Synthesis and Characterization of Short α and β-Mixed Peptides with Excellent Anti-Lipase Activities
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Pancreatic Lipase (PL) Inhibitory Activity of Peptides
3. Materials and Methods
3.1. General
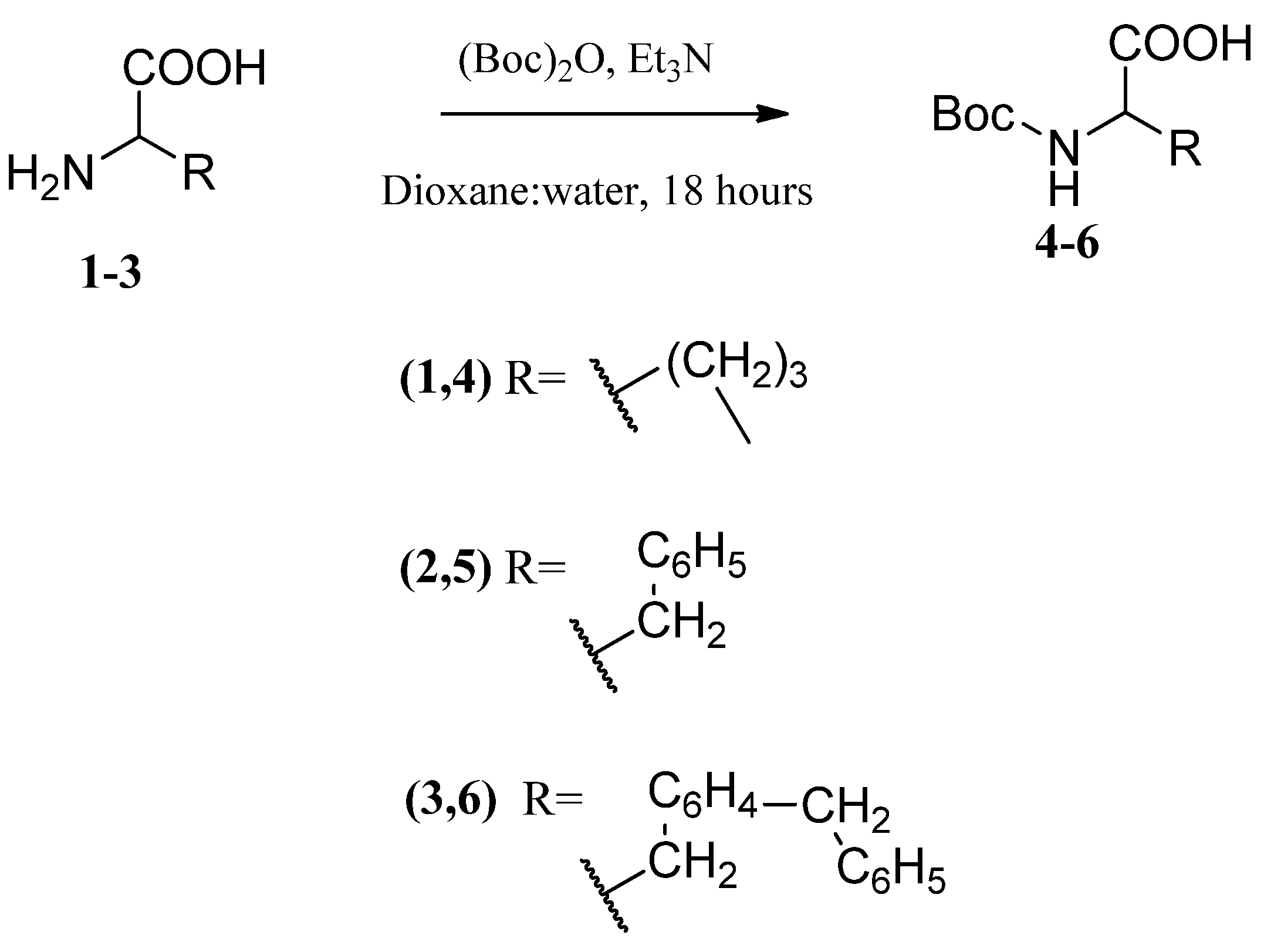
3.2. NH2-Group Protection of L-Amino Acids (4–6)
- N-(Boc)-L-Proline (4)
- N-(Boc)-L-Phenylalanine (5)
- N-Boc-O-Bn-L-Tyr (6)
3.3. Generation of Diazomethane Ethereal Solution
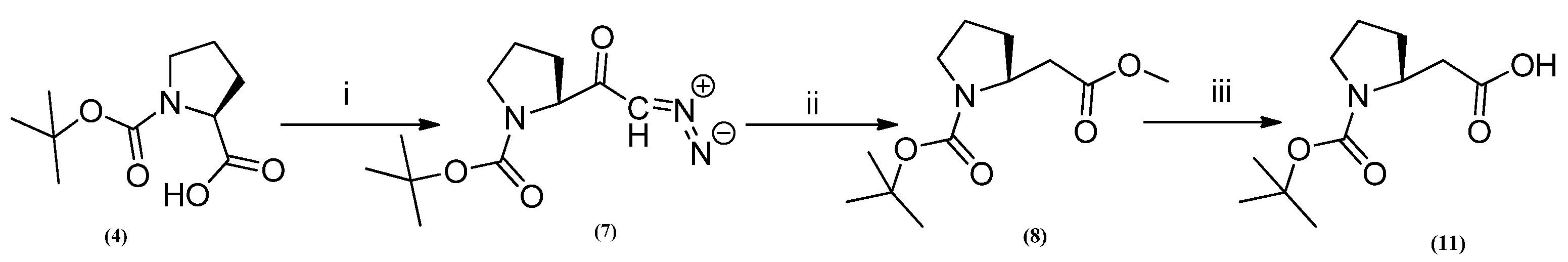
3.4. Synthesis of N-Boc-Proline Diazoketone (7)
3.5. Synthesis of N-Boc-Pro-β-Methyl Ester (8)
3.6. Boc-Deprotection of N-Boc-Amino Ester (9) and (10)
3.7. Boc-Deprotection of N-Boc-Peptide Ester (16)
3.8. Synthesis of N-Boc-β-Amino Acid (11)
- (N-Boc-L-Proline-β-Acid) (11)
3.9. Synthesis of N-Boc-Peptide Acid (15)
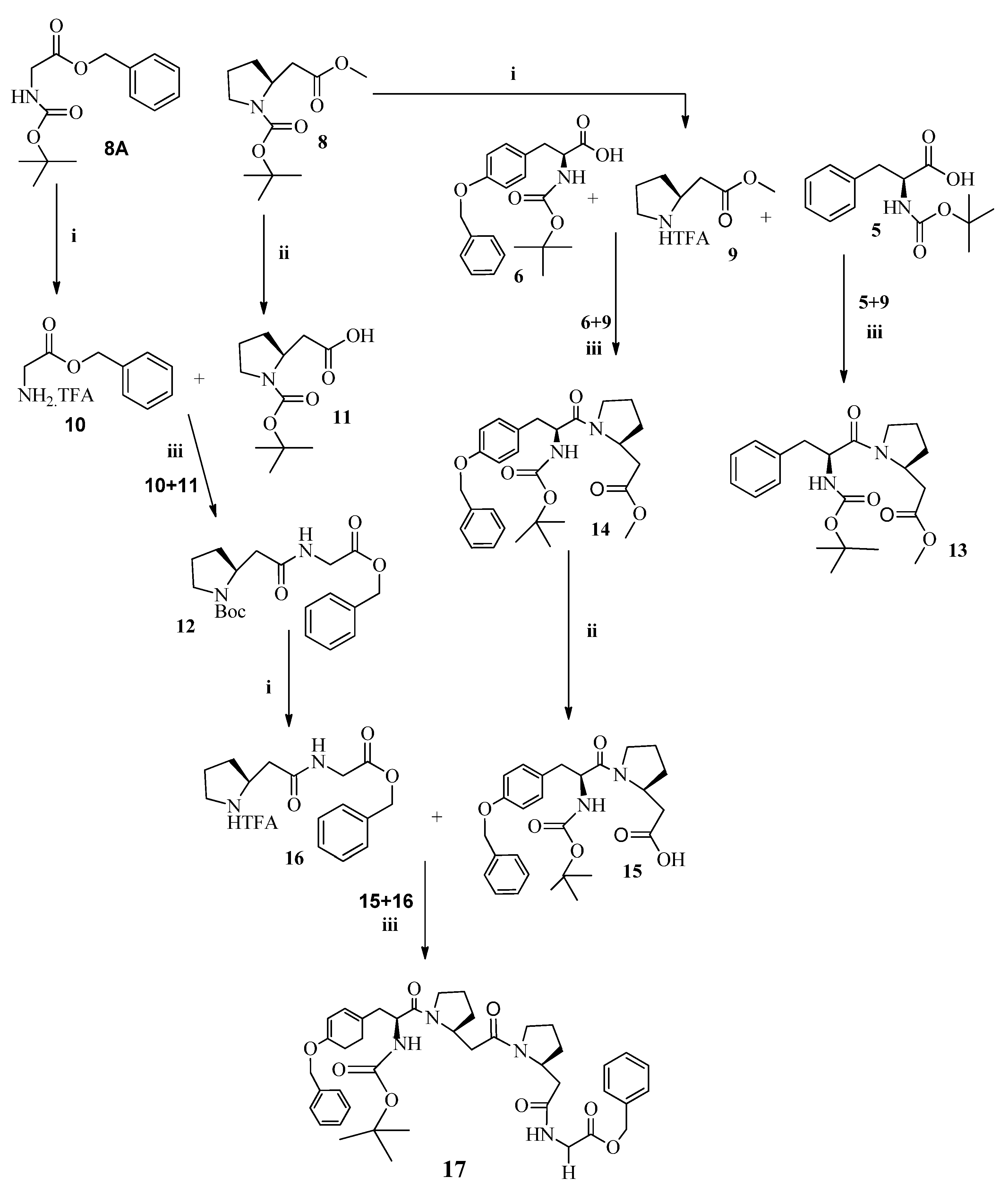
3.10. General Procedure for the Synthesis of Short α,β-Mixed Peptides (12–14)
- Dipeptide (N-Boc-β-Pro-α-Gly-OBn) (12)
- Dipeptide (N-Boc-α-Phe-β-Pro-OCH3) (13)
- Dipeptide (N-Boc-O-Bn-α-Tyr-β-Pro-OCH3) (14)
3.11. Tetrapeptide (N-Boc-O-Bn-α-Tyr-β-Pro-β-Pro-α-Gly-OBn) (17)
4. Lipase Inhibition Assay
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chia, T.Y.; Gan, C.-Y.; Shafie, M.H.; Yap, P.G.; Rodhi, A.M.; Ahmad, A.; Murugaiyah, V.; Abdulla, M.H.; Johns, E.J. A comprehensive review on the pancreatic lipase inhibitory peptides: A future anti-obesity strategy. Electron. J. Gen. Med. 2023, 20, em470. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Dietz, W.H.; Baur, L.A.; Hall, K.; Puhl, R.M.; Taveras, E.M.; Uauy, R.; Kopelman, P. Management of obesity: Improvement of health-care training and systems for prevention and care. Lancet 2015, 385, 2521–2533. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Krentz, A.J.; Fujioka, K.; Hompesch, M. Evolution of pharmacological obesity treatments: Focus on adverse side-effect profiles. Diabetes Obes. Metab. 2016, 18, 558–570. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Kimura, Y. Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J. Pharm. Pharmacol. 2006, 58, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lairon, D.; Lafont, H.; Vigne, J.-L.; Nalbone, G.; Léonardi, J.; Hauton, J.C. Effects of dietary fibers and cholestyramine on the activity of pancreatic lipase in vitro. Am. J. Clin. Nutr. 1985, 42, 629–638. [Google Scholar] [CrossRef]
- Tsujita, T.; Takaichi, H.; Takaku, T.; Aoyama, S.; Hiraki, J. Antiobesity action of ɛ-polylysine, a potent inhibitor of pancreatic lipase. J. Lipid Res. 2006, 47, 1852–1858. [Google Scholar] [CrossRef]
- Tsujita, T.; Matsuura, Y.; Okuda, H. Studies on the inhibition of pancreatic and carboxylester lipases by protamine. J. Lipid Res. 1996, 37, 1481–1487. [Google Scholar] [CrossRef]
- Roy, D.M.; Schneeman, B.O. Effect of soy protein, casein and trypsin inhibitor on cholesterol, bile acids and pancreatic enzymes in mice. J. Nutr. 1981, 111, 878–885. [Google Scholar] [CrossRef]
- Point, V.; Pavan Kumar, K.V.P.; Marc, S.; Delorme, V.; Parsiegla, G.; Amara, S.; Carrière, F.; Buono, G.; Fotiadu, F.; Canaan, S.; et al. Analysis of the discriminative inhibition of mammalian digestive lipases by 3-phenyl substituted 1,3,4-oxadiazol-2(3H)-ones. Eur. J. Med. Chem. 2012, 58, 452–463. [Google Scholar] [CrossRef]
- Jayanna, N.D.; Vagdevi, H.M.; Dharshan, J.C.; Prashith Kekuda, T.R.; Hanumanthappa, B.C.; Gowdarshivannanavar, B.C. Synthesis and Biological Evaluation of Novel 5,7-Dichloro-1,3-benzoxazole Derivatives. J. Chem. 2013, 2013, 864385. [Google Scholar] [CrossRef]
- Kahveci, B.; Menteşe, E.; Özil, M.; Ülker, S.; Ertürk, M. An efficient synthesis of benzimidazoles via a microwave technique and evaluation of their biological activities. Monatshefte Chem.-Chem. Mon. 2013, 144, 993–1001. [Google Scholar] [CrossRef]
- Huo, P.C.; Hu, Q.; Shu, S.; Zhou, Q.H.; He, R.J.; Hou, J.; Guan, X.Q.; Tu, D.Z.; Hou, X.D.; Liu, P.; et al. Design, synthesis, and biological evaluation of novel chalcone-like compounds as potent and reversible pancreatic lipase inhibitors. Bioorg. Med. Chem. 2021, 29, 115853. [Google Scholar] [CrossRef] [PubMed]
- Al-Hiari, Y.M.; Kasabri, V.N.; Shakya, A.K.; Alzweiri, M.H.; Afifi, F.U.; Bustanji, Y.K.; Al-Masri, I.M. Fluoroquinolones: Novel class of gastrointestinal dietary lipid digestion and absorption inhibitors. Med. Chem. Res. 2014, 23, 3336–3346. [Google Scholar] [CrossRef]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Koosha, S.; Mohamed, Z. Medicinal Plants and Their Inhibitory Activities against Pancreatic Lipase: A Review. Evid.-Based Complement. Altern. Med. 2015, 2015, 973143. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Glazer, G. Long-term pharmacotherapy of obesity 2000: A review of efficacy and safety. Arch. Intern. Med. 2001, 161, 1814–1824. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713. [Google Scholar] [CrossRef]
- James, W.P.T.; Caterson, I.D.; Coutinho, W.; Finer, N.; Van Gaal, L.F.; Maggioni, A.P.; Torp-Pedersen, C.; Sharma, A.M.; Shepherd, G.M.; Rode, R.A. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N. Engl. J. Med. 2010, 363, 905–917. [Google Scholar] [CrossRef]
- Ballinger, A.; Peikin, S.R. Orlistat: Its current status as an anti-obesity drug. Eur. J. Pharmacol. 2002, 440, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P.; St-Pierre, S.; Tremblay, A. Currently available drugs for the treatment of obesity: Sibutramine and orlistat. Mini Rev. Med. Chem. 2007, 7, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Garzón, A.G.; Cian, R.E.; Aquino, M.E.; Drago, S.R. Isolation and identification of cholesterol esterase and pancreatic lipase inhibitory peptides from brewer's spent grain by consecutive chromatography and mass spectrometry. Food Funct. 2020, 11, 4994–5003. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Baba, W.N.; Kamal, H.; FitzGerald, R.J.; Hassan, H.M.; Ayoub, M.A.; Gan, C.Y.; Maqsood, S. A comparative investigation into novel cholesterol esterase and pancreatic lipase inhibitory peptides from cow and camel casein hydrolysates generated upon enzymatic hydrolysis and in-vitro digestion. Food Chem. 2022, 367, 130661. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L.; Wade, J.D. Current challenges in peptide-based drug discovery. Front. Chem. 2014, 2, 62. [Google Scholar] [CrossRef]
- Cabrele, C.; Martinek, T.A.; Reiser, O.; Berlicki, Ł. Peptides containing β-amino acid patterns: Challenges and successes in medicinal chemistry. J. Med. Chem. 2014, 57, 9718–9739. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, F.; Martinek, T.A.; Tóth, G.K. Application of alicyclic β-amino acids in peptide chemistry. Chem. Soc. Rev. 2006, 35, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Bodanszky, M.; Bodanszky, A. The Practice of Peptide Synthesis; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Meier, H.; Zeller, K.P. The Wolff rearrangement of α-diazo carbonyl compounds. Angew. Chem. Int. Ed. Engl. 1975, 14, 32–43. [Google Scholar] [CrossRef]
- Kirmse, W. 100 Years of the Wolff Rearrangement. Eur. J. Org. Chem. 2002, 2002, 2193–2256. [Google Scholar] [CrossRef]
- Bose, D.S.; Lakshminarayana, V. Lewis acid-mediated selective removal of N-tert-butoxycarbonyl protective group (t-Boc). Synthesis 1999, 1999, 66–68. [Google Scholar] [CrossRef]
- Carter, J.D.; LaBean, T.H. Coupling strategies for the synthesis of peptide-oligonucleotide conjugates for patterned synthetic biomineralization. J. Nucleic Acids 2011, 2011, 926595. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, C.; Wang, S.; Du, M.; Zhu, B. Efficient screening of pancreatic lipase inhibitors from cod meat hydrolysate through ligand fishing strategy. Front. Nutr. 2022, 9, 969558. [Google Scholar] [CrossRef]
- Takashiro, E.; Hayakawa, I.; Nitta, T.; Kasuya, A.; Miyamoto, S.; Ozawa, Y.; Yagi, R.; Yamamoto, I.; Shibayama, T.; Nakagawa, A.; et al. Structure–activity relationship of HIV-1 protease inhibitors containing α-hydroxy-β-amino acids. Detailed study of P1 site. Bioorg. Med. Chem. 1999, 7, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Tørfoss, V.; Isaksson, J.; Ausbacher, D.; Brandsdal, B.-O.; Flaten, G.E.; Anderssen, T.; Cavalcanti-Jacobsen, C.d.A.; Havelkova, M.; Nguyen, L.T.; Vogel, H.J.; et al. Improved anticancer potency by head-to-tail cyclization of short cationic anticancer peptides containing a lipophilic β2,2-amino acid. J. Pept. Sci. 2012, 18, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-R.; Raman, N.; Gellman, S.H.; Lynn, D.M.; Palecek, S.P. Incorporation of β-Amino Acids Enhances the Antifungal Activity and Selectivity of the Helical Antimicrobial Peptide Aurein 1.2. ACS Chem. Biol. 2017, 12, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Singh, G.; Shankar, S.; Sharma, A.; Katoch, M.; Rai, R. Short hybrid peptides incorporating β- and γ-amino acids as antimicrobial agents. Peptides 2017, 97, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Han, M.-H.; Gu, Q.; Wang, J.-D.; Zhao, H.; Zhai, B.-W.; Nie, S.-M.; Liu, Z.-G.; Fu, Y.-J. Identification of pancreatic lipase inhibitors from Eucommia ulmoides tea by affinity-ultrafiltration combined UPLC-Orbitrap MS and in vitro validation. Food Chem. 2023, 426, 136630. [Google Scholar] [CrossRef] [PubMed]
- Deora, N.; Venkatraman, K. Lipase activity inhibited by aloenin A: Glycoside from Aloe vera (L.) Burm. f.—In vitro and molecular docking studies. J. Mol. Recognit. 2023, 36, e3002. [Google Scholar] [CrossRef] [PubMed]
- Abouzied, A.; Break, M.; Huwaimel, B.; Hussein, W.; Alafnan, A.; Younes, K.; Amr, S.; Abouzied, A. Discovery of A Novel Synthetic Thiazole-benzimidazole Conjugate that Acts as a Potent Pancreatic Lipase Inhibitor using in silico and in vitro Approaches CC-BY 4.0. Indian J. Pharm. Educ. 2023, 57, 218–227. [Google Scholar] [CrossRef]
- George, G.; Yadav, N.; Auti, P.S.; Paul, A.T. Molecular modelling, synthesis and in vitro evaluation of quinazolinone hybrid analogues as potential pancreatic lipase inhibitors. J. Biomol. Struct. Dyn. 2023, 41, 9583–9601. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Paul, A.T. Synthesis of amide warhead containing coumarin derivatives as potential pancreatic lipase inhibitors: In silico and in vitro evaluation for obesity treatment. Med. Chem. Res. 2023, 32, 2219–2233. [Google Scholar] [CrossRef]
- George, G.; Auti, P.S.; Sengupta, P.; Yadav, N.; Paul, A.T. Synthesis, molecular modelling and pharmacological evaluation of novel indole-thiazolidinedione based hybrid analogues as potential pancreatic lipase inhibitors. J. Biomol. Struct. Dyn. 2023, 14, 1–20. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Hou, X.; Han, J.; Qin, X.; Yang, Y.; Song, Y.; Liu, Z.; Zhang, Y.; Xu, Z.; et al. Design, synthesis and biological evaluation of salicylanilides as novel allosteric inhibitors of human pancreatic lipase. Bioorg. Med. Chem. 2023, 91, 117413. [Google Scholar] [CrossRef]
- Ketprayoon, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. An in vitro study of lipase inhibitory peptides obtained from de-oiled rice bran. RSC Adv. 2021, 11, 18915–18929. [Google Scholar] [CrossRef]
- Esfandi, R.; Seidu, I.; Willmore, W.; Tsopmo, A. Antioxidant, pancreatic lipase, and α-amylase inhibitory properties of oat bran hydrolyzed proteins and peptides. J. Food Biochem. 2022, 46, e13762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Rui, X.; Simpson, B.K. Interactions of C. frondosa-derived inhibitory peptides against angiotensin I-converting enzyme (ACE), α-amylase and lipase. Food Chem. 2022, 367, 130695. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B.; Jakubczyk, A. Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects. Eur. Food Res. Technol. 2020, 246, 1361–1369. [Google Scholar] [CrossRef]
- Siow, H.-L.; Choi, S.-B.; Gan, C.-Y. Structure–activity studies of protease activating, lipase inhibiting, bile acid binding and cholesterol-lowering effects of pre-screened cumin seed bioactive peptides. J. Funct. Foods 2016, 27, 600–611. [Google Scholar] [CrossRef]
- Vermaak, I.; Viljoen, A.M.; Hamman, J.H. Natural products in anti-obesity therapy. Nat. Prod. Rep. 2011, 28, 1493–1533. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-Associated Adverse Effects and Drug Interactions. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dang, Y. Advances in the stability challenges of bioactive peptides and improvement strategies. Curr. Res. Food Sci. 2022, 5, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.D. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Endeavour: London, UK, 1990; Volume 14, p. 148. [Google Scholar]
- Chankeshwara, S.V.; Chakraborti, A.K. Catalyst-free chemoselective N-tert-butyloxycarbonylation of amines in water. Org. Lett. 2006, 8, 3259–3262. [Google Scholar] [CrossRef]
- Deming, T.J. The Practice of Peptide Synthesis; Bodansky, M., Bodansky, A., Eds.; Springer: New York, NY, USA, 1995; Volume 33, p. 2289. [Google Scholar]
- Asghar, M.; Sajjad, A.; Hanif, S.; Ali, J.S.; Ali, Z.; Zia, M. Comparative analysis of synthesis, characterization, antimicrobial, antioxidant, and enzyme inhibition potential of roses petal based synthesized copper oxide nanoparticles. Mater. Chem. Phys. 2022, 278, 125724. [Google Scholar] [CrossRef]
| Codes | Short α, β-Mixed Peptides | % Lipase Inhibition |
|---|---|---|
| 12 | N-Boc-β-Pro-Gly-OBz | 93 |
| 13 | N-Boc-Phe-β-Pro-OCH3 | 90 |
| 14 | N-Boc-O-Bz-Tyr-β-Pro-OCH3 | 89 |
| 15 | N-Boc-O-Bz-Tyr-β-Pro-COOH | 91 |
| 17 | N-Boc-O-Bz-Tyr-β-Pro-β-Pro-Gly-OBz | 92 |
| Standard | Orlistat | 98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, N.; Asif, S.; Arfan, M.; Mahmood, Q.; Islam, A.; Gatasheh, M.K.; Zia, M. Synthesis and Characterization of Short α and β-Mixed Peptides with Excellent Anti-Lipase Activities. Molecules 2024, 29, 765. https://doi.org/10.3390/molecules29040765
Ahmed N, Asif S, Arfan M, Mahmood Q, Islam A, Gatasheh MK, Zia M. Synthesis and Characterization of Short α and β-Mixed Peptides with Excellent Anti-Lipase Activities. Molecules. 2024; 29(4):765. https://doi.org/10.3390/molecules29040765
Chicago/Turabian StyleAhmed, Naeem, Sabahat Asif, Muhammad Arfan, Qaiser Mahmood, Amjad Islam, Mansour K. Gatasheh, and Muhammad Zia. 2024. "Synthesis and Characterization of Short α and β-Mixed Peptides with Excellent Anti-Lipase Activities" Molecules 29, no. 4: 765. https://doi.org/10.3390/molecules29040765
APA StyleAhmed, N., Asif, S., Arfan, M., Mahmood, Q., Islam, A., Gatasheh, M. K., & Zia, M. (2024). Synthesis and Characterization of Short α and β-Mixed Peptides with Excellent Anti-Lipase Activities. Molecules, 29(4), 765. https://doi.org/10.3390/molecules29040765








