Effect of the Side-Chain Length in Polycarboxylic Superplasticizer on the Competition Adsorption in the Presence of Montmorillonite: A Density Functional Theory Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorption Energies of PCEs on CaMMT and CH
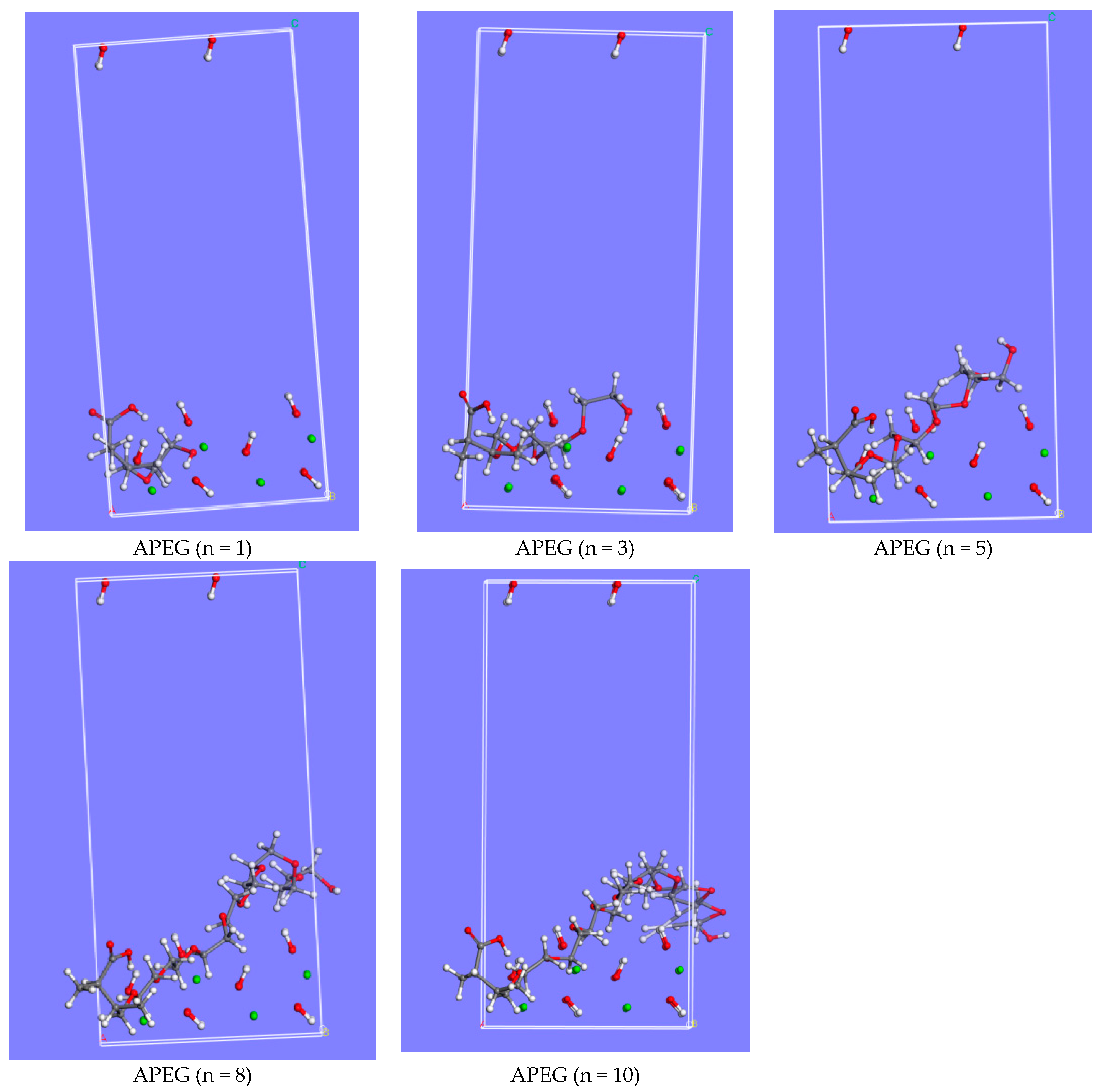
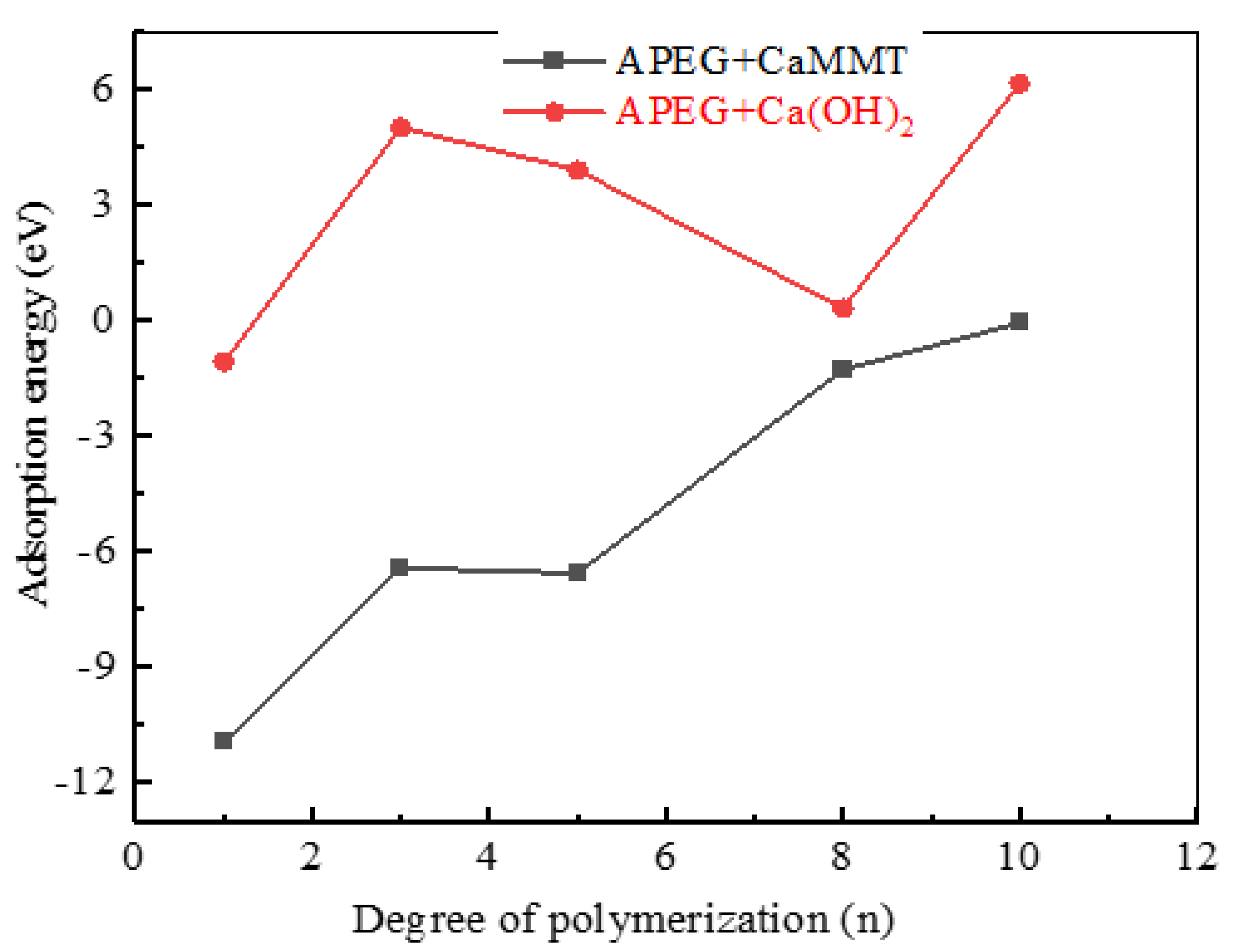
2.1.1. APEGs with Different Side-Chain Polymerization Degrees
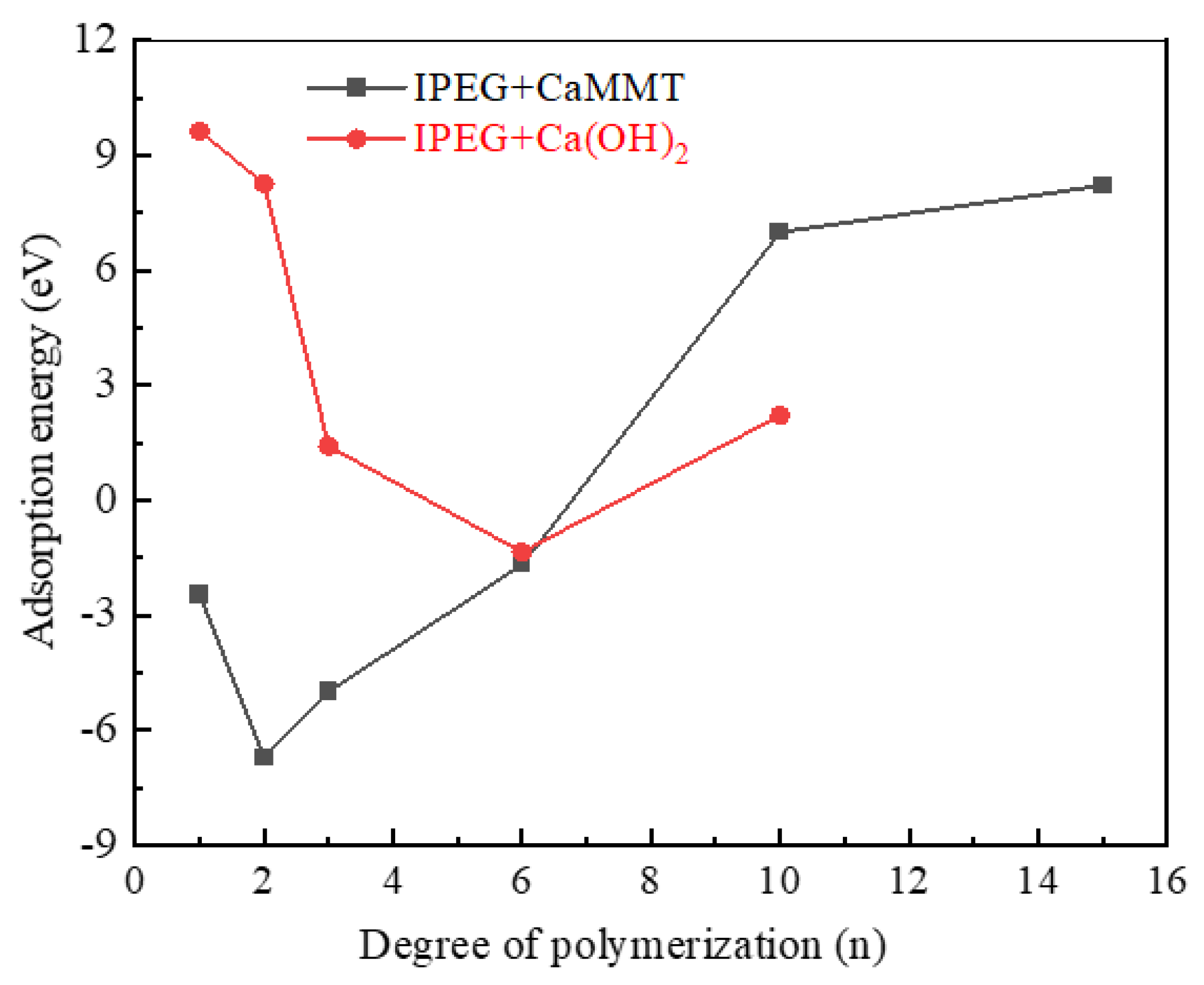
2.1.2. IPEGs with Different Side-Chain Polymerization Degrees
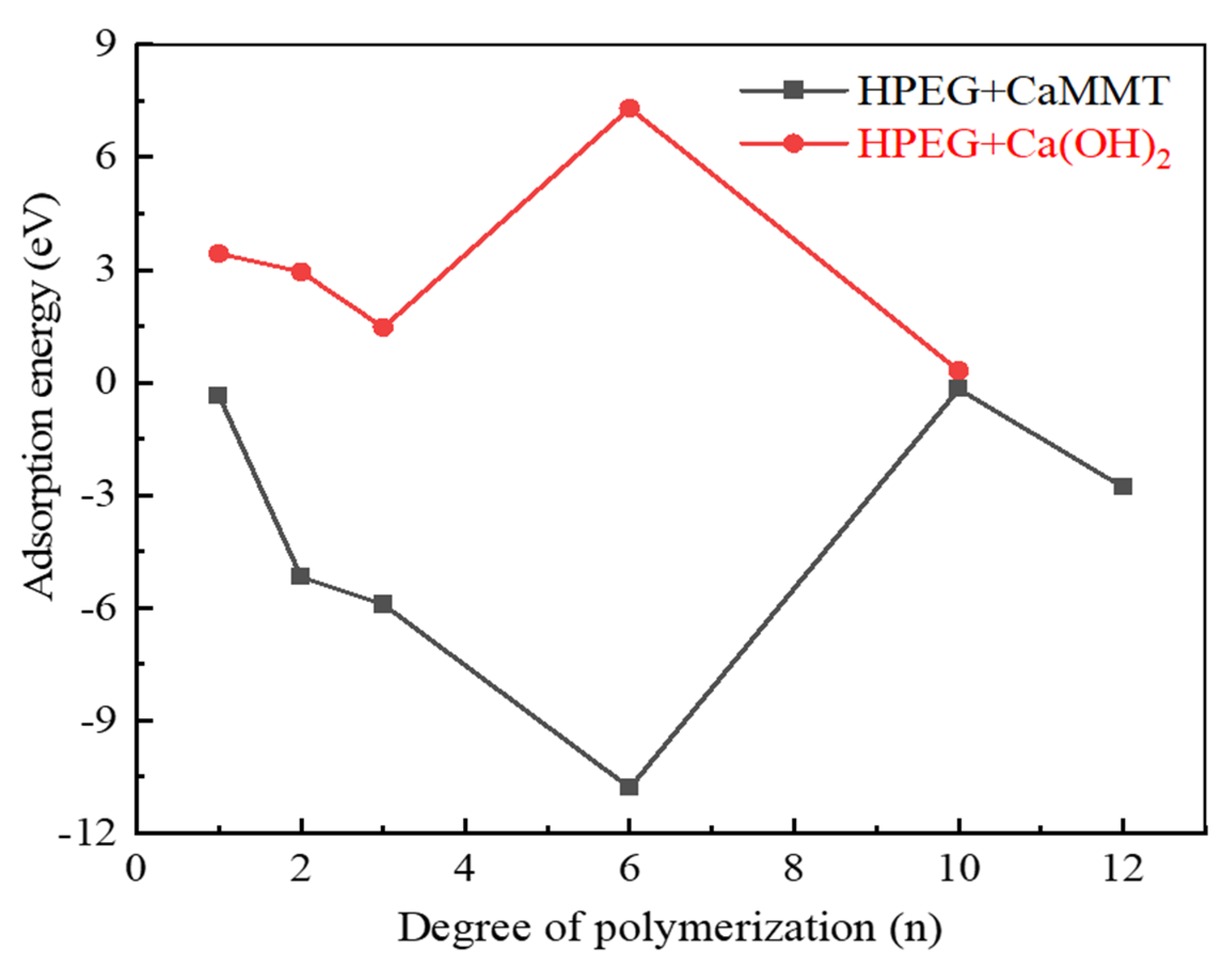
2.1.3. HPEGs with Different Side-Chain Polymerization Degrees
2.2. Electronic Structures and Charge Distribution Analysis
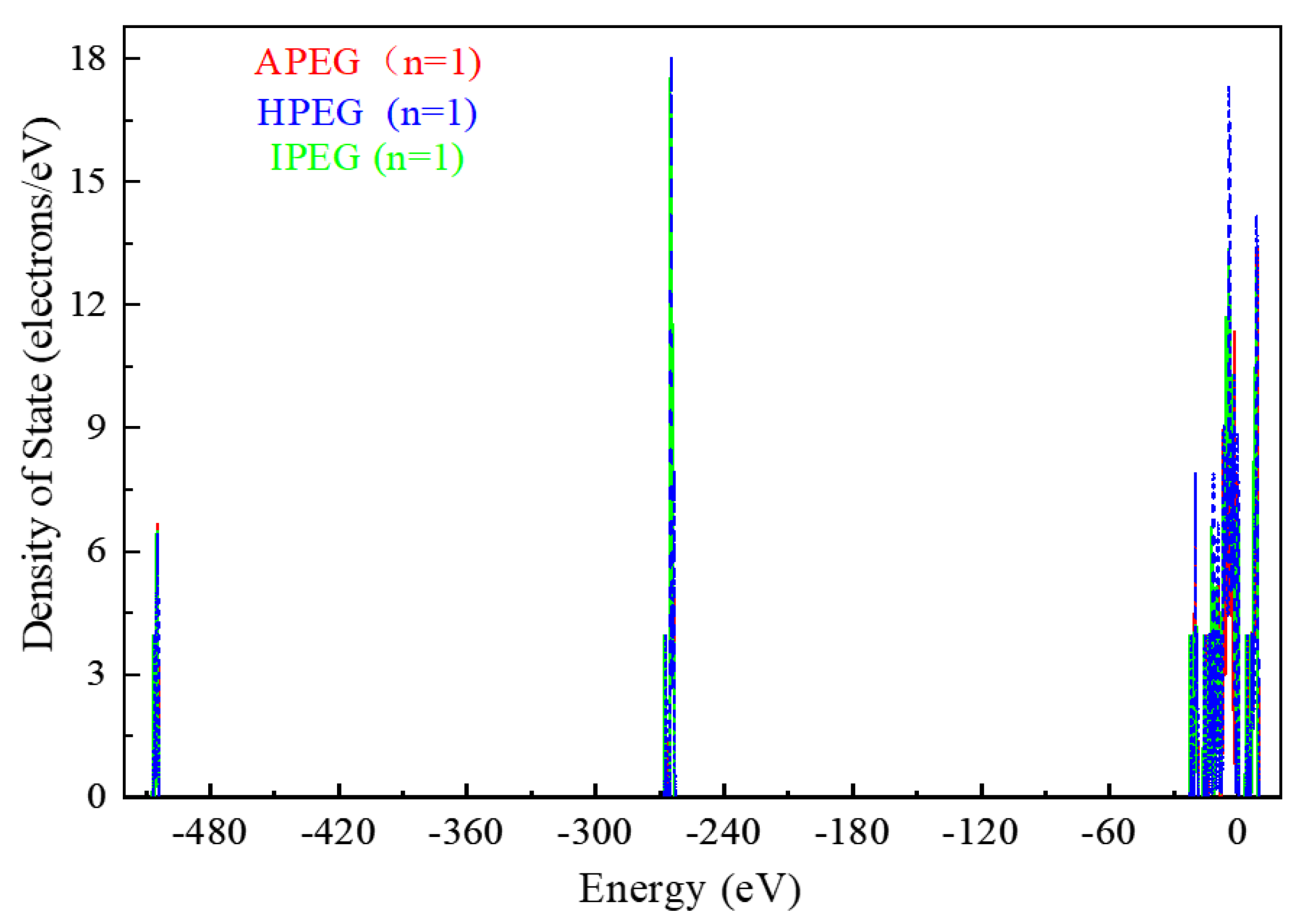
2.2.1. DOS of Different PCEs before and after Adsorption
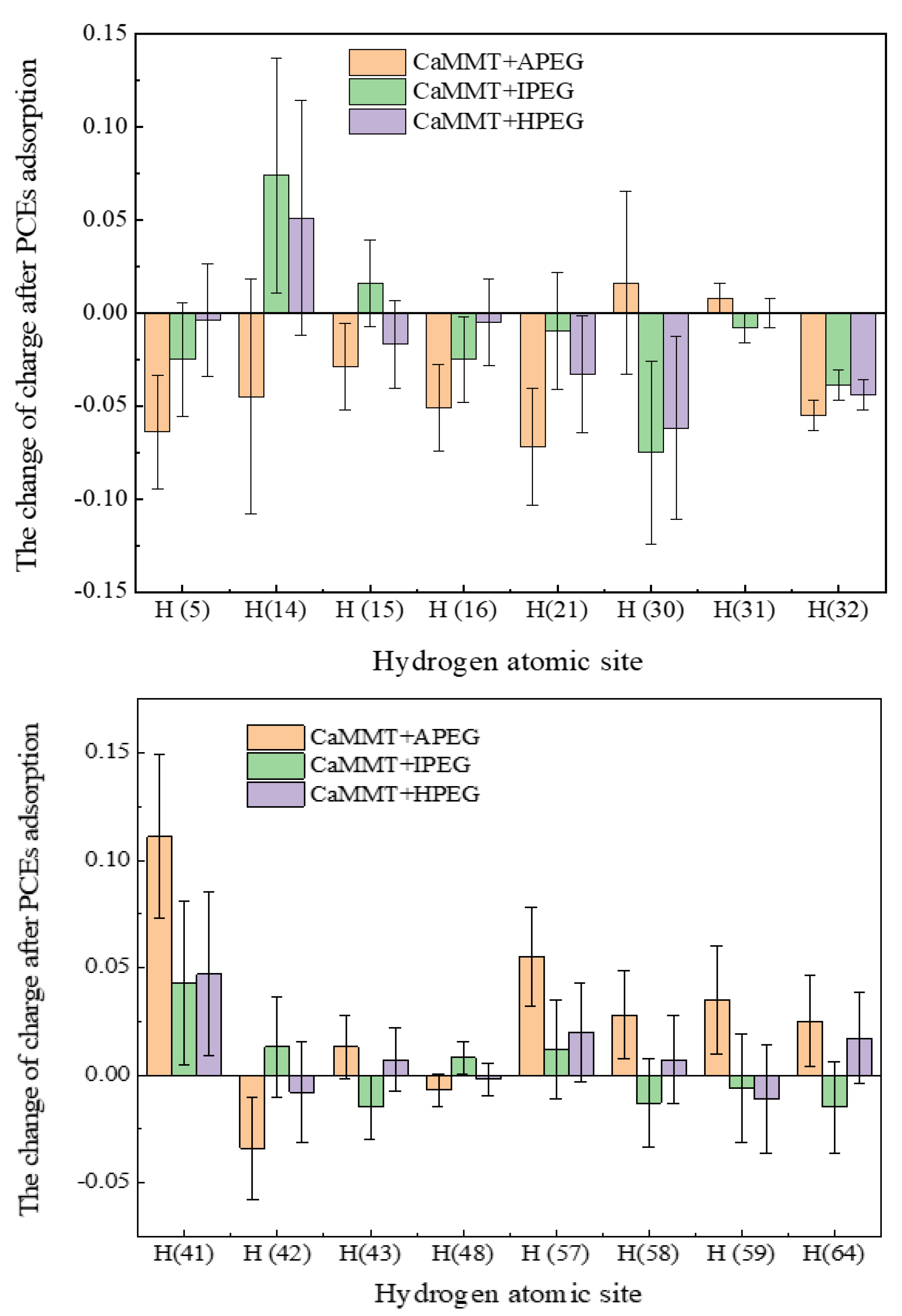
2.2.2. Charge Distribution of Different Atoms in PCEs
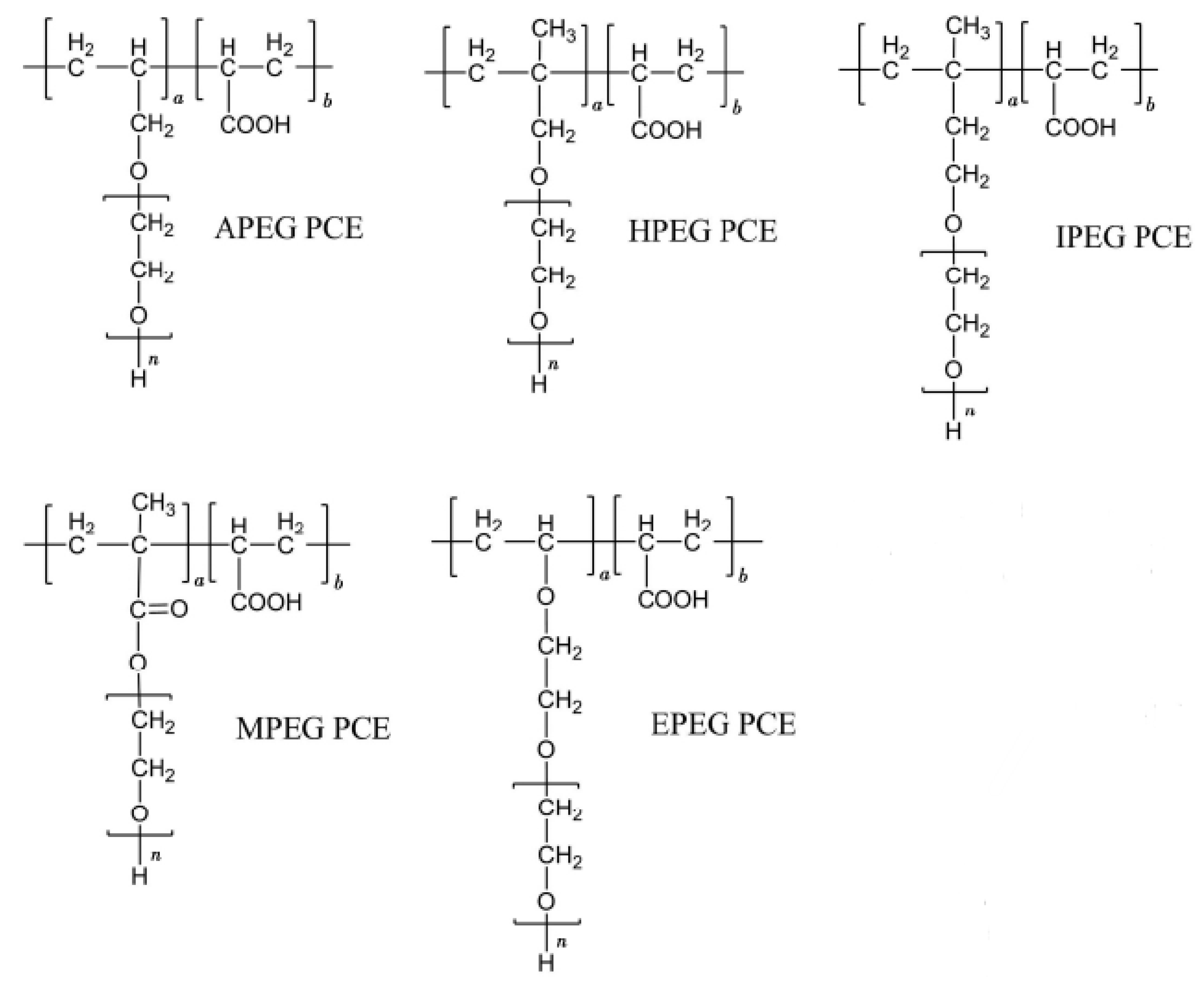
3. Models and the Calculation Method
4. Conclusions
- (1)
- Competitive adsorption between CaMMT and CH was evident when comparing the adsorption energies of PCEs with different degrees of polymerization. Given that the adsorption energy on CaMMT is lower than that on CH, the PCEs exhibited a preference for adsorption on clay.
- (2)
- For the PCEs studied in this article, the adsorption difficulty of PCEs on CaMMT increased gradually with the degree of polymerization, with a less pronounced effect observed for the adsorption of HPEGs. When n ≥ 10, the adsorption energy remained negative, indicating that the adsorption on CaMMT was more likely to occur.
- (3)
- After adsorption with PCEs, the DOS of CaMMT may undergo varying degrees of change. The peak values of APEG and IPEG are 46.65 and 46.55 electrons/eV, respectively, higher than that before the adsorption, while the peak area is also larger for APEG at n = 1. The total number of transferred electrons in APEG was 0.648, significantly exceeding those in IPEG and HPEG. The stronger changes in the density of states corresponded to more pronounced alterations in the transferred electrons, signifying a more substantial adsorption between the PCEs and CaMMT. Ultimately, these findings offer a crucial theoretical foundation for the anti-clay modification and structural design of mud-resistant PCEs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCE | Polycarboxylic superplasticizers |
| HPEG | methylallyl polyoxyethylene ether |
| DOS | density of states |
| APEG | allyl polyethylene ether |
| IPEG | isopentenol polyoxyethylene ether |
| DFT | Density functional theory |
| MS | Material Studio |
| BP | Becke–Perdew |
| GGA | generalized gradient approximation |
| TDOS | total density of states |
References
- Li, Y.; Yang, C.; Zhang, Y.; Zheng, J.; Guo, H.; Lu, M. Study on dispersion, adsorption and flow retaining behaviors of cement mortars with TPEG-type polyether kind polycarboxylate superplasticizers. Constr. Build. Mater. 2014, 64, 324–332. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, X.; Chen, X.; Guo, H.; Li, X. Progress on the Clay Tolerance of Polycarboxylate Superplasticizers. Mater. Rep. 2022, 36, 22040251. (In Chinese) [Google Scholar]
- Feng, H.; Pan, L.; Zheng, Q.; Li, J.; Xu, N.; Pang, S. Effects of molecular structure of polycarboxylate superplasticizers on their dispersion and adsorption behavior in cement paste with two kinds of stone powder. Construct. Build. Mater. 2018, 170, 182–192. [Google Scholar] [CrossRef]
- Xing, G.; Wang, W.; Fang, G. Cement dispersion performance of superplasticisers in the presence of clay and interaction between superplasticisers and clay. Adv. Cem. Res. 2017, 29, 194–205. [Google Scholar] [CrossRef]
- Kong, F.-R.; Pan, L.-S.; Wang, C.-M.; Zhang, D.-L.; Xu, N. Effects of polycarboxylate superplasticizers with different molecular structure on the hydration behavior of cement paste. Constr. Build. Mater. 2016, 105, 545–553. [Google Scholar] [CrossRef]
- Ran, Q.; Liu, J.; Yang, Y.; Shu, X.; Zhang, J.; Mao, Y. Effect of molecular weight of polycarboxylate superplasticizer on its dispersion, adsorption, and hydration of a cementitious system. J. Mater. Civ. Eng. 2016, 28, 04015184. [Google Scholar] [CrossRef]
- Wen, X.-D.; Feng, L.; Hu, D.-Y.; Wang, K.; Zhang, Z. Effect of side-chain length in polycarboxylic superplasticizer on the early-age performance of cement-based materials. Constr. Build. Mater. 2019, 211, 26–32. [Google Scholar] [CrossRef]
- Tang, S. Improvements on Effect of Mud on Polycarboxylate Superplasticizer in Concrete. Master’s Thesis, Chongqing University, Chongqing, China, 2018. (In Chinese). [Google Scholar]
- Chen, G.; Lei, J.; Du, Y.; Du, X.; Chen, X. A polycarboxylate as a superplasticizer for montmorillonite clay in cement: Adsorption and tolerance studies. Arab. J. Chem. 2018, 11, 747–755. [Google Scholar] [CrossRef]
- Li, X.K.; Zheng, D.F.; Zheng, T.; Lin, X.L.; Lou, H.M.; Qiu, X.Q. Enhancement clay tolerance of PCE by lignin-based polyoxyethylene ether in montmorillonite-contained paste. J. Ind. Eng. Chem. 2017, 49, 168–175. [Google Scholar] [CrossRef]
- Zhao, H.; Liao, B.; Nian, F.; Wang, K.; Guo, Y.; Pang, H. Investigation of the Clay Sensitivity and Cement Hydration Process of Modified HPEG-type Polycarboxylate Superplasticizers. J. Appl. Polym. Sci. 2018, 135, 46572. [Google Scholar] [CrossRef]
- Teng, H.; He, Z.; Meiben, G.; Zhang, J. DFT Study on the Compatibility Between Bentonite Clay Mineral and Hydration Products With the Polycarboxylate Water Reducer in the Cement Hydration Process. Front. Earth Sci. 2022, 10, 890968. [Google Scholar] [CrossRef]
- Liu, Z. Research on Synthesis, Properties and Mechanism of Different Carboxyl Density and Functionallizing Polycarboxylate Superplasticizer. Ph.D. Thesis, China University of Mining & Technology, Beijing, China, 2013. (In Chinese). [Google Scholar]
- Qu, H.; Fu, C.E.; Yang, W.; Yang, Z.; Zhang, L. Preparation, application and water reducing mechanism of a novel fluorescent superplasticizer with improved flow retaining ability and clay tolerance. J. Disper. Sci. Technol. 2018, 39, 1829–1839. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Zheng, Y.; Wang, Z.; Cui, S.; Lan, M.; Li, H. Novel designs of polycarboxylate superplasticizers for improving resistance in clay-contaminated concrete. J. Ind. Eng. Chem. 2017, 55, 80–90. [Google Scholar] [CrossRef]
- Xing, G.; Wang, W.; Xu, J. Grafting tertiary amine groups into the molecular structures of polycarboxylate superplasticizers lowers their clay sensitivity. RSC Adv. 2016, 6, 106921–106927. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Ma, B.; Gu, B.; Zou, F. Effect of sodium gluconate on clay tolerance of polycarboxylate superplasticiser. Adv. Cem. Res. 2017, 29, 278–286. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.; Scherb, S.; Köberl, M.; Thienel, K.-C. Acceleration of cement blended with calcined clays. Constr. Build. Mater. 2020, 245, 118439. [Google Scholar] [CrossRef]
- Erzengin, S.G.; Kaya, K.; Özkorucuklu, S.P.; Özdemir, V.; Yıldırım, G. The properties of cement systems superplasticized with methacrylic ester-based polycarboxylates. Constr. Build. Mater. 2018, 166, 96–109. [Google Scholar] [CrossRef]
- Mudaser, A.H.; Shahzad, K.M.; Al-Harthi, M. Polymeric and low molecular weight shale inhibitors: A review. Fuel 2019, 251, 187–217. [Google Scholar]
- Yong, D. Study on Modification and Chemical Mechanism of Polycarboxylate Superplasticizers for Enhanced Clay Tolerance. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2019. (In Chinese). [Google Scholar]
- Ma, Y.; Shi, C.; Lei, L.; Sha, S.; Zhou, B.; Liu, Y.; Xiao, Y. Research progress on polycarboxylate based superplasticizers with tolerance to clays-a review. Constr. Build. Mater. 2020, 255, 119386. [Google Scholar] [CrossRef]
- Peng, C.; Min, F.; Liu, L. Effect of pH on the Adsorption of Dodecylamine on Montmorillonite: Insights from Experiments and Molecular Dynamics Simulations. App. Surf. Sci. 2017, 425, 996–1005. [Google Scholar] [CrossRef]
- He, Z.; Pei, X.; Zhang, J.; Huang, R.; Deng, M.; Gao, Y.; Gao, M.; Xie, L. Molecular simulation for the relationship between the functional groups of chemical admixtures and cement hydration product Ca (OH)2 in the grouting process. Ecol. Indic. 2023, 153, 110404. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, G.; Wang, P.; Liu, X.; Zhang, G. Study on the retardation of re-dispersible polymer powders on cement hydration based on morphologies of Ca(OH)2. J. Chin. Electron Microsc. Soc. 2016, 35, 490–495. (In Chinese) [Google Scholar]
- Song, Y.; Zhang, Y.; Shen, S.; Pan, C.; Yan, D.; Wang, Z. Effects of Ca(OH)2 on the reinforcement corrosion of sulfoaluminate cement mortar. Mater. Struct. 2023, 56, 26. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, Y.; Li, R. Specific molecular design of polycarboxylate polymers exhibiting optimal compatibility with clay contaminants in concrete. Cem. Concr. Res. 2021, 147, 106504. [Google Scholar] [CrossRef]
- Koteeswaran, S.; Pashin, J.C.; Ramsey, J.D.; Clark, P.E. Quantitative characterization of polyacrylamide–shale interaction under various saline conditions. Pet. Sci. 2017, 14, 586–596. [Google Scholar] [CrossRef]
- Babaei, Z.; Najafi Chermahini, A.; Dinari, M.; Dinari, M. Glycerol Adsorption and Mechanism of Dehydration to Acrolein over TiO2 Surface: A Density Functional Theory Study. J. Colloid Interface Sci. 2020, 563, 1–7. [Google Scholar] [CrossRef]
- Kürkçü, C.; Merdan, Z.; Yamçiçier, Ҫ. Structural phase transition and electronic properties of CaO under high pressure. Mater. Res. Express 2018, 5, 125903. [Google Scholar] [CrossRef]
- Taifan, W.E.; Bučko, T.; Baltrusaitis, J. Catalytic conversion of ethanol to 1,3-butadiene on MgO: A comprehensive mechanism elucidation using DFT calculations. J. Catal. 2017, 346, 78–91. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, J.; Kang, T. Effect of water on methane adsorption on the kaolinite (0 0 1) surface based on molecular simulations. Appl. Surf. Sci. 2018, 439, 792–800. [Google Scholar] [CrossRef]
- Peng, C.; Zhong, Y.; Min, F. Adsorption of alkylamine cations on montmorillonite (001) surface: A density functional theory study. Appl. Clay Sci. 2018, 152, 249–258. [Google Scholar] [CrossRef]
- Chen, J.; Min, F.-F.; Liu, L.; Liu, C.; Lu, F. Experimental investigation and DFT calculation of different amine/ammonium salts adsorption on kaolinite. Appl. Surf. Sci. 2017, 419, 241–251. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Guo, J.; Zhang, L. Interaction between low rank coal and kaolinite particles: A DFT simulation. Appl. Surf. Sci. 2018, 456, 215–220. [Google Scholar] [CrossRef]
- Fan, Y.; Zhuo, Y.; Li, L. SeO2 adsorption on CaO surface: DFT and experimental study on the adsorption of multiple SeO2 molecules. App. Surf. Sci. 2017, 420, 465–471. [Google Scholar] [CrossRef]
- Sodeifian, G.; Nikooamal, H.R.; Yousefi, A.A. Molecular dynamics study of epoxy/clay nanocomposites: Rheology and molecular confinement. J. Polym. Res. 2012, 19, 9897. [Google Scholar] [CrossRef]
- Weng, Z.; Song, Y.; Cheng, C.; Tong, D.; Xu, M.; Wang, M.; Xie, Y. Possible underestimation of the coal-fired power plants to air pollution in China. Resour. Conserv. Recycl. 2023, 198, 107208. [Google Scholar] [CrossRef]
- Orazi, V.; Juan, A.; González, E.A.; Marchetti Jorge, M.; Jasen, P.V. DFT study of ethanol adsorption on CaO (0 0 1) surface. App. Surf. Sci. 2020, 500, 144254. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Liu, Y.; Qu, Y. Adsorption mechanisms of metal ions on the potassium dihydrogen phosphate (1 0 0) surface: A density functional theory-based investigation. J. Colloid Interface Sci. 2018, 522, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, C.; Zou, C.; Zhang, Y. DFT Study of Se and SeO2 adsorbed on CaO (001) Surface: Role of Oxygen. Appl. Surf. Sci. 2020, 510, 145488. [Google Scholar] [CrossRef]
- Sodeifian, G. Non-Linear Rheology of Polymer Melts: Constitutive Equations, Rheological Properties of Polymer Blends, Shear Flow, Sliding Plate Rheometers; LAP Lambert Academic: London, UK, 2011. [Google Scholar]
- Logsdail, A.J.; Mora-Fonz, D.; Scanlon, D.O.; Catlow CR, A.; Sokol, A.A. Structural, energetic and electronic properties of (100) surfaces for alkaline earth metal oxides as calculated with hybrid density functional theory. Surf. Sci. 2015, 642, 58–65. [Google Scholar] [CrossRef]

| Before and after Adsorption | ||||
|---|---|---|---|---|
| Atoms | CaMMT | CaMMT + APEG (n = 1) | CaMMT + IPEG (n = 1) | CaMMT + HPEG (n = 1) |
| H (5) | 0.479 | 0.415 | 0.454 | 0.475 |
| H (14) | 0.442 | 0.397 | 0.516 | 0.493 |
| H (15) | 0.003 | −0.026 | 0.019 | −0.014 |
| H (16) | 0.496 | 0.445 | 0.471 | 0.491 |
| H (21) | 0.479 | 0.407 | 0.469 | 0.446 |
| H (30) | 0.442 | 0.458 | 0.367 | 0.380 |
| H (31) | 0.003 | 0.011 | −0.005 | 0.003 |
| H (32) | 0.496 | 0.441 | 0.457 | 0.452 |
| H (41) | 0.404 | 0.515 | 0.447 | 0.451 |
| H (42) | −0.000 | −0.034 | 0.013 | −0.008 |
| H (43) | 0.421 | 0.434 | 0.406 | 0.428 |
| H (48) | −0.003 | −0.010 | 0.005 | −0.005 |
| H (57) | 0.404 | 0.459 | 0.416 | 0.424 |
| H (58) | −0.000 | 0.028 | −0.013 | 0.007 |
| H (59) | 0.421 | 0.456 | 0.415 | 0.410 |
| H (64) | −0.003 | 0.022 | −0.018 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Huang, T.; Gao, M.; Kong, D.; Li, M. Effect of the Side-Chain Length in Polycarboxylic Superplasticizer on the Competition Adsorption in the Presence of Montmorillonite: A Density Functional Theory Study. Molecules 2024, 29, 752. https://doi.org/10.3390/molecules29040752
He Z, Huang T, Gao M, Kong D, Li M. Effect of the Side-Chain Length in Polycarboxylic Superplasticizer on the Competition Adsorption in the Presence of Montmorillonite: A Density Functional Theory Study. Molecules. 2024; 29(4):752. https://doi.org/10.3390/molecules29040752
Chicago/Turabian StyleHe, Zhihao, Teng Huang, Meiben Gao, Desong Kong, and Meng Li. 2024. "Effect of the Side-Chain Length in Polycarboxylic Superplasticizer on the Competition Adsorption in the Presence of Montmorillonite: A Density Functional Theory Study" Molecules 29, no. 4: 752. https://doi.org/10.3390/molecules29040752
APA StyleHe, Z., Huang, T., Gao, M., Kong, D., & Li, M. (2024). Effect of the Side-Chain Length in Polycarboxylic Superplasticizer on the Competition Adsorption in the Presence of Montmorillonite: A Density Functional Theory Study. Molecules, 29(4), 752. https://doi.org/10.3390/molecules29040752




