Porous Electropolymerized Films of Ruthenium Complex: Photoelectrochemical Properties and Photoelectrocatalytic Synthesis of Hydrogen Peroxide
Abstract
1. Introduction
2. Results and Discussions
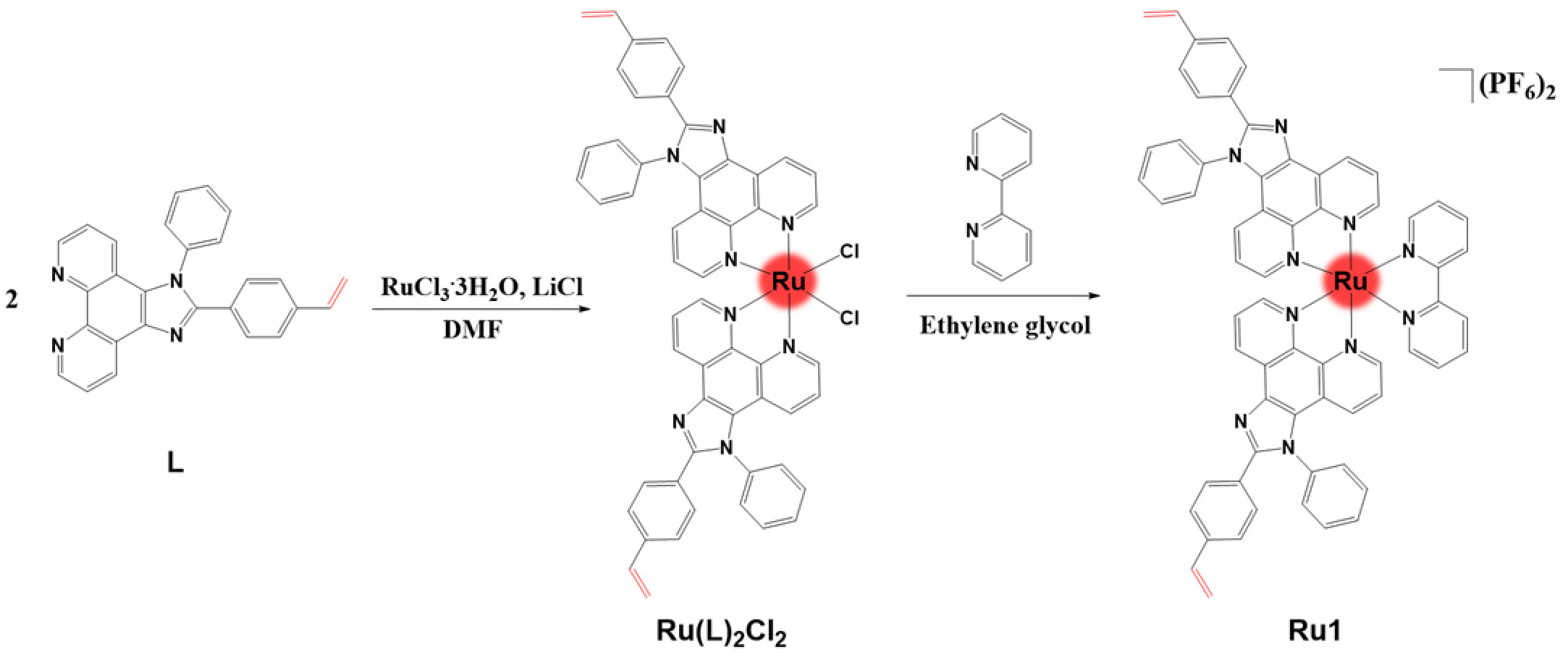
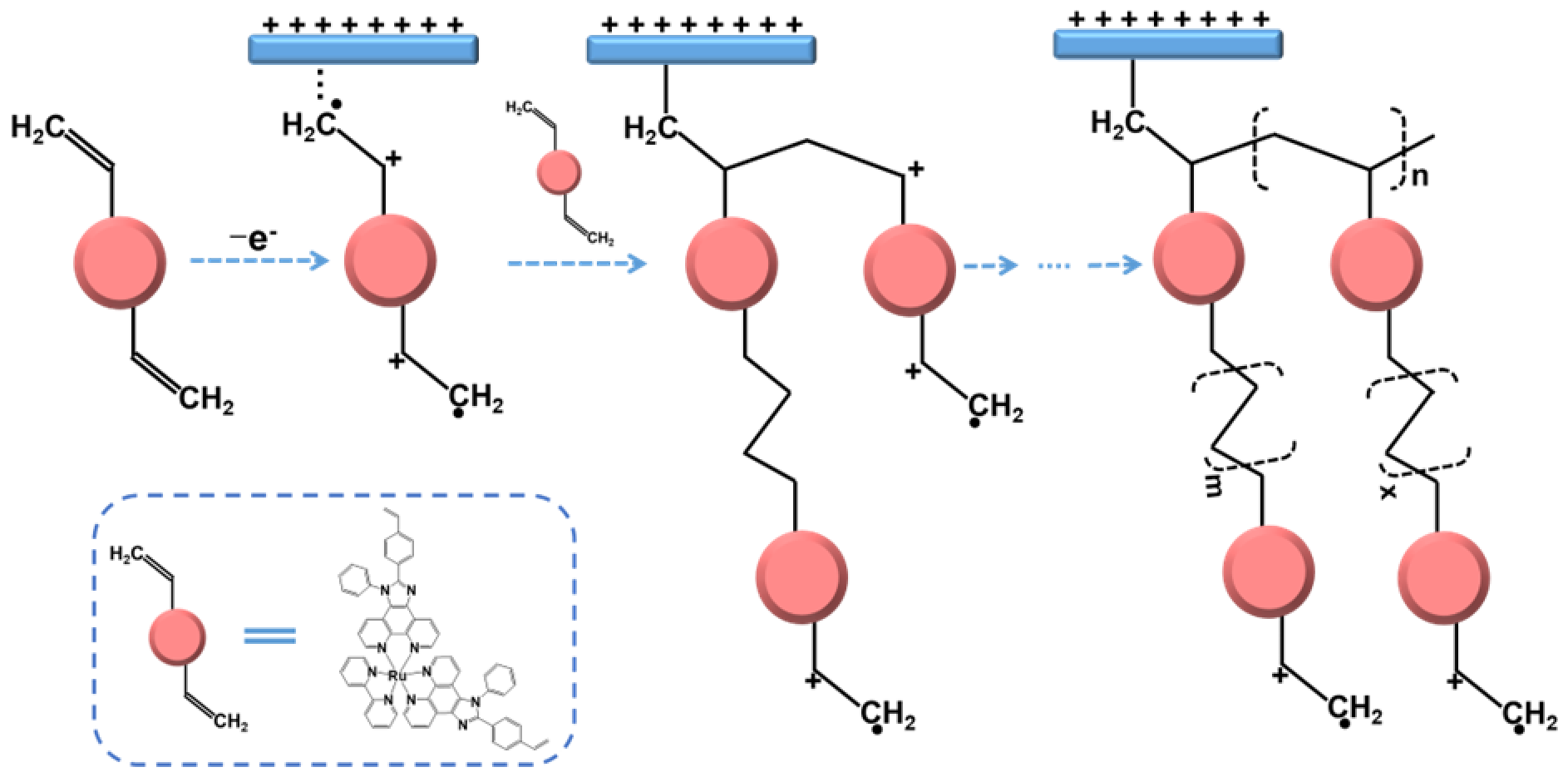
2.1. Synthesis and Electropolymerization
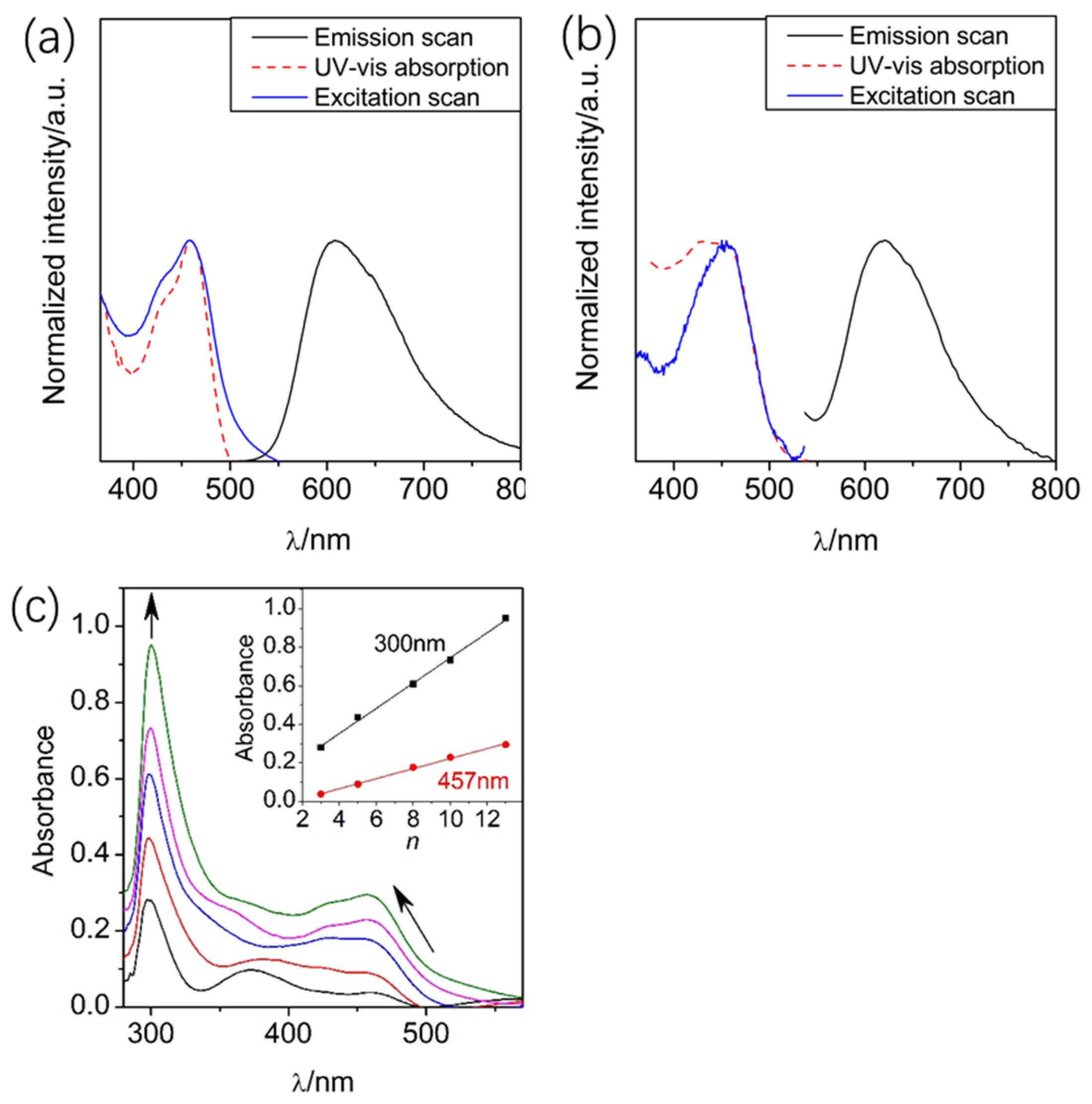
2.2. UV–Vis Absorption and Emission Spectroscopy
2.3. X-Ray Photoelectron Spectroscopy (XPS)
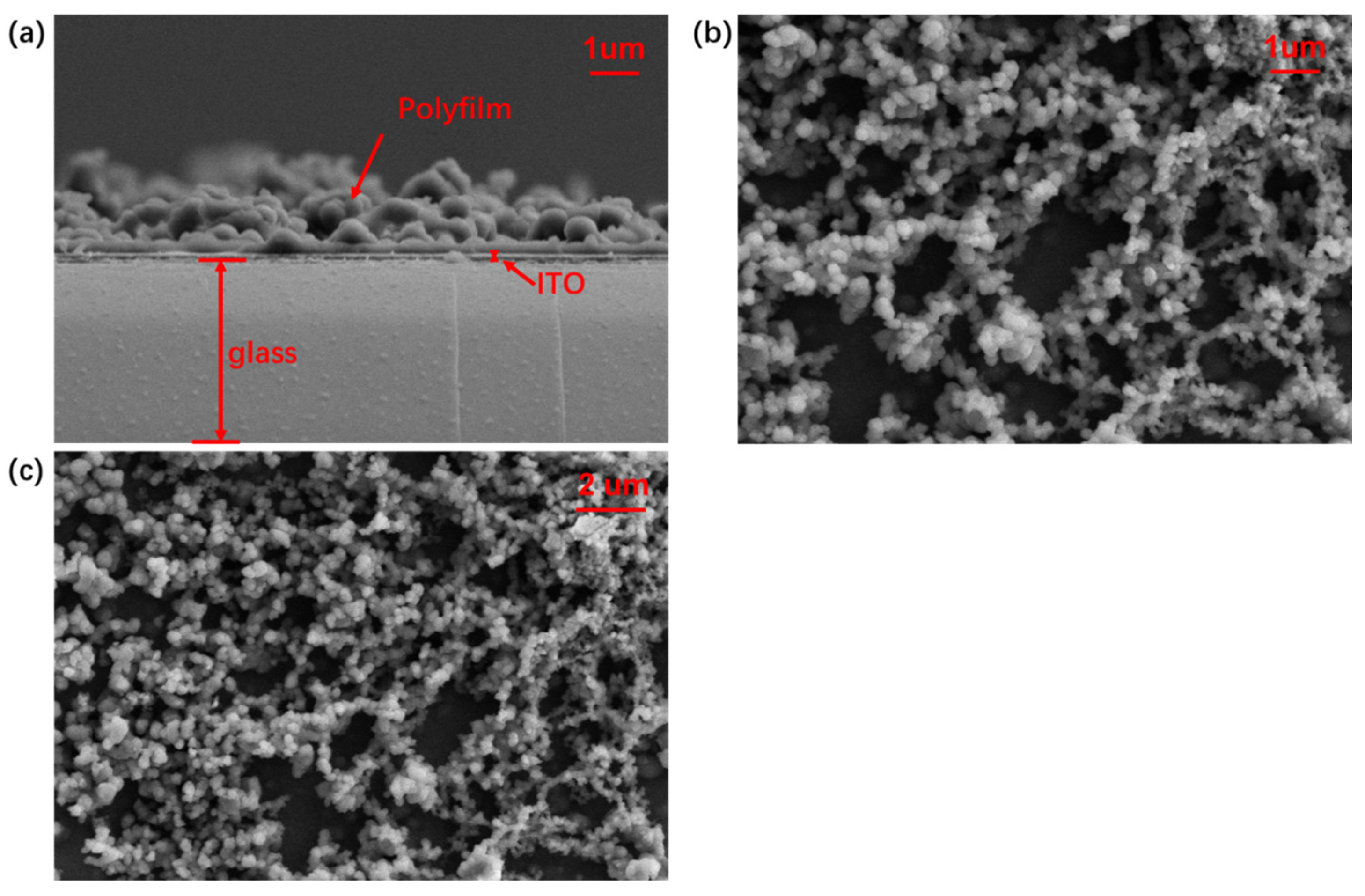
2.4. Scanning Electron Microscopy (SEM)
2.5. Electrochemical Studies
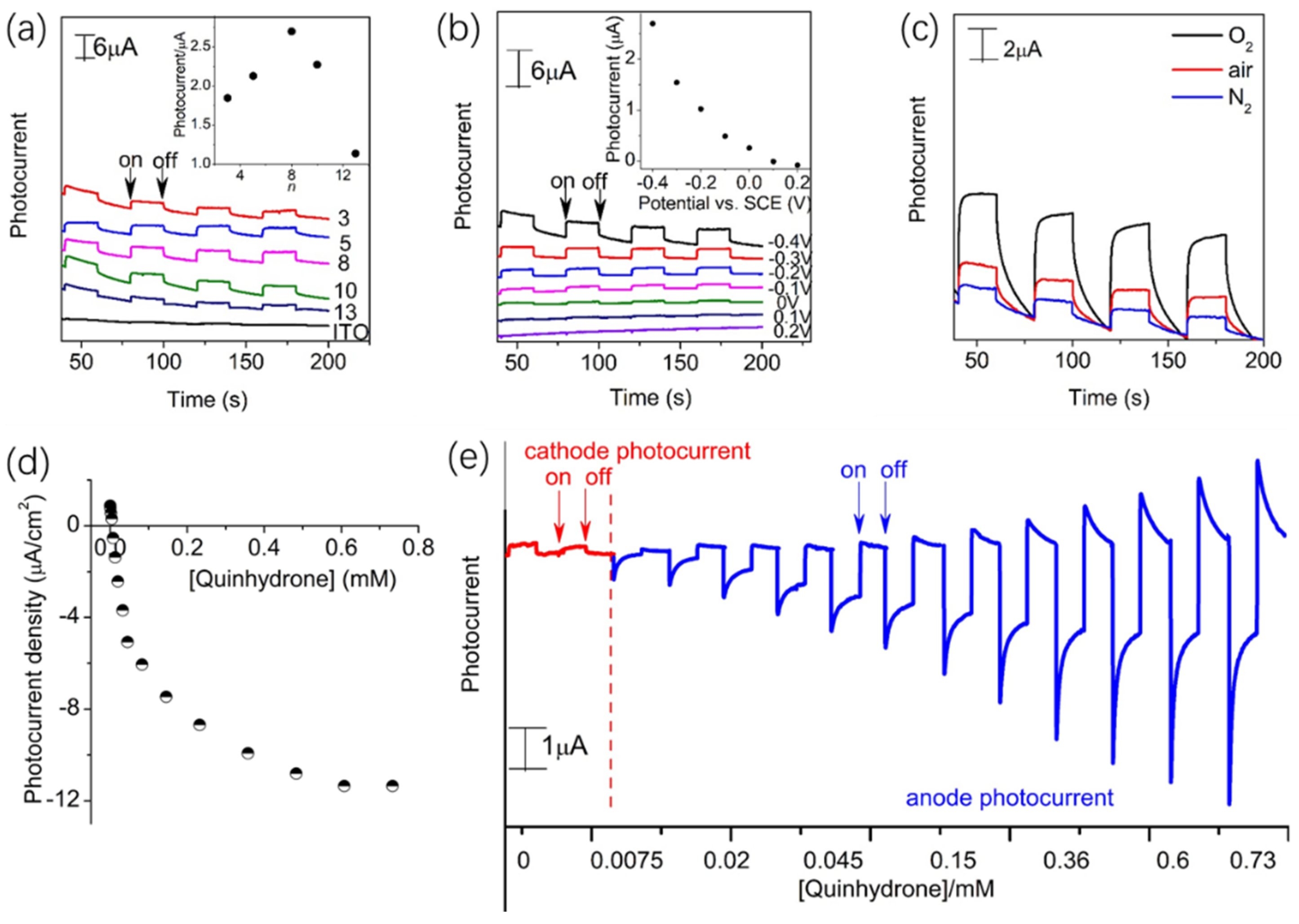
2.6. Photocurrent Generation Behaviors
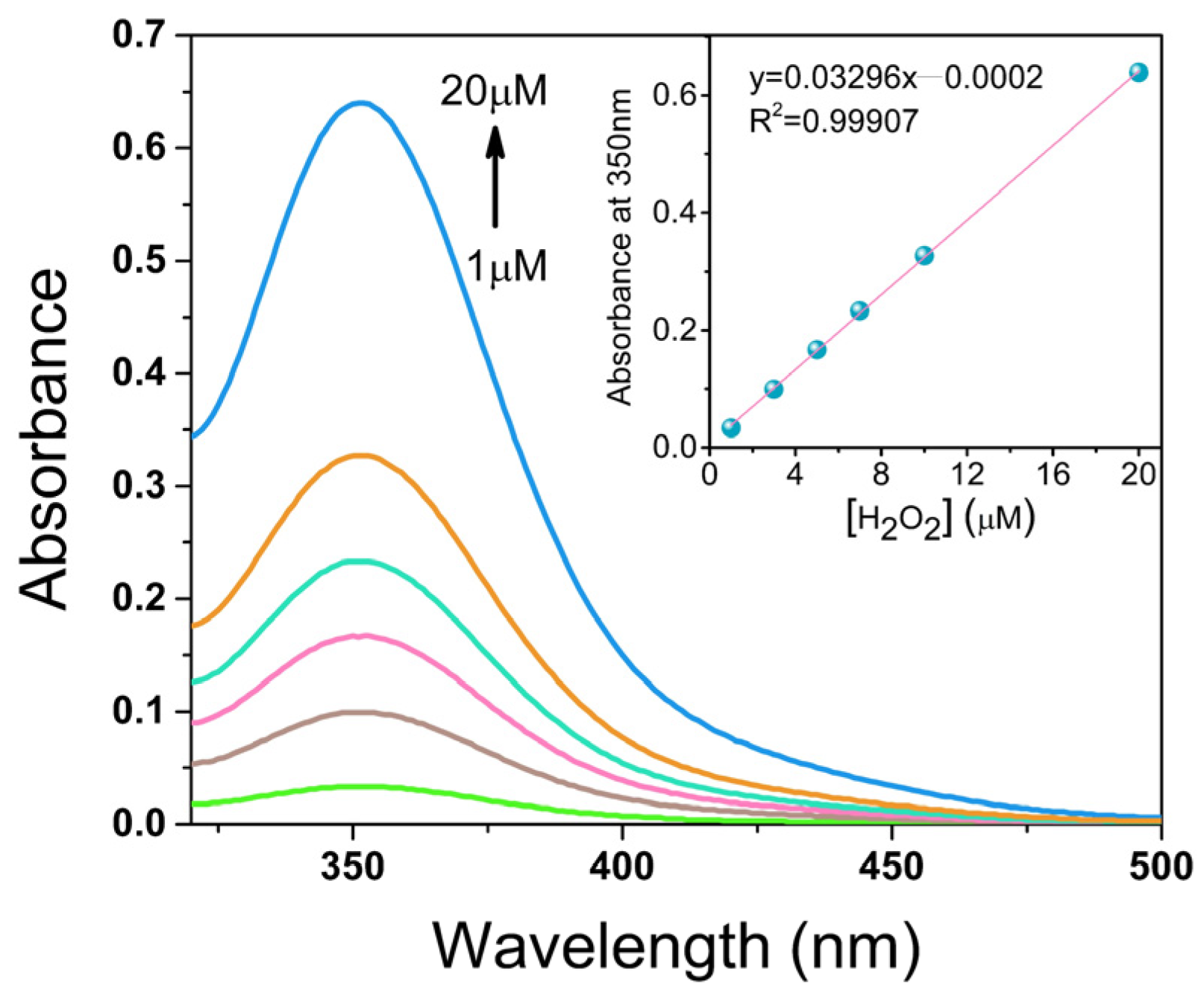
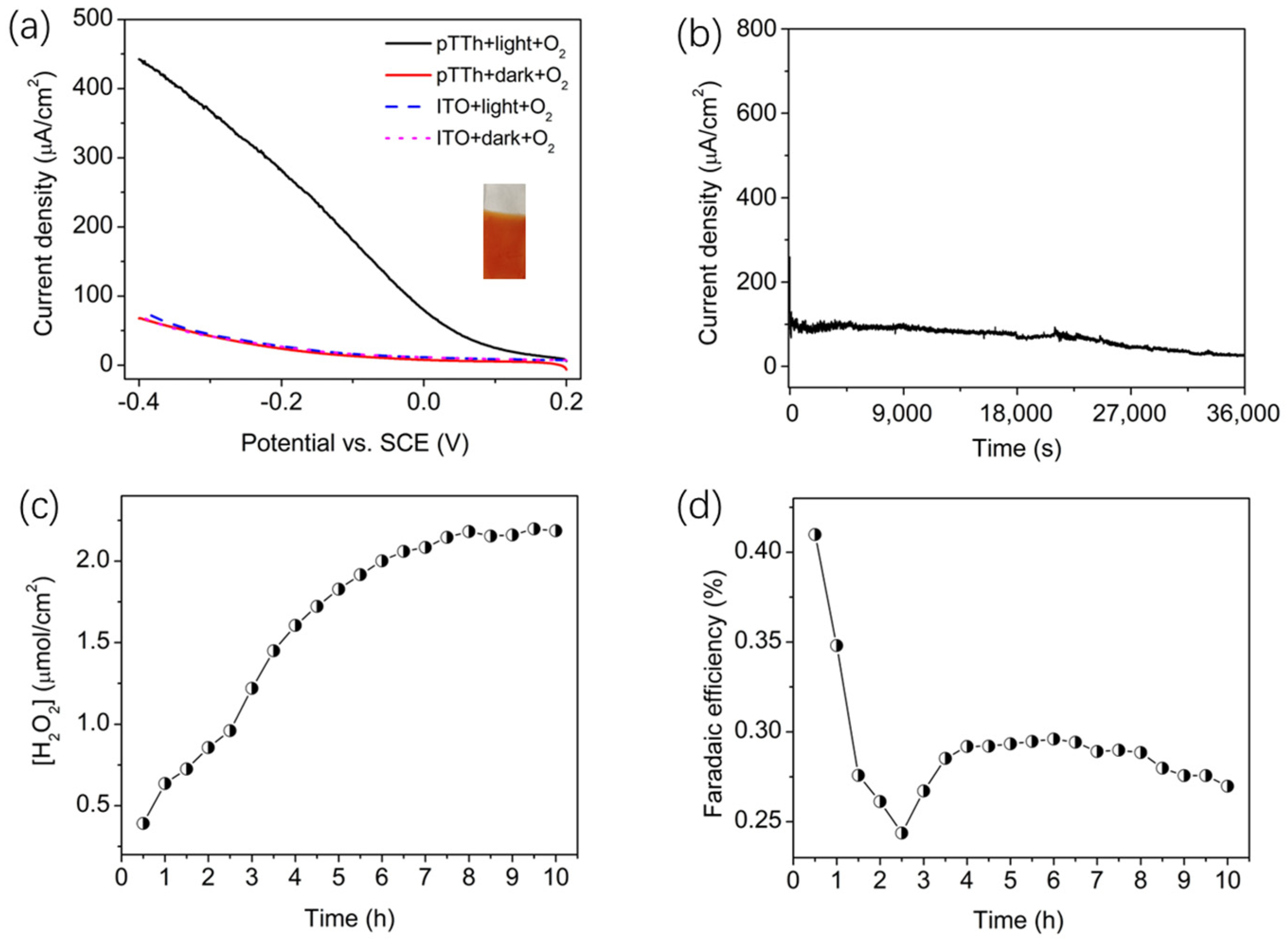
2.7. Photoelectrocatalytic Oxygen Reduction to Hydrogen Peroxide
3. Materials and Methods
3.1. Materials and Common Instrumentation
3.2. Synthesis of Ru(L)2Cl2
3.3. Synthesis of [Ru(L)2(bpy)](PF6)2 (bpy = 2,2′-bipyridine) Ru1
3.4. Electrochemical and Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, M.; Lim, J.S.; Jang, J.W.; Joo, S.H. Bias-Free photoelectrochemical H2O2 production and its in situ applications. ACS EST Eng. 2023, 3, 910–922. [Google Scholar] [CrossRef]
- Han, G.-F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J.-P.; Kim, S.-W.; Kim, S.-J.; Bu, Y.; Fu, Z.; Lu, Y.; et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020, 11, 2209. [Google Scholar] [CrossRef]
- Zhao, X.; Lojewski, B.; Yang, W.; Zhu, T.; Mi, B.; Gao, Z.; Huang, W.; Deng, W. Electrospray as a fabrication tool in organic photovoltaics. Rev. Nanosci. Nanotechnol. 2012, 1, 172–186. [Google Scholar] [CrossRef]
- Materna, K.L.; Jiang, J.; Crabtree, R.H.; Brudvig, G.W. Silatrane anchors for metal oxide surfaces: Optimization for potential photocatalytic and electrocatalytic applications. ACS Appl. Mater. Interfaces 2019, 11, 5602–5609. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, Y.; Du, T.; Wang, Z.; Liang, Z.; Han, Y.; Deng, Y.; Tian, H.; Geng, Y. Diketopyrrolopyrrole-based small molecules for solution-processed n-channel organic thin film transistors. J. Mater. Chem. C 2019, 7, 13939–13946. [Google Scholar] [CrossRef]
- Kumar, R.S.; Govindan, K.; Ramakrishnan, S.; Kim, A.R.; Kim, J.-S.; Yoo, D.J. Fe3O4 nanorods decorated on polypyrrole/reduced graphene oxide for electrochemical detection of dopamine and photocatalytic degradation of acetaminophen. Appl. Surf. Sci. 2021, 556, 149765–149779. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Gimbert-Suriñach, C.; Matheu, R.; Sala, X.; Llobet, A. How to make an efficient and robust molecular catalyst for water oxidation. Chem. Soc. Rev. 2017, 46, 6088–6098. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Wang, R.; Hu, T.; Zou, K.; Liang, R.; Liu, B.; Chen, Z.; Menezes, P.W. Dynamic piezoelectric response facilitating lead-free ceramic to efficiently electrosynthesize hydrogen peroxide via oxygen reduction. Electrochim. Acta 2023, 469, 143215–143223. [Google Scholar] [CrossRef]
- Kim, C.; O Park, S.; Kwak, S.K.; Xia, Z.; Kim, G.; Dai, L. Concurrent oxygen reduction and water oxidation at high ionic strength for scalable electrosynthesis of hydrogen peroxide. Nat. Commun. 2023, 14, 5822–5833. [Google Scholar] [CrossRef]
- Gabunada, J.C.; Vinothkannan, M.; Kim, D.H.; Kim, A.R.; Yoo, D.J. Magnetite nanorods stabilized by polyaniline/reduced graphene oxide as a sensing platform for selective and sensitive non-enzymatic hydrogen peroxide detection. Electroanalysis 2019, 31, 1507–1516. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Bedford, N.M.; Han, Z.; Thomsen, L.; Smith, S.; Amal, R.; Lu, X. Direct insights into the role of epoxy groups on cobalt sites for acidic H2O2 production. Nat. Commun. 2020, 11, 4181–4192. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, P.; Mostaghimi, A.H.B.; Zhao, X.; Denny, S.R.; Lee, J.H.; Gao, H.; Zhang, Y.; Xin, H.L.; Siahrostami, S.; et al. Promoting H2O2 production via 2-electron oxygen reduction by coordinating partially oxidized Pd with defect carbon. Nat. Commun. 2020, 11, 2178–2187. [Google Scholar] [CrossRef]
- Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 2019, 366, 226–231. [Google Scholar] [CrossRef]
- Jakešová, M.; Apaydin, D.H.; Sytnyk, M.; Oppelt, K.; Heiss, W.; Sariciftci, N.S.; Głowacki, E.D. Hydrogen-bonded organic semiconductors as stable photoelectrocatalysts for efficient hydrogen peroxide photosynthesis. Adv. Funct. Mater. 2016, 26, 5248–5254. [Google Scholar] [CrossRef]
- Mase, K.; Yoneda, M.; Yamada, Y.; Fukuzumi, S. Efficient photocatalytic production of hydrogen peroxide from water and dioxygen with bismuth vanadate and a cobalt(II) chlorin complex. ACS Energy Lett. 2016, 1, 913–919. [Google Scholar] [CrossRef]
- Gryszel, M.; Markov, A.; Vagin, M.; Głowacki, E.D. Organic heterojunction photocathodes for optimized photoelectrochemical hydrogen peroxide production. J. Mater. Chem. A 2018, 6, 24709–24716. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Wang, L.; Jin, B.; Yang, X.; Zhang, S.; Park, J.H. Near-complete suppression of oxygen evolution for photoelectrochemical H2O oxidative H2O2 synthesis. J. Am. Chem. Soc. 2020, 142, 8641–8648. [Google Scholar] [CrossRef]
- Jeon, T.H.; Kim, B.; Kim, C.; Xia, C.; Wang, H.; Alvarez, P.J.; Choi, W. Solar photoelectrochemical synthesis of electrolyte-free H2O2 aqueous solution without needing electrical bias and H2. Energy Environ. Sci. 2021, 14, 3110–3119. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Q.; Gou, F.; He, B.; Chen, W.; Zheng, W.; Jiang, X.; Chen, K.; Qi, C.; Ma, D. Gd-doped CuBi2O4/CuO heterojunction film photocathodes for photoelectrochemical H2O2 production through oxygen reduction. Nano Res. 2021, 14, 3439–3445. [Google Scholar] [CrossRef]
- Jung, O.; Pegis, M.L.; Wang, Z.; Banerjee, G.; Nemes, C.T.; Hoffeditz, W.L.; Hupp, J.T.; Schmuttenmaer, C.A.; Brudvig, G.W.; Mayer, J.M. Highly active NiO photocathodes for H2O2 production enabled via outer-sphere electron transfer. J. Am. Chem. Soc. 2018, 140, 4079–4084. [Google Scholar] [CrossRef]
- Li, C.L.; He, J.F.; Xiao, Y.Q.; Li, Y.B.; Delaunay, J.J. Earth-abundant Cu-based metal oxide photocathodes for photoelectrochemical water splitting. Energy Environ. Sci. 2020, 13, 3269–3306. [Google Scholar] [CrossRef]
- Day, N.U.; Wamser, C.C. Poly-tetrakis-5,10,15,20-(4-aminophenyl)porphyrin Films as Two Electron Oxygen Reduction Photoelectrocatalysts for the Production of H2O2. J. Phys. Chem. C 2017, 121, 11076–11082. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, B.; Wang, X.; Ma, W.; Li, D.; Wang, Z.; Dupuis, M.; Shi, J.; Liao, S.; Li, C. Efficient hydrogen peroxide synthesis by metal-free polyterthiophene via photoelectrocatalytic dioxygen reduction. Energy Environ. Sci. 2020, 13, 238–245. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Duan, H.M.; Wang, N.; Wei, D.D.; Mo, L.M.; Wang, N.; Wang, X.P.; Lei, S.L.; Zhang, Y.; Wang, H.Y. Developing positively surface-charged carbon dots as “semiconductor electrolyte” for photo-electrochemical H2O2 production based on oxygen reduction. Chem. Eng. J. 2023, 477, 146903. [Google Scholar] [CrossRef]
- Yin, H.-J.; Zhang, C.; Yang, T.; Yan, D.-P.; Wang, K.-Z. Oxidative electropolymerization films of a styrene-appending ruthenium complex with highly performed electrochemical, solar photoelectric conversion and photoelectrochemical oxygen reduction properties. Electrochim. Acta 2022, 403, 139672–139686. [Google Scholar] [CrossRef]
- Zhong, Y.-W.; Yao, C.-J.; Nie, H.-J. Electropolymerized films of vinyl-substituted polypyridine complexes: Synthesis, characterization, and applications. Coord. Chem. Rev. 2013, 257, 1357–1372. [Google Scholar] [CrossRef]
- Cabrera, C.R.; Abruña, H.D. Electrocatalysis of CO2 reduction at surface modified metallic and semiconducting electrodes. J. Electroanal. Chem. 1986, 209, 101–107. [Google Scholar] [CrossRef]
- Nie, H.-J.; Shao, J.-Y.; Wu, J.; Yao, J.; Zhong, Y.-W. Synthesis and reductive electropolymerization of metal complexes with 5,5′-divinyl-2,2′-bipyridine. Organometallics 2012, 31, 6952–6959. [Google Scholar] [CrossRef]
- Braglia, M.; Ferrari, I.; Pasquini, L.; Djenizian, T.; Sette, M.; Di Vona, M.; Knauth, P. Electrochemical synthesis of thin, dense, and conformal anion exchange membranes with quaternary ammonium groups. Electrochim. Acta 2018, 265, 78–88. [Google Scholar] [CrossRef]
- Akbulut, U.; Fernandez, J.E.; Birke, R.L. Electroinitiated cationic polymerization of styrene by direct electron transfer. J. Polym. Sci. Polym. Chem. 1975, 13, 133–149. [Google Scholar] [CrossRef]
- Torres, G.R.; Dupart, E.; Mingotaud, C.; Ravaine, S. Electrochemical and photoelectrochemical properties of new hybrid langmuir-Blodgett films containing prussian blue and a tris-(bipyridine) ruthenium derivative. J. Phys. Chem. B 2000, 104, 9487–9490. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H.T.; Zhang, Y.Y.; Zhang, C.C.; Cheng, Q.R.; Hou, S.M.; Liao, J.H.; Wang, K.Z. Three-dimensional high-rate electropolymerized thin film with exceptionally high photocurrent based on a triphenylamine-containing ruthenium complex. Electrochim. Acta 2019, 298, 265–278. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, C.X.; Li, Y.J.; Fu, Y.H.; Yin, Z.H.; Gao, L.H.; Wang, K.Z. 3D electropolymerized thin film based on a thiophene-functionalized Ru(II) complex: Electrochemical and photoelectrochemical insights. Inorg. Chem. Front. 2019, 6, 3518–3528. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, S.; Li, X.; Xu, W. Dual-phase phosphorin-glass based on a Sn-P-F-O ultralow-melting glass for warm white light-emitting diodes. RSC Adv. 2017, 7, 36168–36174. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Ferapontova, E.E. Sequence-specific electron transfer mediated by DNA duplexes attached to gold through the alkanethiol linker. J. Phys. Chem. B 2018, 122, 10077–10085. [Google Scholar] [CrossRef]
- Roberts, G.I.; Crowell, C.R. Capacitance energy level spectroscopy of deep-lying semiconductor impurities using schottky barriers. J. Appl. Phys. 1970, 41, 1767–1776. [Google Scholar] [CrossRef]
- Yin, H.; Yang, T.; Wang, K.Z.; Tong, J.; Yu, S.Y. Unusual photoelectrochemical properties of electropolymerized films of a triphenylamine-containing organic small molecule. Langmuir 2019, 35, 12620–12629. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cheng, T.; Li, F.; Huang, C.-H.; Hou, T.; Yu, A.; Zhao, X.; Xu, X. Photophysical studies on the mono- and dichromophoric hemicyanine dyes I photoelectric conversion from the dye modified ITO electrodes. J. Phys. Chem. B 2002, 106, 10020–10030. [Google Scholar] [CrossRef][Green Version]
- Wang, K.; Huang, L.; Gao, L.; Jin, L.; Huang, C. Synthesis, crystal structure, and photoelectric properties of Re(CO)3ClL (L = 2-(1-ethylbenzimidazol-2-yl)pyridine). Inorg. Chem. 2002, 41, 3353–3358. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Muller, E.; Liska, P.; Vlachopoulos, N.; Gratzel, M. Conversion of light to electricity by cis-X2Bis(2,2′-bipyridyl-4,4′-dicarboxylate)-ruthenium(II) charge-transfer sensitizers (X = Cl−, Br−, I−, CN−, and SCN−) on nanocrystalline TiO2 electrodes. J. Am. Chem. Soc. 1993, 115, 6382. [Google Scholar] [CrossRef]
- Imahori, H.; Norieda, H.; Nishimura, Y.; Yamazaki, I.; Higuchi, K.; Kato, N.; Motohiro, T.; Yamada, H.; Tamaki, K.; Arimura, M.; et al. Chain length effect on the structure and photoelectrochemical properties of self-assembled monolayers of porphyrins on gold electrodes. J. Phys. Chem. B 2000, 104, 1253–1260. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic production of H2O2 and organic peroxides in aqueous suspensions of TiO2, ZnO, and desert sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef]
- Lockhart, P.; Little, B.K.; Slaten, B.L.; Mills, G. Photogeneration of H2O2 in water-swollen SPEEK/PVA polymer films. J. Phys. Chem. A 2016, 120, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, C.J.; Dodsworth, E.S.; Monteiro, M.A.; Lever, A.B.P. Bis(2,2′-bipyridine)(1,2-diimino-9,10-anthraquinone)-ruthenium(II) derivatives: A ZINDO analysis of a redox series involving coupled proton and electron transfers. Inorg. Chem. 1999, 38, 5399–5409. [Google Scholar] [CrossRef]
- Meng, T.T.; Xue, L.X.; Wang, H.; Wang, K.Z.; Haga, M.A. pH controllable photocurrent switching and molecular half-subtractor calculation based on a monolayer composite film of a dinuclear Ru(II) complex and graphene oxide. J. Mater. Chem. C 2017, 5, 3390–3396. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Z.B.; Meng, T.T.; Wang, K.Z. Synergistically enhanced photoelectrochemical properties of a layer-by-layer hybrid film based on graphene oxide and a free terpyridyl-grafted ruthenium complex. J. Mater. Chem. A 2015, 3, 3441–3449. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.-J.; Wang, K.-Z. Porous Electropolymerized Films of Ruthenium Complex: Photoelectrochemical Properties and Photoelectrocatalytic Synthesis of Hydrogen Peroxide. Molecules 2024, 29, 734. https://doi.org/10.3390/molecules29030734
Yin H-J, Wang K-Z. Porous Electropolymerized Films of Ruthenium Complex: Photoelectrochemical Properties and Photoelectrocatalytic Synthesis of Hydrogen Peroxide. Molecules. 2024; 29(3):734. https://doi.org/10.3390/molecules29030734
Chicago/Turabian StyleYin, Hong-Ju, and Ke-Zhi Wang. 2024. "Porous Electropolymerized Films of Ruthenium Complex: Photoelectrochemical Properties and Photoelectrocatalytic Synthesis of Hydrogen Peroxide" Molecules 29, no. 3: 734. https://doi.org/10.3390/molecules29030734
APA StyleYin, H.-J., & Wang, K.-Z. (2024). Porous Electropolymerized Films of Ruthenium Complex: Photoelectrochemical Properties and Photoelectrocatalytic Synthesis of Hydrogen Peroxide. Molecules, 29(3), 734. https://doi.org/10.3390/molecules29030734




