RETRACTED: Synthesis and Properties of Novel Alkyl-Substituted Hexaazacyclophanes and Their Diradical Dications
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Identification
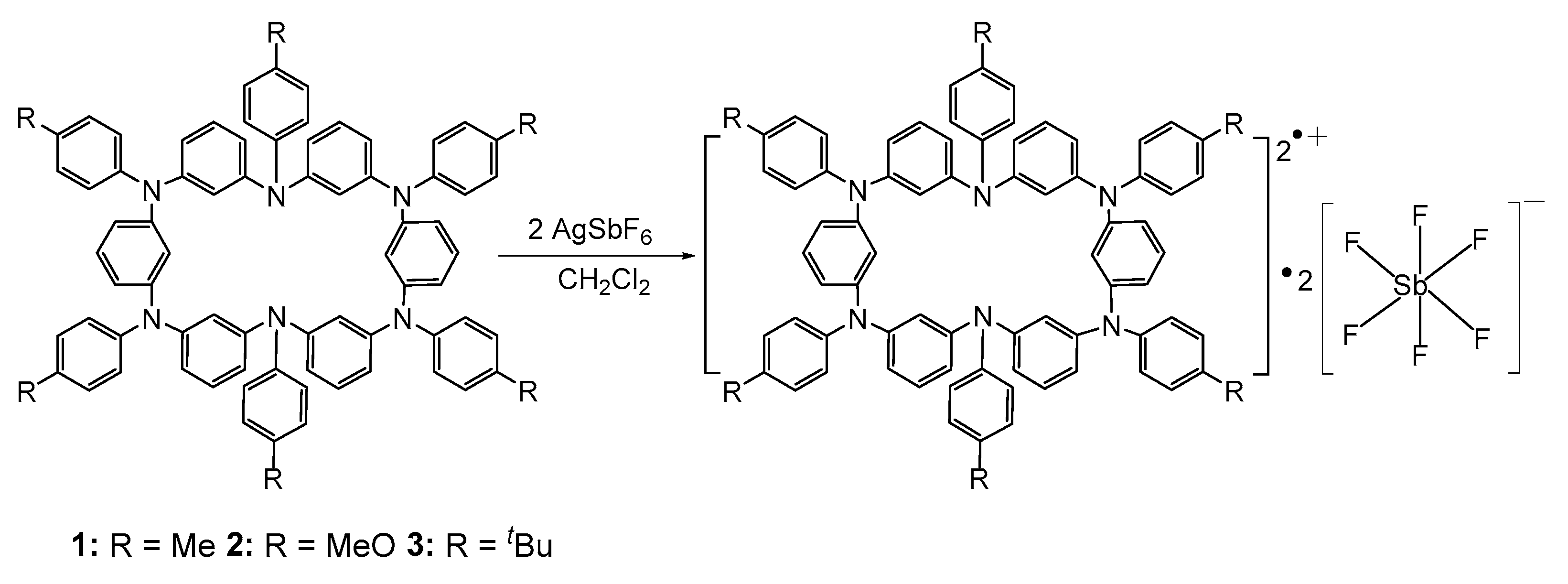
2.1.1. Synthesis of Neutral Hexaazacyclophane Compounds (1–3)
2.1.2. Synthesis of Hexaazacyclophane Diradical Dications 12·+•2[SbF6]−, 22·+•2[SbF6]−, and 32·+•2[SbF6]−
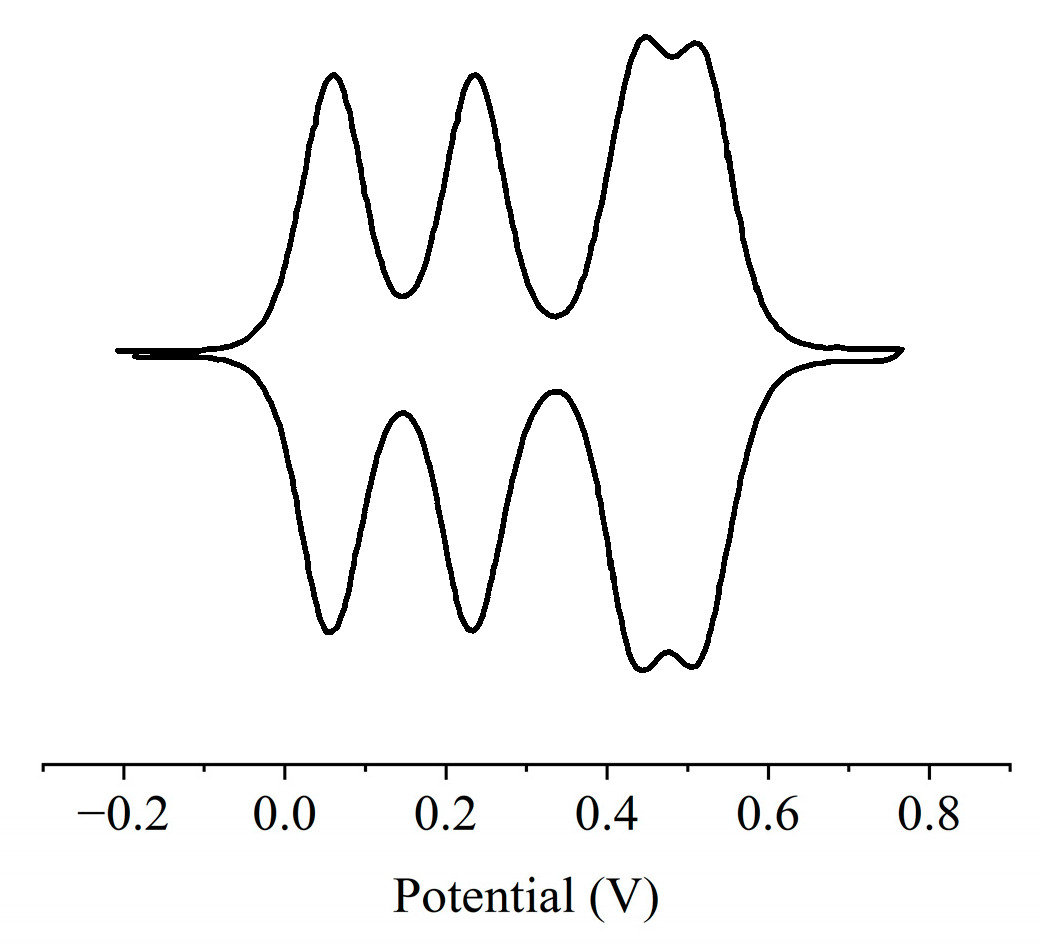
2.2. Electrochemical Properties
2.3. Electronic Properties
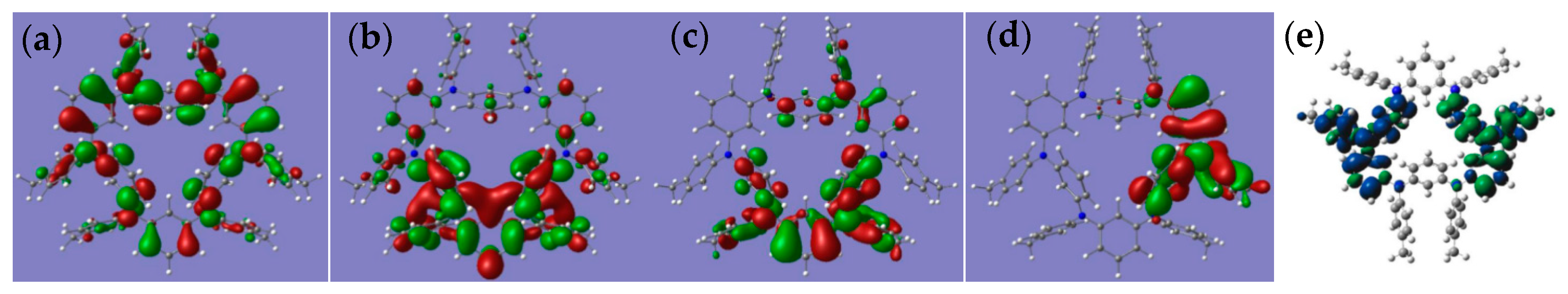
2.4. DFT Calculations
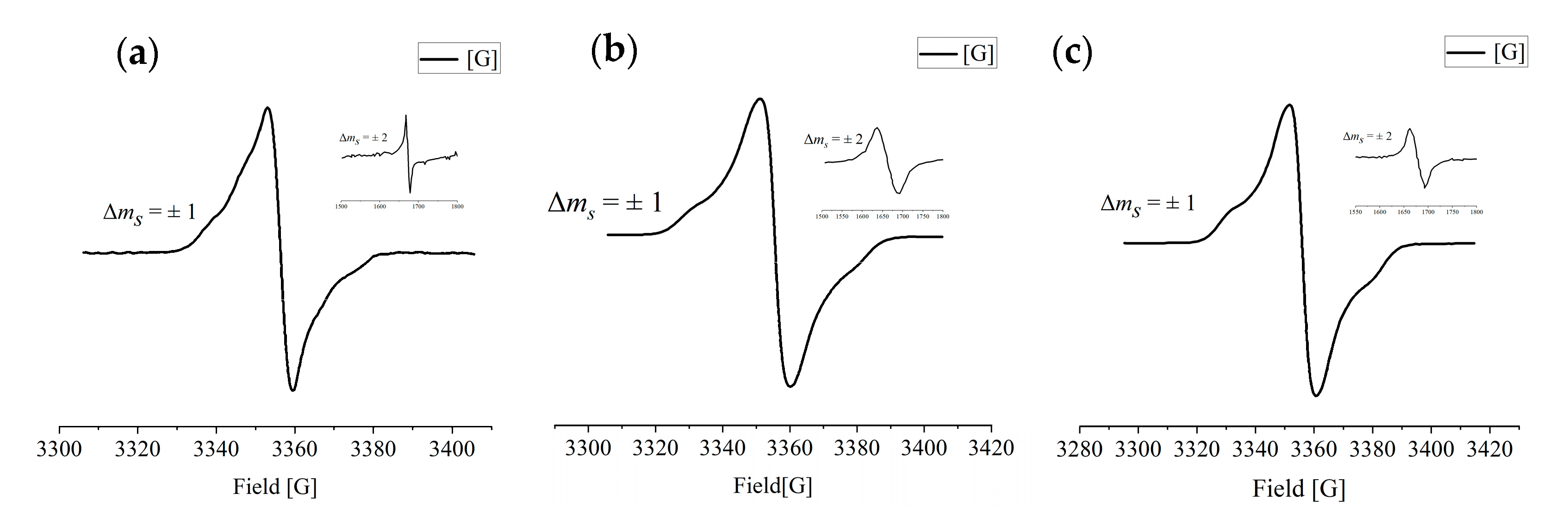
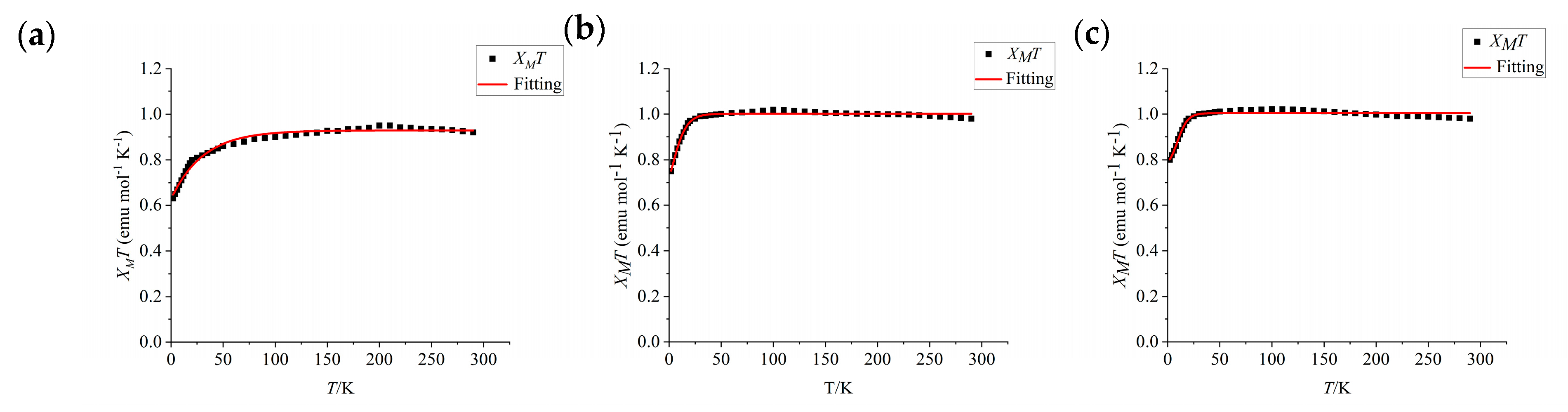
2.5. Magnetic Properties
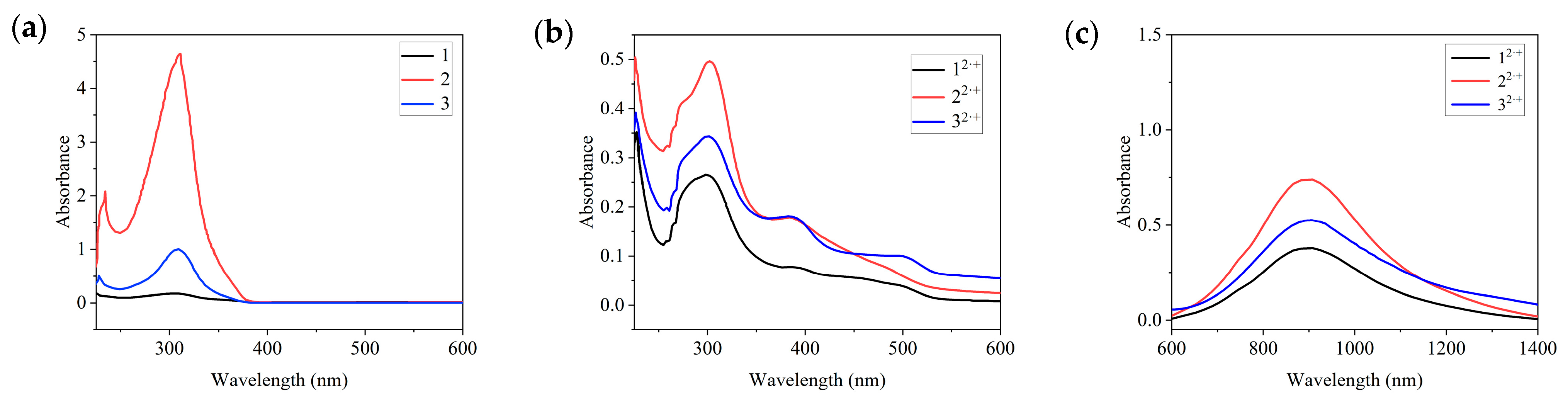
2.6. Spectral Properties
3. Materials and Methods
4. Experiment
4.1. Synthesis of Netural Compounds
4.1.1. Synthesis of A–C
- N,N′-bis(3-bromophenyl)-N,N′-bis(4-methylphenyl)benzene-1,3-diamine A
- N,N′-bis(3-bromophenyl)-N,N′-bis(4-methoxylphenyl)benzene-1,3-diamine B
- N,N′-bis(3-bromophenyl)-N,N′-bis(4-tert-butylphenyl)benzene-1,3-diamine C
4.1.2. Synthesis of Netural Hexaazacyclophanes (1–3)
- 2,4,6,8,10,12-hexakis(4-methylphenyl)-2,4,6,8,10,12-hexaaza-1,3,5,7,9,11(1,3)-hexabenzenacyclododecaphane 1
- 2,4,6,8,10,12-hexakis(4-methoxyphenyl)-2,4,6,8,10,12-hexaaza-1,3,5,7,9,11(1,3)-hexabenzenacyclododecaphane 2
- 2,4,6,8,10,12-hexakis(4-tbutylphenyl)-2,4,6,8,10,12-hexaaza-1,3,5,7,9,11(1,3)-hexabenzenacyclododecaphane 3
4.2. Synthesis of Hexaazacyclophane Diradical Dications 12·+•2[SbF6]−, 22·+•2[SbF6]−, and 32·+•2[SbF6]−
- Synthesis of 12·+•2[SbF6]−
- Synthesis of 22·+•2[SbF6]−
- Synthesis of 32·+•2[SbF6]−
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iyoda, M.; Yamakawa, J.; Rahman, M.J. Conjugated Macrocycles: Concepts and Applications. Angew. Chem. Int. Ed. 2011, 50, 10522–10553. [Google Scholar] [CrossRef]
- Höger, S. Shape-Persistent Macrocycles: From Molecules to Materials. Chem. Eur. J. 2004, 10, 1320–1329. [Google Scholar] [CrossRef]
- Li, G.; Matsuno, T.; Han, Y.; Phan, H.; Wu, S.; Jiang, Q.; Zou, Y.; Isobe, H.; Wu, J. Benzidine/Quinoidal-Benzidine-Linked, Superbenzene-Based π-Conjugated Chiral Macrocycles and Cyclophanes. Angew. Chem. Int. Ed. 2020, 59, 9727–9735. [Google Scholar] [CrossRef] [PubMed]
- Widera, A.; Filbeck, E.; Wadepohl, H.; Kaifer, E.; Himmel, H.J. Electron-Rich, Lewis Acidic Diborane Meets N-Heterocyclic Aromatics: Formation and Electron Transfer in Cyclophane Boranes. Chem. Eur. J. 2020, 26, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- Badía-Domínguez, I.; Perez-Guardiola, A.; Sancho-García, J.C.; Navarrete, J.T.L.; Jolín, V.H.; Li, H.; Sakamaki, D.; Seki, S.; Delgado, M.C.R. Formation of Cyclophane Macrocycles in Carbazole-Based Biradicaloids: Impact of the Dicyanomethylene Substitution Position. ACS Omega 2019, 4, 4761–4769. [Google Scholar] [CrossRef]
- Garci, A.; Abid, S.; David, A.H.G.; Jones, L.O.; Azad, C.S.; Ovalle, M.; Brown, P.J.; Stern, C.L.; Zhao, X.; Malaisrie, L.; et al. Exciplex Emission and Förster Resonance Energy Transfer in Polycyclic Aromatic Hydrocarbon-Based Bischromophoric Cyclophanes and Homo[2]catenanes. J. Am. Chem. Soc. 2023, 145, 18391–18401. [Google Scholar] [CrossRef] [PubMed]
- Shear, T.A.; Johnson, D.W. Main Group Supramolecular Chemistry Led to Surprising New Directions in the Self-Assembly of Organic Macrocycles, Cages, and Cyclophanes. Synlett 2021, 32, 1702–1710. [Google Scholar]
- Eder, S.; Ding, B.; Thornton, D.B.; Sammut, D.; White, A.J.P.; Plasser, F.; Stephens, E.L.I.; Heeney, M.; Mezzavilla, S.; Glöcklhofer, F. Squarephaneic Tetraanhydride: A Conjugated Square-Shaped Cyclophane for the Synthesis of Porous Organic Materials. Angew. Chem. Int. Ed. 2022, 61, e202212623. [Google Scholar] [CrossRef] [PubMed]
- Garci, A.; Abid, S.; David, A.H.G.; Codesal, M.D.; Đorđević, L.; Young, R.M.; Sai, H.; Bras, L.L.; Perrier, A.; Ovalle, M.; et al. Aggregation-Induced Emission and Circularly Polarized Luminescence Duality in Tetracationic Binaphthyl-Based Cyclophanes. Angew. Chem. Int. Ed. 2022, 61, e202208679. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Tobe, Y. Ball-, Bowl-, and Belt-Shaped Conjugated Systems and Their Complexing Abilities: Exploration of the Concave−Convex π−π Interaction. Chem. Rev. 2006, 106, 5274–5290. [Google Scholar] [CrossRef]
- Bulovic, V.; Gu, G.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Transparent light-emitting devices. Nature 1996, 380, 29. [Google Scholar] [CrossRef]
- Lewis, S.E. Cycloparaphenylenes and related nanohoops. Chem. Soc. Rev. 2015, 44, 2221–2304. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Y.; Ikemoto, K.; Takahashi, N.; Izumi, T.; Taka, H.; Kita, H.; Sato, S.; Isobe, H. Cyclo-meta-phenylene Revisited: Nickel-Mediated Synthesis, Molecular Structures, and Device Applications. J. Org. Chem. 2014, 79, 9735–9739. [Google Scholar] [CrossRef] [PubMed]
- Jasti, R.; Bhattacharjee, J.; Neaton, J.B.; Bertozzi, C.R. Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]Cycloparaphenylene: Carbon Nanohoop Structures. J. Am. Chem. Soc. 2008, 130, 17646–17647. [Google Scholar] [CrossRef] [PubMed]
- Takaba, H.; Omachi, H.; Yamamoto, Y.; Bouffard, J.; Itami, K. Selective Synthesis of Cycloparaphenylene. Angew. Chem. Int. Ed. 2009, 48, 6112–6116. [Google Scholar] [CrossRef] [PubMed]
- Yamago, S.; Watanabe, Y.; Iwamoto, T. Synthesis of [8]Cycloparaphenylene from a Square-Shaped Tetranuclear Platinum Complex. Angew. Chem. Int. Ed. 2010, 49, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ono, Y.; Tanaka, K. Tetraaza[1.1.1.1]metacyclophane. New J. Chem. 1998, 22, 779–781. [Google Scholar] [CrossRef]
- Wang, M.X. Heterocalixaromatics, new generation macrocyclic host molecules in supramolecular chemistry. Chem. Commun. 2008, 38, 4541–4551. [Google Scholar] [CrossRef]
- Wang, M.X. Nitrogen and Oxygen Bridged Calixaromatics: Synthesis, Structure, Functionalization, and Molecular Recognition. Acc. Chem. Res. 2012, 45, 182–195. [Google Scholar] [CrossRef]
- Bujak, P.; Kulszewicz-Bajer, I.; Zagorska, M.; Maurel, V.; Wielgus, I.; Pron, A. Polymers for electronics and spintronics. Chem. Soc. Rev. 2013, 42, 8895–8999. [Google Scholar] [CrossRef]
- Ito, A. Acrocyclic oligoarylamines as hole- and spin-containing scaffolds for molecule-based electronics. J. Mater. Chem. C. 2016, 4, 4614–4625. [Google Scholar] [CrossRef]
- Jiao, T.; Cai, K.; Nelson, J.N.; Jiao, Y.; Qiu, Y.; Wu, G.; Zhou, J.; Cheng, C.; Shen, D.; Feng, Y.; et al. A Probe-Enabled Approach for the Selective Isolation and Characterization of Functionally Active Subpopulations in the Gut Microbiome. J. Am. Chem. Soc. 2019, 141, 42–47. [Google Scholar]
- Marshall-Roth, T.; Libretto, N.J.; Wrobel, A.T.; Anderton, K.J.; Pegis, M.L.; Ricke, N.D.; Voorhis, T.V.; Miller, J.T.; Surendranath, Y. A pyridinic Fe-N4 macrocycle models the active sites in Fe/N-doped carbon electrocatalysts. Nat. Commun. 2020, 11, 5283–5298. [Google Scholar] [CrossRef] [PubMed]
- Stawski, W.; Zhu, Y.; Wei, Z.; Petrukhina, M.A.; Anderson, H.L. Crystallographic evidence for global aromaticity in the di-anion and tetra-anion of a cyclophane hydrocarbon. Chem. Sci. 2023, 14, 14109–14114. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Lv, W.; Liu, H.; Liu, Y.; Liao, S.; Wang, X.; Zhu, K. ProBox: A Rigid yet Dynamic Cyclophane Capable of Adaptive and Redox-Switchable Host–Guest Binding. Org. Lett. 2023, 25, 3508–3511. [Google Scholar] [CrossRef] [PubMed]
- David, A.H.G.; Garci, A.; Abid, S.; Li, X.; Young, R.M.; Seale, J.S.W.; Hornick, J.E.; Azad, C.S.; Jiao, Y.; Roy, I.; et al. Divinylanthracene-Containing Tetracationic Organic Cyclophane with Near-Infrared Photoluminescence. J. Am. Chem. Soc. 2023, 145, 9182–9190. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, N.; Li, G.; Qiao, Y.; Zhang, M.; Zhang, L.; Guo, Q.H.; He, G. The Green Box: Selenoviologen-Based Tetracationic Cyclophane for Electrochromism, Host–Guest Interactions, and Visible-Light Photocatalysis. J. Am. Chem. Soc. 2023, 145, 9118–9128. [Google Scholar] [CrossRef]
- Kurata, R.; Tanaka, K.; Ito, A. Isolation and Characterization of Persistent Radical Cation and Dication of 2,7-Bis(dianisylamino)pyrene. J. Org. Chem. 2016, 81, 137–145. [Google Scholar] [CrossRef]
- Ito, A.; Tanaka, K. Macrocyclic oligoarylamine-based spin system. Pure Appl. Chem. 2010, 82, 979–989. [Google Scholar] [CrossRef][Green Version]
- Haddoub, R.; Touil, M. Unprecedented Tunable Tetraazamacrocycles. Org. Lett. 2010, 12, 2722–2725. [Google Scholar] [CrossRef]
- Alam, T.; Tarannum, H.; Viladkar, S.; Kamaluddin. Oxidation of aniline and its derivatives by manganese ferrocyanide. Oxid. Commun. 1999, 22, 599–609. [Google Scholar]
- Yao, Q.; Liu, L.; Li, C. High energy proton beam bombardment of polyaniline. Radiat. Phys. Chem. 1997, 41, 791–795. [Google Scholar] [CrossRef]
- Takemura, H. [1n]Paracyclophanes. Curr. Org. Chem. 2009, 13, 1633–1653. [Google Scholar] [CrossRef]
- Hauck, S.I.; Lakshmi, K.V.; Hartwig, J.F. Tetraazacyclophanes by Palladium-Catalyzed Aromatic Amination. Geometrically Defined, Stable, High-Spin Diradicals. Org. Lett. 1999, 1, 2057–2060. [Google Scholar] [CrossRef]
- Gałecka, M.; Wielgus, I.; Zagórska, M.; Pawłwski, M.; Kulszewicz-Bajer, I. High-Spin Radical Cations of Poly(m−p-anilines) and Poly(m−p−p-anilines): Synthesis and Spectroscopic Properties. Macromolecules 2007, 40, 4924–4932. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]


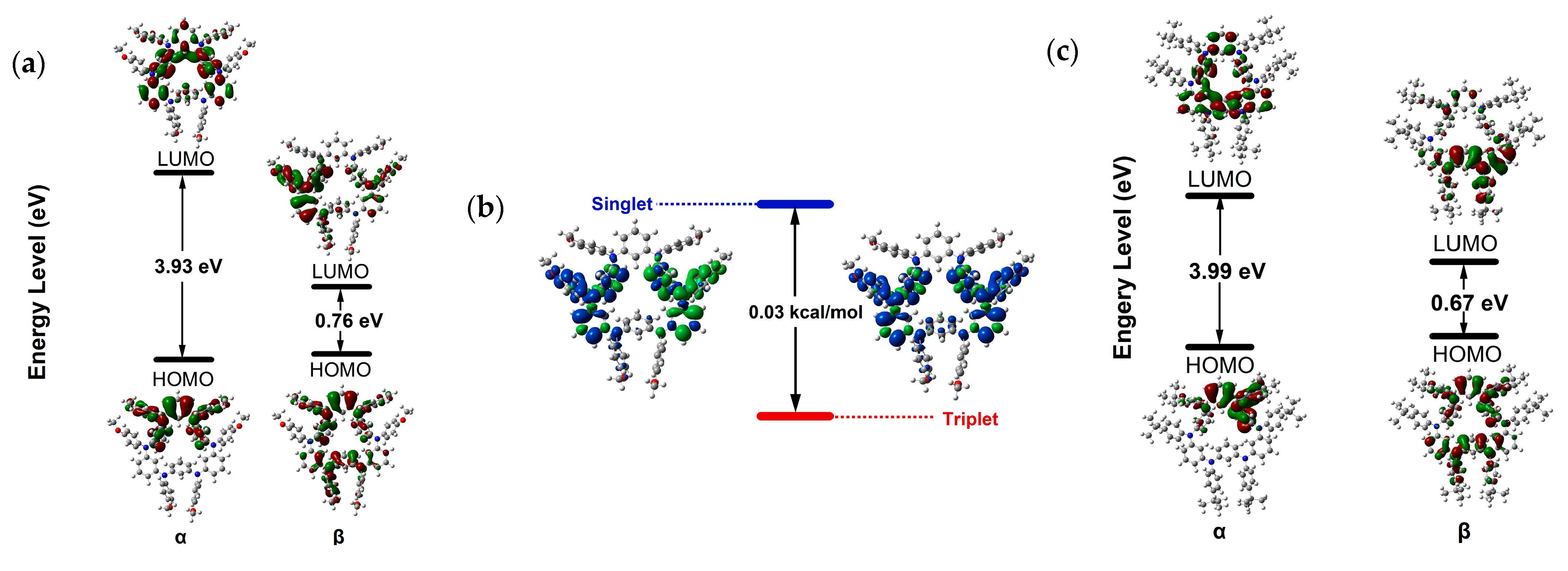
| Functional | Basis Set | ET (a.u.) | EOS (a.u.) | EOS-T (kcal/mol) |
|---|---|---|---|---|
| (U)B3LYP | 6-311g(d,p) | −3341.510047 | −3341.510127 | −0.050 |
| PBE0 | 6-311g(d,p) | −3337.352538 | −3337.352645 | −0.067 |
| CAM-B3LYP | 6-311g(d,p) | −3339.404973 | −3339.405091 | −0.074 |
| M062X | 6-311g(d,p) | −3339.724699 | −3339.724759 | −0.038 |
| wB97xD | 6-311g(d,p) | −3339.977570 | −3339.977521 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Chen, J. RETRACTED: Synthesis and Properties of Novel Alkyl-Substituted Hexaazacyclophanes and Their Diradical Dications. Molecules 2024, 29, 789. https://doi.org/10.3390/molecules29040789
Li S, Chen J. RETRACTED: Synthesis and Properties of Novel Alkyl-Substituted Hexaazacyclophanes and Their Diradical Dications. Molecules. 2024; 29(4):789. https://doi.org/10.3390/molecules29040789
Chicago/Turabian StyleLi, Shunjie, and Jian Chen. 2024. "RETRACTED: Synthesis and Properties of Novel Alkyl-Substituted Hexaazacyclophanes and Their Diradical Dications" Molecules 29, no. 4: 789. https://doi.org/10.3390/molecules29040789
APA StyleLi, S., & Chen, J. (2024). RETRACTED: Synthesis and Properties of Novel Alkyl-Substituted Hexaazacyclophanes and Their Diradical Dications. Molecules, 29(4), 789. https://doi.org/10.3390/molecules29040789







