium Di- and Monofluoroalkanesulfinates. Org. Lett. 2015, 17, 1838–1841. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Xu, H.; Qing, F. Copper-Catalyzed Regioselective Borylfluoromethylation of Alkenes. ACS Catal. 2019, 9, 5726–5731. [Google Scholar] [CrossRef]
- Tang, X.; Thomoson, C.S.; Dolbier Jr, W.R. Photoredox-Catalyzed Tandem Radical Cyclization of N-Arylacrylamides: General Methods to Construct Fluorinated 3,3-Disubstituted 2-Oxindoles Using Fluoroalkylsulfonyl Chlorides. Org. Lett. 2014, 16, 4594–4597. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Jr, W. Efficient Cu-catalyzed Atom Transfer Radical Addition Reactions of Fluoroalkylsulfonyl Chlorides with Electron-deficient Alkenes Induced by Visible Light. Angew. Chem. Int. Ed. 2015, 54, 4246–4249. [Google Scholar] [CrossRef]
- Tian, S.; Chen, M.; Tang, Y.; Cheng, K.; Tsui, G.C.; Wang, Q. Acylmonofluoromethylation of alkenes via dual NHC/photoredox catalysis. Org. Chem. Front. 2023, 10, 6124–6130. [Google Scholar] [CrossRef]
- Ramkumar, N.; Baumane, L.; Zacs, D.; Veliks, J. Merging Copper(I) Photoredox Catalysis and Iodine(III) Chemistry for the Oxy-monofluoromethylation of Alkenes. Angew. Chem. Int. Ed. 2023, 62, e202219027. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, Y.; Sheng, H.; Zhang, C.; Su, X.; Wang, Z.; Chen, X. Visible-Light-Induced Selective Photolysis of Phosphonium Iodide Salts for Monofluoromethylations. Angew. Chem. Int. Ed. 2021, 60, 25477–25484. [Google Scholar] [CrossRef]
- Hell, S.M.; Meyer, C.F.; Ortalli, S.; Sap, J.B.I.; Chen, X.; Gouverneur, V. Hydrofluoromethylation of alkenes with fluoroiodomethane and beyond. Chem Sci. 2021, 12, 12149–12155. [Google Scholar] [CrossRef]
- Deneny, P.J.; Kumar, R.; Gaunt, M.J. Visible light-mediated radical fluoromethylation via halogen atom transfer activation of fluoroiodomethane. Chem Sci. 2021, 12, 12812–12818. [Google Scholar] [CrossRef]
- Noto, N.; Koike, T.; Akita, M. Visible-Light-Triggered Monofluoromethylation of Alkenes by Strongly Reducing 1,4-Bis(diphenylamino)naphthalene Photoredox Catalysis. ACS Catal. 2019, 9, 4382–4387. [Google Scholar] [CrossRef]
- Noto, N.; Takahashi, K.; Goryo, S.; Takakado, A.; Iwata, K.; Koike, K.; Akita, M. Laser Flash Photolysis Studies on Radical Monofluoromethylation by (Diarylamino)naphthalene Photoredox Catalysis: Long Lifetime of the Excited State is Not Always a Requisite. J. Org. Chem. 2020, 85, 13220–13227. [Google Scholar] [CrossRef] [PubMed]
- Guin, J.; De Sarkar, S.; Grimme, S.; Studer, A. Biomimetic Carbene-Catalyzed Oxidations of Aldehydes Using TEMPO. Angew. Chem. Int. Ed. 2008, 47, 8727–8730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Mück-Lichtenfeld, C.; Studer, A. Cooperative N-Heterocyclic Carbene (NHC) and Ruthenium Redox Catalysis: Oxidative Esterification of Aldehydes with Air as the Terminal Oxidant. Adv. Synth. Catal. 2013, 355, 1098–1106. [Google Scholar] [CrossRef]
- Ishii, T.; Kakeno, Y.; Nagao, K.; Ohmiya, H. N-Heterocyclic Carbene-Catalyzed Decarboxylative Alkylation of Aldehydes. J. Am. Chem. Soc. 2019, 141, 3854–3858. [Google Scholar] [CrossRef]
- Ishii, T.; Ota, K.; Nagao, K.; Ohmiya, H. N-Heterocyclic Carbene-Catalyzed Radical Relay Enabling Vicinal Alkylacylation of Alkenes. J. Am. Chem. Soc. 2019, 141, 14073–14077. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Y.; Li, X.; Shao, Y.; Li, G.; Webster, R.D.; Chi, Y.R. N-Heterocyclic Carbene Organocatalytic Reductive β,β-Coupling Reactions of Nitroalkenes via Radical Intermediates. Org. Lett. 2014, 16, 5678–5681. [Google Scholar] [CrossRef]
- Regnier, V.; Romero, E.A.; Molton, F.; Jazzar, R.; Bertrand, G.; Martin, D. What Are the Radical Intermediates in Oxidative N-Heterocyclic Carbene Organocatalysis. J. Am. Chem. Soc. 2019, 141, 1109–1117. [Google Scholar] [CrossRef]
- Delfau, L.; Nichilo, S.; Molton, F.; Broggi, J.; Tomás-Mendivil, E.; Martin, D. Critical Assessment of the Reducing Ability of Breslow-type Derivatives and Implications for Carbene-Catalyzed Radical Reactions. Angew. Chem. Int. Ed. 2021, 60, 26783–26789. [Google Scholar] [CrossRef]
- Li, X.; Ren, X.; Chen, C.; Sun, L.; Han, Y. Isolation and characterization of an annelated N-heterocyclic carbene stabilized Breslow enolate. Org. Chem. Front. 2024, 10. [Google Scholar] [CrossRef]
- Liu, K.; Schwenzer, M.; Studer, A. Radical NHC Catalysis. ACS Catal. 2022, 12, 11984–11999. [Google Scholar] [CrossRef]
- Ishii, T.; Nagao, K.; Ohmiya, H. Recent advances in N-heterocyclic carbene-based radical catalysis. Chem. Sci. 2020, 11, 5630–5636. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, L.; Zheng, G.; Zhang, Q. Recent advances in three-component radical acylative difunctionalization of unsaturated carbon–carbon bonds. Org. Chem. Front. 2023, 10, 4488–4515. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Yang, R.; Song, H.; Liu, Y.; Wang, Q. Recent advances in combining photo- and N-heterocyclic carbene catalysis. Chem. Sci. 2023, 14, 13367–13383. [Google Scholar] [CrossRef]
- Patra, T.; Das, M.; Daniliuc, C.G.; Glorius, F. Metal-free photosensitized oxyimination of unactivated alkenes with bifunctional oxime carbonates. Nat. Catal. 2021, 4, 54–61. [Google Scholar] [CrossRef]
- Tan, G.; Das, M.; Kleinmans, R.; Katzenburg, F.; Daniliuc, C.; Glorius, F. Energy transfer-enabled unsymmetrical diamination using bifunctional nitrogen-radical precursors. Nat. Catal. 2022, 5, 1120–1130. [Google Scholar] [CrossRef]
- Tan, G.; Das, M.; Keum, H.; Bellotti, P.; Daniliuc, C.; Glorius, F. Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes. Nat. Chem. 2022, 14, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.Y.; Döben, N.; Studer, A. Cooperative NHC and Photoredox Catalysis for the Synthesis of β-Trifluoromethylated Alkyl Aryl Ketones. Angew. Chem. Int. Ed. 2020, 59, 19956–19960. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Studer, A. Direct α-Acylation of Alkenes via N-Heterocyclic Carbene, Sulfinate, and Photoredox Cooperative Triple Catalysis. J. Am. Chem. Soc. 2021, 143, 4903–4909. [Google Scholar] [CrossRef] [PubMed]
- Doeben, N.; Reimler, J.; Studer, A. Cooperative NHC/Photoredox Catalysis: Three Component Radical Coupling of Aroyl Fluorides, Styrenes and Alcohols. Adv. Synth. Catal. 2022, 364, 3348–3353. [Google Scholar] [CrossRef]
- Yu, X.; Meng, Q.Y.; Daniliuc, C.G.; Studer, A. Aroyl Fluorides as Bifunctional Reagents for Dearomatizing Fluoroaroylation of Benzofurans. J. Am. Chem. Soc. 2022, 144, 7072–7079. [Google Scholar] [CrossRef]
- Yu, X.; Maity, A.; Studer, A. Cooperative Photoredox and N-Heterocyclic Carbene Catalyzed Fluoroaroylation for the Synthesis of α-Trifluoromethyl-Substituted Ketones. Angew. Chem. Int. Ed. 2023, 62, e202310288. [Google Scholar] [CrossRef]
- Lezius, L.; Reimler, J.; Döben, J.; Hamm, M.; Daniliuc, C.J.; Studer, A. Aminoacylation of Alkenes by Cooperative NHC and Photoredox Catalysis. Synlett. 2023, 34. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Lezius, L.; Studer, A. Benzylic C−H acylation by cooperative NHC and photoredox catalysis. Nat. Commun. 2021, 12, 2068. [Google Scholar] [CrossRef]
- Zuo, Z.; Daniliuc, C.G.; Studer, A. Cooperative NHC/Photoredox Catalyzed Ring-Opening of Aryl Cyclopropanes to 1-Aroyloxylated-3-Acylated Alkanes. Angew. Chem. Int. Ed. 2021, 60, 25252–25257. [Google Scholar] [CrossRef] [PubMed]
- Reimler, J.; Yu, X.; Spreckelmeyer, N.; Daniliuc, C.; Studer, A. Regiodivergent C−H Acylation of Arenes by Switching from Ionic- to Radical-Type Chemistry Using NHC Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202303222. [Google Scholar] [CrossRef] [PubMed]
- Bay, A.V.; Fitzpatrick, K.P.; Betori, R.C.; Scheidt, K.A. Combined Photoredox and Carbene Catalysis for the Synthesis of Ketones from Carboxylic Acids. Angew. Chem. Int. Ed. 2020, 59, 9143–9148. [Google Scholar] [CrossRef] [PubMed]
- Baylya, A.-A.; McDonalda, B.-R.; Mrksicha, M.; Scheidt, K.A. High-throughput photocapture approach for reaction discovery. Proc. Natl. Acad. Sci. USA 2020, 117, 13261–13266. [Google Scholar] [CrossRef] [PubMed]
- Bay, A.V.; Fitzpatrick, K.P.; González-Montiel, G.A.; Farah, A.O.; Cheong, P.H.Y.; Scheidt, K.A. Light-Driven Carbene Catalysis for the Synthesis of Aliphatic and α-Amino Ketones. Angew. Chem. Int. Ed. 2021, 60, 17925–17931. [Google Scholar] [CrossRef] [PubMed]
- Bay, A.V.; Farnam, E.J.; Scheidt, K.A. Synthesis of Cyclohexanones by a Tandem Photocatalyzed Annulation. J. Am. Chem. Soc. 2022, 144, 7030–7037. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Schull, C.R.; Tam, A.T.; Renteria-Gomez, A.; Gogoi, A.R.; Gutierrez, O.; Scheidt, K.A. Photoinduced Acylations Via Azolium-Promoted Intermolecular Hydrogen Atom Transfer. J. Am. Chem. Soc. 2023, 145, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Hwang, M.U.; Wise, H.R.; Bay, A.V.; Cheong, P.H.-Y.; Scheidt, K.A. Light-Driven Enantioselective Carbene-Catalyzed Radical-Radical Coupling. Angew. Chem. Int. Ed. 2023, 62, e202312829. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Zhu, J.L.; Valencia, O.L.; Schull, C.R.; Scheidt, K.A. Cooperative Carbene Photocatalysis for β-Amino Ester Synthesis. J. Am. Chem. Soc. 2023, 145, 24486–24492. [Google Scholar] [CrossRef]
- Ren, S.C.; Lv, W.X.; Yang, X.; Yan, J.L.; Xu, J.; Wang, F.X.; Hao, L.; Chai, H.; Jin, Z.; Chi, Y.R. Carbene-Catalyzed Alkylation of Carboxylic Esters via Direct Photoexcitation of Acyl Azolium Intermediates. ACS Catal. 2021, 11, 2925–2934. [Google Scholar] [CrossRef]
- Ren, S.-C.; Yang, X.; Mondal, B.; Mou, C.; Tian, W.; Jin, Z.; Chi, Y.R. Carbene and photocatalyst-catalyzed decarboxylative radical coupling of carboxylic acids and acyl imidazoles to form ketones. Nat. Commun. 2022, 13, 2846. [Google Scholar] [CrossRef]
- Sato, Y.; Goto, Y.; Nakamura, K.; Miyamoto, Y.; Sumida, Y.; Ohmiya, H. Light-Driven N-Heterocyclic Carbene Catalysis Using Alkylborates. ACS Catal. 2021, 11, 12886–12892. [Google Scholar] [CrossRef]
- Takemura, N.; Sumida, Y.; Ohmiya, H. Organic Photoredox-Catalyzed Silyl Radical Generation from Silylboronate. ACS Catal. 2022, 12, 7804–7810. [Google Scholar] [CrossRef]
- Tan, C.-Y.; Kim, M.; Hong, S. Photoinduced Electron Transfer from Xanthates to Acyl Azoliums: Divergent Ketone Synthesis via N-Heterocyclic Carbene Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202306191. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, R.; Zhu, B.; Liu, Y.; Song, H.; Dong, J.; Wang, Q. Direct allylic acylation via cross-coupling involving cooperative N-heterocyclic carbene, hydrogen atom transfer, and photoredox catalysis. Nat. Commun. 2023, 14, 2951. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Sano, M.; Sumida, Y.; Ohmiya, H. N-heterocyclic carbene- and organic photoredox-catalysed meta-selective acylation of electron-rich arenes. Nat. Synth. 2023, 2, 1037–1045. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Sun, J.; Zheng, G.; Zhang, Q. NHC and visible light-mediated photoredox co-catalyzed 1,4-sulfonylacylation of 1,3-enynes for tetrasubstituted allenyl ketones. Chem. Sci. 2022, 13, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, J.; Xia, J.; Li, M.; Zhang, L.; Ma, R.; Zheng, G.; Zhang, Q. Visible light-mediated NHCs and photoredox co-catalyzed radical 1,2-dicarbonylation of alkenes for 1,4-diketones. Sci. China Chem. 2022, 65, 1938–1944. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Sun, J.; Zheng, G.; Zhang, Q. Synthesis of Axially Chiral Aldehydes by N-Heterocyclic-Carbene-Catalyzed Desymmetrization Followed by Kinetic Resolution. Angew. Chem. Int. Ed. 2022, 61, e202117340. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, J.; Xia, J.; Ma, R.; Zheng, G.; Zhang, Q. Visible light-mediated NHC and photoredox co-catalyzed 1,2-sulfonylacylation of allenes via acyl and allyl radical cross-coupling. Org. Chem. Front. 2023, 10, 1047–1055. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Xia, J.; Liu, X.; Sun, J.; Zheng, G.; Zhang, Q. DBU-Mediated Isomerization/6-pi Electro-Cyclization/Oxidation Cascade of Sulfonyl-Substituted Allenyl Ketones for the Construction of Hetero-1,3,5-Trisubstituted Benzene. Chem. Eur. J. 2023, 29, e202203309. [Google Scholar] [CrossRef]
- Liang, T.; Wu, Y.; Sun, J.; Li, M.; Zhao, H.; Zhang, J.; Zheng, G.; Zhang, Q. Visible Light-Mediated Cobalt and Photoredox Dual-Catalyzed Asymmetric Reductive Coupling for Axially Chiral Secondary Alcohols. Chin. J. Chem. 2023, 41, 3253–3260. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Y.; Xia, J.; Ma, R.; Zheng, G.; Zhang, Q. Catalyst-Free Trans-Selective Oxyiodination and Oxychlorination of Alkynes Employing N–X (Halogen) Reagents. Molecules 2023, 28, 7420. [Google Scholar] [CrossRef] [PubMed]
- Leifert, D.; Studer, A. The Persistent Radical Effect in Organic Synthesis. Angew. Chem. Int. Ed. 2020, 59, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Lv, G.; Meng, Q.; Zhang, G.; Xiong, T.; Zhang, Q. Cobalt-Catalyzed Radical Hydroamination of Alkenes with N-Fluorobenzenesulfonimides. Angew. Chem. Int. Ed. 2021, 60, 25949–25957. [Google Scholar] [CrossRef]
- MacKenzie, I.A.; Wang, L.; Onuska, N.P.R.; Williams, O.F.; Begam, K.; Moran, A.M.; Dunietz, B.D.; Nicewicz, D.A. Discovery and characterization of an acridine radical photoreductant. Nature 2020, 580, 76–80. [Google Scholar] [CrossRef]
- Molander, G.A.; Brown, A.R. Suzuki-Miyaura Cross-Coupling Reactions of Potassium Vinyltrifluoroborate with Aryl and Heteroaryl Electrophiles. J. Org. Chem. 2006, 71, 9681–96864. [Google Scholar] [CrossRef] [PubMed]
 | |||||
|---|---|---|---|---|---|
| Entry | NHCs (15 mol%) | PC (1.5 mol%) | Solvent (X mL) | Base (2.0 equiv) | Yield (%) |
| 1 | NHC-1 | PC-1 | DCM | Cs2CO3 | 50 |
| 2 | NHC-1 | PC-2 | DCM | Cs2CO3 | 38 |
| 3 | NHC-1 | PC-3 | DCM | Cs2CO3 | 49 |
| 4 | NHC-1 | PC-4 | DCM | Cs2CO3 | 37 |
| 5 | NHC-2 | PC-1 | DCM | Cs2CO3 | 60 |
| 6 | NHC-3 | PC-1 | DCM | Cs2CO3 | 47 |
| 7 | NHC-4 | PC-1 | DCM | Cs2CO3 | 6 |
| 8 | NHC-5 | PC-1 | DCM | Cs2CO3 | 55 |
| 9 | NHC-2 | PC-1 | PhCF3 | Cs2CO3 | 36 |
| 10 | NHC-2 | PC-1 | THF | Cs2CO3 | 20 |
| 11 | NHC-2 | PC-1 | DCE | Cs2CO3 | 55 |
| 12 | NHC-2 | PC-1 | Acetone | Cs2CO3 | 61 |
| 13 | NHC-2 | PC-1 | Toluene | Cs2CO3 | 64 |
| 14 | NHC-2 | PC-1 | CH3CN | Cs2CO3 | 89 |
| 15 | NHC-2 | PC-1 | CHCl3 | Cs2CO3 | 65 |
| 16 | NHC-2 | PC-1 | CH3CN | K2CO3 | 32 |
| 17 | NHC-2 | PC-1 | CH3CN | K3PO4 | 25 |
| 18 | rac-NHC-2 | PC-1 | CH3CN | Cs2CO3 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Guo, Y.; Lv, Z.; Sun, J.; Zheng, G.; Zhang, Q. Visible Light-Mediated Monofluoromethylation/Acylation of Olefins by Dual Organo-Catalysis. Molecules 2024, 29, 790. https://doi.org/10.3390/molecules29040790
Xia J, Guo Y, Lv Z, Sun J, Zheng G, Zhang Q. Visible Light-Mediated Monofluoromethylation/Acylation of Olefins by Dual Organo-Catalysis. Molecules. 2024; 29(4):790. https://doi.org/10.3390/molecules29040790
Chicago/Turabian StyleXia, Jiuli, Yunliang Guo, Zhiguang Lv, Jiaqiong Sun, Guangfan Zheng, and Qian Zhang. 2024. "Visible Light-Mediated Monofluoromethylation/Acylation of Olefins by Dual Organo-Catalysis" Molecules 29, no. 4: 790. https://doi.org/10.3390/molecules29040790
APA StyleXia, J., Guo, Y., Lv, Z., Sun, J., Zheng, G., & Zhang, Q. (2024). Visible Light-Mediated Monofluoromethylation/Acylation of Olefins by Dual Organo-Catalysis. Molecules, 29(4), 790. https://doi.org/10.3390/molecules29040790







