Trimethylamine N-Oxide Improves Exercise Performance by Reducing Oxidative Stress through Activation of the Nrf2 Signaling Pathway
Abstract
1. Introduction
2. Results
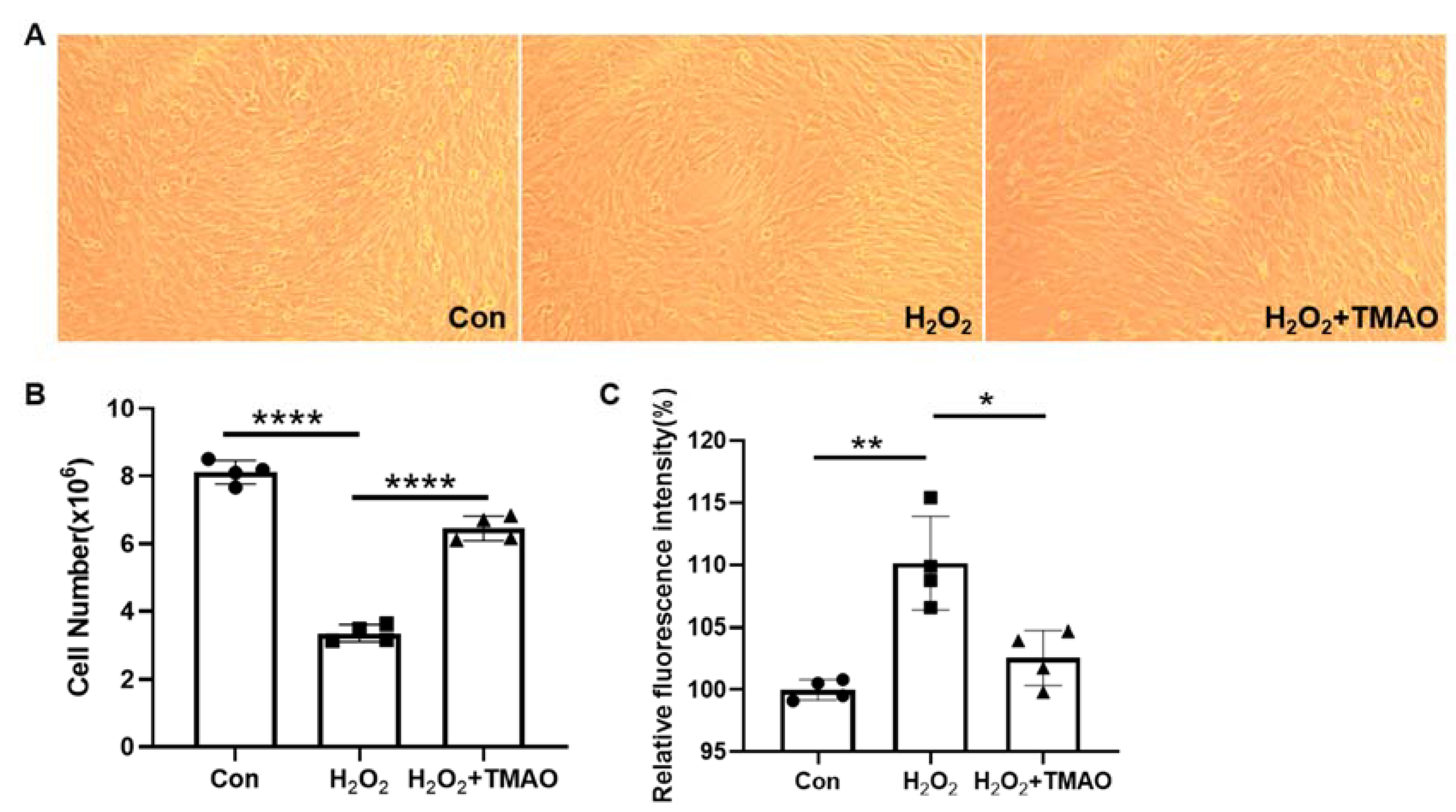
2.1. TMAO Prevented H2O2-Induced Damage in C2C12 Cells
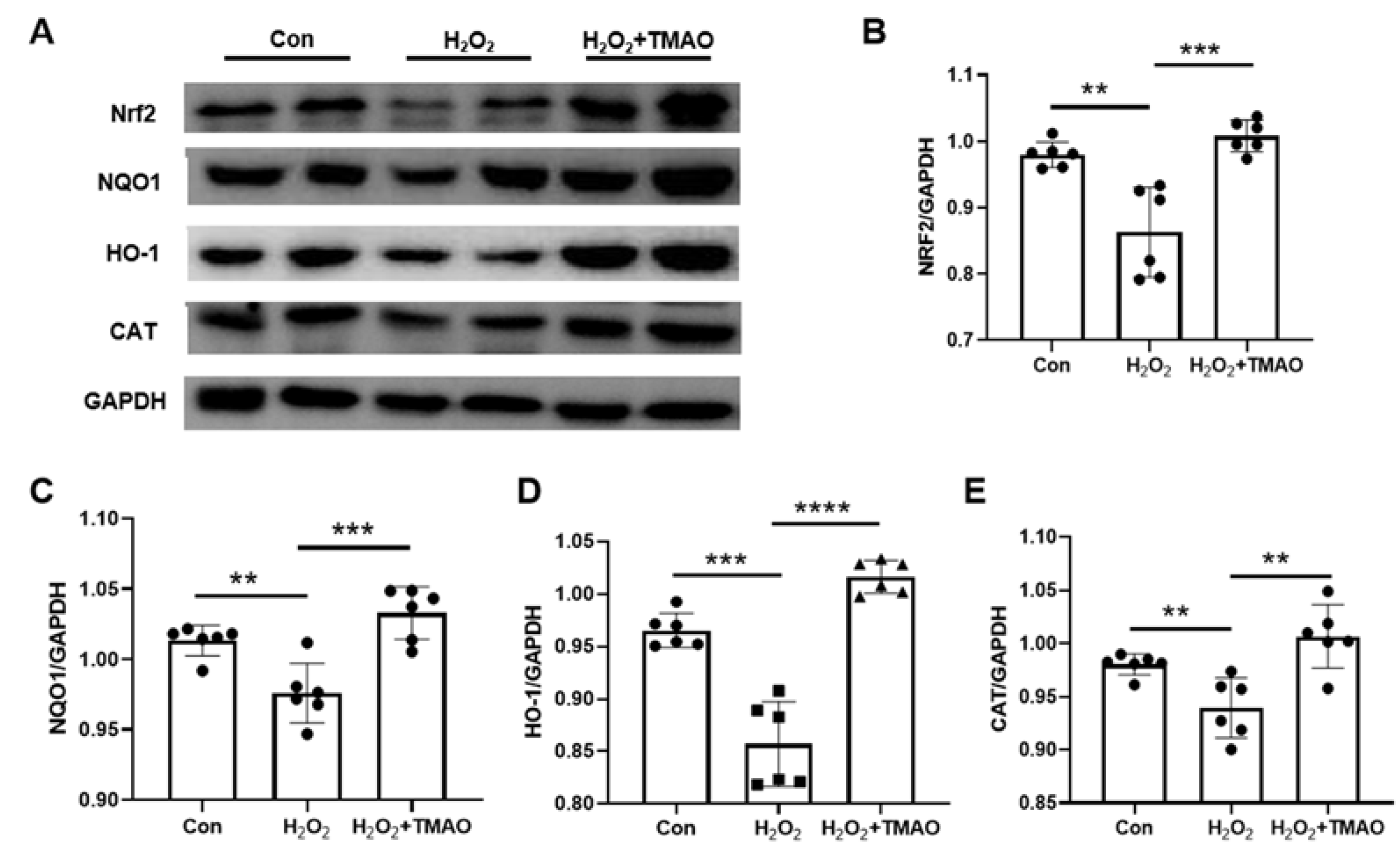
2.2. TMAO Promoted Expression Levels of Nrf2, HO-1, NQO1, and CAT in H2O2-Impaired C2C12 Cells
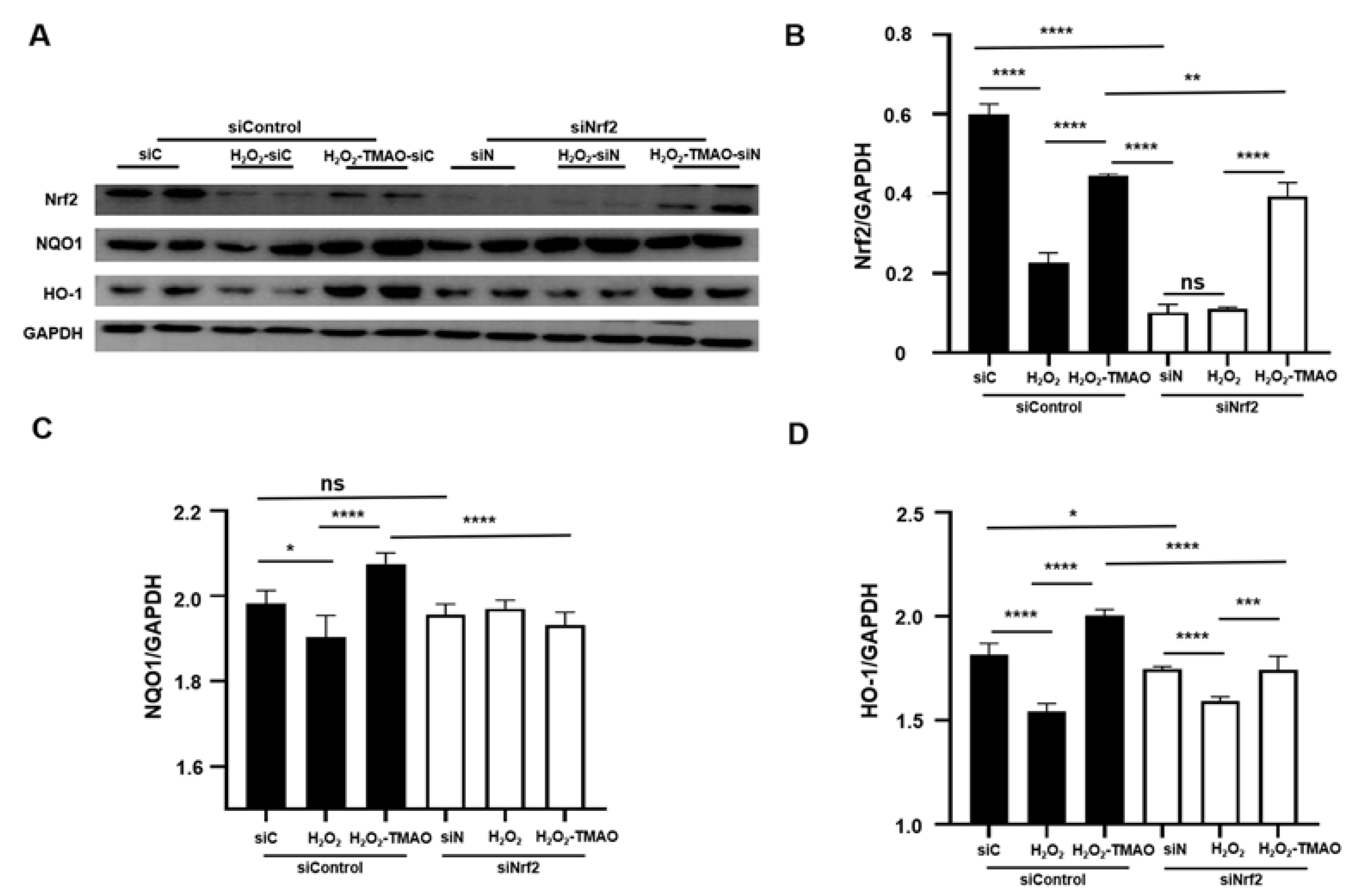
2.3. Knockdown of Nrf2 Decreased the Expression of Nrf2 and Its Downstream Genes in C2C12 Cells
2.4. TMAO Increased the Exhaustive Swimming Times of Mice
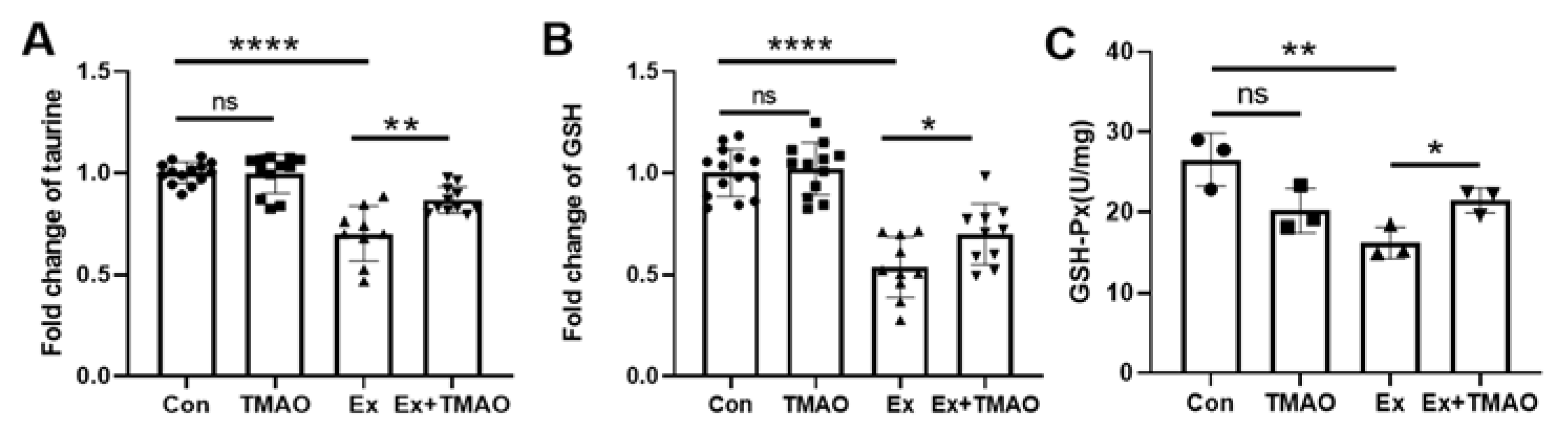
2.5. TMAO Enhanced Antioxidant Activity in the Mouse Gastrocnemius
2.6. TMAO Promoted the Expression of Nrf2 and Its Downstream Genes in the Mouse Gastrocnemius
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Treatments
4.3. Cell Transfection for Assessing the Impact of siRNA Nrf2
4.4. Cell Viability and Proliferation Assay
4.5. ROS Level
4.6. Animals and Ethical Approval
4.7. Western Blotting
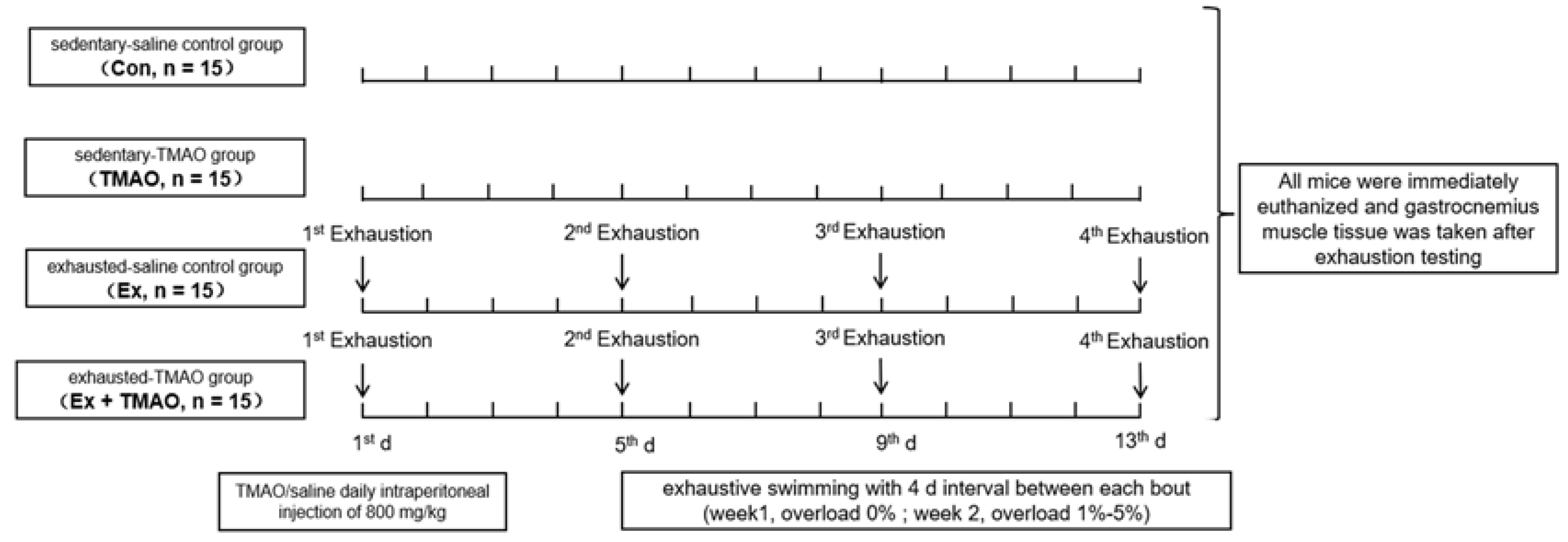
4.8. Animal Experimental Design
4.9. Preparation of NMR Samples
4.10. NMR Measurements
4.11. Measurement of GSH-Px Activity in the Mouse Gastrocnemius
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, W.W.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef]
- Oellgaard, J.; Winther, S.A.; Hansen, T.S.; Rossing, P.; von Scholten, B.J. Trimethylamine N-oxide (TMAO) as a New Potential Therapeutic Target for Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3699–3712. [Google Scholar] [CrossRef]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe—Generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; De Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; More, M.; Bellamine, A. Trimethylamine N-Oxide in Relation to Cardiometabolic Health-Cause or Effect? Nutrients 2020, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Krueger, E.S.; Beales, J.L.; Russon, K.B.; Elison, W.S.; Davis, J.R.; Hansen, J.M.; Neilson, A.P.; Hansen, J.M.; Tessem, J.S. Gut Metabolite Trimethylamine N-Oxide Protects INS-1 beta-Cell and Rat Islet Function under Diabetic Glucolipotoxic Conditions. Biomolecules 2021, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Woltjer, R.L.; McMahan, W.; Milatovic, D.; Kjerulf, J.D.; Shie, F.-S.; Rung, L.G.; Montine, K.S.; Montine, T.J. Effects of chemical chaperones on oxidative stress and detergent-insoluble species formation following conditional expression of amyloid precursor protein carboxy-terminal fragment. Neurobiol. Dis. 2007, 25, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Lupachyk, S.; Watcho, P.; Stavniichuk, R.; Shevalye, H.; Obrosova, I.G. Endoplasmic Reticulum Stress Plays a Key Role in the Pathogenesis of Diabetic Peripheral Neuropathy. Diabetes 2013, 62, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Huang, C.; Zhou, L.; Lu, R.; Zhang, Y.; Lin, D. NMR-Based Metabolomic Analysis for the Effects of Trimethylamine N-Oxide Treatment on C2C12 Myoblasts under Oxidative Stress. Biomolecules 2022, 12, 1288. [Google Scholar] [CrossRef] [PubMed]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Li, W.; Kong, A. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef]
- Alfredo, S.I.C.; Perla, M. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar]
- Goldstein, A.; Soroka, Y.; Frušić-Zlotkin, M.; Lewis, A.; Kohen, R. The bright side of plasmonic gold nanoparticles; activation of Nrf2, the cellular protective pathway. Nanoscale 2016, 8, 11748–11759. [Google Scholar] [CrossRef]
- Yamamoto, T.; Suzuki, T.; Kobayashi, A.; Wakabayashi, J.; Maher, J.; Motohashi, H.; Yamamoto, M. Physiological Significance of Reactive Cysteine Residues of Keap1 in Determining Nrf2 Activity. Mol. Cell. Biol. 2008, 28, 2758–2770. [Google Scholar] [CrossRef]
- Lee, J.-M.; Johnson, J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J. Biochem. Mol. Biol. 2004, 37, 139–143. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The Carboxy-Terminal Neh3 Domain of Nrf2 Is Required for Transcriptional Activation. Mol. Cell. Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef]
- Jarmi, T.; Agarwal, A. Heme oxygenase and renal disease. Curr. Hypertens. Rep. 2009, 11, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, R.; Jaiswal, A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase(1) gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative stress in exercise training: The involvement of inflammation and peripheral signals. Free Radic. Res. 2019, 53, 1155–1165. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Powers, S.K.; Hamilton, K. Antioxidants and exercise. Clin. Sports Med. 1999, 18, 525–536. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Lundsgaard, A.-M.; Kiens, B. Dietary Fuels in Athletic Performance. Annu. Rev. Nutr. 2019, 39, 45–73. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ji, L.L.; Leeuwenburgh, C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: A brief review. Med. Sci. Sports Exerc. 1999, 31, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Antioxidants and oxidative stress in exercise. Proc. Soc. Exp. Biol. Med. 1999, 222, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Palazzetti, S.; Rousseau, A.-S.; Richard, M.-J.; Favier, A.; Margaritis, I. Antioxidant supplementation preserves antioxidant response in physical training and low antioxidant intake. Br. J. Nutr. 2004, 91, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef] [PubMed]
- Mann, G.E.; Forman, H.J. Introduction to Special Issue on ‘Nrf2 Regulated Redox Signaling and Metabolism in Physiology and Medicine. Free Radic. Biol. Med. 2015, 88 Pt B, 91–92. [Google Scholar] [CrossRef]
- Warpsinski, G.; Smith, M.J.; Srivastava, S.; Keeley, T.P.; Siow, R.C.; Fraser, P.A.; Mann, G.E. Nrf2-regulated redox signaling in brain endothelial cells adapted to physiological oxygen levels: Consequences for sulforaphane mediated protection against hypoxia-reoxygenation. Redox Biol. 2020, 37, 101708. [Google Scholar] [CrossRef] [PubMed]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-induced oxidative stress and dietary antioxidants. Asian J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef]
- Alway, S.E.; Myers, M.J.; Mohamed, J.S. Regulation of Satellite Cell Function in Sarcopenia. Front. Aging Neurosci. 2014, 6, 246. [Google Scholar] [CrossRef]
- Kurosaka, M.; Ogura, Y.; Funabashi, T.; Akema, T. Early Growth Response 3 (Egr3) Contributes a Maintenance of C2C12 Myoblast Proliferation. J. Cell. Physiol. 2017, 232, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Enea, C.; Seguin, F.; Petitpas-Mulliez, J.; Boildieu, N.; Boisseau, N.; Delpech, N.; Diaz, V.; Eugène, M.; Dugué, B. 1H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Anal. Bioanal. Chem. 2010, 396, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Papaioannou, K.G.; Tsalis, G.; Saraslanidis, P.; Mougios, V.; Theodoridis, G.A. Monitoring the Response of the Human Urinary Metabolome to Brief Maximal Exercise by a Combination of RP-UPLC-MS and 1H NMR Spectroscopy. J. Proteome Res. 2015, 14, 4610–4622. [Google Scholar] [CrossRef] [PubMed]
- Taesuwan, S.; Cho, C.E.; Malysheva, O.V.; Bender, E.; King, J.H.; Yan, J.; Thalacker-Mercer, A.E.; Caudill, M.A. The metabolic fate of isotopically labeled trimethylamine-N-oxide (TMAO) in humans. J. Nutr. Biochem. 2017, 45, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.-J.; Liu, W.-W.; Shi, A.-W.; Gu, N. Salidroside Suppresses HUVECs Cell Injury Induced by Oxidative Stress through Activating the Nrf2 Signaling Pathway. Molecules 2016, 21, 1033. [Google Scholar] [CrossRef]
- Choi, Y.H. The cytoprotective effect of isorhamnetin against oxidative stress is mediated by the upregulation of the Nrf2-dependent HO-1 expression in C2C12 myoblasts through scavenging reactive oxygen species and ERK inactivation. Gen. Physiol. Biophys. 2016, 35, 145–154. [Google Scholar] [CrossRef]
- Rajasekaran, N.S.; Shelar, S.B.; Jones, D.P.; Hoidal, J.R. Reductive stress impairs myogenic differentiation. Redox Biol. 2020, 34, 101492. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Elkalaf, M.; Anděl, M.; Trnka, J. Low Glucose but Not Galactose Enhances Oxidative Mitochondrial Metabolism in C2C12 Myoblasts and Myotubes. PLoS ONE 2013, 8, e70772. [Google Scholar] [CrossRef] [PubMed]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The role of oxidative stress in skeletal muscle injury and regeneration: Focus on antioxidant enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Eckl, P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.J.; Jude, B.; Lanner, J.T. Intramuscular mechanisms of overtraining. Redox Biol. 2020, 35, 101480. [Google Scholar] [CrossRef] [PubMed]
- Scirè, A.; Cianfruglia, L.; Minnelli, C.; Bartolini, D.; Torquato, P.; Principato, G.; Galli, F.; Armeni, T. Glutathione compartmentalization and its role in glutathionylation and other regulatory processes of cellular pathways. BioFactors 2019, 45, 152–168. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Spriet, L.L.; Whitfield, J. Taurine and skeletal muscle function. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 96–101. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Steckling, F.M.; Lima, F.D.; Farinha, J.B.; Rosa, P.C.; Royes, L.F.F.; Cuevas, M.J.; Bresciani, G.; Soares, F.A.; González-Gallego, J.; Barcelos, R.P. Diclofenac attenuates inflammation through TLR4 pathway and improves exercise performance after exhaustive swimming. Scand. J. Med. Sci. Sports 2020, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]


| Date | Training Time (min) | Overload (%) |
|---|---|---|
| Week 1 | ||
| Day 1 | 10 | no |
| Day 2 | 20 | no |
| Day 3 | 30 | no |
| Day 4 | 40 | no |
| Day 5 | 50 | no |
| Week 2 | ||
| Day 1 | 30 | 1 |
| Day 2 | 30 | 2 |
| Day 3 | 30 | 3 |
| Day 4 | 30 | 4 |
| Day 5 | 30 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Zhou, Y.; Gong, L.; Huang, C.; Liu, X.; Lu, R.; Yu, J.; Kong, Z.; Zhang, Y.; Lin, D. Trimethylamine N-Oxide Improves Exercise Performance by Reducing Oxidative Stress through Activation of the Nrf2 Signaling Pathway. Molecules 2024, 29, 759. https://doi.org/10.3390/molecules29040759
Zou H, Zhou Y, Gong L, Huang C, Liu X, Lu R, Yu J, Kong Z, Zhang Y, Lin D. Trimethylamine N-Oxide Improves Exercise Performance by Reducing Oxidative Stress through Activation of the Nrf2 Signaling Pathway. Molecules. 2024; 29(4):759. https://doi.org/10.3390/molecules29040759
Chicago/Turabian StyleZou, Hong, Yu Zhou, Lijing Gong, Caihua Huang, Xi Liu, Ruohan Lu, Jingjing Yu, Zhenxing Kong, Yimin Zhang, and Donghai Lin. 2024. "Trimethylamine N-Oxide Improves Exercise Performance by Reducing Oxidative Stress through Activation of the Nrf2 Signaling Pathway" Molecules 29, no. 4: 759. https://doi.org/10.3390/molecules29040759
APA StyleZou, H., Zhou, Y., Gong, L., Huang, C., Liu, X., Lu, R., Yu, J., Kong, Z., Zhang, Y., & Lin, D. (2024). Trimethylamine N-Oxide Improves Exercise Performance by Reducing Oxidative Stress through Activation of the Nrf2 Signaling Pathway. Molecules, 29(4), 759. https://doi.org/10.3390/molecules29040759







