Luminescent Properties of (Ca7ZrAl6O18-Ca3Al2O6-CaZrO3):Eu3+ Composite Ceramics and Tracing in the Hydration Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Phase Composition (XRD) and Microstructural Characterizations (SEM-EDS)
2.2. Structural Characterization (FT-IR)
2.3. Particle Size Distribution of Eu-Containing Ceramic Powders
2.4. Luminescent Properties of Eu-Containing Ceramic Powders
2.5. Tracing in the Hydration Process of Eu-Containing Ceramic Powder via Luminescence and XRD Techniques
3. Materials and Methods
3.1. Synthesis of Eu-Containing Composite Ceramics, Cement Powders, and Methods of Investigation
3.2. Cement Paste Preparation and Methods of Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madej, D.; Rajska, M.; Kruk, A. Synthesis and hydration behaviour of calcium zirconium aluminate powders by modifying co-precipitation method. Ceram. Int. 2020, 46, 2373–2383. [Google Scholar] [CrossRef]
- Madej, D.; Kruk, A. Tracing the early and long-term hydration of fast setting cementitious material (Ca7ZrAl6O18) and calcium aluminate cement (CAC) pastes by means of electrochemical impedance spectroscopy and other methods. Constr. Build. Mater. 2018, 164, 94–102. [Google Scholar] [CrossRef]
- Szczerba, J.; Madej, D.; Dul, K.; Bobowska, P. Ca7ZrAl6O18 acting as a hydraulic and ceramic bonding in the MgO-CaZrO3 dense refractory composite. Ceram. Int. 2014, 40, 7315–7320. [Google Scholar] [CrossRef]
- Madej, D.; Sieroń, K.; Kruk, A. Synthesis and performance of aluminous cements containing zirconium and strontium as al-ternatives to the calcium aluminate cements designed for the production of high performance refractories. Cem. Concr. Compos. 2022, 130, 104518. [Google Scholar] [CrossRef]
- Madej, D. Synthesis, formation mechanism and hydraulic activity of novel composite cements belonging to the system CaO-Al2O3-ZrO2. J. Therm. Anal. Calorim. 2017, 130, 1913–1924. [Google Scholar] [CrossRef]
- Murayama, Y.; Watanabe, S.; Akase, M.; Matsui, K. Effects of composition and reduction conditions on persistent luminescence of SrAl2O4: Eu,Dy prepared via a solid-state reaction. J. Lumin. 2022, 251, 119248. [Google Scholar] [CrossRef]
- Kumar, K.; Singh, A.K.; Rai, S.B. Laser excited long lasting luminescence in CaAl2O4: Eu3+/Eu2++Nd3+ phosphor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 212–218. [Google Scholar] [CrossRef]
- Freeda, M.; Suresh, G. Structural and Luminescent properties of Eu-doped CaAl2O4 Nanophosphor by sol-gel method. Mater. Today Proc. 2017, 4 Pt B, 4260–4265. [Google Scholar] [CrossRef]
- Teng, X.; Zhuang, W.; He, H. Influence of La3+ and Dy3+ on the properties of the long afterglow phosphor CaAl2O4: Eu2+, Nd3+. Rare Met. 2008, 27, 335–339. [Google Scholar] [CrossRef]
- Peng, H.; Gao, Q.; Meng, L.; Zhang, L.; Pang, Q.; Liang, L. Sol-gel method and optical properties of Ca12Al14O32F2: Eu3+ red phosphors. J. Rare Earths 2015, 33, 927–932. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Wang, T.; Hao, W. Preparation and characterization of a new long afterglow indigo phosphor Ca12Al14O33: Nd, Eu. Mater. Lett. 2003, 57, 4315–4318. [Google Scholar] [CrossRef]
- Verma, N.; Singh, K.C.; Marí, B.; Mollar, M.; Jindal, J. Luminescence properties of CaAl2O4: Eu3+, Gd3+ phosphors synthesized by combustion synthesis method. Acta Phys. Pol. A 2017, 132, 1261–1264. [Google Scholar] [CrossRef]
- Kalita, J.; Chithambo, M. Temperature dependence of persistent luminescence in CaAl2O4: Eu2+, Nd3+ related to beta irradiation and optical excitation. J. Lumin. 2019, 206, 27–32. [Google Scholar] [CrossRef]
- Wiglusz, R.; Grzyb, T.; Lukowiak, A.; Bednarkiewicz, A.; Lis, S.; Strek, W. Tuning luminescence properties of Eu3+ doped CaAl2O4 nanophosphores with Na+ co-doping. J. Lumin. 2013, 133, 102–109. [Google Scholar] [CrossRef]
- Kaur, P.; Khanna, A. Structural, thermal and light emission properties of Eu, Sm, Dy, Er and Mn doped CaAl2O4 and SrAl2O4. Ceram. Int. 2021, 47 Pt A, 14655–14664. [Google Scholar] [CrossRef]
- Mabelane, T.S.; Koao, L.F.; Motloung, S.V.; Motaung, T.E.; Kroon, R.E.; Mhlongo, M.R. Effect of annealing period on the structure, morphology, and optical properties of CaAl2O4: 0.1% Sm3+ prepared by citrate sol-gel method. J. Mol. Struct. 2022, 1260, 132751. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, S.; Jayasimhadri, M. Luminescence properties of orange emitting CaAl4O7: Sm3+ phosphor for solid state lighting applications. Solid State Sci. 2020, 101, 106049. [Google Scholar] [CrossRef]
- Puchalska, M.; Zych, E. The effect of charge compensation by means of Na+ ions on the luminescence behavior of Sm3+-doped CaAl4O7 phosphor. J. Lumin. 2012, 132, 826–831. [Google Scholar] [CrossRef]
- Afshani, J.; Delgado, T.; Paveliuc, G.; Hagemann, H. Luminescence spectroscopy of CaAl12O19: Eu3+ and SrAl12O19:Eu3+ nanoparticles. J. Lumin. 2022, 246, 118805. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, G. Influence of Mg2+ on luminescence efficiency and charge compensating mechanism in phosphor CaAl12O19: Mn4+. J. Lumin. 2011, 131, 465–468. [Google Scholar] [CrossRef]
- Burek, K.; Dengler, J.; Emmerling, F.; Feldmann, I.; Kumke, M.U.; Stroh, J. Lanthanide luminescence revealing the phase compo-sition in hydrating cementitious systems. Chem. Open 2019, 8, 1441–1452. [Google Scholar]
- Pointeau, I.; Piriou, B.; Fedoroff, M.; Barthes, M.-G.; Marmier, N.; Fromage, F. Sorption Mechanisms of Eu3+ on CSH Phases of Hydrated Cements. J. Colloid Interface Sci. 2001, 236, 252–259. [Google Scholar] [CrossRef]
- Burek, K.; Krause, F.; Schwotzer, M.; Nefedov, A.; Suessmuth, J.; Haubitz, T.; Kumke, M.U.; Thissen, P. Hydrophobic Properties of Calcium-Silicate Hydrates Doped with Rare-Earth Elements. ACS Sustain. Chem. Eng. 2018, 6, 14669–14678. [Google Scholar] [CrossRef]
- dos Santos, V.; Tonoli, G.H.D.; Mármol, G.; Frías, M.; Savastano, H. Monitoring the dynamics of Portland cement hydration through photoluminescence and other correlated spectroscopy techniques. Constr. Build. Mater. 2020, 252, 119073. [Google Scholar] [CrossRef]
- Meng, W.; Bachilo, S.M.; Parol, J.; Nagarajaiah, S.; Weisman, R.B. Near-infrared photoluminescence of Portland cement. Sci. Rep. 2022, 12, 1197. [Google Scholar] [CrossRef]
- Koehler, A.; Neubauer, J.; Goetz-Neunhoeffer, F. How C12A7 influences the early hydration of calcium aluminate cement at different temperatures. Cem. Concr. Res. 2022, 162, 106972. [Google Scholar] [CrossRef]
- Goergens, J.; Belli, R.; Schulbert, C.; Goetz-Neunhoeffer, F. Influence of different CA2/CA-ratios on hydration degree, AH3 content and flexural strength investigated for a binder formulation of calcium aluminate cement with calcite. Cem. Concr. Res. 2023, 165, 107090. [Google Scholar] [CrossRef]
- Goergens, J.; Manninger, T.; Goetz-Neunhoeffer, F. In-situ XRD study of the temperature-dependent early hydration of calcium aluminate cement in a mix with calcite. Cem. Concr. Res. 2020, 136, 106160. [Google Scholar] [CrossRef]
- Abd-El-Raoof, F.; Youssef, H.; El-Sokkary, T.; El-Shakour, Z.A.; Tawfik, A. Fabrication and characterization of calcium aluminates cement via microwave-hydrothermal route: Mayenite, katoite, and hydrocalumite. Constr. Build. Mater. 2023, 401, 132988. [Google Scholar] [CrossRef]
- Idrees, M.; Ekincioglu, O.; Sonyal, M.S. Hydration behavior of calcium aluminate cement mortars with mineral admixtures at different curing temperatures. Constr. Build. Mater. 2021, 285, 122839. [Google Scholar] [CrossRef]
- Kruk, A.; Madej, D. A new approach to time-resolved electrochemical impedance spectroscopy using the Impedance Camera to track fast hydration processes in cement-based materials. Measurement 2022, 205, 112199. [Google Scholar] [CrossRef]
- Amer, A.A.; El-Didamony, H.; El-Sokkary, T.M.; Wahdan, M.I. Synthesis and characterization of some calcium aluminate phases from nano-size starting materials. Boletín Soc. Española Cerámica Y Vidr. 2020, 61, 98–106. [Google Scholar] [CrossRef]
- Parauha, Y.R.; Dhoble, S. Thermoluminescence study and evaluation of trapping parameter of rare earth activated Ca3Al2O6: RE (RE = Eu2+, Ce3+) phosphors. J. Mol. Struct. 2020, 1211, 127993. [Google Scholar] [CrossRef]
- Suarez, M.; Rocha, V.; Fernandez, A.; Menendez, J.; Torrecillas, R. Synthesis and processing of spinel powders for transparent ceramics. Ceram. Int. 2014, 40, 4065–4069. [Google Scholar] [CrossRef][Green Version]
- Yu, Z.Q.; Li, C.; Zhang, N. Size dependence of the luminescence spectra of nanocrystal alumina. J. Lumin. 2002, 99, 29–34. [Google Scholar] [CrossRef]
- Lenus, A.J.; Rajan, K.G.; Yousuf, M.; Sornadurai, D.; Purniah, B. Luminescence behaviour of rare earth doped alkaline earth aluminates prepared by the halide route. Mater. Lett. 2002, 54, 70–74. [Google Scholar] [CrossRef]
- Wen, H.; Jia, G.; Duan, C.-K.; Tanner, P.A. Understanding Eu3+ emission spectra in glass. Phys. Chem. Chem. Phys. 2010, 2, 9933–9937. [Google Scholar] [CrossRef]
- Mondal, P.; Jeffery, J.W. The crystal structure of tricalcium aluminate, Ca3Al2O6. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 689–697. [Google Scholar] [CrossRef]
- Fukuda, K.; Iwata, T.; Nishiyuki, K. Crystal structure, structural disorder, and hydration behavior of calcium zirconium alu-minate, Ca7ZrAl6O18. Chem. Mater. 2007, 19, 3726–3731. [Google Scholar] [CrossRef]
- Zikmund, T.; Bulíř, J.; Novotný, M.; Jiříček, P.; Houdková, J.; Lančok, J. Electric and magnetic dipole emission of Eu3+: Effect of proximity to a thin aluminum film. J. Lumin. 2022, 246, 118778. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A.; Nieuwpoort, W.C. On the Eu3+ fluorescence in mixed metal oxides: Part I-The crystal structure sensitivity of the intensity ratio of electric and magnetic dipole emission. J. Phys. Chem. Solids 1966, 27, 1587–1592. [Google Scholar] [CrossRef]
- Parchura, A.K.; Ningthoujam, R.S. Behaviour of electric and magnetic dipole transitions of Eu3+, 5D0 → 7F0 and Eu-O charge transfer band in Li+ co-doped YPO4:Eu3+. RSC Adv. 2012, 2, 10859–10868. [Google Scholar] [CrossRef]
- Cortecchia, D.; Mróz, W.; Folpini, G.; Borzda, T.; Leoncino, L.; Alvarado-Leaños, A.L.; Speller, E.M.; Petrozza, A. Layered per-ovskite doping with Eu3+ and β-diketonate Eu3+ complex. Chem. Mater. 2021, 33, 2289–2297. [Google Scholar] [CrossRef]
- Singh, D.K.; Baitha, P.K.; Manam, J. Enhancement of luminescence intensity and spectroscopic analysisof Eu3+-activated and Li+ charge-compensated CaTiO3 colortunable phosphors for solid-state lighting. Appl. Phys. A Mater. Sci. Process. 2016, 122, 668. [Google Scholar] [CrossRef]
- Madej, D.; Kruk, A. Monitoring hydration of Sr-doped calcium zirconium aluminate (Ca,Sr)7ZrAl6O18 cement via electrochemical impedance spectroscopy (EIS) and supported techniques. Constr. Build. Mater. 2019, 206, 307–320. [Google Scholar] [CrossRef]

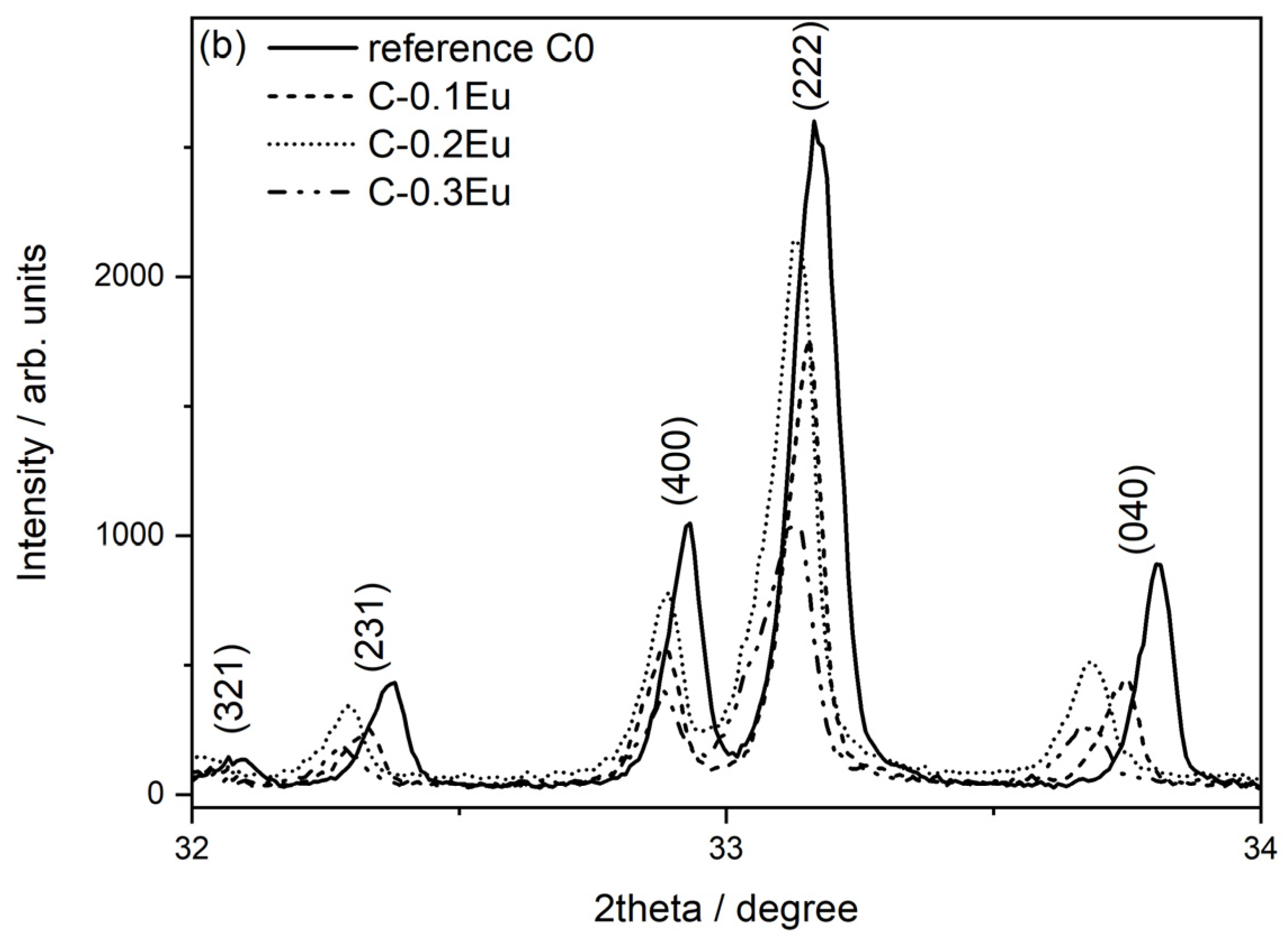

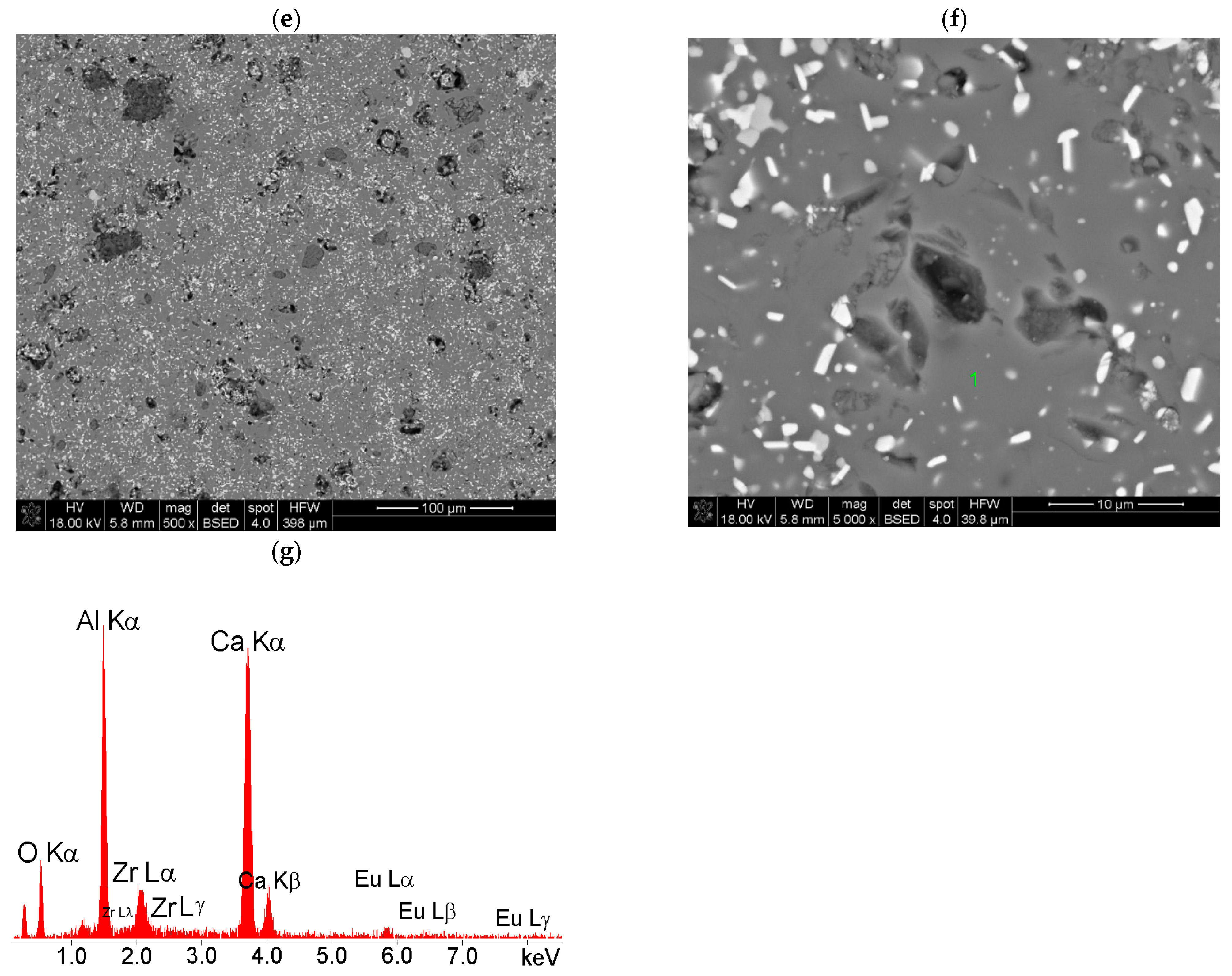
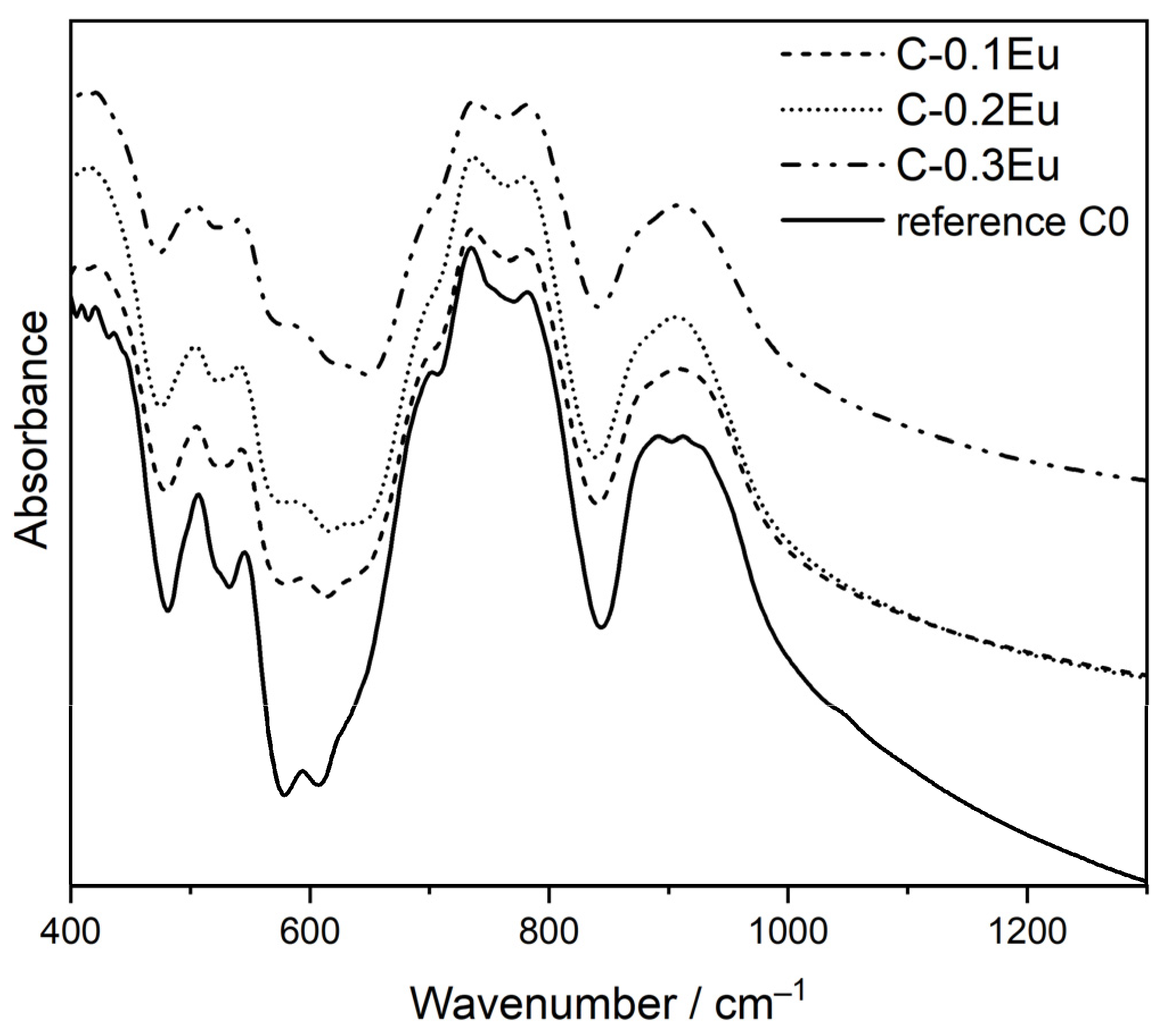

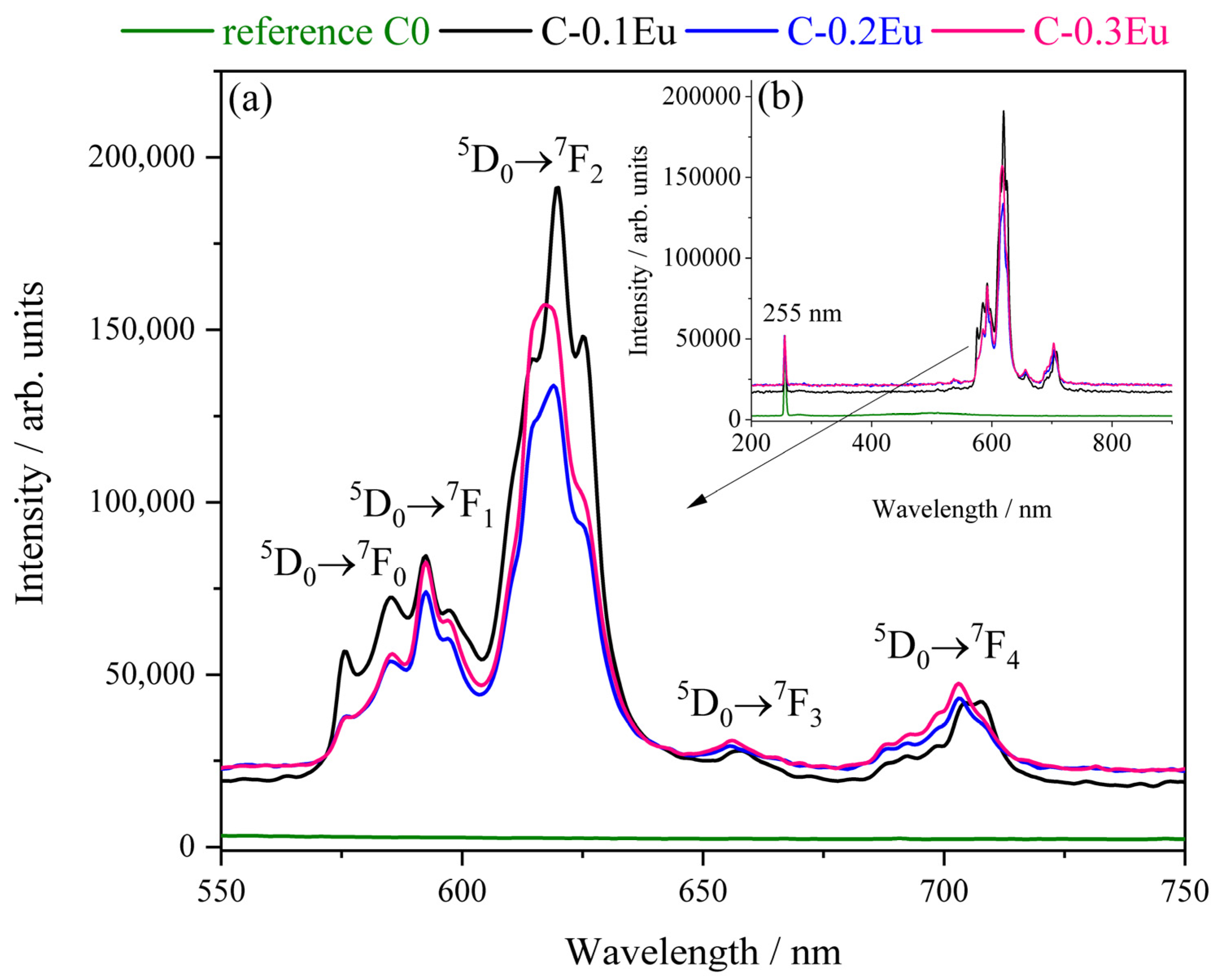

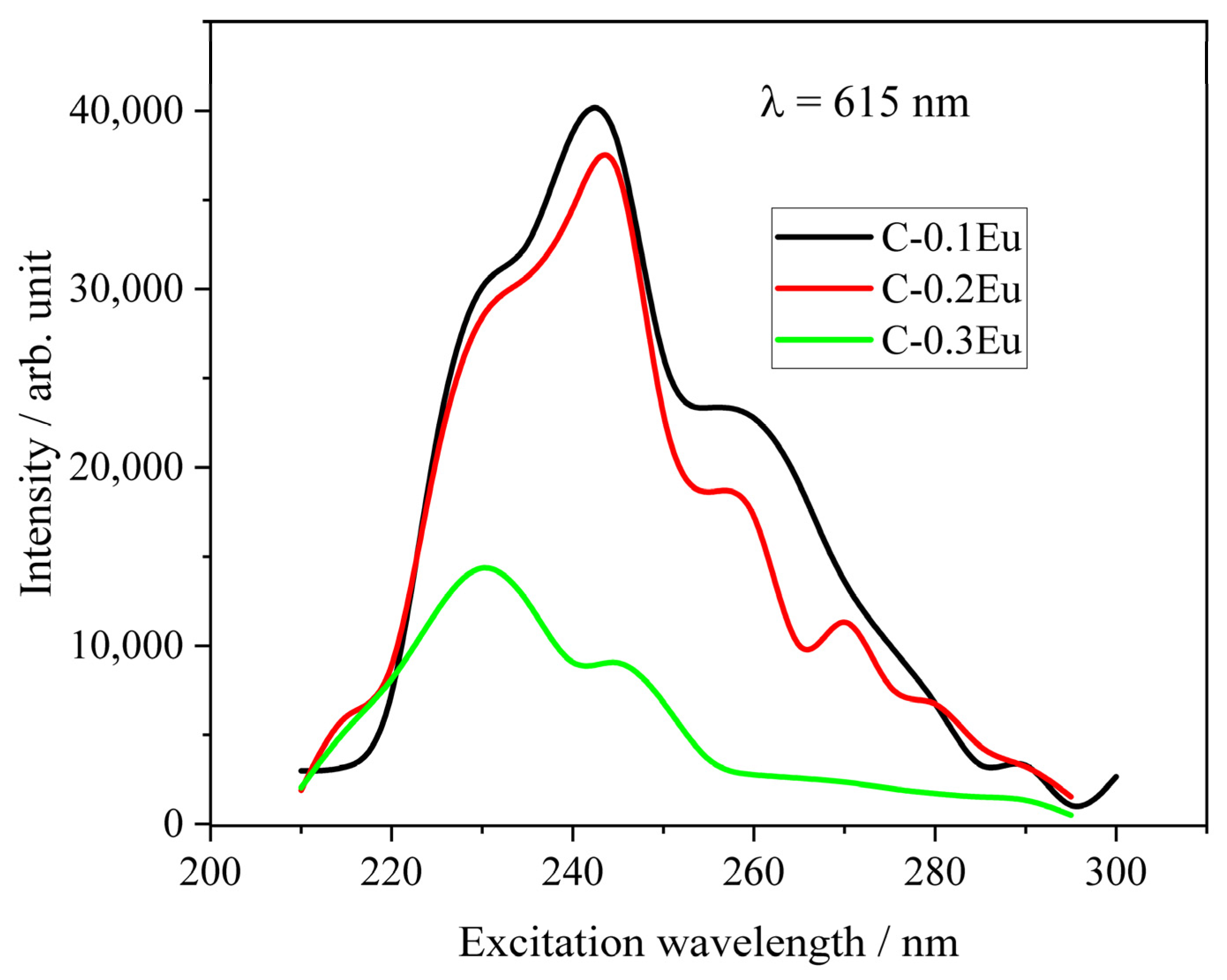

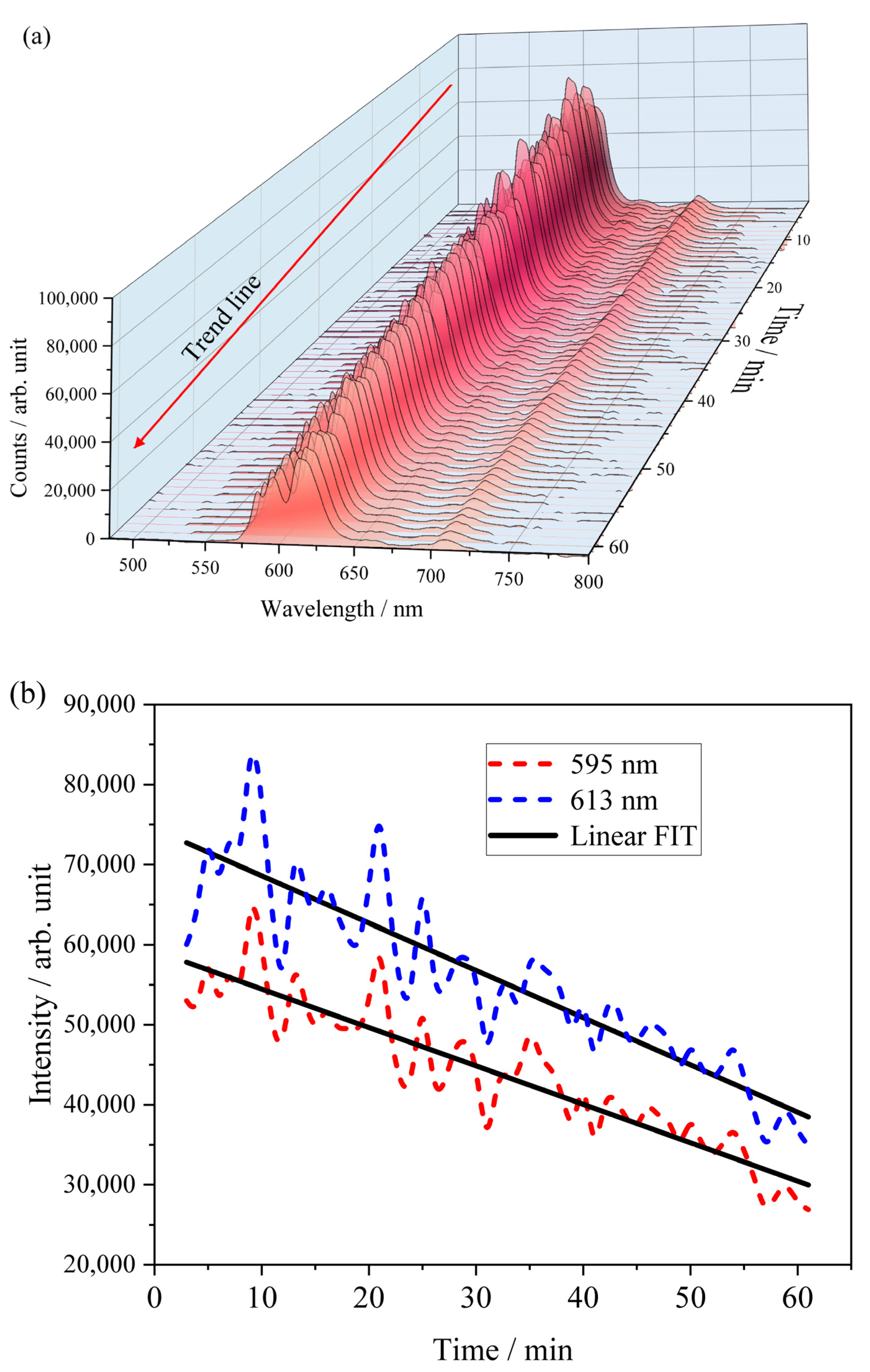
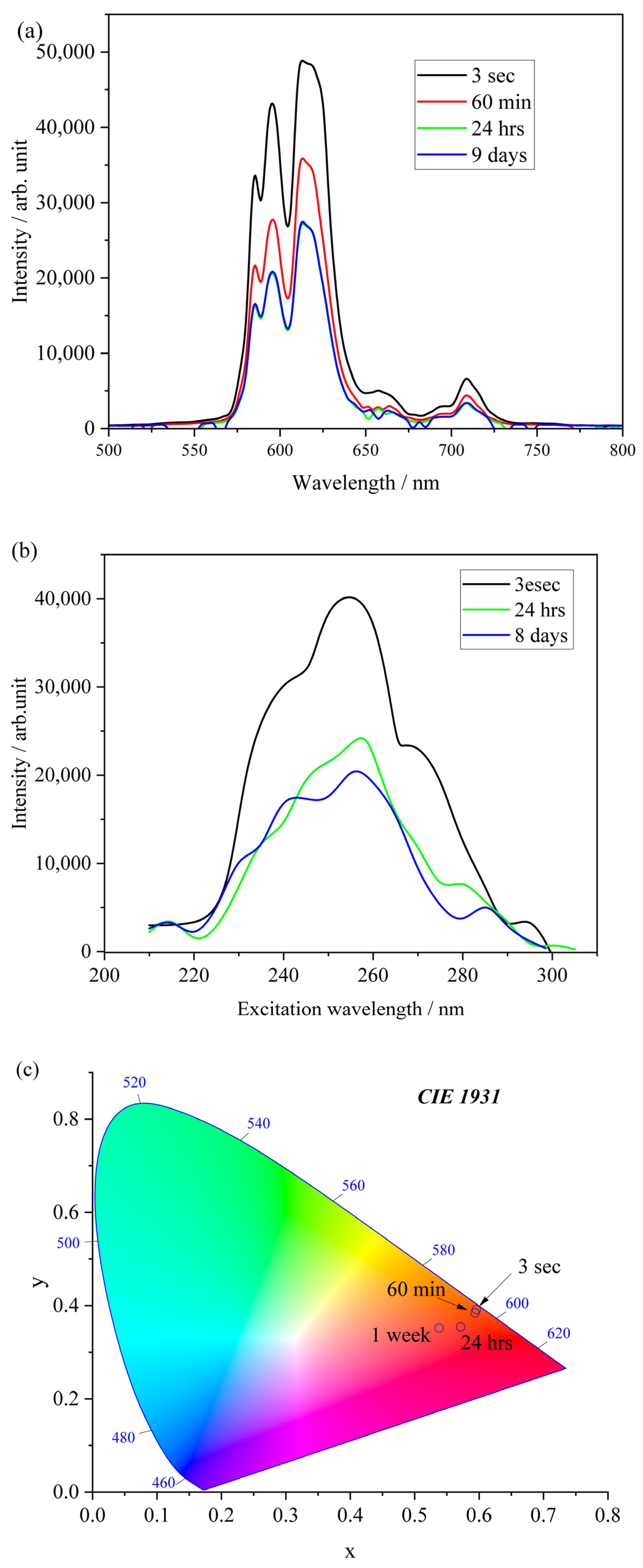
| Sample | Rietveld quantitative phase analysis, wt.% | Phase | Unit Cell Parameters/Å | ||
| a | b | c | |||
| Ca7ZrAl6O18 (ICSD PDF No. 98-018-2622) | 10.8490 | 10.5910 | 7.6690 | ||
| CaZrO3 (ICSD PDF No. 98-009-7465) | 5.7610 | 8.0200 | 5.5940 | ||
| Ca3Al2O6 (ICSD PDF No. 98-015-1369) | 7.6240 | 7.6240 | 7.6240 | ||
| C-0.1Eu | 95.0 | Ca7ZrAl6O18 | 10.87890 ± 0.00275 | 10.6100 ± 0.00179 | 7.6698 ± 0.0008 |
| 4.9 | CaZrO3 | 5.7489 ± 0.0021 | 8.0159 ± 0.0005 | 5.5954 ± 0.00025 | |
| 0.1 | Ca3Al2O6 | 7.1995 ± 0.0556 | 7.1995 ± 0.00025 | 7.1995 ± 0.00025 | |
| C-0.2Eu | 92.2 | Ca7ZrAl6O18 | 10.8761 ± 0.00249 | 10.6265 ± 0.00335 | 7.6709 ± 0.00025 |
| 7.1 | CaZrO3 | 5.7527 ± 0.00144 | 8.0159 ± 0.0005 | 5.5961 ± 0.00037 | |
| 0.7 | Ca3Al2O6 | 8.2747 ± 0.0853 | 8.2747 ± 0.0853 | 8.2747 ± 0.0853 | |
| C-0.3Eu | 89.5 | Ca7ZrAl6O18 | 10.8745 ± 0.0023 | 10.6265 ± 0.0033 | 7.6705 ± 0.0061 |
| 8.9 | CaZrO3 | 5.7522 ± 0.00153 | 8.0173 ± 0.00034 | 5.5971 ± 0.00055 | |
| 1.6 | Ca3Al2O6 | 7.3354 ± 0.0393 | 7.3354 ± 0.03785 | 7.3354 ± 0.03785 | |
| Sample | Elements in wt.% | ||||
|---|---|---|---|---|---|
| Ca | Eu | Al | Zr | O | |
| Theoretical composition of pure Ca7ZrAl6O18 | 34.17 | - | 19.65 | 11.11 | 35.07 |
| C0 | 33.85 | - | 23.86 | 11.38 | 30.91 |
| C-0.1Eu | 33.30 | 3.29 | 19.36 | 11.01 | 33.04 |
| C-0.2Eu | 32.70 | 5.21 | 18.70 | 10.95 | 32.44 |
| C-0.3Eu | 31.85 | 8.45 | 19.49 | 10.97 | 29.24 |
| Sample | Elements in wt.% | |||
|---|---|---|---|---|
| Ca | Eu | Al | O | |
| Theoretical composition of pure Ca3Al2O6 | 44.50 | - | 19.97 | 35.53 |
| C0 | 42.89 | - | 20.65 | 36.46 |
| C-0.1Eu | 41.82 | 2.54 | 19.27 | 36.37 |
| C-0.2Eu | 40.76 | 3.55 | 19.58 | 36.11 |
| C-0.3Eu | 40.19 | 3.83 | 19.86 | 36.12 |
| Sample | Elements in wt.% | |||
|---|---|---|---|---|
| Ca | Eu | Zr | O | |
| Theoretical composition of pure CaZrO3 | 22.35 | - | 50.88 | 26.77 |
| C0 | 20.51 | - | 48.13 | 31.36 |
| C-0.1Eu | 20.07 | 1.82 | 47.14 | 30.97 |
| C-0.2Eu | 19.50 | 2.60 | 47.62 | 30.28 |
| C-0.3Eu | 19.08 | 3.21 | 46.80 | 30.91 |
| Sample Code | Composite Type |
|---|---|
| C0 | Ca7ZrAl6O18 |
| C-0.1Eu * | (Ca7ZrAl6O18-Ca3Al2O6-CaZrO3):0.1Eu3+ |
| C-0.2Eu * | (Ca7ZrAl6O18-Ca3Al2O6-CaZrO3):0.2Eu3+ |
| C-0.3Eu * | (Ca7ZrAl6O18-Ca3Al2O6-CaZrO3):0.3Eu3+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madej, D.; Kruk, A. Luminescent Properties of (Ca7ZrAl6O18-Ca3Al2O6-CaZrO3):Eu3+ Composite Ceramics and Tracing in the Hydration Process. Molecules 2023, 28, 7799. https://doi.org/10.3390/molecules28237799
Madej D, Kruk A. Luminescent Properties of (Ca7ZrAl6O18-Ca3Al2O6-CaZrO3):Eu3+ Composite Ceramics and Tracing in the Hydration Process. Molecules. 2023; 28(23):7799. https://doi.org/10.3390/molecules28237799
Chicago/Turabian StyleMadej, Dominika, and Andrzej Kruk. 2023. "Luminescent Properties of (Ca7ZrAl6O18-Ca3Al2O6-CaZrO3):Eu3+ Composite Ceramics and Tracing in the Hydration Process" Molecules 28, no. 23: 7799. https://doi.org/10.3390/molecules28237799
APA StyleMadej, D., & Kruk, A. (2023). Luminescent Properties of (Ca7ZrAl6O18-Ca3Al2O6-CaZrO3):Eu3+ Composite Ceramics and Tracing in the Hydration Process. Molecules, 28(23), 7799. https://doi.org/10.3390/molecules28237799







