An AIE Metal Iridium Complex: Photophysical Properties and Singlet Oxygen Generation Capacity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis of Photophysical Properties of Complexes
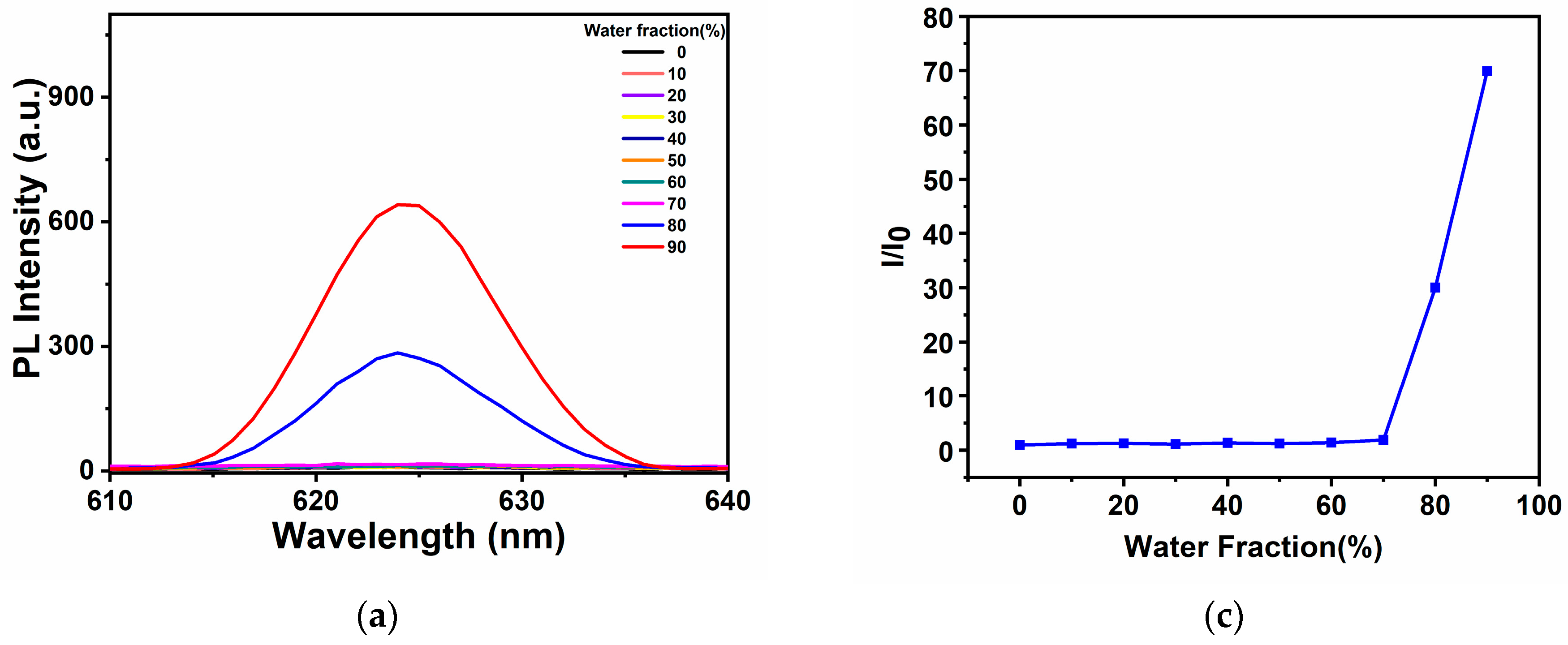
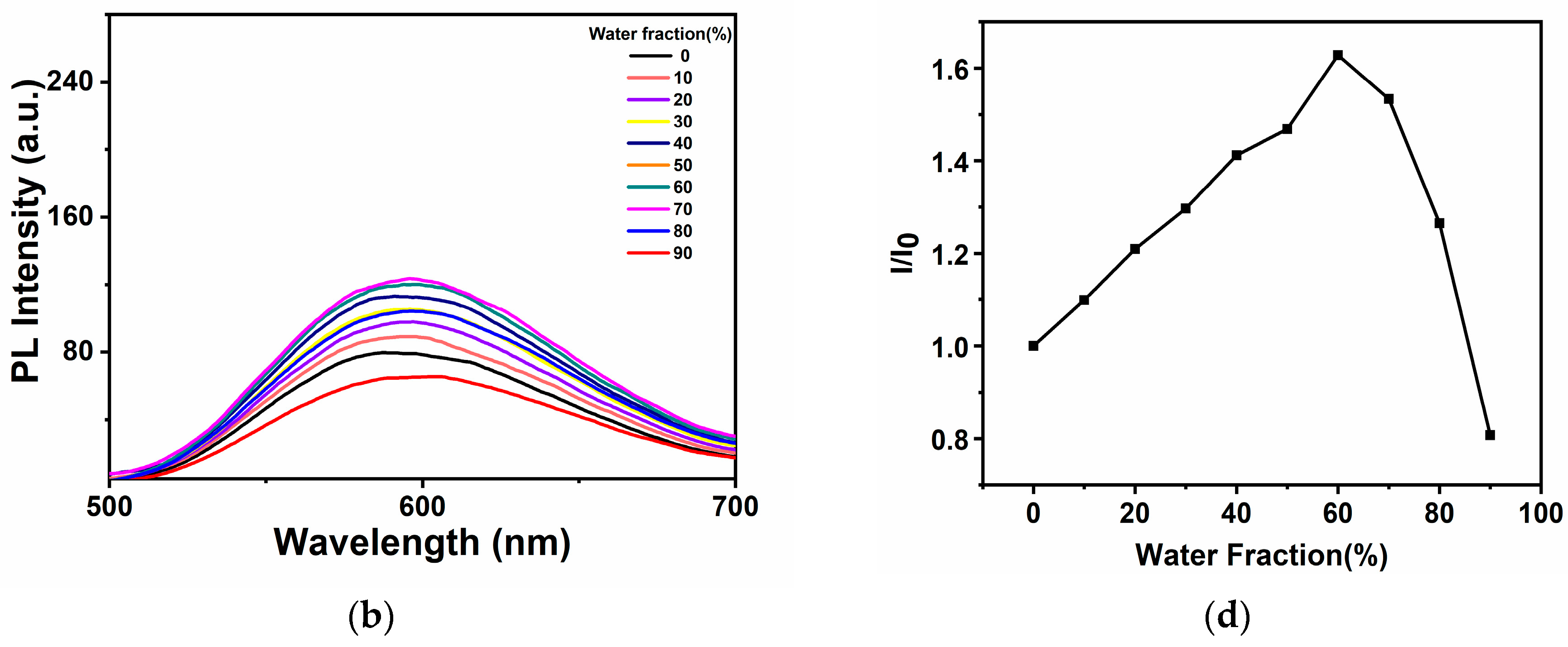
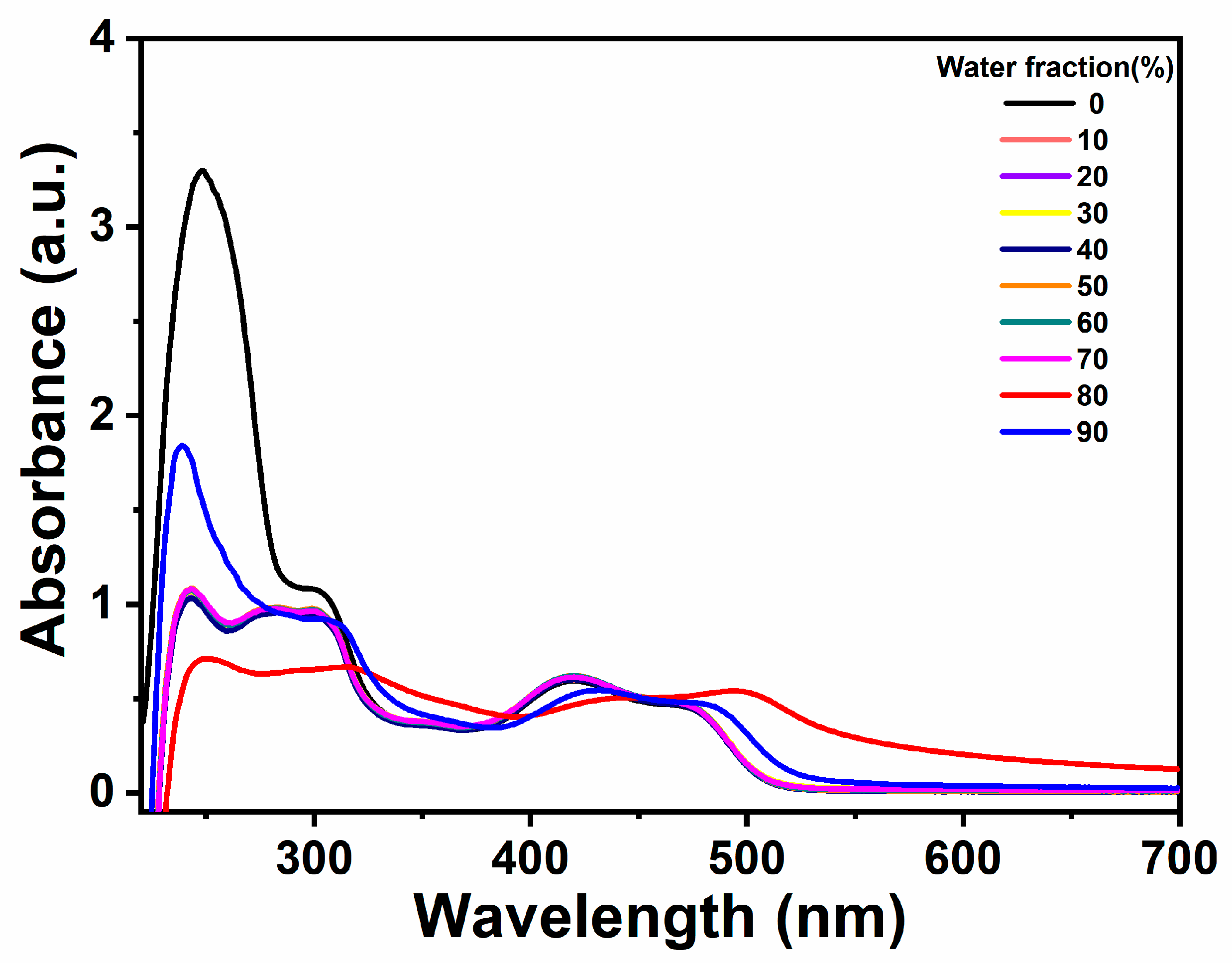
2.2. Analysis of AIE Properties of Complexes
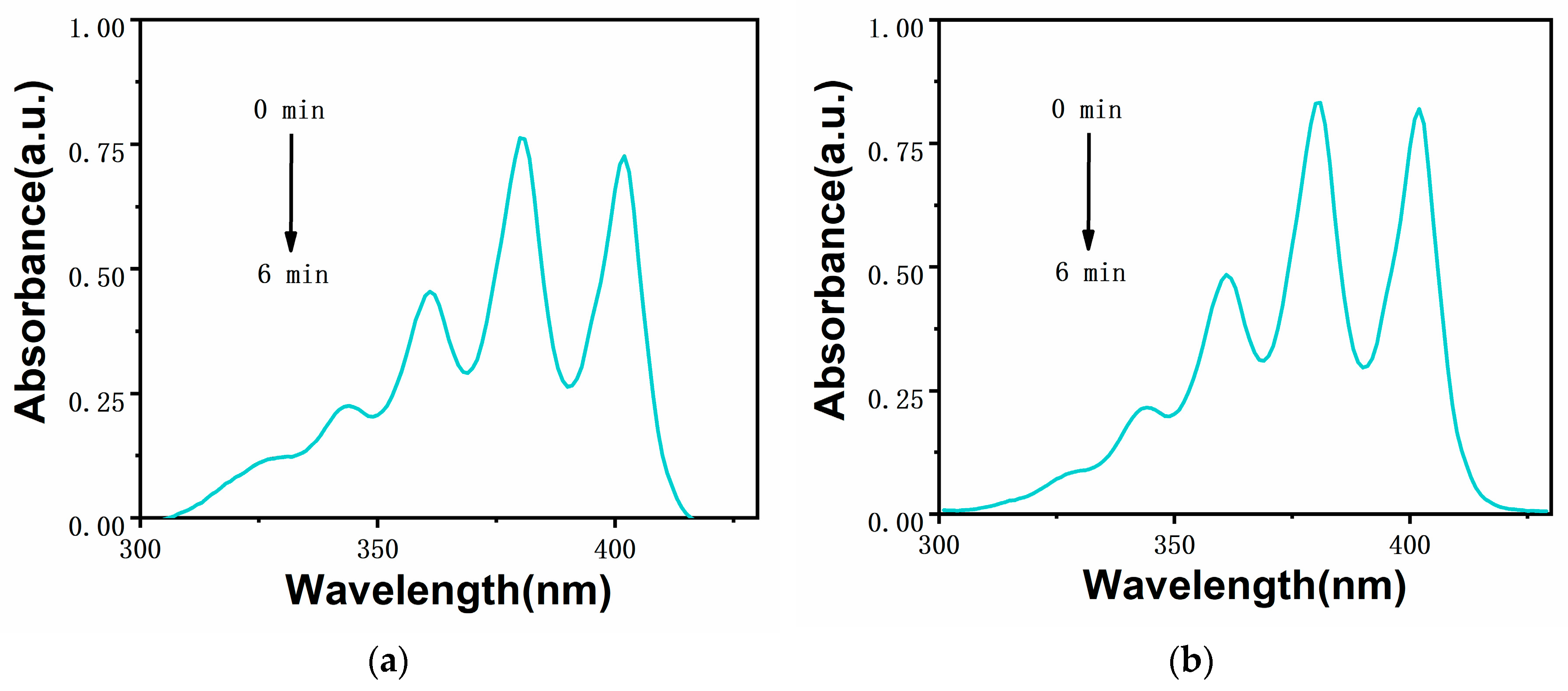
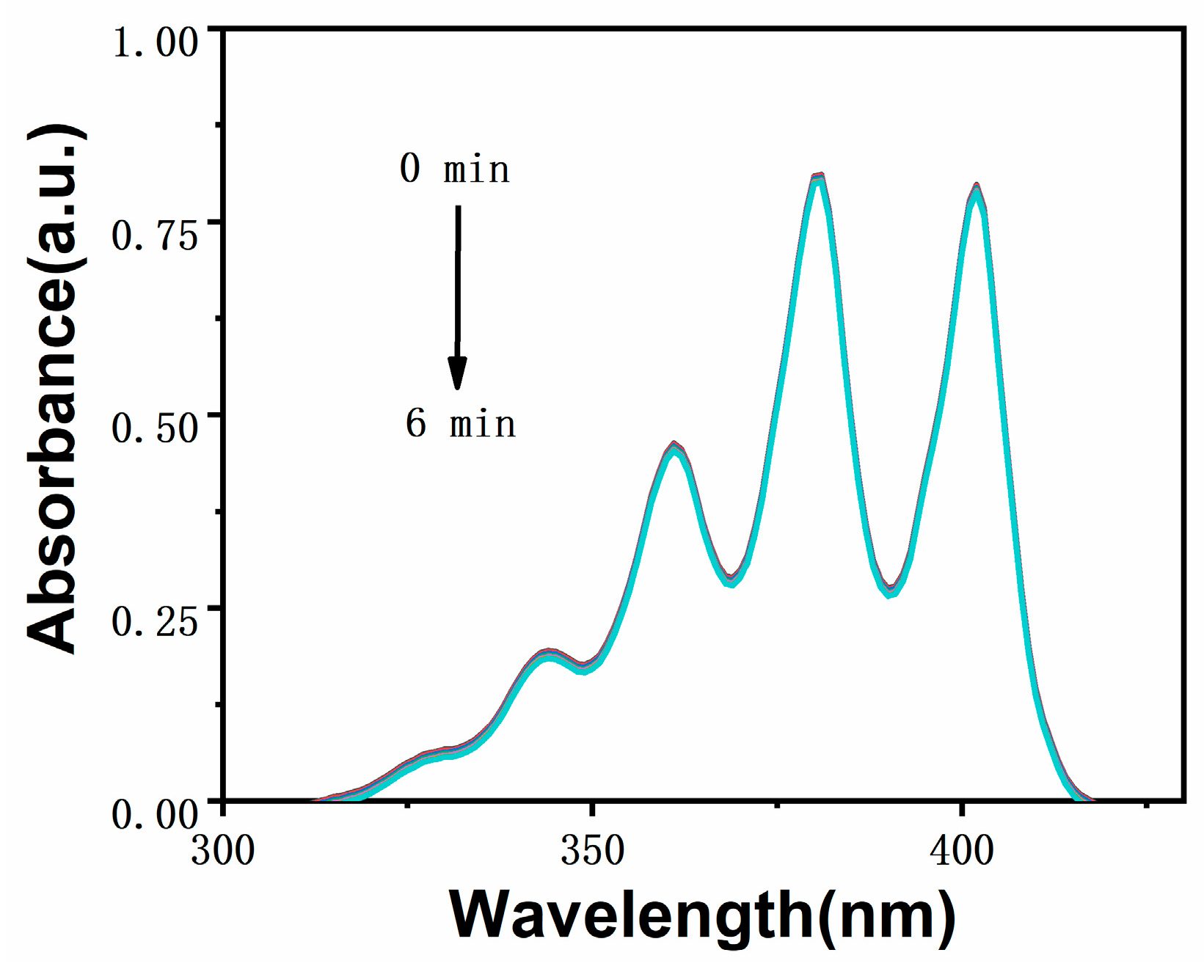
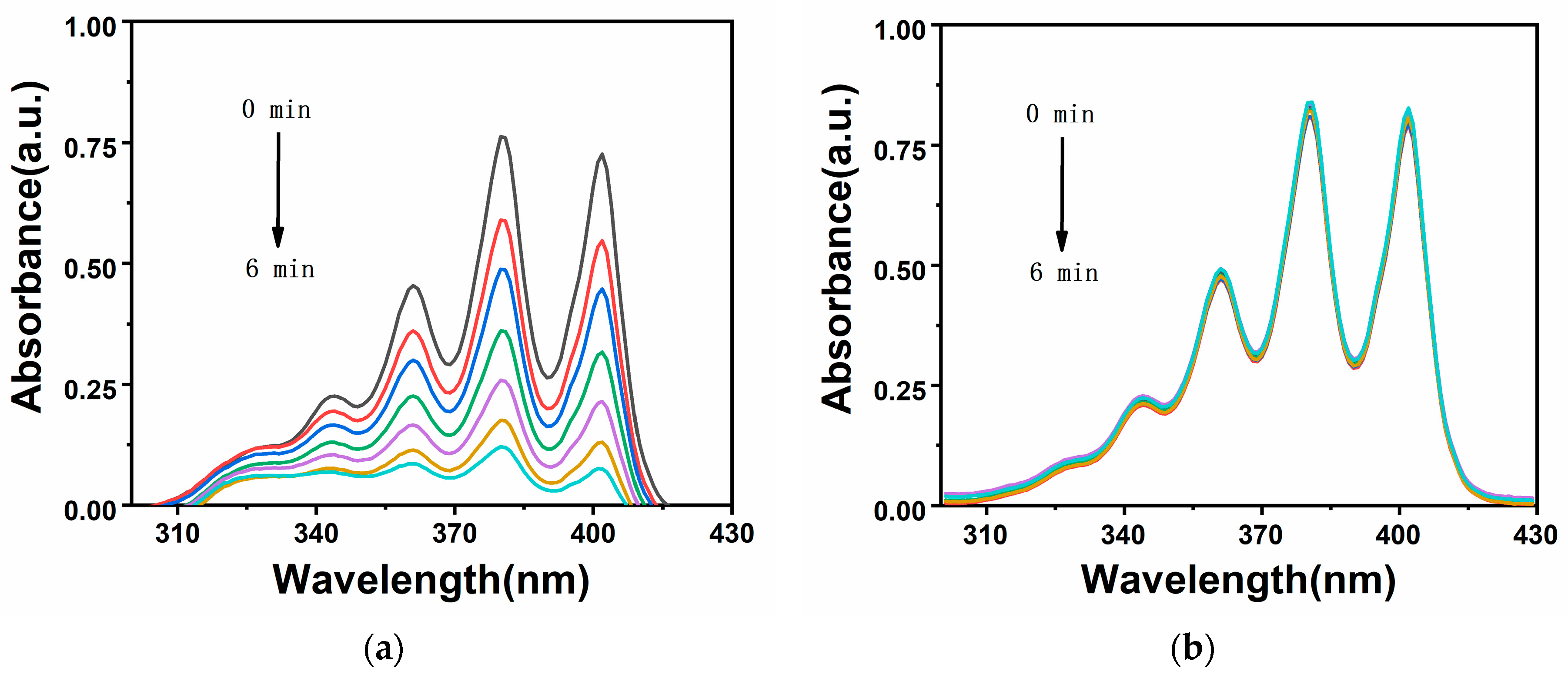
2.3. Analysis of Singlet Oxygen Generation Capacity in Solution
3. Materials and Methods
3.1. General Information
3.2. Solution Preparation Method
3.3. Fluorescent Spectrum Test Method
3.4. Test Method for Singlet Oxygen in Solution
3.5. Synthesis and Characterization of Complexes
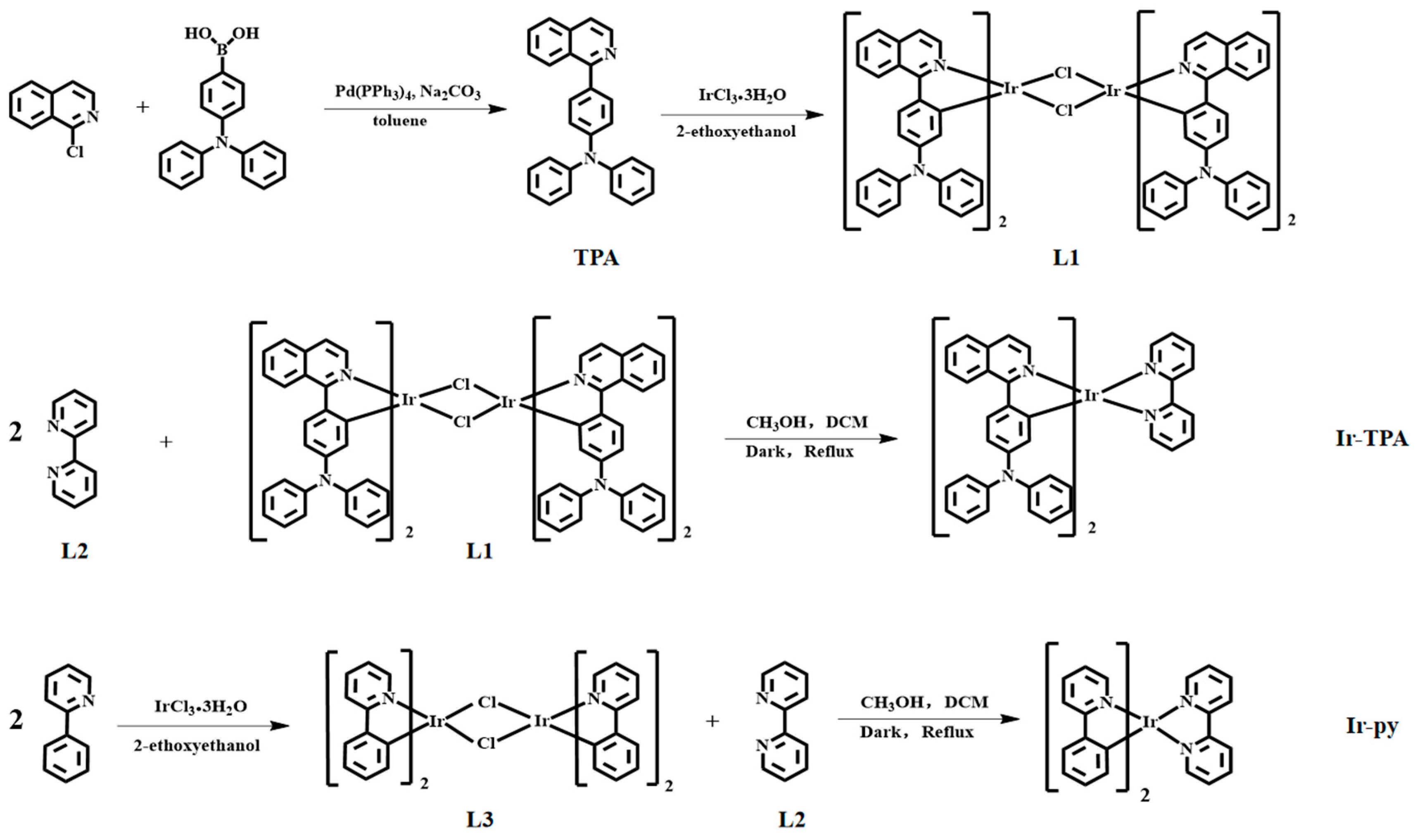
3.5.1. Synthesis of Cyclometalated Ligand TPA
3.5.2. Synthesis of L1
3.5.3. Synthesis of Ir-TPA
3.5.4. Synthesis of Ir-py
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, H.-B.; Qiao, B.; Li, H.; Cao, J.; Luo, Y.; Swamy, K.M.K.; Zhao, J.; Wang, Z.; Lee, J.Y.; Liang, X.-J.; et al. Protein-Activatable Diarylethene Monomer as a Smart Trigger of Noninvasive Control Over Reversible Generation of Singlet Oxygen: A Facile, Switchable, Theranostic Strategy for Photodynamic-Immunotherapy. J. Am. Chem. Soc. 2021, 143, 2413–2422. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińskab, J.; Michel, O.; Kotowski, K.; Kulbacka, J.; et al. Photodynamic therapy—mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 10, 1098–1107. [Google Scholar] [CrossRef]
- Liu, S.; Feng, G.; Tang, B.Z.; Liu, B. Recent advances of AIE light-up probes for photodynamic therapy. Chem. Sci. 2021, 12, 6488–6506. [Google Scholar] [CrossRef]
- Kang, M.; Zhou, C.; Wu, S.; Yu, B.; Zhang, Z.; Song, N.; Lee, M.M.S.; Wang, D.; Wang, L.; Tang, B.Z.; et al. Evaluation of structure-function relationships of aggregation-induced emission luminogens for simultaneous dual applications of specific discrimination and efficient photodynamic killing of gram-positive bacteria. J. Am. Chem. Soc. 2019, 141, 16781–16789. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Xue, K.; Xiao, M.; Suna, Z.; Zhu, C. A receptor-targeting AIE photosensitizer for selective bacterial killing and real-time monitoring of photodynamic therapy outcome. Chem. Commun. 2022, 58, 7058–7061. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cai, X.; Wang, C.; Du, K.; Chen, W.; Feng, F.; Wang, S. Cascade reactions by nitric oxide and hydrogen radical for anti-hypoxia photodynamic therapy using an activatable photosensitizer. J. Am. Chem. Soc. 2021, 143, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Zhang, Z.; Wang, L.; Wang, D.; Tang, B.Z. Triple-jump photodynamic theranostics: MnO2 combined upconversion nanoplatforms involving a Type-I photosensitizer with aggregation-induced emission characteristics for potent cancer treatment. Adv. Mater. 2021, 33, 2103748. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Dai, X.; Dong, C.; Huang, H.; Ding, L.; Chen, Y.; Feng, W. Two-dimensional persistent luminescence “optical battery” for autophagy inhibition-augmented photodynamic tumor nanotherapy. Nano Today 2022, 42, 101362. [Google Scholar] [CrossRef]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Yoon, J. Phthalocyanines as Medicinal Photosensitizers: Developments in the Last Five Years. Coordin. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Wu, J.; Yu, L.; Singh, N.; Yang, K.; Lan, M.; Wang, P.; Kim, J.S. Photodynamic Therapy for Hypoxic Tumors: Advances and Perspectives. Coordin. Chem. Rev. 2021, 438, 213888. [Google Scholar] [CrossRef]
- McAdam, C.A.; McLaughlin, M.G.; Cook, M.J. Functionalization of cyclometalated iridium(iii) polypyridine complexes for the design of intracellular sensors, organelle-targeting imaging reagents, and metallodrugs. Inorg. Chem. Front. 2015, 2, 510–524. [Google Scholar] [CrossRef]
- You, Y.; Nam, W. Photofunctional triplet excited states of cyclometalated Ir(III) complexes: Beyond electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef] [PubMed]
- Kenry; Liu, B. Enhancing the Theranostic Performance of Organic Photosensitizers with Aggregation-Induced Emission. Acc. Mater. Res. 2022, 3, 721–734. [Google Scholar] [CrossRef]
- Ma, D.; Duan, L. Recent Progress in Sublimable Cationic Iridium(III) Complexes for Organic Light-Emitting Diodes. Chem. Rec. 2019, 19, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
- De Soricellis, G.; Fagnani, F.; Colombo, A.; Dragonetti, C.; Roberto, D.; Marinotto, D.; Hartnell, D.H.; Hackett, M.J.; Massi, M.; Carboni, B.; et al. An attractive family of cyclometalated Ir(III) dyes functionalized with tryptophan for potential neuroimaging applications. Dye. Pigment. 2023, 210, 111012. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Yiu, S.-M.; Tang, B.Z.; Lo, K.K.-W. Luminescent neutral cyclometalated iridium(III) complexes featuring a cubic polyhedral oligomeric silsesquioxane for lipid droplet imaging and photocytotoxic applications. Inorg. Chem. 2021, 60, 11672–11683. [Google Scholar] [CrossRef] [PubMed]
- Minaev, B.; Baryshnikovb, G.; Agren, H. Principles of phosphorescent organic light emitting devices. Phys. Chem. Chem. Phys. 2014, 16, 1719. [Google Scholar] [CrossRef]
- Baryshnikov, G.; Minaev, B.; Ågren, H. Theory and Calculation of the Phosphorescence Phenomenon. Chem. Rev. 2017, 117, 6500–6537. [Google Scholar] [CrossRef]
- Jiang, J.; Qian, Y.; Xu, Z.; Lv, Z.; Tao, P.; Xie, M.; Liu, S.; Huang, W.; Zhao, Q. Enhancing Singlet Oxygen Generation in Semiconducting Polymer Nanoparticles Through Fluorescence Resonance Energy Transfer for Tumor Treatment. Chem. Sci. 2019, 10, 5085–5094. [Google Scholar] [CrossRef]
- Cabrera-González, J.; Soriano, J.; Conway-Kenny, R.; Wang, J.; Lu, Y.; Zhao, J.; Nogués, C.; Draper, S.M. Multinuclear Ru(ii) and Ir(iii) Decorated Tetraphenylporphyrins as Efficient PDT Agents. Biomater. Sci. 2019, 7, 3287–3296. [Google Scholar] [CrossRef]
- Liu, S.; Han, J.; Chang, Y.; Wang, W.; Wang, R.; Wang, Z.; Li, G.; Zhu, D.; Bryce, M.R. AIE-active iridium(III) complex integrated with upconversion nanoparticles for NIR-irradiated photodynamic therapy. Chem. Commun. 2022, 58, 10056–10059. [Google Scholar] [CrossRef]
- Hao, B.; Wang, J.; Wang, C.; Xue, K.; Xiao, M.; Lva, S.; Zhu, C. Bridging D-A type photosensitizers with the azo group to boost intersystem crossing for efficient photodynamic therapy. Chem. Sci. 2022, 13, 4139–4149. [Google Scholar] [CrossRef]
- Watson, W.F.; Livingston, R. Concentration quenching of fluorescence in chlorophyll solutions. Nature 1948, 162, 452–453. [Google Scholar] [CrossRef]
- Jia, S.; Yuan, H.; Hu, R. Design and structural regulation of AIE photosensitizers for imaging-guided photodynamic anti-tumor application. Biomater. Sci. 2022, 10, 4443–4457. [Google Scholar] [CrossRef]
- Yan, D.; Wu, Q.; Wang, D.; Tang, B.Z. Innovative Synthetic Procedures for Luminogens Showing Aggregation-Induced Emission. Angew. Chem. Int. Ed. 2021, 60, 15724–15742. [Google Scholar] [CrossRef]
- Luby, B.M.; Walsh, C.D.; Zheng, G. Advanced Photosensitizer Activation Strategies for Smarter Photodynamic Therapy Beacons. Angew. Chem. Int. Ed. Engl. 2019, 58, 2558–2569. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shao, X.; Zhao, J.; Wu, M. Controllable Photodynamic Therapy Implemented by Regulating Singlet Oxygen Efficiency. Adv. Sci. 2017, 4, 1700113–1700134. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Yang, G.; Li, Y.; Zhang, C.; Li, B.; Li, K. Recent advances in AIEgen-based photodynamic therapy and immunotherapy. Adv. Healthc. Mater. 2021, 10, 2101066. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Wang, C.; Wang, J.; Lv, S.; Hao, B.; Zhu, C.; Tang, B.Z. A sensitive and reliable organic fluorescent nanothermometer for noninvasive temperature sensing. J. Am. Chem. Soc. 2021, 143, 14147–14157. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Beyer, B.; Friebe, C.; Winter, A.; Schubert, U.S. Recent Developments in the Application of Phosphorescent Iridium(III) Complex Systems. Adv. Mater. 2009, 21, 4418–4441. [Google Scholar] [CrossRef]
- Xu, B.; Li, W.; He, J.; Wu, S.; Zhu, Q.; Yang, Z.; Wu, Y.-C.; Zhang, Y.; Jin, C.; Lu, P.-Y.; et al. Achieving very bright mechanoluminescence from purely organic luminophores with aggregation-induced emission by crystal design. Chem. Sci. 2016, 7, 5307–5312. [Google Scholar] [CrossRef]
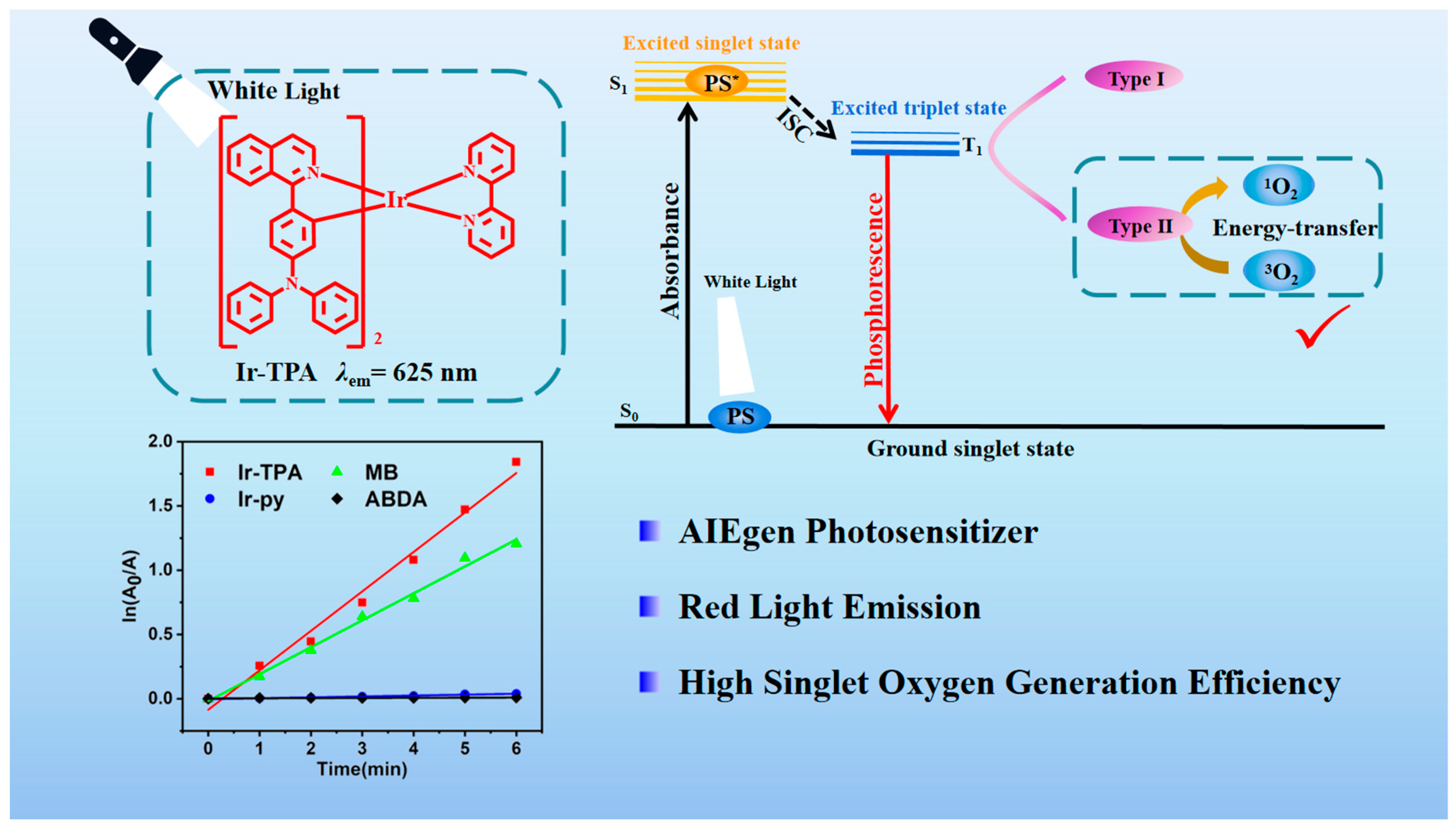
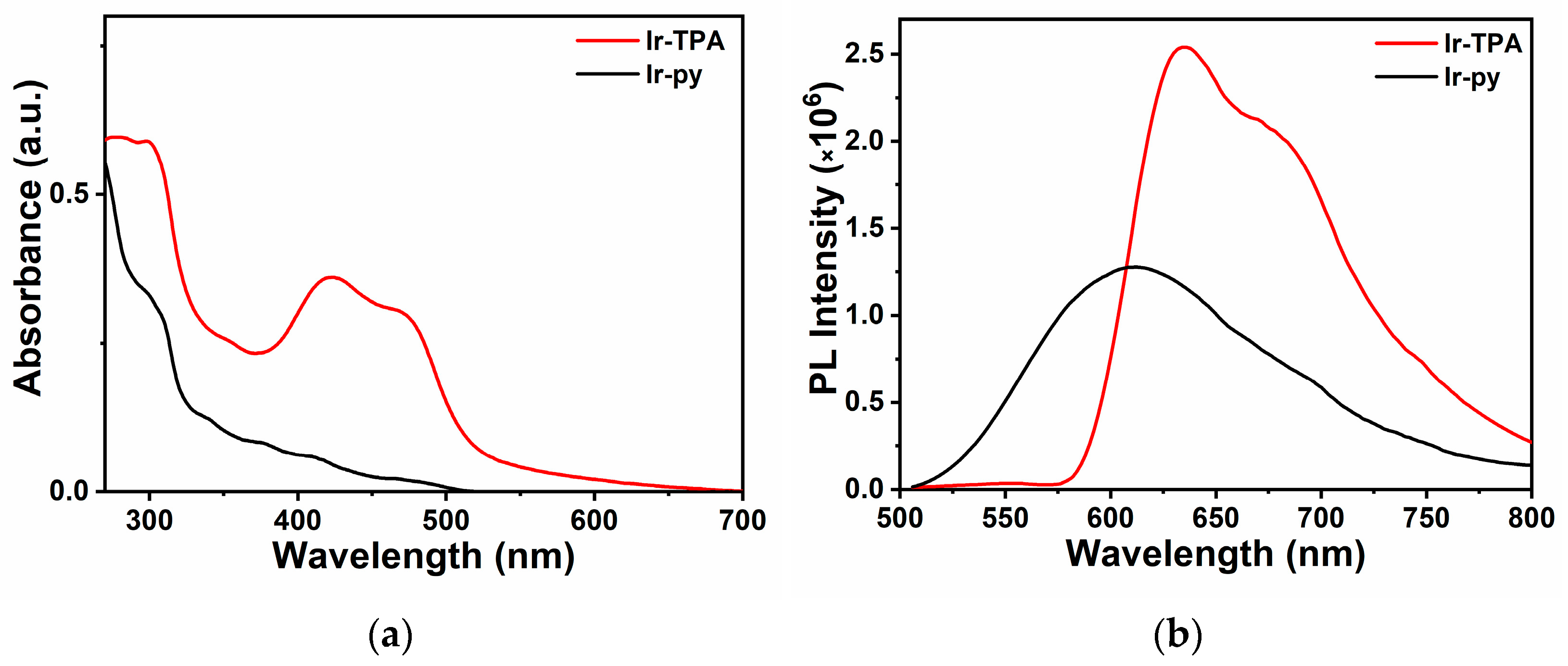



| Λabs (nm) | λem (nm) | Фp (%) | Τp (ns) | Ɛ (m−1·cm−1) | |
|---|---|---|---|---|---|
| Ir-TPA 1 | 300; 425; 470 | 625 | 16.34 | 218.6 | 62,513; 41,680; 34,300 |
| Ir-py 2 | 310 | 590 | 4.29 | 69.5 | 30,247 |
| Linear Fit Slope | Intercept | Singlet Oxygen Yield (%) | |
|---|---|---|---|
| Ir-TPA | 0.30687 | 0.08544 | 83.5 |
| Ir-py | 0.00701 | 0.00288 | 24.0 |
| MB | 0.20943 | 0.01792 | 52.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Liu, S.; Wang, Z.; Shi, C.; Zhang, Q.; Wu, Z.; Li, G.; Zhu, D. An AIE Metal Iridium Complex: Photophysical Properties and Singlet Oxygen Generation Capacity. Molecules 2023, 28, 7914. https://doi.org/10.3390/molecules28237914
Zhu W, Liu S, Wang Z, Shi C, Zhang Q, Wu Z, Li G, Zhu D. An AIE Metal Iridium Complex: Photophysical Properties and Singlet Oxygen Generation Capacity. Molecules. 2023; 28(23):7914. https://doi.org/10.3390/molecules28237914
Chicago/Turabian StyleZhu, Weijin, Shengnan Liu, Ziwei Wang, Chunguang Shi, Qiaohua Zhang, Zihan Wu, Guangzhe Li, and Dongxia Zhu. 2023. "An AIE Metal Iridium Complex: Photophysical Properties and Singlet Oxygen Generation Capacity" Molecules 28, no. 23: 7914. https://doi.org/10.3390/molecules28237914
APA StyleZhu, W., Liu, S., Wang, Z., Shi, C., Zhang, Q., Wu, Z., Li, G., & Zhu, D. (2023). An AIE Metal Iridium Complex: Photophysical Properties and Singlet Oxygen Generation Capacity. Molecules, 28(23), 7914. https://doi.org/10.3390/molecules28237914




