Current Status of Novel Multifunctional Targeted Pt(IV) Compounds and Their Reductive Release Properties
Abstract
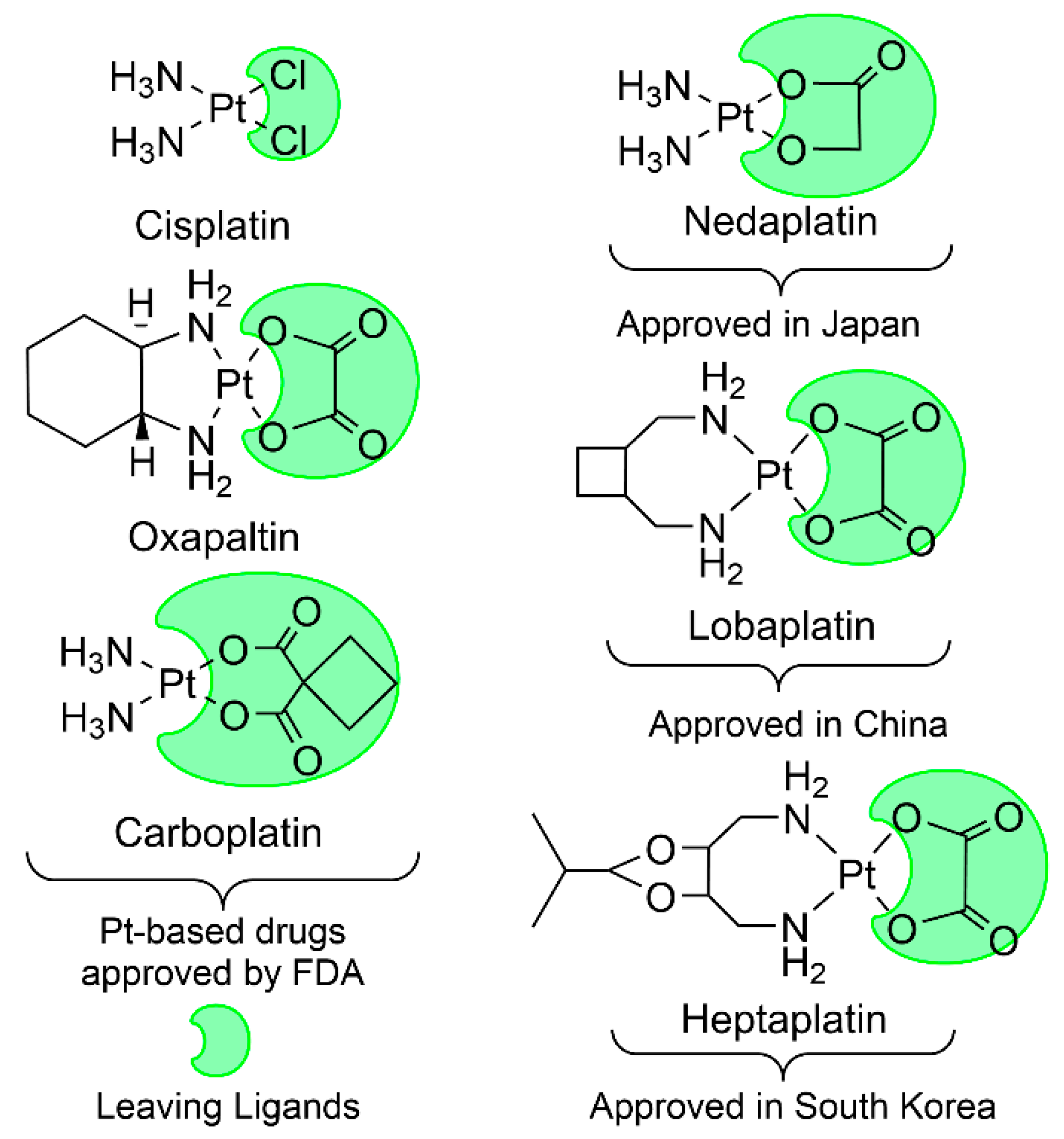
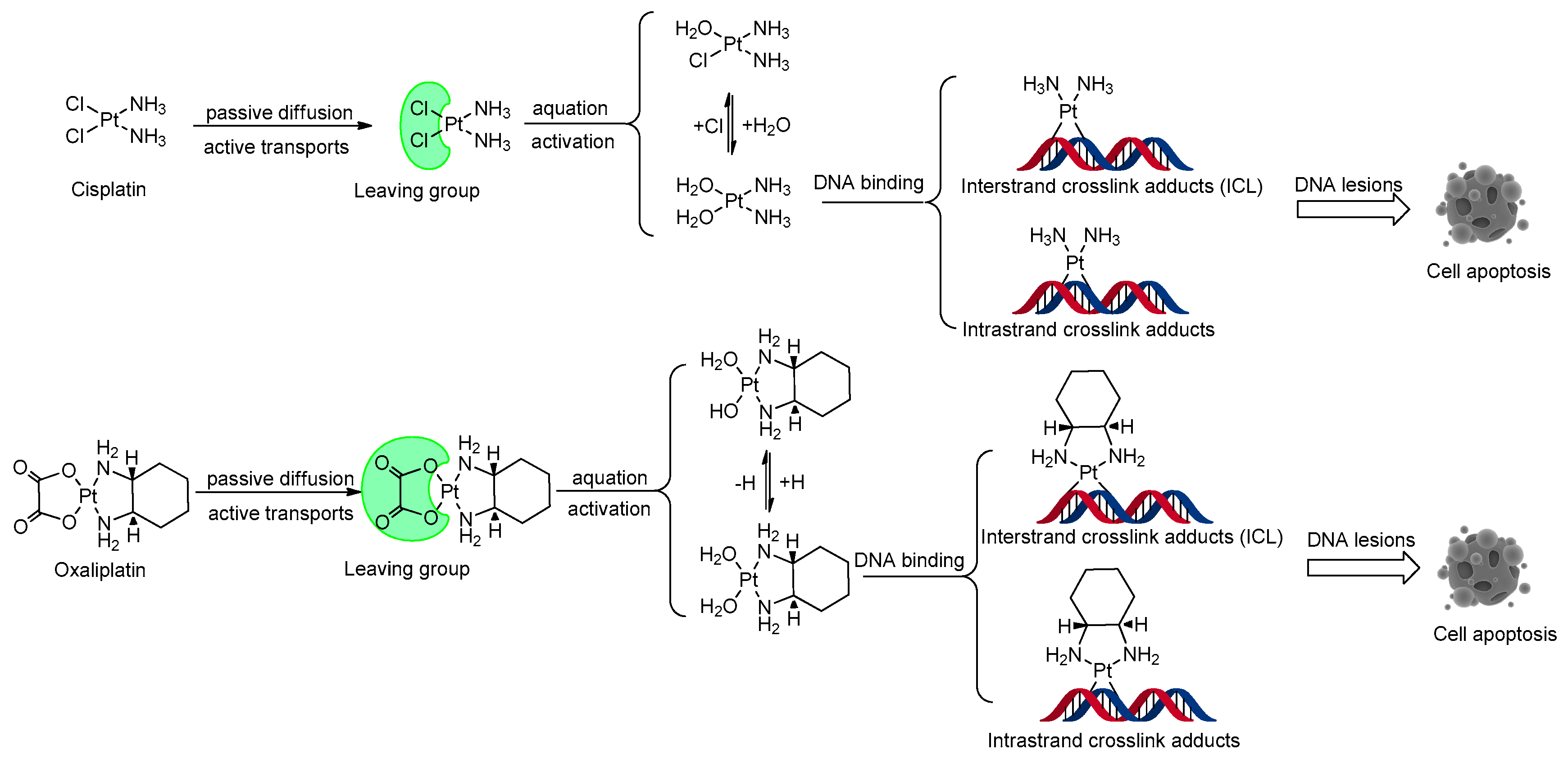
1. Introduction
2. Novel Multifunctional Targeted Pt(II) Compounds
2.1. Targeting Glucose Receptors with Pt(II) Compounds
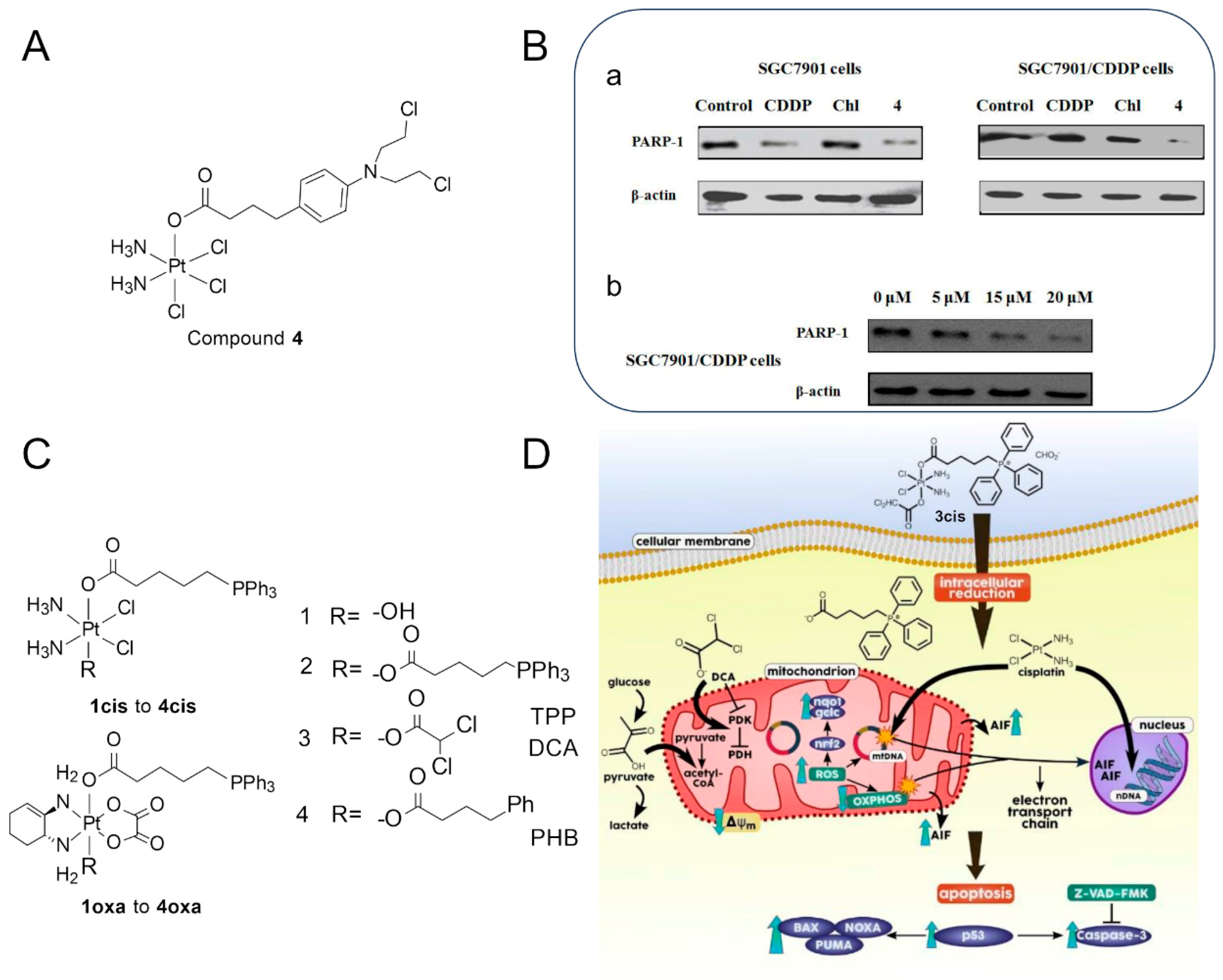
2.2. Targeting of Bile Acids with Pt(II) Compounds
2.3. Targeting Folate Receptor with Pt(II) Compounds
2.4. Targeting Mitochondria with Pt(II) Compounds
2.5. Targeting Telomerase with Pt(II) Compounds
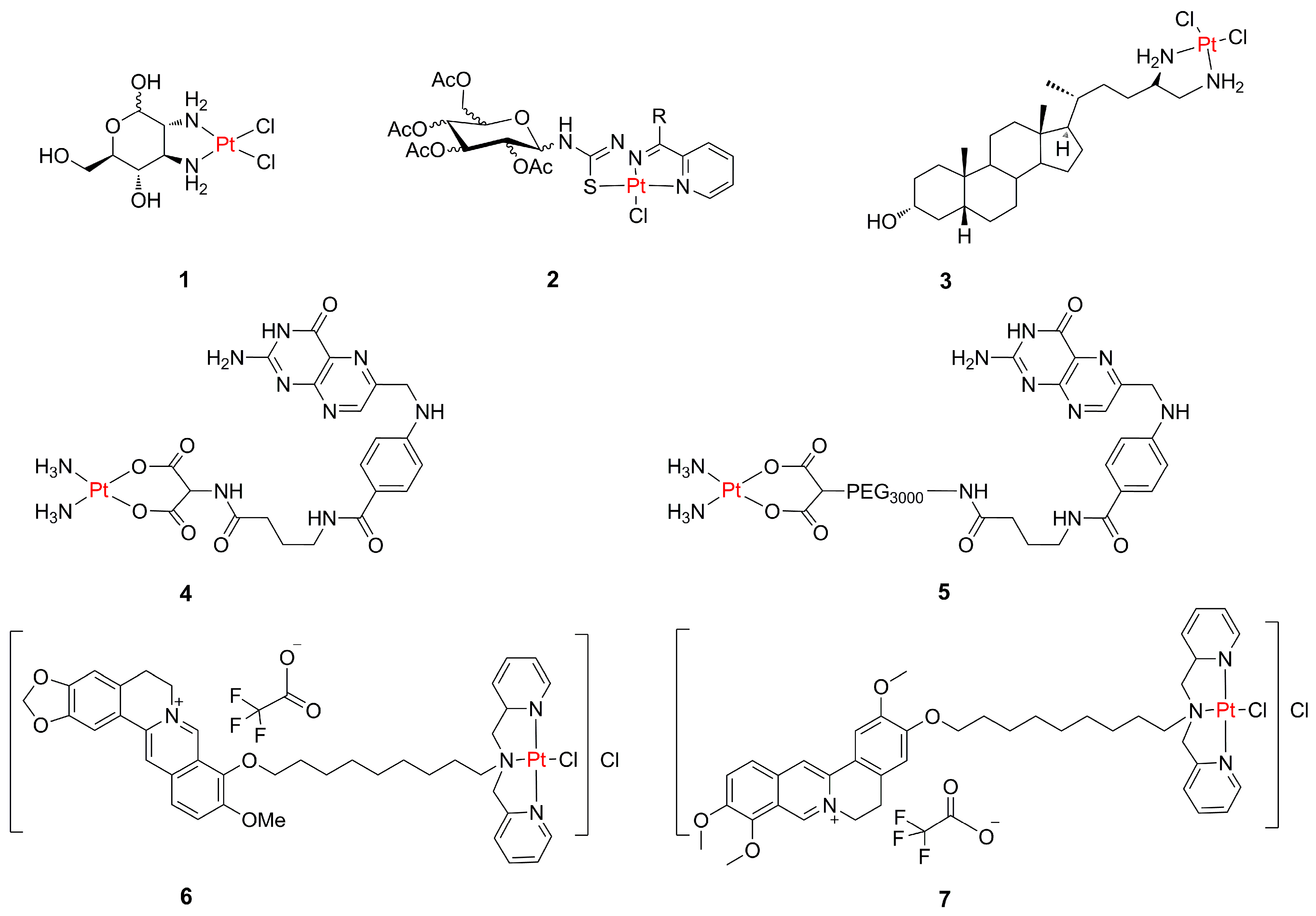
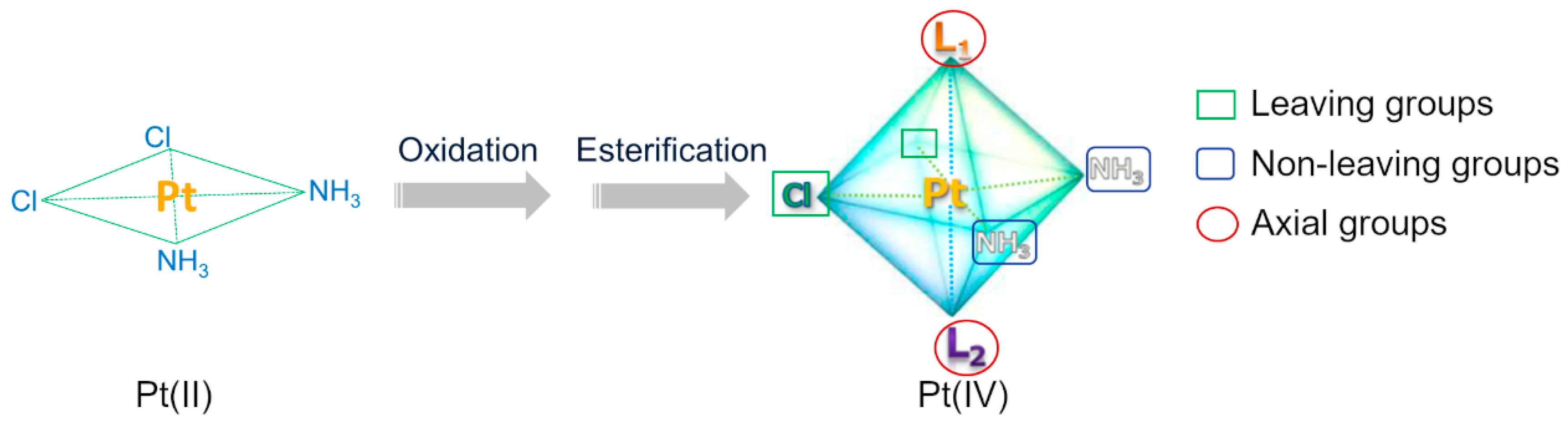
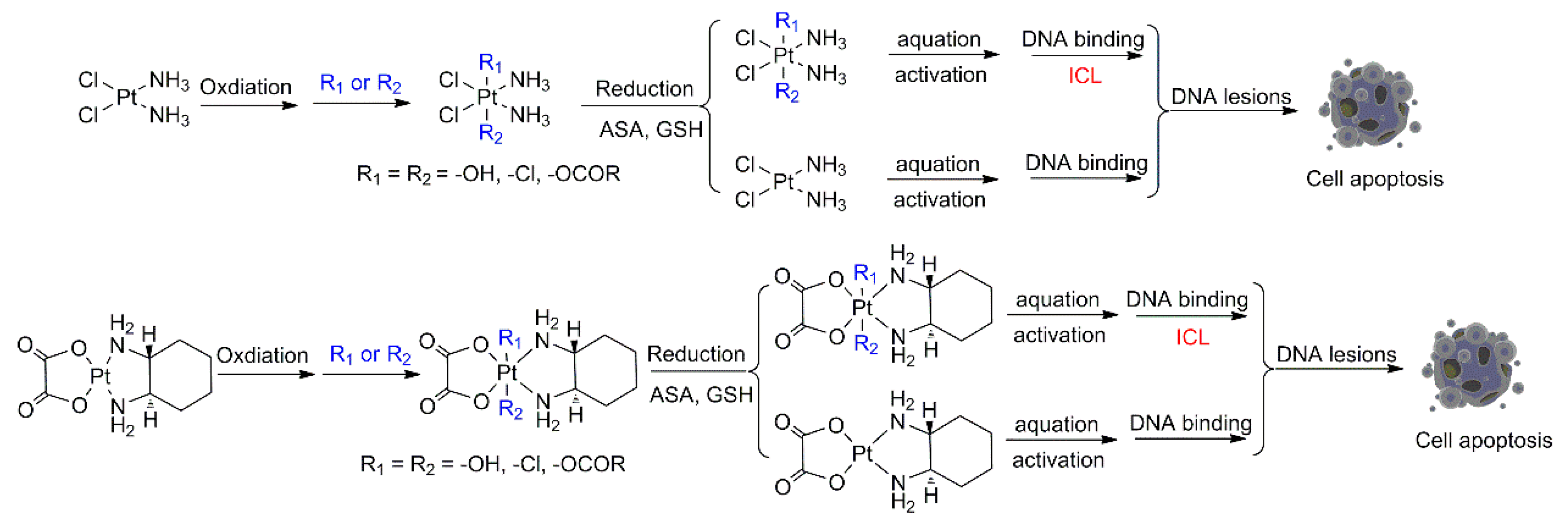
3. Novel Multifunctional Targeted Pt(IV) Compounds and Reduction Releasing
3.1. Targeting p53-MDM2 Interaction with Pt(IV) Compounds
3.2. Targeting Cyclooxygenase-2 with Pt(IV) Compounds
3.3. Targeting Lipid Metabolism with Pt(IV) Compounds
3.4. Novel Multifunctional Pt(IV) Dual-Prodrug Compounds
4. Effect of Different Reducing Agents on the Reductive Release Rate of Pt(IV) Compounds
5. Other Multifunctional Targeted Pt(IV) Compounds
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khoury, A.; Deo, K.M.; Aldrich-Wright, J.R. Recent advances in platinum-based chemotherapeutics that exhibit inhibitory and targeted mechanisms of action. J. Inorg. Biochem. 2020, 207, 111070. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zheng, J.; Zhang, J.; Lin, J.; Lai, J.; Lyu, Z.; Feng, H.; Wang, J.; Wu, D.; Li, Y. A Novel Protein Encoded by Exosomal CircATG4B Induces Oxaliplatin Resistance in Colorectal Cancer by Promoting Autophagy. Adv. Sci. 2022, 9, 2204513. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J.; Lippard, S.J. Synthetic Methods for the Preparation of Platinum Anti-cancer Complexes. Chem. Rev. 2013, 114, 4470–4495. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Pro-drugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- da Costa, A.A.B.A.; Baiocchi, G. Genomic profiling of platinum-resistant ovarian cancer: The road into druggable targets. Semin. Cancer Biol. 2021, 77, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D. Multi-action Pt(IV) anti-cancer agents; do we understand how they work? J. Inorg. Biochem. 2019, 191, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Reed, E. Platinum-DNA adduct, nucleotide excision repair and platinum based anti-cancer chemotherapy. Cancer Treat. Rev. 1998, 24, 331–344. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anti-cancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological Effects of Cisplatin Combination with Natural Products in Cancer Chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Kleih, M.; Böpple, K.; Dong, M.; Gaißler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 4, 6645–6653. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Deng, Z.; Tang, M.; Xiao, D.; Cai, P. Impact of genetic factors on platinum-induced gastrointestinal toxicity. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108324. [Google Scholar] [CrossRef] [PubMed]
- Canil, G.; Braccini, S.; Marzo, T.; Marchetti, L.; Pratesi, A.; Biver, T.; Funaioli, T.; Chiellini, F.; Hoeschele, J.D.; Gabbiani, C. Photocytotoxic Pt(iv) complexes as prospective anti-cancer agents. Dalton Trans. 2019, 48, 10933–10944. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kong, Y.; Li, X.; Cheng, D.; Hou, Y.; Li, Y.; Li, T.; Xiao, Y.; Zhang, Q.; Rong, R. Novel Pt(IV) pro-drug self-assembled nanoparticles with enhanced blood circulation stability and improved anti-tumor capacity of oxaliplatin for cancer therapy. Drug Deliv. 2023, 30, 2171158. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, X.; Li, C.; Xu, Z.; Chan, C.Y.; Tse, M.K.; Shi, P.; Zhu, G. A Cancer Cell-Selective and Low-Toxic Bifunctional Heterodinuclear Pt(IV)–Ru(II) Anti-cancer Pro-drug. Inorg. Chem. 2018, 57, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, G.; Wang, S.; Xu, J.; Fang, Q.; Xue, Y.; Yang, L.; Xu, X.; Tang, R. Dynamic precise dual-drug-backboned nano-prodrugs for selective chemotherapy. Acta Biomater. 2021, 129, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, T.; Zhang, M.; Feng, P.; Wang, X.; Cheng, X.; Tang, R. A sequentially responsive nanogel via Pt(IV) cross-linking for overcoming GSH-mediated platinum resistance. J. Colloid Interface Sci. 2021, 601, 85–97. [Google Scholar] [CrossRef]
- Arnesano, F.; Natile, G. Interference between copper transport systems and platinum drugs. Semin. Cancer Biol. 2021, 76, 173–188. [Google Scholar] [CrossRef]
- Spector, D.; Erofeev, A.; Gorelkin, P.; Skvortsov, D.; Trigub, A.; Markova, A.; Nikitina, V.; Ul’Yanovskiy, N.; Shtil’, A.; Semkina, A.; et al. Biotinylated Pt(iv) pro-drugs with elevated lipophilicity and cytotoxicity. Dalton Trans. 2023, 52, 866–871. [Google Scholar] [CrossRef]
- van der Vijgh, W.J. Clinical Pharmacokinetics of Carboplatin. Clin. Pharmacokinet. 1991, 21, 242–261. [Google Scholar] [CrossRef]
- Han, X.; Sun, J.; Wang, Y.; He, Z. Recent Advances in Platinum (IV) Complex-Based Delivery Systems to Improve Platinum (II) Anti-cancer Therapy. Med. Res. Rev. 2015, 35, 1268–1299. [Google Scholar] [CrossRef]
- Choroba, K.; Machura, B.; Szlapa-Kula, A.; Malecki, J.G.; Raposo, L.; Roma-Rodrigues, C.; Cordeiro, S.; Baptista, P.V.; Fernandes, A.R. Square planar Au(III), Pt(II) and Cu(II) complexes with quinoline-substituted 2,2′:6′,2″-terpyridine ligands: From in vitro to in vivo biological properties. Eur. J. Med. Chem. 2021, 218, 113404. [Google Scholar] [CrossRef]
- Date, T.; Kuche, K.; Ghadi, R.; Kumar, P.; Jain, S. Understanding the Role of Axial Ligands in Modulating the Biopharmaceutical Outcomes of Cisplatin(IV) Derivatives. Mol. Pharm. 2022, 19, 1325–1337. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Y.; Bian, M.; Yang, Z.; Ma, X.; Liu, W. Pt(II) and Au(III) complexes containing Schiff-base ligands: A promising source for anti-tumor treatment. Eur. J. Med. Chem. 2021, 211, 6485. [Google Scholar] [CrossRef]
- Bai, L.; Gao, C.; Liu, Q.; Yu, C.; Zhang, Z.; Cai, L.; Yang, B.; Qian, Y.; Yang, J.; Liao, X. Research progress in modern structure of platinum complexes. Eur. J. Med. Chem. 2017, 140, 349–382. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Sanche, L.; Hunting, D.J. Cisplatin Enhances the Formation of DNA Single- and Double-Strand Breaks by Hydrated Electrons and Hydroxyl Radicals. Radiat. Res. 2013, 179, 323–331. [Google Scholar] [CrossRef]
- Sava, G.; Jaouen, G.; Hillard, E.A.; Bergamo, A. Targeted therapy vs. DNA-adduct formation-guided design: Thoughts about the future of metal-based anti-cancer drugs. Dalton Trans. 2012, 41, 8226–8234. [Google Scholar] [CrossRef] [PubMed]
- Boldinova, E.O.; Yudkina, A.V.; Shilkin, E.S.; Gagarinskaya, D.I.; Baranovskiy, A.G.; Tahirov, T.H.; Zharkov, D.O.; Makarova, A.V. Translesion activity of PrimPol on DNA with cisplatin and DNA–protein cross-links. Sci. Rep. 2021, 11, 17588. [Google Scholar] [CrossRef]
- Ang, W.H.; Myint, M.; Lippard, S.J. Transcription Inhibition by Platinum-DNA Cross-Links in Live Mammalian Cells. J. Am. Chem. Soc. 2010, 132, 7429–7435. [Google Scholar] [CrossRef]
- Todd, R.C.; Lippard, S.J. Structure of duplex DNA containing the cisplatin 1,2-{Pt(NH3)2}2+-d(GpG) cross-link at 1.77 Å resolution. J. Inorg. Biochem. 2010, 104, 902–908. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, G.; Huang, X.; Lippard, S.J. X-ray structure and mechanism of RNA polymerase II stalled at an antineoplastic monofunctional platinum-DNA adduct. Proc. Natl. Acad. Sci. USA 2010, 107, 9584–9589. [Google Scholar] [CrossRef]
- Ponte, F.; Scoditti, S.; Mazzone, G.; Sicilia, E. The current status in computational exploration of Pt(iv) pro-drug activation by reduction. Phys. Chem. Chem. Phys. 2023, 25, 15586–15599. [Google Scholar] [CrossRef] [PubMed]
- Wexselblatt, E.; Gibson, D. What do we know about the reduction of Pt(IV) pro-drugs? J. Inorg. Biochem. 2012, 117, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Muhammad, N.; Sun, Y.; Tan, Y.; Yuan, H.; Song, D.; Guo, Z.; Wang, X. Multispecific Platinum(IV) Complex Deters Breast Cancer via Interposing Inflammation and Immunosuppression as an Inhibitor of COX-2 and PD-L1. Angew. Chem. Int. Ed. 2020, 59, 23313–23321. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Gao, Y.Y.; Zheng, L.X.; Ding, X.J.; Xu, L.W.; Hu, J.J.; Gao, W.Z.; Xu, J.Y. Targeting ROS-AMPK pathway by multiaction Platinum(IV) pro-drugs containing hypolipidemic drug bezafibrate. Eur. J. Med. Chem. 2021, 223, 113730. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Q.; Liu, R.P.; Wang, S.Q.; Li, Z.; Ma, Z.Y.; Zhang, R.; Xie, C.Z.; Qiao, X.; Xu, J.Y. Anti-cancer Melatplatin Pro-drugs: High Effect and Low Toxicity, MT1-ER-Target and Immune Response In Vivo. J. Med. Chem. 2020, 63, 6096–6106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Qiao, X.; Liu, X.M.; Song, X.Q.; Zou, Y.H.; Li, D.Q.; Yu, X.W.; Bao, W.G.; Xu, J.Y. Rapid DNA interstrand cross-linking of Pt(IV) compound. Eur. J. Pharmacol. 2022, 925, 174985. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Qiu, J.; O’Sullivan, D.; Buck, M.D.; Noguchi, T.; Curtis, J.D.; Chen, Q.; Gindin, M.; Gubin, M.M.; van der Windt, G.J.W.; et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015, 162, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Nazarov, A.A.; Hartinger, C.G.; Groessl, M.; Valiahdi, S.M.; Jakupec, M.A.; Keppler, B.K. A glucose derivative as natural alternative to the cyclohexane-1,2-diamine ligand in the anti-cancer drug oxaliplatin? ChemMedChem 2007, 2, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shao, J.; Wang, J.; Gong, X.-J.; Liu, W.-X.; Wang, S.; Zhang, Y.; Yang, S.; Zhang, Q.-S.; Wei, J.-X.; et al. Anti-tumor effects of new glycoconjugated PtII agents dual-targeting GLUT1 and Pgp proteins. Dalton Trans. 2022, 51, 16082–16092. [Google Scholar] [CrossRef] [PubMed]
- Criado, J.J.; Garcia-Moreno, M.; Macias, R.R.; Marin, J.J.; Medarde, M.; Rodriguez-Fernandez, E. Synthesis and characterization of Sodium cis-dichlorochenodeoxycholylglycinato(O,N) platinum(II)—Cytostatic activity. BioMetals 1999, 12, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Criado, J.J.; Manzano, J.L.; Rodríguez-Fernández, E. New organotropic compounds. J. Inorg. Biochem. 2003, 96, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Trauner, M.; Boyer, J.L. Bile Salt Transporters: Molecular Characterization, Function, and Regulation. Physiol. Rev. 2003, 83, 633–671. [Google Scholar] [CrossRef]
- Barbara, C.; Orlandi, P.; Bocci, G.; Fioravanti, A.; Di Paolo, A.; Natale, G.; Del Tacca, M.; Danesi, R. In vitro and in vivo antitumour effects of novel, orally active bile acid-conjugated platinum complexes on rat hepatoma. Eur. J. Pharmacol. 2006, 549, 27–34. [Google Scholar] [CrossRef]
- Seroka, B.; Łotowski, Z.; Hryniewicka, A.; Rárová, L.; Sicinski, R.R.; Tomkiel, A.M.; Morzycki, J.W. Synthesis of New Cisplatin Derivatives from Bile Acids. Molecules 2020, 25, 655. [Google Scholar] [CrossRef]
- Nakhaei, E.; Kim, C.W.; Funamoto, D.; Sato, H.; Nakamura, Y.; Kishimura, A.; Mori, T.; Katayama, Y. Design of a ligand for cancer imaging with long blood circulation and an enhanced accumulation ability in tumors. MedChemComm 2017, 8, 1190–1195. [Google Scholar] [CrossRef]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R., Jr.; Kamen, B.A. Distribution of the Folate Receptor GP38 in Normal and Malignant Cell Lines and Tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar]
- Jennifer-Sudimack, B.A.; Lee, R.J. Targeted drug delivery via the folate receptor. Adv. Drug Deliv. Rev. 2002, 41, 147–162. [Google Scholar] [CrossRef]
- Morales-Cruz, M.; Cruz-Montañez, A.; Figueroa, C.M.; González-Robles, T.; Davila, J.; Inyushin, M.; Loza-Rosas, S.A.; Molina, A.M.; Muñoz-Perez, L.; Kucheryavykh, L.Y.; et al. Combining Stimulus-Triggered Release and Active Targeting Strategies Improves Cytotoxicity of Cytochrome c Nanoparticles in Tumor Cells. Mol. Pharm. 2016, 13, 2844–2854. [Google Scholar] [CrossRef]
- Nahire, R.; Haldar, M.K.; Paul, S.; Ambre, A.H.; Meghnani, V.; Layek, B.; Katti, K.S.; Gange, K.N.; Singh, J.; Sarkar, K.; et al. Multifunctional polymersomes for cytosolic delivery of gemcitabine and doxorubicin to cancer cells. Biomaterials 2014, 35, 6482–6497. [Google Scholar] [CrossRef]
- Wen, Y.; Graybill, W.S.; Previs, R.A.; Hu, W.; Ivan, C.; Mangala, L.S.; Zand, B.; Nick, A.M.; Jennings, N.B.; Dalton, H.J.; et al. Immunotherapy Targeting Folate Receptor Induces Cell Death Associated with Autophagy in Ovarian Cancer. Clin. Cancer Res. 2015, 21, 448–459. [Google Scholar] [CrossRef]
- Vitols, K.; Montejano, Y.; Duffy, T.; Pope, L.; Grundler, G.; Huennekens, F. Platinum-Folate Compounds: Synthesis, Properties and Biological Activity. Adv. Enzyme Regul. 1987, 26, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Pierroz, V.; Joshi, T.; Leonidova, A.; Mari, C.; Schur, J.; Ott, I.; Spiccia, L.; Ferrari, S.; Gasser, G. Molecular and Cellular Characterization of the Biological Effects of Ruthenium(II) Complexes Incorporating 2-Pyridyl-2-pyrimidine-4-carboxylic Acid. J. Am. Chem. Soc. 2012, 134, 20376–20387. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Zou, Y.; Chen, D.; Yang, L.; Rao, J.; Chen, H.; Chan, M.C.W.; Li, L.; Guo, Z.; et al. Mitochondria-targeted platinum(ii) complexes: Dual inhibitory activities on tumor cell proliferation and migration/invasion via intracellular trafficking of β-catenin. Metallomics 2017, 9, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.P.; Wang, Z.F.; Huang, X.L.; Tan, M.X.; Luo, Z.H.; Wang, S.L.; Zou, B.Q.; Liang, H. Two telomerase-targeting Pt(ii) complexes of jatrorrhizine and berberine derivatives induce apoptosis in human bladder tumor cells. Dalton Trans. 2019, 48, 15247–15254. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Liu, J.-G.; Jiang, C.-W.; Ji, L.-N.; Li, X.-Y.; Feng, C.-L. Stereoisomerically controlled supramolecular architectures: A new strategy for the construction of enantio- and diastereomerically pure multinuclear RuII complexes. Inorg. Chem. Commun. 2001, 4, 45–48. [Google Scholar] [CrossRef]
- Xiong, K.; Ouyang, C.; Liu, J.; Karges, J.; Lin, X.; Chen, X.; Chen, Y.; Wan, J.; Ji, L.; Chao, H. Chiral RuII-PtIIComplexes Inducing Telomere Dysfunction against Cisplatin-Resistant Cancer Cells. Angew. Chem. Int. Ed. 2022, 61, e202204866. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Vaishampayan, U.N. Satraplatin: Leading the new generation of oral platinum agents. Expert Opin. Investig. Drugs 2009, 18, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Choy, H.; Park, C.; Yao, M. Current Status and Future Prospects for Satraplatin, an Oral Platinum Analogue. Clin. Cancer Res. 2008, 14, 1633–1638. [Google Scholar] [CrossRef]
- Doshi, G.; Sonpavde, G.; Sternberg, C.N. Clinical and pharmacokinetic evaluation of satraplatin. Expert Opin. Drug Metab. Toxicol. 2012, 8, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Tian, H.; Song, C.; Shi, T.; Elding, L.I. Reduction of ormaplatin by an extended series of thiols unravels a remarkable correlation. Dalton Trans. 2018, 47, 5548–5552. [Google Scholar] [CrossRef]
- Rischin, D.; Ling, V. Ormaplatin resistance is associated with decreased accumulation of its platinum (II) analogue, dichloro(D,L-trans)1,2-diaminocyclohexaneplatinum(II). Br. J. Cancer 1996, 74, 590–596. [Google Scholar] [CrossRef][Green Version]
- Trask, C.; Silverstone, A.; Ash, C.M.; Earl, H.; Irwin, C.; Bakker, A.; Tobias, J.S.; Souhami, R.L. A Randomized Trial of Carboplatin Versus Iproplatin in Untreated Advanced Ovarian Cancer. J. Clin. Oncol. 1991, 9, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Tian, H. Current Developments in Pt(IV) Pro-drugs Conjugated with Bioactive Ligands. Bioinorg. Chem. Appl. 2018, 2018, 8276139. [Google Scholar] [CrossRef]
- Mu, M.; Zhan, J.; Dai, X.; Gao, H. Research progress of azido-containing Pt(IV) anti-tumor compounds. Eur. J. Med. Chem. 2022, 227, 113927. [Google Scholar] [CrossRef]
- Spector, D.; Krasnovskaya, O.; Pavlov, K.; Erofeev, A.; Gorelkin, P.; Beloglazkina, E.; Majouga, A. Pt(IV) Pro-drugs with NSAIDs as Axial Ligands. Int. J. Mol. Sci. 2021, 22, 3817. [Google Scholar] [CrossRef]
- Spector, D.; Pavlov, K.; Beloglazkina, E.; Krasnovskaya, O. Recent Advances in Light-Controlled Activation of Pt(IV) Pro-drugs. Int. J. Mol. Sci. 2022, 23, 14511. [Google Scholar] [CrossRef]
- Siddik, Z.; Hagopian, G.; Thai, G.; Tomisaki, S.; Toyomasu, T.; Khokhar, A. Role of p53 in the ability of 1,2-diaminocyclohexane-diacetato-dichloro-Pt(IV) to circumvent cisplatin resistance. J. Inorg. Biochem. 1999, 77, 65–70. [Google Scholar] [CrossRef]
- Zheng, S.; Li, G.; Shi, J.; Liu, X.; Li, M.; He, Z.; Tian, C.; Kamei, K.I. Emerging platinum(IV) pro-drug nanotherapeutics: A new epoch for platinum-based cancer therapy. J. Control. Release 2023, 361, 819–846. [Google Scholar] [CrossRef] [PubMed]
- Ravera, M.; Gabano, E.; McGlinchey, M.J.; Osella, D. Pt(iv) anti-tumor pro-drugs: Dogmas, paradigms, and realities. Dalton Trans. 2022, 51, 2121–2134. [Google Scholar] [CrossRef]
- Tsvetkova, D.; Ivanova, S. Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases. Molecules 2022, 27, 2466. [Google Scholar] [CrossRef] [PubMed]
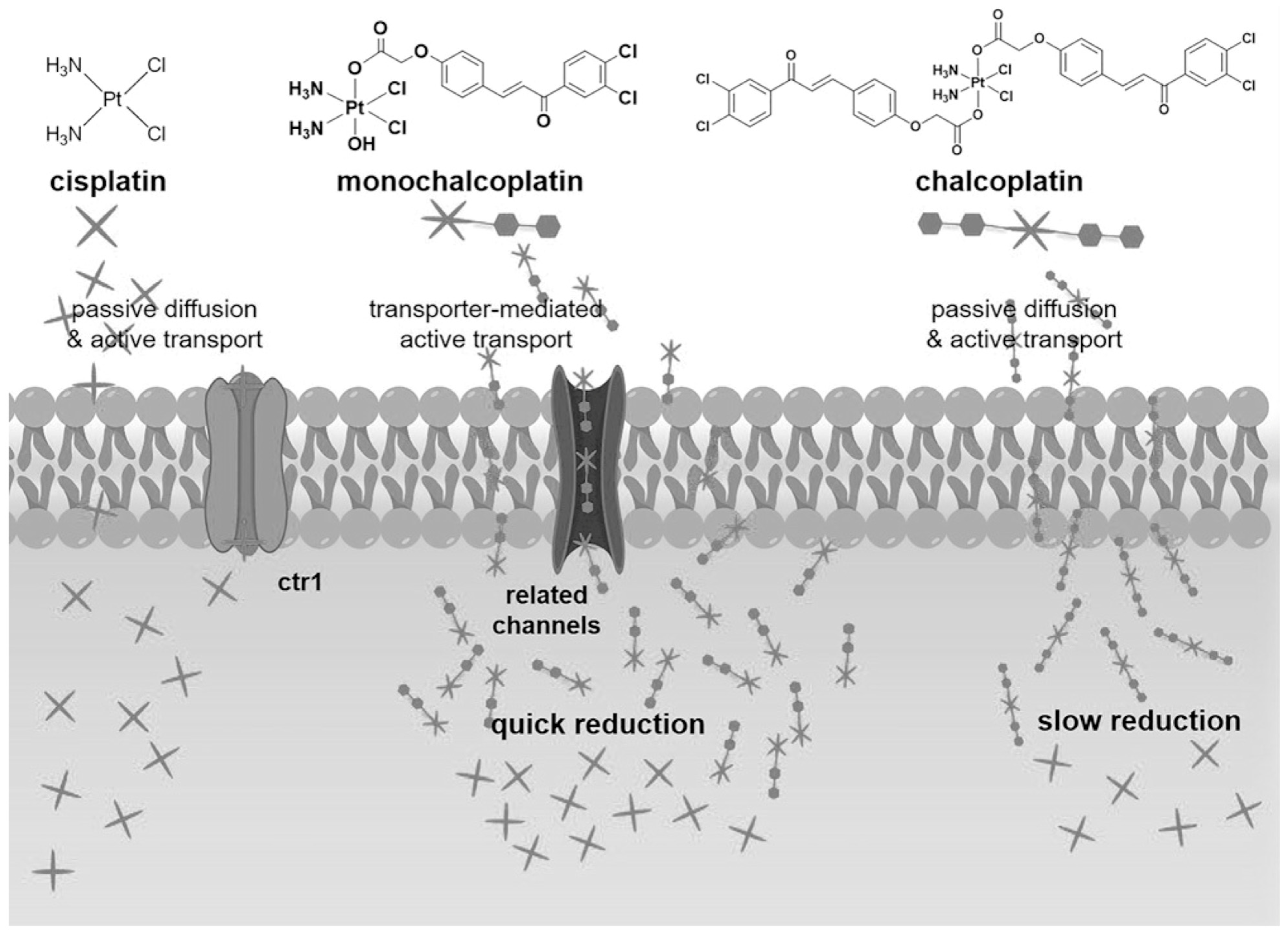
- Ma, L.; Wang, N.; Ma, R.; Li, C.; Xu, Z.; Tse, M.K.; Zhu, G. Monochalcoplatin: An Actively Transported, Quickly Reducible, and Highly Potent PtIV Anti-cancer Pro-drug. Angew. Chem. Int. Ed. Engl. 2018, 57, 9098–9102. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Zhao, Y.; Zhang, J.; Liu, M.; Wang, Z.; Li, D.; Han, J. Naproxen platinum(iv) hybrids inhibiting cycloxygenases and matrix metalloproteinases and causing DNA damage: Synthesis and biological evaluation as anti-tumor agents in vitro and in vivo. Dalton Trans. 2020, 49, 5192–5204. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Marrache, S.; Choi, J.H.; Berding, T.B.; Dhar, S. The Pro-drug Platin-A: Simultaneous Release of Cisplatin and Aspirin. Angew. Chem. Int. Ed. Engl. 2014, 53, 1963–1967. [Google Scholar] [CrossRef]
- Song, X.Q.; Ma, Z.Y.; Wu, Y.G.; Dai, M.L.; Wang, D.B.; Xu, J.Y.; Liu, Y. New NSAID-Pt(IV) pro-drugs to suppress metastasis and invasion of tumor cells and enhance anti-tumor effect in vitro and in vivo. Eur. J. Med. Chem. 2019, 167, 377–387. [Google Scholar] [CrossRef]
- Białek, A.; Jelińska, M.; Białek, M.; Lepionka, T.; Czerwonka, M.; Czauderna, M. The Effect of Diet Supplementation with Pomegranate and Bitter Melon on Lipidomic Profile of Serum and Cancerous Tissues of Rats with Mammary Tumours. Antioxidants 2020, 9, 243. [Google Scholar] [CrossRef]
- Valentín-Guillama, G.; López, S.; Kucheryavykh, Y.V.; Chorna, N.E.; Pérez, J.; Ortiz-Rivera, J.; Inyushin, M.; Makarov, V.; Valentín-Acevedo, A.; Quinones-Hinojosa, A.; et al. HIV-1 Envelope Protein gp120 Promotes Proliferation and the Activation of Glycolysis in Glioma Cell. Cancers 2018, 10, 301. [Google Scholar] [CrossRef]
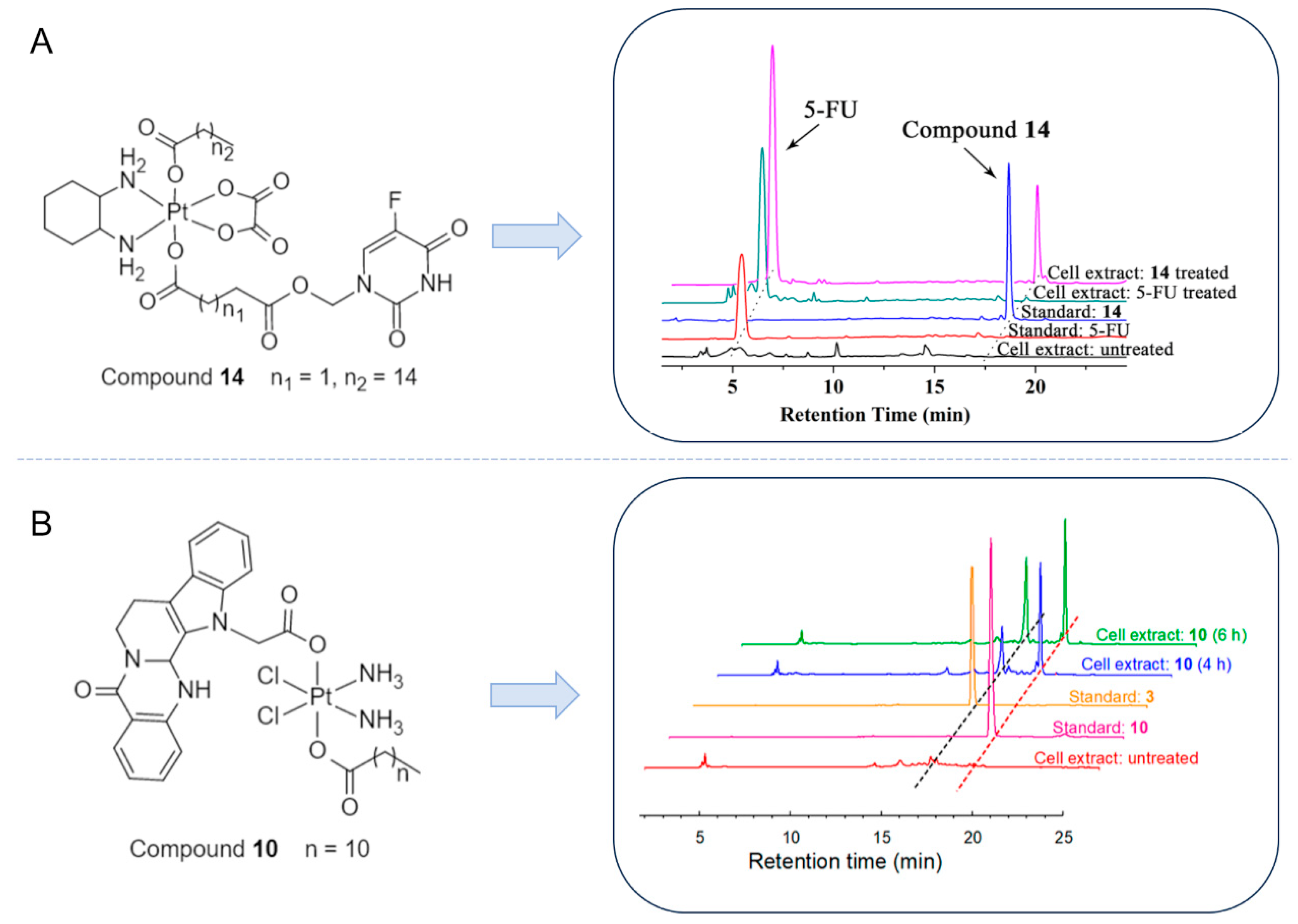
- Zhang, R.; Song, X.Q.; Liu, R.P.; Ma, Z.Y.; Xu, J.Y. Fuplatin: An Efficient and Low-Toxic Dual-Prodrug. J. Med. Chem. 2019, 62, 4543–4554. [Google Scholar] [CrossRef]
- Liu, X.M.; Li, Z.; Xie, X.R.; Wang, J.Q.; Qiao, X.; Qiao, X.; Xie, C.Z.; Xu, J.Y. Combination of DNA Damage, Autophagy, and ERK Inhibition: Novel Evodiamine-Inspired Multi-Action Pt(IV) Pro-drugs with High-Efficiency and Low-Toxicity Anti-tumor Activity. J. Med. Chem. 2023, 66, 1852–1872. [Google Scholar] [CrossRef]
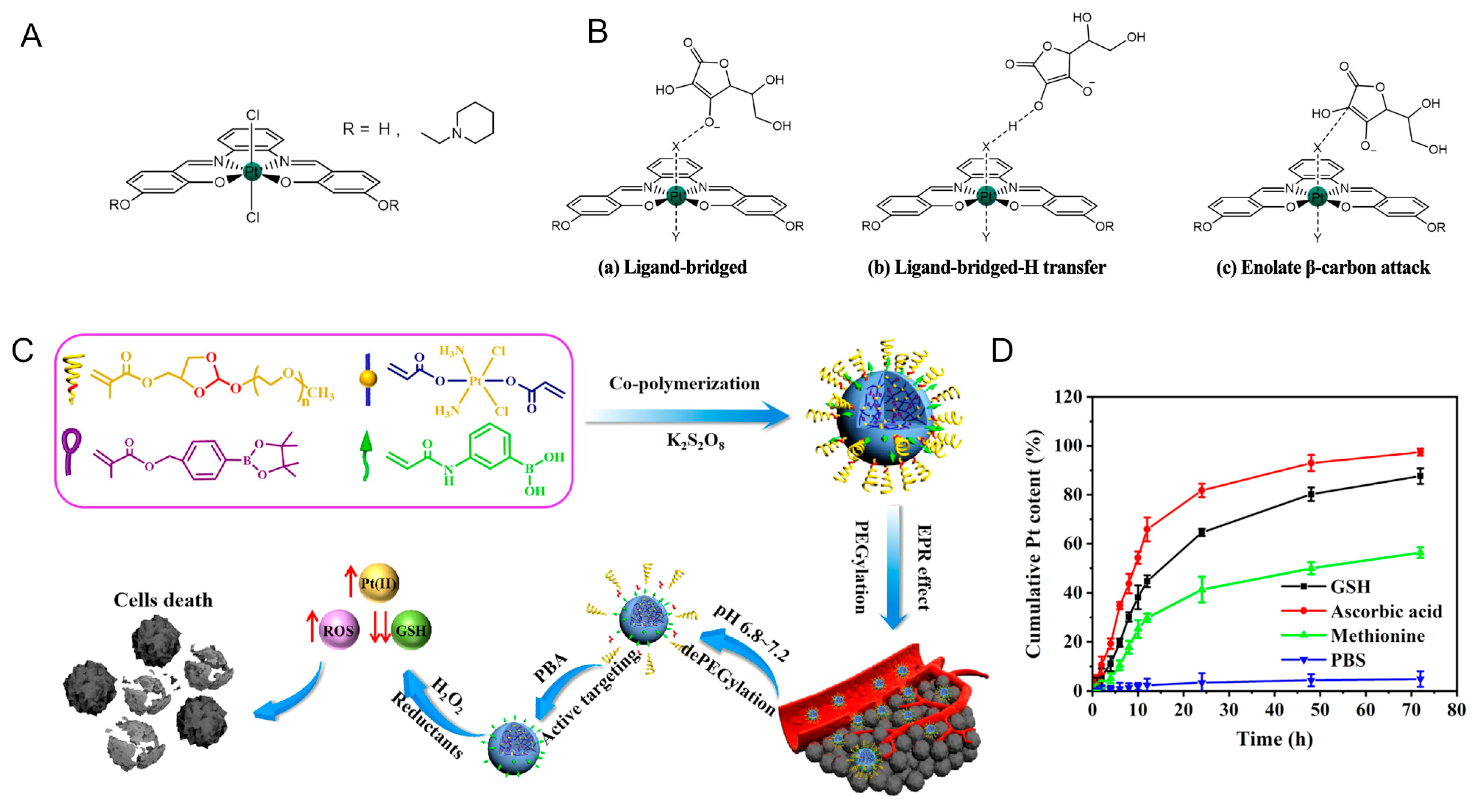
- Vigna, V.; Scoditti, S.; Spinello, A.; Mazzone, G.; Sicilia, E. Anti-cancer Activity, Reduction Mechanism and G-Quadruplex DNA Binding of a Redox-Activated Platinum(IV)–Salphen Complex. Int. J. Mol. Sci. 2022, 23, 15579. [Google Scholar] [CrossRef]
- Bodappa, N. Rapid assessment of platinum disk ultramicroelectrodes’ sealing quality by a cyclic voltammetry approach. Anal. Methods 2020, 12, 3545–3550. [Google Scholar] [CrossRef]
- Ponte, F.; Russo, N.; Sicilia, E. Insights from Computations on the Mechanism of Reduction by Ascorbic Acid of PtIV Pro-drugs with Asplatin and Its Chlorido and Bromido Analogues as Model Systems. Chem.-Eur. J. 2018, 24, 9572–9580. [Google Scholar] [CrossRef]
- Tanimoto, S.; Ichimura, A. Discrimination of Inner- and Outer-Sphere Electrode Reactions by Cyclic Voltammetry Experiments. J. Chem. Educ. 2013, 90, 778–781. [Google Scholar] [CrossRef]
- Ejehi, Z.; Ariafard, A. A computational mechanistic investigation into the reduction of Pt(iv) pro-drugs with two axial chlorides by biological reductants. Chem. Comm. 2017, 53, 1413–1416. [Google Scholar] [CrossRef]
- Dong, J.; Ren, Y.; Huo, S.; Shen, S.; Xu, J.; Tian, H.; Shi, T. Reduction of ormaplatin and cis-diamminetetrachloroplatinum(iv) by ascorbic acid and dominant thiols in human plasma: Kinetic and mechanistic analyses. Dalton Trans. 2016, 45, 11326–11337. [Google Scholar] [CrossRef] [PubMed]
- Lemma, K.; Berglund, J.; Farrell, N.; Elding, L.I. Kinetics and mechanism for reduction of anticancer-active tetrachloroam(m)ine platinum(IV) compounds by glutathione. J. Biol. Inorg. Chem. 2000, 5, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.L.; Bose, R.N. Platinum(II) catalysis and radical intervention in reductions of platinum(IV) anti-tumor drugs by ascorbic acid. J. Inorg. Biochem. 2003, 95, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Poona, G.K.; Mistry, P.; Raynaud, F.I.; Harrap, K.R.; Murrer, B.A.; Barnardb, C.F.J. Determination of metabolites of a novel platinum anti-cancer drug JM216 in human plasma ultrafiltrates. J. Pharm. Biomed. Anal. 1995, 13, 1493–1498. [Google Scholar] [CrossRef]
- Raynaud, F.I.; Mistry, P.; Donaghue, A.; Poon, G.K.; Kelland, L.R.; Barnard, C.F.J.; Murrer, B.A.; Harrap, K.R. Biotransformation of the platinum drug JM216 following oral administration to cancer patients. Cancer Chemother. Pharmacol. 1996, 38, 155–162. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Wexselblatt, E.; Hambley, T.W.; Gibson, D. Pt(iv) analogs of oxaliplatin that do not follow the expected correlation between electrochemical reduction potential and rate of reduction by ascorbate. Chem. Commun. 2012, 48, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Lasorsa, A.; Stuchlíková, O.; Brabec, V.; Natile, G.; Arnesano, F. Activation of Platinum(IV) Pro-drugs by Cytochrome c and Characterization of the Protein Binding Sites. Mol. Pharm. 2016, 13, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Pace, P.; Mosedale, G.; Hodskinson, M.R.; Rosado, I.V.; Sivasubramaniam, M.; Patel, K.J. Ku70 Corrupts DNA Repair in the Absence of the Fanconi Anemia Pathway. Science 2010, 329, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Fang, L.; Chen, F.; Gou, S. Conjugation of platinum(IV) complexes with chlorambucil to overcome cisplatin resistance via a “joint action” mode toward DNA. Eur. J. Med. Chem. 2017, 137, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Babak, M.V.; Zhi, Y.; Czarny, B.; Toh, T.B.; Hooi, L.; Chow, E.K.H.; Ang, W.H.; Gibson, D.; Pastorin, G. Dual-Targeting Dual-Action Platinum(IV) Platform for Enhanced Anti-cancer Activity and Reduced Nephrotoxicity. Angew. Chem. Int. Ed. 2019, 58, 8109–8114. [Google Scholar] [CrossRef]
- Abdelgawwad, A.M.A.; Monari, A.; Tuñón, I.; Francés-Monerris, A. Spatial and Temporal Resolution of the Oxygen-Independent Photoinduced DNA Interstrand Cross-Linking by a Nitroimidazole Derivative. J. Chem. Inf. Model. 2022, 62, 3239–3252. [Google Scholar] [CrossRef]
- Kazmierczak, D.; Jopek, K.; Sterzynska, K.; Nowicki, M.; Rucinski, M.; Januchowski, R. The Profile of MicroRNA Expression and Potential Role in the Regulation of Drug-Resistant Genes in Cisplatin- and Paclitaxel-Resistant Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 526. [Google Scholar] [CrossRef] [PubMed]
- Krasnovskaya, O.O.; Akasov, R.A.; Spector, D.V.; Pavlov, K.G.; Bubley, A.A.; Kuzmin, V.A.; Kostyukov, A.A.; Khaydukov, E.V.; Lopatukhina, E.V.; Semkina, A.S.; et al. Photoinduced Reduction of Novel Dual-Action Riboplatin Pt(IV) Pro-drug. ACS Appl. Mater. Interfaces 2023, 15, 12882–12894. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Kong, X.; Li, X.; Zhang, B.; Deng, Y.; Wang, J.; Duan, C.; Zhang, D.; Liu, W. Current Status of Novel Multifunctional Targeted Pt(IV) Compounds and Their Reductive Release Properties. Molecules 2024, 29, 746. https://doi.org/10.3390/molecules29040746
Xu L, Kong X, Li X, Zhang B, Deng Y, Wang J, Duan C, Zhang D, Liu W. Current Status of Novel Multifunctional Targeted Pt(IV) Compounds and Their Reductive Release Properties. Molecules. 2024; 29(4):746. https://doi.org/10.3390/molecules29040746
Chicago/Turabian StyleXu, Lingwen, Xiangyu Kong, Xinzhi Li, Bin Zhang, Yuxiao Deng, Jinhu Wang, Chonggang Duan, Daizhou Zhang, and Wentao Liu. 2024. "Current Status of Novel Multifunctional Targeted Pt(IV) Compounds and Their Reductive Release Properties" Molecules 29, no. 4: 746. https://doi.org/10.3390/molecules29040746
APA StyleXu, L., Kong, X., Li, X., Zhang, B., Deng, Y., Wang, J., Duan, C., Zhang, D., & Liu, W. (2024). Current Status of Novel Multifunctional Targeted Pt(IV) Compounds and Their Reductive Release Properties. Molecules, 29(4), 746. https://doi.org/10.3390/molecules29040746





