Non-Volatile Component and Antioxidant Activity: A Comparative Analysis between Litsea cubeba Branches and Leaves
Abstract
1. Introduction
2. Results
2.1. UPLC-HRMS Analysis of Non-Volatile Components from L. cubeba Branches and Leaves
2.1.1. Analysis of Non-Volatile Components in Branches
2.1.2. Analysis of Non-Volatile Components in Leaves
2.2. Comparative Analysis of Non-Volatile Components in Branches and Leaves
2.3. Antioxidant Activity
Results of Four In Vitro Antioxidant Activity Tests
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.1.1. Plant Material
4.1.2. Preparation of Liquid-Sample Solution for Mass Spectrometry
4.1.3. Preparation of Samples for Antioxidant Activity Testing
4.2. The Main Chemicals and Reagents
4.3. UPLC-HRMS Analysis
4.3.1. Instrumentation and Conditions
4.3.2. Data Analysis and Identification of Compounds
4.4. Antioxidant Activity
4.4.1. ABTS Radical Scavenging Assay
4.4.2. DPPH Radical Scavenging Assay
4.4.3. Ferric Reducing Ability of Plasma (FRAP) Assay
4.4.4. Cupric Reducing Antioxidant Capacity (CUPRAC) Assay
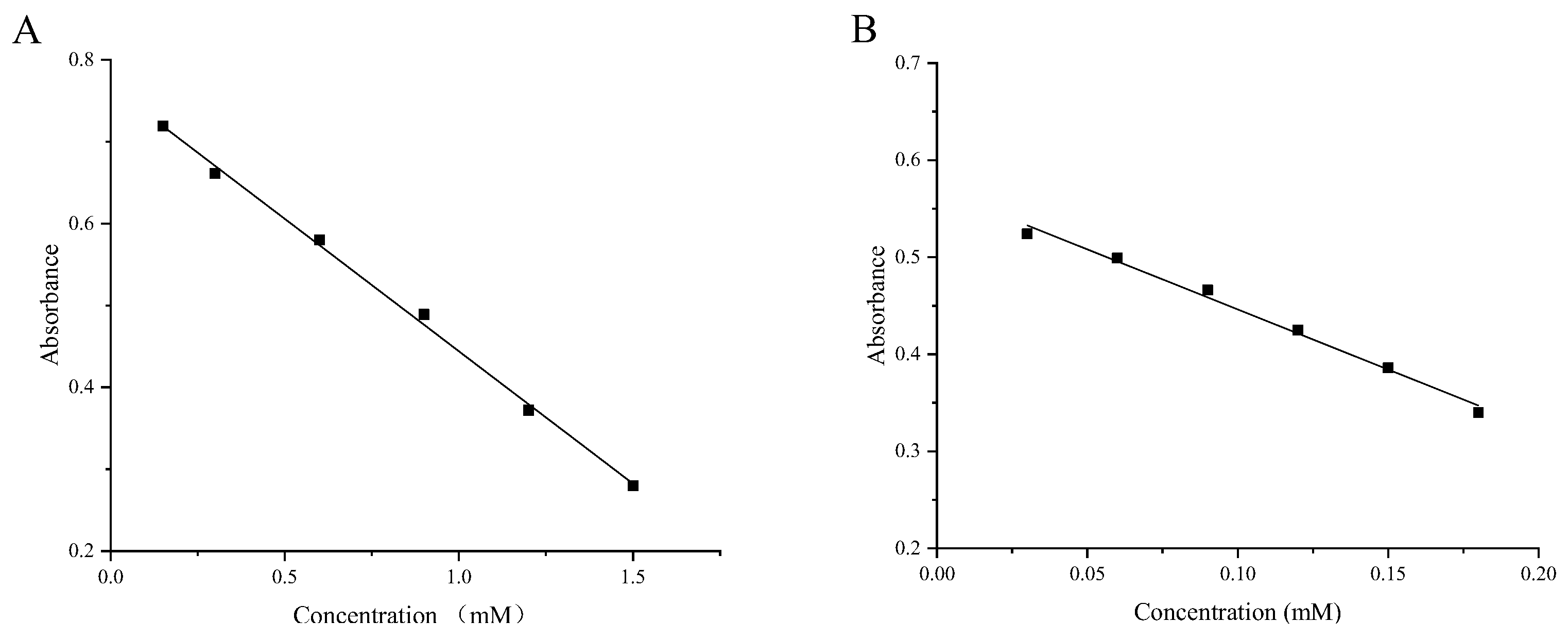
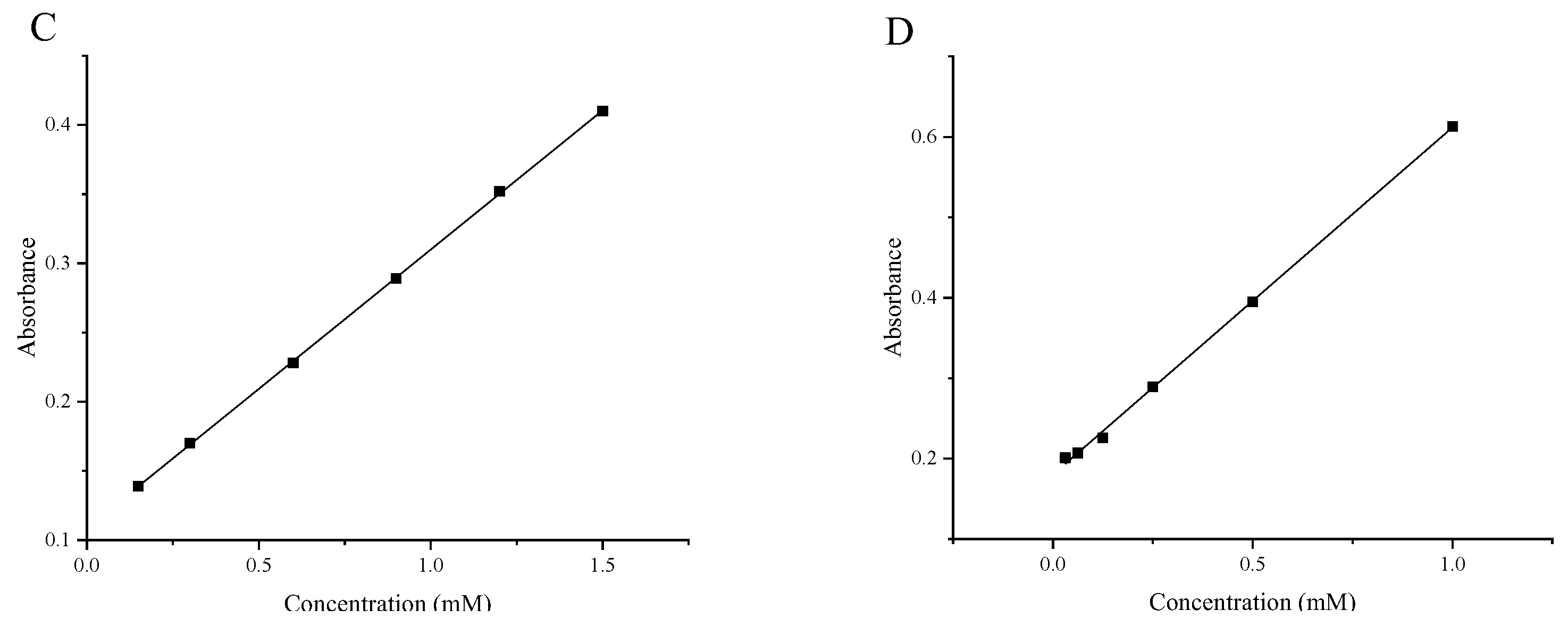
4.4.5. Construction of Standard Curves
4.4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Editorial Committee of Flora of China, Chinese Academy of Science. Flora of China, 1st ed.; Science Press: Beijing, China, 1993; Volume 31, pp. 271–272. [Google Scholar]
- Ru, H.; Liu, M.; Liao, T.; Zhu, J.J. Analysis on technological innovation ability and competitive situation of Litsea cubeba industry from the perspective of patent, Hunan. For. Sci. Tec. 2022, 49, 89–94. [Google Scholar]
- Yu, L.Y.; Jia, D.; Shen, H.; Sun, X.L.; Qin, L.P.; Han, T. Anti-inflammatory study on 9,9′-O-di-(E)-feruloyl-meso-5,5′-dimethoxysecoisolariciresinol (LCA), an active ingredient in Litsea cubeba (Lour.) Pers. J. Pha. Pra. 2020, 38, 216–220. [Google Scholar]
- Zhang, L.; Chen, X.S.; Fan, G.R.; Liao, S.L.; Wang, Z.D. Research progress in application and functions of Litsea cubeba essential oil components. Agric. Univ. Jiangxiensis 2021, 43, 355–363. [Google Scholar]
- Li, X.Y.; Gou, Y.H.; Shen, H.J.; Li, Y.H.; Wang, T.; Yin, L.Z. Antibacterial mechanism of Litsea cubeba essential oil on Salmonella. J. Sci. Agric. Uni. 2021, 39, 385–390. [Google Scholar]
- Liu, T.; Yang, T.S. Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems. Int. J. Food. Mic. 2012, 156, 68–75. [Google Scholar] [CrossRef]
- Xiang, Y.J.; Wang, H.H.; Sun, Y.D. Advances in the application of Litsea cubeba oil. Chin. J. Cer. 2020, 35, 186–195. [Google Scholar]
- Zhang, S.Y.; Guo, Q.; Gao, X.L.; Guo, Z.Q.; Zhao, Y.F.; Chai, X.Y.; Tu, O.F. A phytochemical and pharmacological advance on medicinal plant Litsea cubeba (Lauraceae). Chin. Herb. Med. 2014, 39, 769–776. [Google Scholar]
- Feng, T.; Xu, Y.; Cai, X.H.; Du, Z.Z.; Luo, X.D. Antimicrobially Active Isoquinoline Alkaloids from Litsea cubeba. Planta Med. 2009, 75, 76–79. [Google Scholar] [CrossRef]
- Guo, Q.; Bai, R.F.; Su, G.Z.; Zhu, Z.X.; Zeng, K.W.; Tu, P.F.; Chai, X.Y. Chemical Constituents from the Roots and Stems of Litsea cubeba. J. Asian Nat. Prod. Res. 2016, 18, 51–58. [Google Scholar] [CrossRef]
- Lee, S.S.; Chen, C.K.; Huang, F.M.; Chen, C.H. Two dibenzopyrrocoline alkaloids from Litsea cubeba. J. Nat. Prod. 1996, 59, 80–82. [Google Scholar] [CrossRef]
- Zhang, S.S.; Tan, Q.W.; Guan, L.P. Antioxidant, anti-inflammatory, antibacterial, and analgesic activities and mechanisms of quinolines, indoles and related derivatives. Mini Rev. Med. Chem. 2021, 21, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Diniz Vilela, D.; Gomes Peixoto, L.; Teixeira, R.R.; Belele Baptista, N.; Carvalho Caixeta, D.; de Souza, A.V.; Machado, H.L.; Pereira, M.N.; Sabino–Silva, R.; Espindola, F.S. The Role of Metformin in Controlling Oxidative Stress in Muscle of Diabetic Rats. Oxid. Med. Cell Longev. 2016, 2016, 6978625. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, C.; Cao, Y.; Li, Y.; Zhang, Z.; Nie, D.; Tang, W.; Li, Y. Comparison of Chemical Compositions and Antioxidant Activity of Essential Oils from Litsea cubeba, Cinnamon, Anise, and Eucalyptus. Molecules 2023, 28, 5051. [Google Scholar] [CrossRef] [PubMed]
- She, Q.H.; Li, W.S.; Jiang, Y.Y.; Wu, Y.C.; Zhou, Y.H.; Zhang, L. Chemical Composition, Antimicrobial Activity, and Antioxidant Activity of Litsea cubeba Essential Oils in Different Months. Nat. Prod. Res. 2020, 34, 3285–3288. [Google Scholar] [CrossRef]
- Hwang, J.K.; Choi, E.M.; Lee, J.H. Antioxidant activity of Litsea cubeba. Fitoterapia 2005, 76, 684–686. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, H.; Zhang, Y. Study on formation of acrylamide in asparagine-sugar microwave heating systems using UPLC–MS/MS analytical method. Food Chem. 2008, 108, 542–550. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.Y.; Guo, J.Z.; Xia, Q.L.; Zhao, G.; Zhou, H.N.; Xie, F.W. Metabolic profiling of Chinese tobacco leaf of different geographical origins by GC–MS. J. Agric. Food Chem. 2013, 61, 2597–2605. [Google Scholar] [CrossRef]
- Shen, Q.Y.; Yang, R.J.; Hua, X.; Ye, F.Y.; Wang, H.; Zhao, W.; Wang, K. Enzymatic synthesis and identification of oligosaccharides obtained by transgalactosylation of lactose in the presence of fructose using β-galactosidase from Kluyveromyces lactis. Food Chem. 2012, 135, 1547–1554. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Gale, T.V.; Schieffelin, J.S.; Branco, L.M.; Garry, R.F.; Grant, D.S. Elevated L-threonine is a biomarker for Lassa fever and Ebola. Virol. J. 2020, 17, 188. [Google Scholar] [CrossRef]
- Song, M.; Hang, T.J.; Wang, C.; Yang, L.; Wen, A.D. Precolumn derivatization LC-MS/MS method for the determination and pharmacokinetic study of glucosamine in human plasma and urine. J. Pharm. Anal. 2012, 2, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.S.; Xu, R.Y.; Wang, B.K.; Qi, Q.; Zhang, C.N. Metabolomics analysis reveals the protective effect of fructooligosaccharide on abnormal metabolism of liver in Megalobrama amblycephala induced by Aeromonas hydrophila. Aquacult. Int. 2023, 2023, 1–18. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, R.; Lopez-Martinez, J.C.; Romero-Gonzalez, R.; Martinez-Vidal, J.L.; Flores, M.I.A.; Garrido Frenich, A. Simple LC–MS determination of citric and malic acids in fruits and vegetables. Chromatographia 2010, 72, 55–62. [Google Scholar] [CrossRef]
- Moussa, S.; Van Horn, M.R.; Shah, A.; Pollegioni, L.; Thibodeaux, C.J.; Ruthazer, E.S.; Mauzeroll, J. Editors’ choice—A miniaturized enzymatic biosensor for detection of sensory–evoked D–serine release in the brain. J. Electrochem. Soc. 2021, 168, 025502. [Google Scholar] [CrossRef]
- Klupczynska, A.; Misiura, M.; Miltyk, W.; Oscilowska, I.; Palka, J.; Kokot, Z.; Matysiak, J. Development of an LC-MS targeted metabolomics methodology to study proline metabolism in mammalian cell cultures. Molecules 2020, 25, 4639. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.G.; Day, C.S.; Bierbach, U. Duplex–Promoted Platination of Adenine–N3 in the Minor Groove of DNA: Challenging a Longstanding Bioinorganic Paradigm. J. Am. Chem. Soc. 2005, 127, 1160–1169. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, L.y.; Ding, L.P.; Liu, Y.; Liang, H.; Tu, P.F.; Li, L.; Zhang, Q.Y. Condensation derivatives of 4-isopropylbenzaldehyde with acetophenone from the red alga Laurencia tristicha. Phytochemistry. 2021, 192, 112960. [Google Scholar] [CrossRef]
- Wang, R.; Xiong, X.P.; Yang, M.; He, S.R.; Xu, X.P. A pharmacokinetics study of orally administered higenamine in rats using LC–MS/MS for doping control analysis. Drug Test Anal. 2020, 12, 485–495. [Google Scholar] [CrossRef]
- Larsen, J.; Cornett, C.; Jaroszewski, J.W.; Hansen, S.H. Reaction between drug substances and pharmaceutical excipients: Formation of citric acid esters and amides of carvedilol in the solid state. J. Pharmaceut. Biomed. 2009, 49, 11–17. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, D.; Wang, X.L.; Zhang, L.N.; Han, J.; Yang, M.; Xiao, X.; Zhang, Y.N.; Liu, H.C. Simultaneous quantification of niacin and its three main metabolites in human plasma by LC–MS/MS. J. Chromatogr. B 2012, 904, 107–114. [Google Scholar] [CrossRef]
- Sadilkova, K.; Gospe, S.M.; Hahn, S.H. Simultaneous determination of alpha–aminoadipic semialdehyde, piperideine-6-carboxylate and pipecolic acid by LC–MS/MS for pyridoxine–dependent seizures and folinic acid–responsive seizures. J. Neurosci. 2009, 184, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Magera, M.J.; Helgeson, J.K.; Matern, D.; Rinaldo, P. Methylmalonic acid measured in plasma and urine by stable-isotope dilution and electrospray tandem mass spectrometry. Clin. Chem. 2000, 46, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Rostami–Yazdi, M.; Clement, B.; Schmidt, T.J.; Schinor, D.; Mrowietz, U. Detection of Metabolites of Fumaric Acid Esters in Human Urine: Implications for Their Mode of Action. J. Invest. Dermatol. 2009, 129, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.S.; Vecchi, M.M.; Wen, D.Y. Distinguishing between Leucine and Isoleucine by Integrated LC–MS Analysis Using an Orbitrap Fusion Mass Spectrometer. Anal. Chem. 2016, 88, 10757–10766. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Eris, T.; Lauren, S.L.; Stearns, G.W.; Westcott, K.R.; Lu, H. Use of LC/MS peptide mapping for characterization of isoforms in 14N–labeled recombinant human leptin. Tech. Protein Chem. 1996, 8, 155–163. [Google Scholar]
- Chen, Q.F.; Zhang, B.C.; Hicks, L.M.; Wang, S.P.; Jez, J.M. A liquid chromatography–tandem mass spectrometry-based assay for indole–3–acetic acid–amido synthetase. Anal. Chem. 2009, 390, 149–154. [Google Scholar] [CrossRef]
- Reed, J.D.; Krueger, C.G.; Vestling, M.M. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry 2005, 66, 2248–2263. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.F.; Qi, R.; Jiang, S.W.; Wang, W.; Chen, Z.Q.; Guo, D.A.; Liu, B. Simultaneous determination of ten compounds in Xiangbin Fang by LC-MRM-MS. Zhongguo Zhong Yao Za Zhi 2017, 32, 2226–2229. [Google Scholar]
- Pan, H.F.; Lundgren, L.N. Phenolic extractives from root bark of Picea abies. Phytochemistry 1995, 39, 1423–1428. [Google Scholar] [CrossRef]
- Mollataghi, A.; Hadi, A.H.A.; Awang, K.; Mohamad, J.; Litaudon, M.; Mukhtar, M. (+)–Kunstlerone, a new antioxidant neolignan from the leaves of Beilschmiedia kunstleri Gamble. Molecules 2011, 16, 6582–6590. [Google Scholar] [CrossRef]
- Chiwocha, S.D.S.; Abrams, S.R.; Ambrose, S.J.; Cutler, A.J.; Loewen, M.; Ross, A.R.S.; Kermode, A.R. A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: An analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant Physiol. 2003, 35, 405–417. [Google Scholar] [CrossRef]
- Husna, H.; Muhammad, H.; Sivasothy, Y.; Khaw, K.; Nafiah, M.A.; Hazni, H.; Litaudon, M.; Wan, R.; Wan, A.; Liew, S.Y. N-Methyl Costaricine and Costaricine, Two Potent Butyrylcholinesterase Inhibitors from Alseodaphne pendulifolia Gamb. Int. J. Mol. Sci. 2023, 24, 10699. [Google Scholar] [CrossRef]
- Takemura, T.; Takatsu, Y.; Kasumi, M.; Marubashi, W.; Iwashina, T.H. Flavonoids and their distribution patterns in the flowers of Gladiolus cultivars. Acta Hortic. 2005, 673, 487–493. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, J.K.; Jang, J.M.; Shin, K.H.; Lim, S.S. Rapid identification of the α–glucosidase inhibitory compounds from Thunberg’s Geranium (Geranium thunbergii Sieb. et Zucc.). Food Sci. Biotechnol. 2012, 21, 987–996. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative Evaluation of Quercetin, Isoquercetin and Rutin as Inhibitors of α-Glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.P.; Horst, H.; Sousa, E.; Pizzolatti, M.G.; Silva, F.R.M.B. Insulinomimetic effects of kaempferitrin on glycemia and on 14C–glucose uptake in rat soleus muscle. Chem. Biol. Interact. 2004, 149, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Zhang, Z.Z.; Cain, A.; Wang, B.; Long, M.; Taylor, J. Antifungal Activity of Camptothecin, Trifolin, and Hyperoside Isolated from Camptotheca acuminata. J. Agric. Food Chem. 2005, 53, 32–37. [Google Scholar] [CrossRef]
- Yin, R.; Han, F.; Tang, Z.; Liu, R.; Zhao, X.; Chen, X.H.; Bi, K.S. UFLC–MS/MS method for simultaneous determination of luteolin-7-O-gentiobioside, luteolin-7-O-β-D-glucoside and luteolin-7-O-β-D-glucuronide in beagle dog plasma and its application to a pharmacokinetic study after administration of traditional Chinese medicinal preparation: Kudiezi injection. J. Pharmaceut. Biomed. 2013, 72, 127–133. [Google Scholar]
- Uppugundla, N.; Engelberth, A.; Vandhana, R.S.; Clausen, E.C.; Lay, J.O.; Gidden, J.; Carrier, D.J. Switchgrass Water Extracts: Extraction, Separation and Biological Activity of Rutin and Quercitrin. J. Agric. Food Chem. 2009, 57, 7763–7770. [Google Scholar] [CrossRef] [PubMed]
- Cova, D.; De, A.L.; Giavarini, F.; Palladini, G.; Perego, R. Pharmacokinetics and metabolism of oral diosmin in healthy volunteers. Int J. Clin Pharm Ther. 1992, 30, 29–33. [Google Scholar]
- Zhang, J.K.; Sun, C.D.; Yan, Y.Y.; Chen, Q.J.; Luo, F.L.; Zhu, X.Y.; Li, X.; Chen, K.S. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chem. 2012, 135, 1471–1478. [Google Scholar] [CrossRef]
- Takumi, H.; Nakamura, H.; Simizu, T.; Harada, R.; Kometani, T.; Nadamoto, T.; Mukai, R.; Murota, K.; Kawai, Y.; Terao, J. Bioavailability of orally administered water-dispersible hesperetin and its effect on peripheral vasodilatation in human subjects: Implication of endothelial functions of plasma conjugated metabolites. Food Funct. 2012, 3, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Nectoux, A.M.; Abe, C.; Huang, S.W.; Ohno, N.; Tabata, J.; Miyata, Y.; Tanaka, K.; Tanaka, T.; Yamamura, H.; Matsui, T. Absorption and Metabolic Behavior of Hesperidin (Rutinosylated Hesperetin) after Single Oral Administration to Sprague–Dawley Rats. J. Agric. Food Chem. 2019, 67, 9812–9819. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Kondratyuk, T.P.; Marler, L.E.; Qiu, X.; Choi, Y.; Cao, H.m.; Yu, R.; Sturdy, M.; Pegan, S.; Liu, Y. Isolation and evaluation of kaempferol glycosides from the fern Neocheiropteris palmatopedata. Phytochemistry 2010, 71, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi–Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Nishioka, T.; Watanabe, J.; Kawabata, J.; Niki, R. Isolation and activity of N-p-coumaroyltyramine, an α-glucosidase inhibitor in Welsh onion (Allium fistulosum). Biosci. Biotech. Biochem. 1997, 61, 1138–1141. [Google Scholar] [CrossRef]
- Le, P.M.; Srivastava, V.; Nguyen, T.T.; Pradines, B.; Madamet, M.; Mosnier, J.; Trinh, T.T.; Lee, H. Stephanine from Stephania venosa (Blume) Spreng Showed Effective Antiplasmodial and Anticancer Activities, the Latter by Inducing Apoptosis through the Reverse of Mitotic Exit. Phytother Res. 2017, 31, 1357–1368. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Fan, M.X.; Hu, G.W.; Guo, M.Q. Potential Antioxidative and Anti-Hyperuricemic Components Targeting Superoxide Dismutase and Xanthine Oxidase Explored from Polygonatum Sibiricum Red. Antioxidants 2022, 11, 1651. [Google Scholar] [CrossRef]
- Qin, N.; Li, C.B.; Jin, M.N.; Shi, L.H.; Duan, H.Q.; Niu, W.Y. Synthesis and biological activity of novel tiliroside derivatives. Eur. J. Med. Chem. 2011, 46, 5189–5195. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, C.; David, B.; Fouraste, I.; Vercauteren, J. Acylated kaempferol glycosides from Laurus nobilis leaves. Phytochemistry 1998, 47, 821–824. [Google Scholar] [CrossRef]
- Lotscher, H.R.; DeJong, C.; Capaldi, R.A. Interconversion of high and low ATPase activity forms of ECF1 by the detergent lauryldimethylamine oxide. Biochemistry 1984, 23, 4140–4143. [Google Scholar] [CrossRef]
- Cank, K.B.; Henkin, J.M.; Cook, A.G.; Oberlies, N.H. Droplet probe: A non–destructive residue analysis of Wari ceramics from the imperial heartland. J. Archaeol. Sci. 2021, 134, 105468. [Google Scholar] [CrossRef]
- Hu, Y.W.; Wu, Q.; Qiao, Y.; Zhang, P.; Dai, W.T.; Tao, H.Y.; Chen, S. Disturbances in Metabolic Pathways and the Identification of a Potential Biomarker Panel for Early Cartilage Degeneration in a Rabbit Anterior Cruciate Ligament Transection Model. Cartilage 2021, 13, 1376–1387. [Google Scholar] [CrossRef]
- Caviglioli, G.; Valeria, P.; Brunella, P.; Sergio, C.; Attilia, A.; Gaetano, B. Identification of degradation products of Ibuprofen arising from oxidative and thermal treatments. J. Pharm. Biomed. 2002, 30, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.J.; Peddicord, M.B.; Rinaldi, F.A.; Kelly, K.A.; Bolgar, M.S. Identification of leachables observed in the size exclusion chromatograms of a low concentration product stored in prefilled syringes. J. Pharmceut. Biomed. 2017, 139, 133–142. [Google Scholar] [CrossRef]
- Su, C.H.; Cheng, Y.C.; Chang, Y.C.; Kung, T.H.; Chen, Y.L.; Lai, K.H.; Hsieh, H.L.; Chen, C.Y.; Hwang, T.L.; Yang, Y.L. Untargeted LC-MS/MS-Based Multi-Informative Molecular Networking for Targeting the Antiproliferative Ingredients in Tetradium ruticarpum Fruit. Molecules 2022, 27, 4462. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, Y.Y.; Zhu, H.Q.; Dong, J.; Qiao, J.T.; Kong, L.J.; Zhang, H.C. Two novel markers to discriminate poplar–type propolis from poplar bud extracts: 9-oxo-ODE and 9-oxo-ODA. J. Food Compos. Anal. 2022, 105, 104196. [Google Scholar] [CrossRef]
- Kato, T.; Yamaguchi, Y.; Hirano, T.; Yokoyama, T.; Uyehara, T.; Namai, T.; Yamanaka, S.; Harada, N. Unsaturated hydroxy fatty acids, the self defensive substances in rice plant against rice blast disease. Chem. Lett. 1984, 3, 409–412. [Google Scholar] [CrossRef]
- Zhao, B.; Tomoda, Y.; Mizukami, H.; Makino, T. 9-Oxo-(10E,12E)-octadecadienoic acid, a cytotoxic fatty acid ketodiene isolated from eggplant calyx, induces apoptosis in human ovarian cancer (HRA) cells. J. Nat. Med. Tokyo 2015, 69, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Dong, F.; Sun, Y.Z.; Sun, Z.H.; Song, X.Y.; Dong, Y.G.; Huang, X.C.; Zhong, J.Y.; Zhang, R.; Wang, M.Q. Qualitative and quantitative analysis of six fatty acid amides in 11 edible vegetable oils using liquid chromatography-masss pectrometry. Front Nutr. 2022, 9, 857858. [Google Scholar] [CrossRef] [PubMed]
- Colsch, B.; Afonso, C.; Popa, I.; Portoukalian, J.; Fournier, F.; Tabet, J.; Baumann, N. Characterization of the ceramide moieties of sphingoglycolipids from mouse brain by ESI–MS/MS: Identification of ceramides containing sphingadienine. J. Lipid Res. 2004, 45, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.D.; Chang, H.C.; Huang, S.H. Characterization of allegedly musk-containing medicinal products in Taiwan. J. Forensic Sci. 2004, 49, 1187–1193. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Z.; Wu, K.; Yan, G.; Xu, H.; Gong, Q.; Ni, L. Research Progress on the Chemical Constituents and Pharmacological Activity of Litsea cubeba (Lour) Pers. Rec. Nat. Prod. 2023, 17, 577–594. [Google Scholar]
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.B. Total Phenolic Contents and Antioxidant Capacities of Selected Chinese Medicinal Plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.L.; Zhang, J.C.; Li, C.F.; Liu, S.X.; Wang, L. Morinda citrifolia L. leaves extracts obtained by traditional and eco–friendly extraction solvents: Relation between phenolic compositions and biological properties by multivariate analysis. Ind. Crop. Prod. 2020, 153, 112586. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Liu, Y.; Zhang, Q.; Li, Y.; Wu, Z.Q. Release of phenolics compounds from Rubus idaeus L. dried fruits and seeds during simulated in vitro digestion and their bio-activities. J. Funct. Foods 2018, 46, 57–65. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, L.P.; Xu, C.C.; Mei, J.; Li, Y.F. Effects of germination and high hydrostatic pressure processing on mineral elements, amino acids and antioxidants in vitro bioaccessibility, as well as starch digestibility in brown rice (Oryza sativa L.). Food Chem. 2017, 214, 533–542. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sarikurkcu, C.; Sarikurkcu, R.T.; Wang, M.H. A comparative study on the phenolic composition, antioxidant and enzyme inhibition activities of two Endemic Onosma species. Ind. Crop. Prod. 2019, 142, 111878. [Google Scholar] [CrossRef]
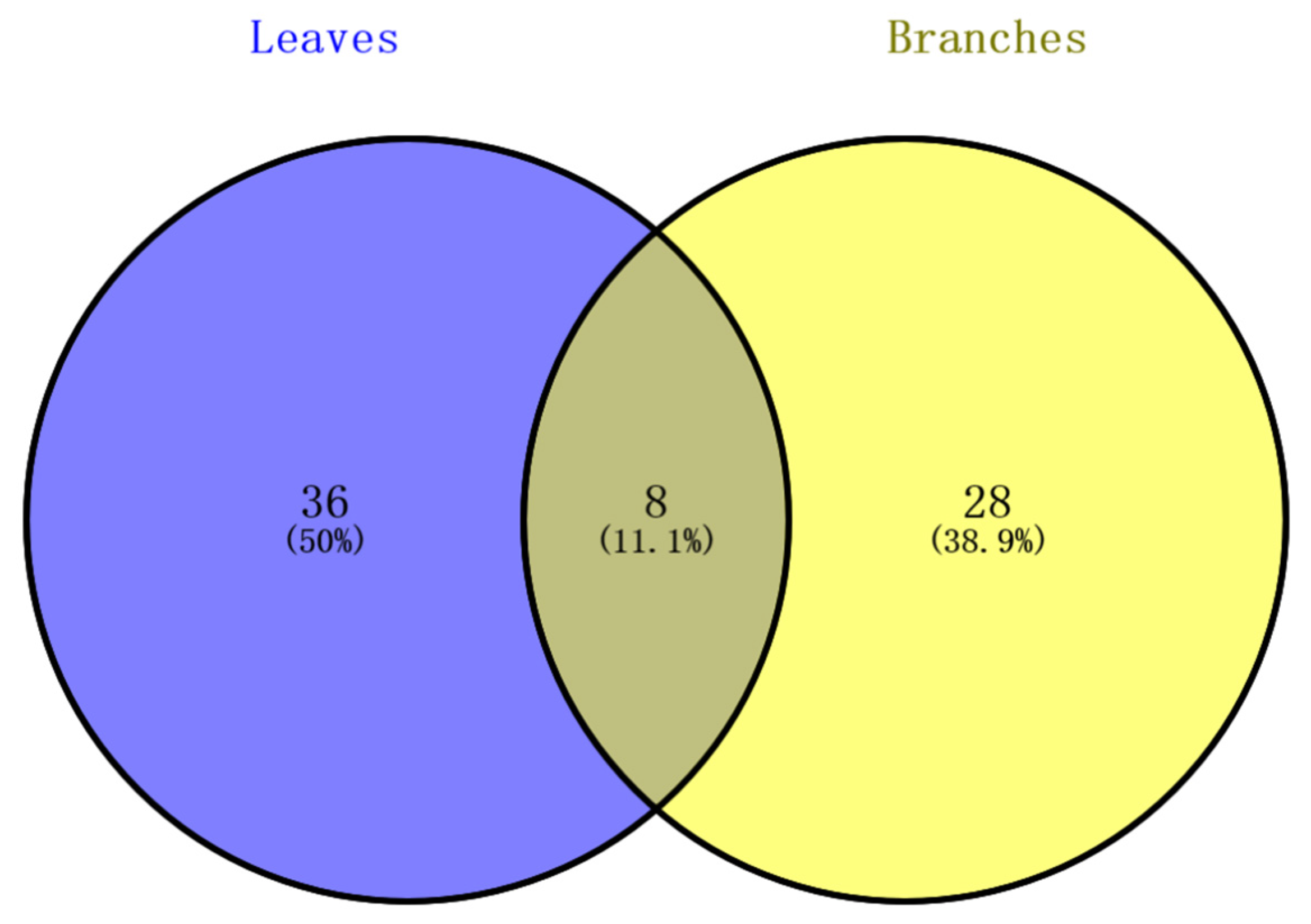
| No. | RT/min | Compounds | Molecular Formula | Error/ppm | m/z | Ion Mode | Compound Types | References | |
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Branches | ||||||||
| 1 | 0.851 | – | 2-Aminosuccinamic acid | C4H8N2O3 | −1.54 | 131.04599 | [M − H]− | alkaloids | [17] |
| 2 | 0.88 | – | Gluconic acid | C6H12O7 | −1.22 | 195.05079 | [M − H]− | carbohydrates | |
| 3 | 0.89 | – | L-Threonic acid | C4H8O5 | −1.26 | 135.02972 | [M − H]− | organic acids | [18] |
| 4 | 0.893 | – | D-(−)-Fructose | C6H12O6 | −0.76 | 179.05597 | [M − H]− | carbohydrates | [19] |
| 5 | 0.904 | – | D-(-)-Quinic acid | C7H12O6 | −0.89 | 191.05595 | [M − H]− | organic acids | [20] |
| 6 | 0.908 | – | L-Threonine | C4H9NO3 | −0.3 | 120.06559 | [M + H]+ | amino acids | [21] |
| 7 | 0.915 | – | D-Glucosamine | C6H13NO5 | 0 | 180.0867 | [M + H]+ | carbohydrates | [22] |
| 8 | 0.918 | – | (2R)-2,3-Dihydroxypropanoic acid ★ | C3H6O4 | −1.37 | 105.01919 | [M − H]− | organic acids | [23] |
| 9 | 0.940 | – | DL-Malic acid | C4H6O5 | −1.53 | 133.01403 | [M − H]− | organic acids | [24] |
| 10 | 0.950 | – | D-Serine ★ | C3H7NO3 | −0.06 | 106.04986 | [M + H]+ | amino acids | [25] |
| 11 | 0.982 | – | Proline | C5H9NO2 | 0.5 | 116.07066 | [M + H]+ | amino acids | [26] |
| 12 | 0.998 | – | Adenine | C5H5N5 | 0.91 | 136.06189 | [M + H]+ | alkaloids | [27] |
| 13 | 1.019 | – | Acetophenone | C8H8O | 0.54 | 121.06486 | [M + H]+ | ketones | [28] |
| 14 | – | 1.024 | 4-Methoxycinnamaldehyde | C10H10O2 | −0.18 | 180.10186 | [M + H]+ | ethers | |
| 15 | – | 1.140 | Higenamine ★ | C16H17NO3 | 0.31 | 272.1282 | [M + H]+ | alkaloids | [29] |
| 16 | 1.219 | – | Citric acid | C6H8O7 | −0.32 | 191.01967 | [M − H]− | organic acids | [30] |
| 17 | 1.232 | – | Nicotinic acid | C6H5NO2 | 0.14 | 124.03932 | [M + NH4]+ | alkaloids | [31] |
| 18 | 1.238 | – | Pipecolic acid | C6H11NO2 | 0.45 | 130.08631 | [M + H]+ | alkaloids | [32] |
| 19 | 1.331 | – | Methylmalonic acid | C4H6O4 | −1.03 | 117.01921 | [M − H]− | organic acids | [33] |
| 20 | 1.335 | – | Fumaric acid | C4H4O4 | −1.05 | 115.00356 | [M − H]− | organic acids | [34] |
| 21 | 1.373 | – | Isoleucine | C6H13NO2 | 0.35 | 132.10195 | [M + H]+ | amino acids | [35] |
| 22 | 1.456 | – | L-Norleucine | C6H13NO2 | 0.41 | 132.10196 | [M + H]+ | amino acids | [36] |
| 23 | – | 1.615 | L-N-Acetylphenylalaninol ★ | C11H15NO2 | −0.04 | 194.11755 | [M + H]+ | alkaloids | |
| 24 | – | 1.989 | (-)-Salsoline | C11H15NO2 | −0.17 | 194.11752 | [M + H]+ | alkaloids | |
| 25 | 2.089 | – | Gentisic acid 5-O-β-glucoside ★ | C13H16O9 | −0.85 | 315.07189 | [M − H]− | organic acids | |
| 26 | – | 2.098 | Indole-3-acetic acid | C10H9NO2 | −0.03 | 176.07061 | [M + H]+ | alkaloids | [37] |
| 27 | – | 3.396 | trans-3-Indoleacrylic acid ★ | C11H9NO2 | −0.39 | 188.07054 | [M + H]+ | alkaloids | [38] |
| 28 | – | 5.066 | Norisoboldine | C18H19NO4 | 0.42 | 314.13882 | [M + H]+ | alkaloids | [39] |
| 29 | 5.091 | – | 3-(4-Hydroxyphenyl)-3-oxopropyl β-D-glucopyranoside | C15H20O8 | −0.82 | 327.10827 | [M − H]− | phenols | [40] |
| 30 | 5.888 | 5.261 | Boldine | C19H21NO4 | −0.07 | 328.15432 | [M + H]+ | alkaloids | [41] |
| 31 | 5.486 | – | Dihydrophaseic acid ★ | C15H22O5 | −0.41 | 281.13933 | [M − H]− | organic acids | [42] |
| 32 | 6.213 | 6.072 | (S)-Boldine | C19H21NO4 | 0.26 | 328.15442 | [M + H]+ | alkaloids | [41] |
| 33 | 6.636 | 6.487 | Glaufinine | C19H21NO4 | 0.07 | 328.15436 | [M + H]+ | alkaloids | [43] |
| 34 | 6.604 | – | Kaempferol 3-sophoroside ★ | C27H30O16 | −0.28 | 609.14594 | [M − H]− | flavonoids | [44] |
| 35 | – | 6.765 | Isocorydine | C20H23NO4 | 0.26 | 342.17007 | [M + H]+ | alkaloids | [45] |
| 36 | 7.526 | 7.817 | Rutin | C27H30O16 | −0.69 | 609.14558 | [M − H]− | flavonoids | [41] |
| 37 | – | 7.117 | Isoquercetin | C21H20O12 | −0.28 | 463.08796 | [M − H]− | flavonoids | [46] |
| 38 | 7.316 | – | Kaempferitrin | C27H30O14 | −0.31 | 577.15588 | [M − H]− | flavonoids | [47] |
| 39 | – | 7.551 | laurotetanine | C19H21NO4 | 0.07 | 328.15436 | [M + H]+ | alkaloids | [41] |
| 40 | 7.557 | – | 5-(4-Hydroxypentyl)-1,3-benzenediol ★ | C11H16O3 | −0.11 | 197.1172 | [M + H]+ | phenols | |
| 41 | – | 7.589 | Corydine ★ | C20H23NO4 | −0.21 | 342.16991 | [M + H]+ | alkaloids | [43] |
| 42 | 7.631 | – | Trifolin ★ | C21H20O11 | −0.34 | 447.09298 | [M − H]− | flavonoids | [48] |
| 43 | 7.951 | – | Cynaroside | C21H20O11 | −0.26 | 447.09299 | [M − H]− | flavonoids | [49] |
| 44 | – | 8.048 | Quercitrin | C21H20O11 | −0.36 | 447.09312 | [M − H]− | flavonoids | [41] |
| 45 | 8.246 | – | Cassythicin | C19H19NO4 | 0.33 | 326.13879 | [M + H]+ | alkaloids | [50] |
| 46 | – | 8.349 | Diosmin ★ | C28H32O15 | −0.77 | 607.16638 | [M − H]− | flavonoids | [51] |
| 47 | – | 8.503 | Neohesperidin ★ | C28H34O15 | −0.78 | 609.18202 | [M − H]− | flavonoids | [52] |
| 48 | – | 8.644 | Hesperetin | C16H14O6 | 0.57 | 303.08649 | [M + H]+ | flavonoids | [53] |
| 49 | – | 8.648 | Hesperidin | C28H34O15 | 0.65 | 611.19739 | [M + H]+ | flavonoids | [54] |
| 50 | 9.196 | – | Afzelin | C21H20O10 | −0.63 | 431.0981 | [M − H]− | flavonoids | [55] |
| 51 | 9.443 | – | Kaempferol ★ | C15H10O6 | 0.05 | 287.05503 | [M + H]+ | flavonoids | [56] |
| 52 | – | 9.930 | N-p-Coumaroyltyramine | C17H17NO3 | 0.32 | 284.12821 | [M + H]+ | phenylpropanoids | [57] |
| 53 | – | 10.391 | Crebanine | C20H21NO4 | 0.3 | 340.15444 | [M + H]+ | alkaloids | [58] |
| 54 | 10.576 | 10.454 | Moupinamide | C18H19NO4 | 0.24 | 314.13876 | [M + H]+ | alkaloids | [59] |
| 55 | 10.869 | – | Tiliroside ★ | C30H26O13 | −0.92 | 593.12952 | [M − H]− | flavonoids | [60] |
| 56 | 14.447 | 14.029 | 15-Hexadecynoic acid ★ | C16H28O2 | −0.2 | 253.21616 | [M + H]+ | organic acids | |
| 57 | 14.508 | – | 3-[[6-Deoxy-3,4-bis-O-[(2E)-3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-α-L-mannopyranosyl]oxy]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | C39H32O14 | −0.9 | 723.17128 | [M − H]− | flavonoids | [61] |
| 58 | 15.609 | 15.177 | Lauryldimethylamine oxide ★ | C14H31NO | −0.09 | 230.24782 | [M + H]+ | alkaloids | [62] |
| 59 | – | 16.099 | Aurantiamide acetate | C27H28N2O4 | 0.05 | 445.21222 | [M + H]+ | alkaloids | [63] |
| 60 | – | 16.286 | 2-Amino-1,3,4-octadecanetriol ★ | C18H39NO3 | 0.55 | 318.30045 | [M + H]+ | alcohols | [64] |
| 61 | – | 16.290 | 4-Ethylbenzaldehyde | C9H10O | 0.59 | 135.08052 | [M + H]+ | esters | [65] |
| 62 | – | 16.297 | 4-Ethoxy ethylbenzoate | C11H14O3 | −0.12 | 195.10155 | [M + H]+ | esters | [66] |
| 63 | 16.529 | – | Schinifoline ★ | C17H23NO | −0.02 | 258.18524 | [M + H]+ | alkaloids | [67] |
| 64 | 16.882 | 16.919 | 1,3:2,4-Di(p-ethylbenzylidene)sorbitol ★ | C24H30O6 | 0.51 | 415.2117 | [M + H]+ | alcohols | |
| 65 | – | 17.097 | 9-Oxo-ODE | C18H30O3 | 0.09 | 295.2268 | [M + H]+ | organic acids | [68] |
| 66 | – | 17.368 | 13(S)-HOTrE | C18H30O3 | 0.08 | 293.21224 | [M − H]− | organic acids | [69] |
| 67 | – | 18.395 | 9-Oxo-10(E),12(E)-octadecadienoic acid | C18H30O3 | 0.4 | 295.22687 | [M + H]+ | organic acids | [70] |
| 68 | – | 19.147 | 12-Oxo-10,15(Z)-phytodienoic acid ★ | C18H28O3 | 0.45 | 293.21124 | [M + H]+ | organic acids | |
| 69 | – | 19.440 | Linoleoyl ethanolamide ★ | C20H37NO2 | 0.28 | 324.28979 | [M + H]+ | alcohols | [71] |
| 70 | – | 20.267 | Sphingosine (d18:1) ★ | C18H37NO2 | 0.6 | 300.28989 | [M + H]+ | alcohols | [72] |
| 71 | 21.806 | – | Muscone | C16H30O | −0.21 | 239.23689 | [M + H]+ | ketones | [73] |
| 72 | 21.829 | – | Pheophorbide A ★ | C35H36N4O5 | 0.14 | 593.27594 | [M + H]+ | alkaloids | |
| Parts | ABTS (TEAC µmol/g) | DPPH (TEAC µmol/g) | FRAP (Fe(Ⅱ) µmol/g) | CUPRAC (TEAC µmol/g) |
|---|---|---|---|---|
| Branches | 42.75 ± 3.94 | 7.55 ± 0.43 | 22.21 ± 0.53 | 42.65 ± 0.35 |
| Leaves | 57.78 ± 1.87 | 9.29 ± 0.39 | 28.68 ± 0.78 | 45.89 ± 0.27 |
| Method | Equation | R2 |
|---|---|---|
| ABTS | y = −0.32338x + 0.76745 | 0.99755 |
| DPPH | y = −1.23810x + 0.57000 | 0.99133 |
| FRAP | y = 0.20124x + 0.10870 | 0.99987 |
| CUPRAC | y = 0.43161x + 0.18027 | 0.99903 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, W.; Li, B.; Xiong, Y.; Dai, L.; Tian, Y.; Zhang, L.; Wang, Q.; Qian, G. Non-Volatile Component and Antioxidant Activity: A Comparative Analysis between Litsea cubeba Branches and Leaves. Molecules 2024, 29, 788. https://doi.org/10.3390/molecules29040788
Dai W, Li B, Xiong Y, Dai L, Tian Y, Zhang L, Wang Q, Qian G. Non-Volatile Component and Antioxidant Activity: A Comparative Analysis between Litsea cubeba Branches and Leaves. Molecules. 2024; 29(4):788. https://doi.org/10.3390/molecules29040788
Chicago/Turabian StyleDai, Wei, Boyi Li, Yanli Xiong, Liping Dai, Yuan Tian, Liangqian Zhang, Qi Wang, and Guoqiang Qian. 2024. "Non-Volatile Component and Antioxidant Activity: A Comparative Analysis between Litsea cubeba Branches and Leaves" Molecules 29, no. 4: 788. https://doi.org/10.3390/molecules29040788
APA StyleDai, W., Li, B., Xiong, Y., Dai, L., Tian, Y., Zhang, L., Wang, Q., & Qian, G. (2024). Non-Volatile Component and Antioxidant Activity: A Comparative Analysis between Litsea cubeba Branches and Leaves. Molecules, 29(4), 788. https://doi.org/10.3390/molecules29040788






