Regioselective C(sp2)-C(sp3) Coupling Mediated by Classical and Rollover Cyclometalation
Abstract
1. Introduction
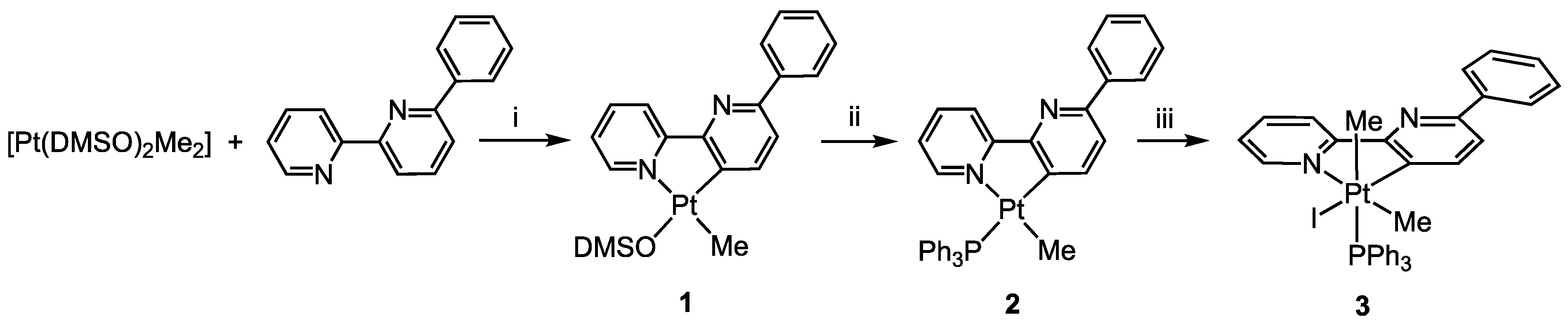
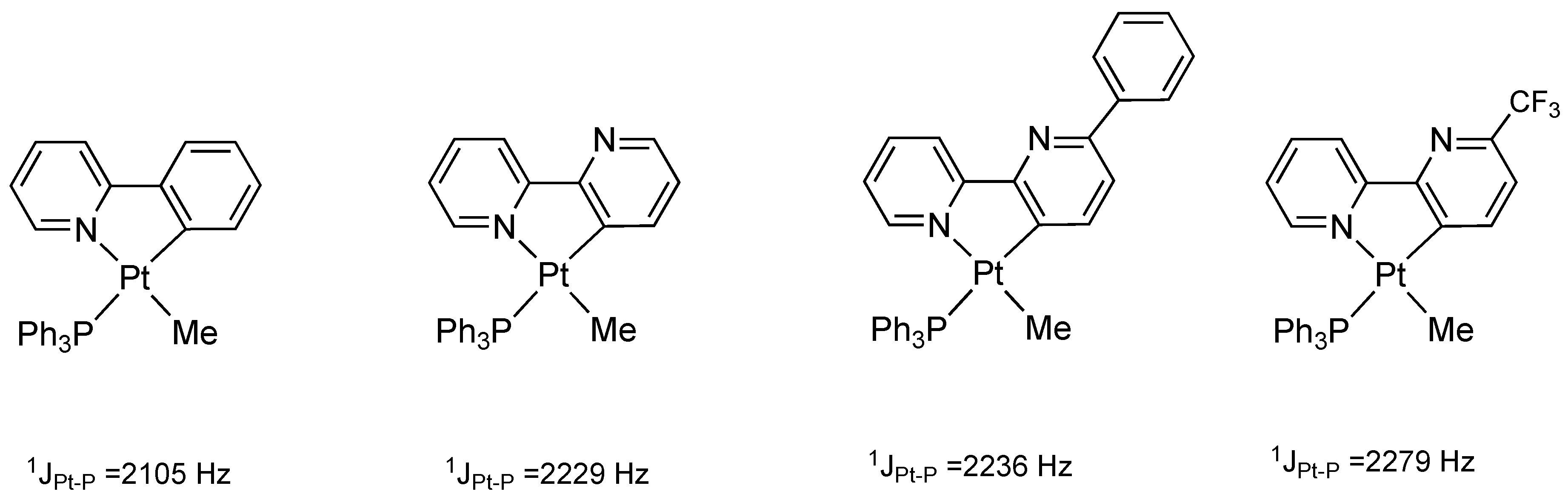
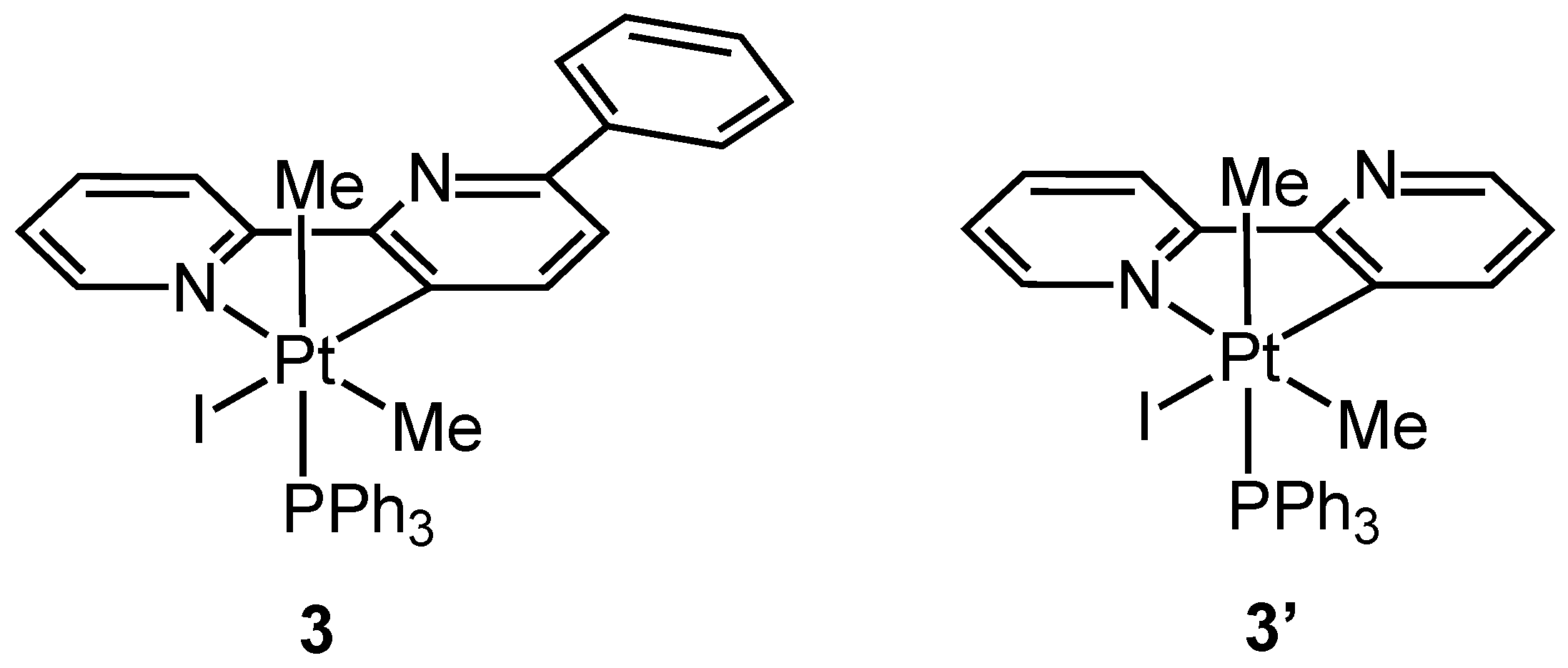
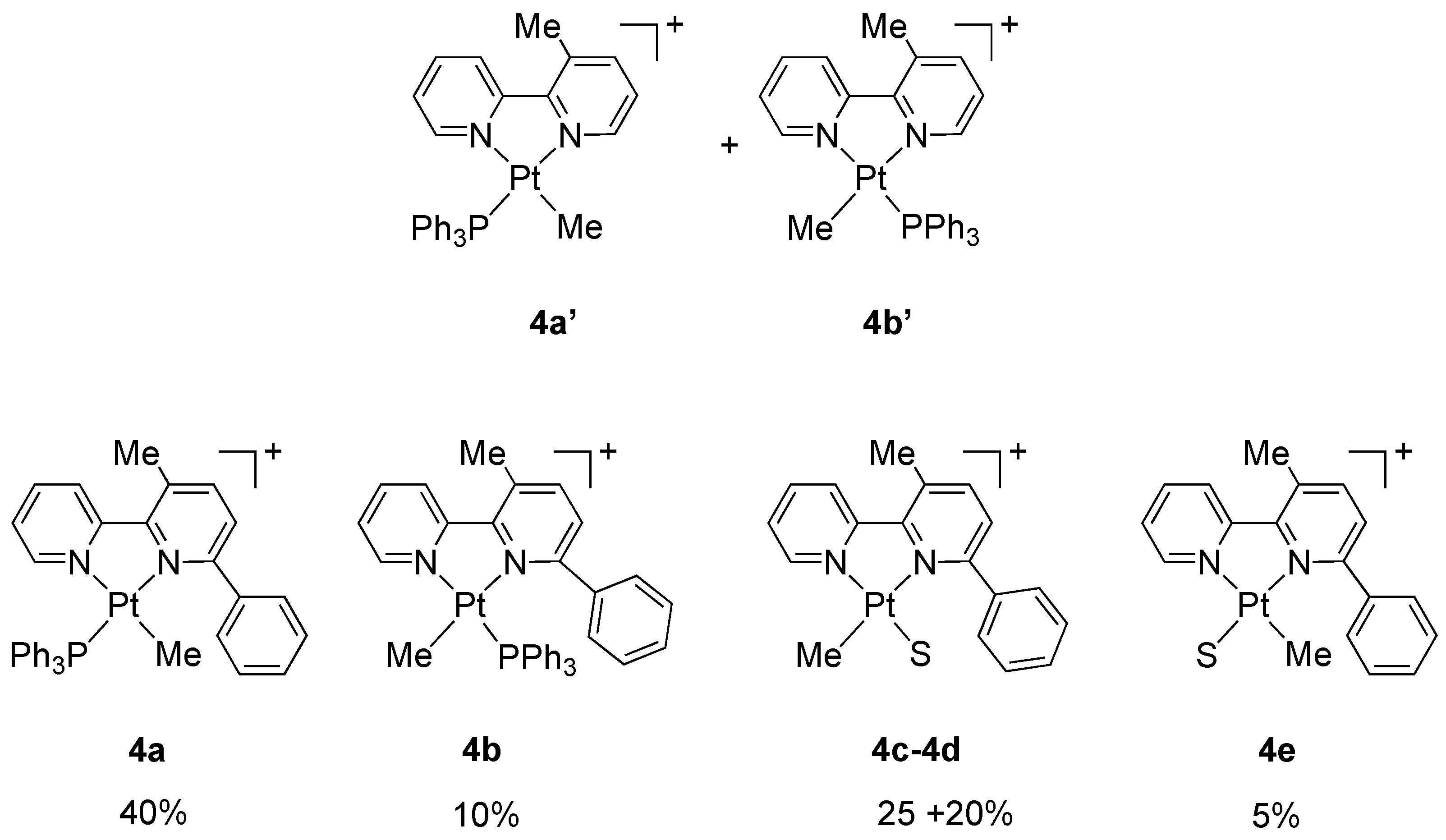
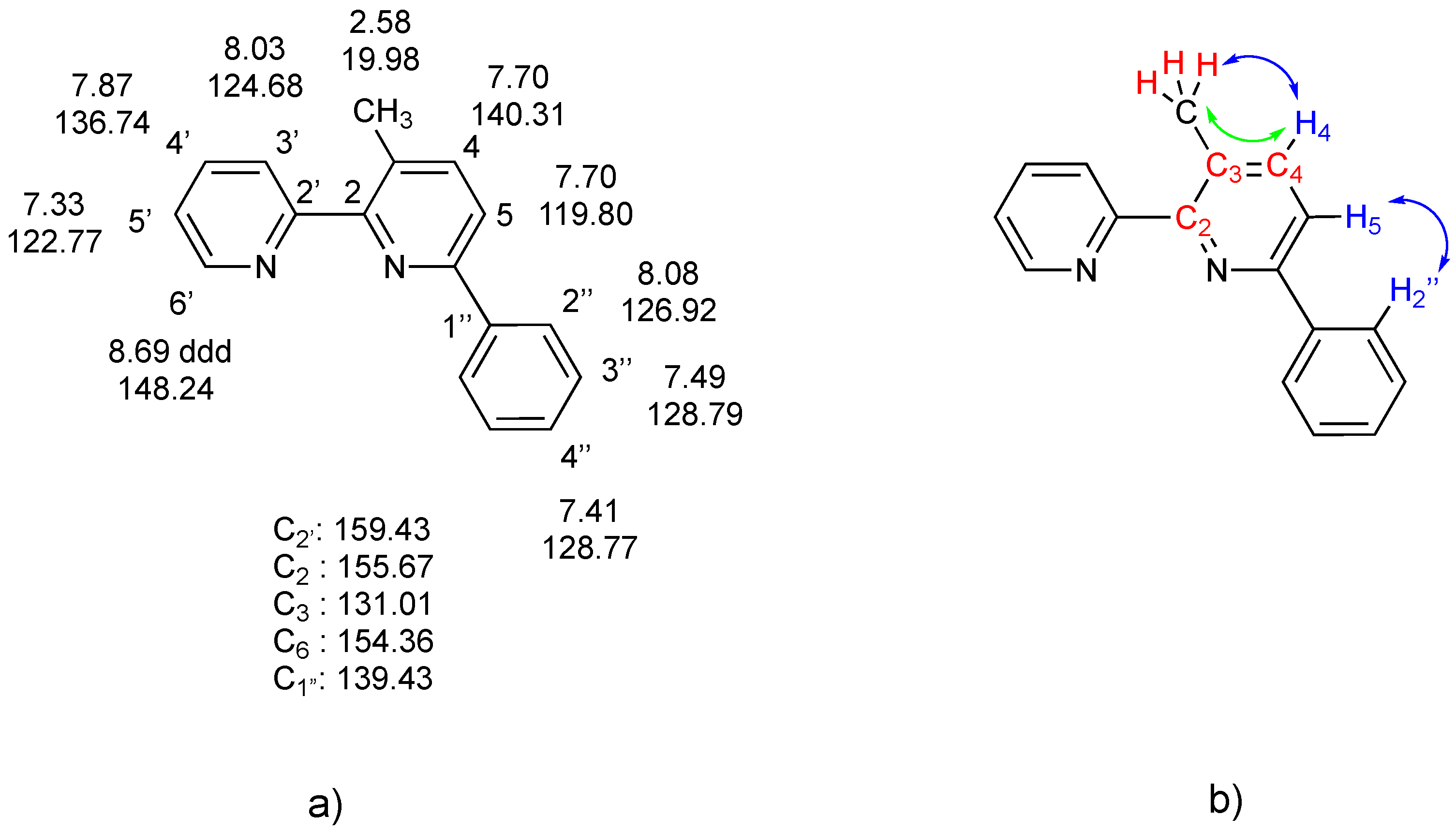
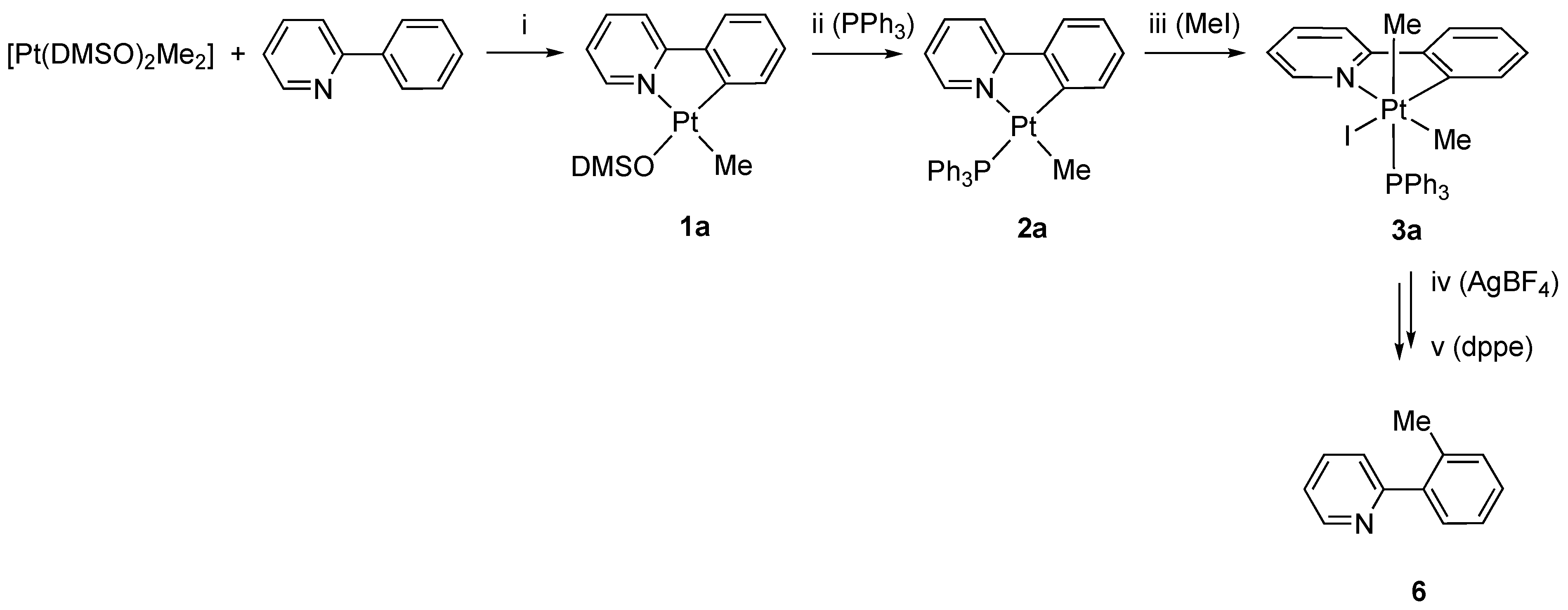
2. Results and Discussion
Treatment of Pt(IV) Complexes with Silver Salts
3. Experimental Section
Preparations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Romine, A.M.; Rubel, C.Z.; Engle, K.M.; Shi, B.F. Transition-Metal-Catalyzed, Coordination-Assisted Functionalization of Nonactivated C(sp3)-H Bonds. Chem. Rev. 2021, 121, 14957–15074. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. The Organometallic Chemistry of the Transition Metals, 7th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2019; ISBN 978-1-119-46588-1. [Google Scholar]
- Albrecht, M. Cyclometalation using d-block transition metals: Fundamental aspects and recent trends. Chem. Rev. 2010, 110, 576–623. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, C.S.; Illam, P.M.; Donthireddy, S.N.R.; Rit, A. Recent Advances in the Syntheses and Catalytic Applications of Homonuclear Ru-, Rh-, and Ir-Complexes of CNHC^C Cyclometalated Ligands. Chem. Eur. J. 2021, 27, 16581–16600. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, S.; Kuhn, F.E.; Casini, A. Cyclometalated complexes of platinum and gold with biological properties: State-of-the-art and future perspectives. Curr. Med. Chem. 2018, 25, 437–461. [Google Scholar] [CrossRef]
- Chakraborty, P.; Oosterhuis, D.; Bonsignore, R.; Casini, A.; Olinga, P.; Scheffers, D.J. An Organogold Compound as Potential Antimicrobial Agent against Drug-Resistant Bacteria: Initial Mechanistic Insights. ChemMedChem 2021, 16, 3060–3070. [Google Scholar] [CrossRef]
- Yoshida, M.; Kato, M. Cation-controlled luminescence behavior of anionic cyclometalated platinum(II) complexes. Coord. Chem. Rev. 2020, 408, 213194. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Lin, S.; Lou, J.; Zhang, Q. Recent development and application of cyclometalated iridium (iii) complexes as chemical and biological probes. Dalton Trans. 2021, 50, 6410–6417. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-catalyzed ligand-directed C-H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Dikova, Y.M.; Yufit, D.S.; Williams, J.A.G. Platinum (IV) Complexes with Tridentate, NNC-Coordinating Ligands: Synthesis, Structures, and Luminescence. Inorg. Chem. 2023, 62, 1306–1322. [Google Scholar] [CrossRef]
- Albrecht, M.; van Koten, G. Platinum Group Organometallics Based on ‘’Pincer’’ Complexes: Sensors, Switches, and Catalysts. Angew. Chem. Int. Ed. 2001, 40, 3750–3781. [Google Scholar] [CrossRef]
- Zucca, A.; Pilo, M.I. Rollover cyclometalation as a valuable tool for regioselective C-H bond activation and functionalization. Molecules 2021, 26, 328. [Google Scholar] [CrossRef] [PubMed]
- Crosby, S.H.; Clarkson, G.J.; Rourke, J.P. Reactions of a Platinum(II) Agostic Complex: Decyclometalation, Dicyclometalation, and Solvent-Switchable Formation of a Rollover Complex. Organometallics 2011, 30, 3603–3609. [Google Scholar] [CrossRef]
- Zucca, A.; Cordeschi, D.; Stoccoro, S.; Cinellu, M.A.; Minghetti, G.; Chelucci, G.; Manassero, M. Platinum(II)-cyclometalated “roll-over” complexes with a chiral pinene-derived 2,2′-bipyridine. Organometallics 2011, 30, 3064–3074. [Google Scholar] [CrossRef]
- Maidich, L.; Zuri, G.; Stoccoro, S.; Cinellu, M.A.; Masia, M.; Zucca, A. Mesoionic complexes of platinum(II) derived from “rollover” cyclometalation: A delicate balance between Pt-C(sp3) and Pt-C(sp2) bond cleavage as a result of different reaction conditions. Organometallics 2013, 32, 438–448. [Google Scholar] [CrossRef]
- Butschke, B.; Schlangen, M.; Schröder, D.; Schwarz, H. “Roll-over” Cyclometalation of 2,2′-Bipyridine Platinum(II) Complexes in the Gas Phase: A Combined Experimental and Computational Study. Chem. Eur. J. 2008, 14, 11050–11060. [Google Scholar] [CrossRef] [PubMed]
- Skapski, A.C.; Sutcliffe, V.F.; Young, G.B. Roll-over’ 3-Metallation of Co-ordinated 2,2′-Bipyridyl in the Thermal Rearrangement of Diaryl(bipyridyl)platinum(II) Complexes: Molecular- Structure of (m-bidyl)[PtPh(Butpy)]2. J. Chem. Soc. Chem. Commun. 1985, 9, 609–611. [Google Scholar] [CrossRef]
- Butschke, B.; Schwarz, H. On the activation of chloromethanes by cyclometalated [Pt(bipy—H)]+ in the gas phase: A mechanistic study. Int. J. Mass Spectrom. 2011, 306, 108–113. [Google Scholar] [CrossRef]
- Butschke, B.; Schlangen, M.; Schröder, D.; Schwarz, H. Platinum (II)-mediated dehydrosulfurization and oxidative carbon—Carbon coupling in the gas-phase decomposition of thioethers. Int. J. Mass Spectrom. 2009, 283, 3–8. [Google Scholar] [CrossRef]
- Kwak, J.; Ohk, Y.; Jung, Y.; Chang, S. Rollover Cyclometalation Pathway in Rhodium Catalysis: Dramatic NHC Effects in the C–H Bond Functionalization. J. Am. Chem. Soc. 2012, 134, 17778–17788. [Google Scholar] [CrossRef]
- Shibata, T.; Takayasu, S.; Yuzawa, S.; Otani, T. Rh(III)-Catalyzed C–H Bond Activation along with “Rollover” for the Synthesis of 4-Azafluorenes. Org. Lett. 2012, 14, 5106–5109. [Google Scholar] [CrossRef]
- Taghizadeh Ghoochany, L.; Kerner, C.; Farsadpour, S.; Menges, F.; Sun, Y.; Niedner-Schatteburg, G.; Thiel, W.R. C–H Activation at a Ruthenium(II) Complex—The Key Step for a Base-Free Catalytic Transfer Hydrogenation? Eur. J. Inorg. Chem. 2013, 24, 4305–4317. [Google Scholar] [CrossRef]
- Zucca, A.; Maidich, L.; Canu, L.; Petretto, G.L.; Stoccoro, S.; Cinellu, M.A.; Clarkson, G.J.; Rourke, J.P. Rollover-assisted C(sp2)-C(sp3) bond formation. Chemistry. A Eur. J. 2014, 20, 5501–5510. [Google Scholar] [CrossRef]
- Maidich, L.; Dettori, G.; Stoccoro, S.; Cinellu, M.A.; Rourke, J.P.; Zucca, A. Electronic and Steric Effects in Rollover C–H Bond Activation. Organometallics 2015, 34, 817–828. [Google Scholar] [CrossRef]
- Minghetti, G.; Stoccoro, S.; Cinellu, M.A.; Soro, B.; Zucca, A. Activation of a C-H Bond in a Pyridine Ring. Reaction of 6-Substituted 2,2′-Bipyridines with Methyl and Phenyl Platinum(II) Derivatives: N,C(3)-“Rollover” Cyclometalation. Organometallics 2003, 22, 4770–4777. [Google Scholar] [CrossRef]
- Anderson, C.M.; Crespo, M.; Jennings, M.C.; Lough, A.J.; Ferguson, G.; Puddephatt, R.J. Competition between Intramolecular Oxidative Addition and Ortho Metalation in Organoplatinum(II) Compounds: Activation of Aryl-Halogen Bonds. Organometallics 1991, 10, 2672–2679. [Google Scholar] [CrossRef]
- Nabavizadeh, S.M.; Habibzadeh, S.; Rashidi, M.; Puddephatt, R.J. Oxidative Addition of Ethyl Iodide to a Dimethylplatinum(II) Complex: Unusually Large Kinetic Isotope Effects and Their Transition-State Implications. Organometallics 2010, 29, 6359–6368. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Rashidi, M.; Masoud Nabavizadeh, S.; Mahmoodi, L.; Hosseini, F.N.; Puddephatt, R.J. Steric and Solvent Effects on the Secondary Kinetic α-Deuterium Isotope Effects in the Reaction of Methyl Iodide with Organoplatinum(II) Complexes: Application of a Second-Order Technique in Measuring the Rates of Rapid Processes. Organometallics 2010, 29, 82–88. [Google Scholar] [CrossRef]
- Rendina, L.M.; Puddephatt, R.J. Oxidative Addition Reactions of Organoplatinum(II) Complexes with Nitrogen-Donor Ligands. Chem. Rev. 1997, 97, 1735–1754. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.J.; Nasrabadi, H.; Nabavizadeh, S.M.; Rashidi, M.; Puddephatt, R.J. Reactivity and Mechanism in the Oxidative Addition of Allylic Halides to a Dimethylplatinum(II) Complex. Organometallics 2012, 31, 2357–2366. [Google Scholar] [CrossRef]
- Nabavizadeh, S.M.; Amini, H.; Jame, F.; Khosraviolya, S.; Shahsavari, H.R.; Hosseini, F.N.; Rashidi, M. Oxidative Addition of MeI to Some Cyclometalated Organoplatinum(II) Complexes: Kinetics and Mechanism. J. Organomet. Chem. 2012, 698, 53–61. [Google Scholar] [CrossRef]
- Maidich, L.; Pilo, M.I.; Rourke, J.P.; Clarkson, G.J.; Canu, P.; Stoccoro, S.; Zucca, A. Classical vs. non-classical cyclometalated Pt(II) complexes. Molecules 2022, 27, 7249. [Google Scholar] [CrossRef]
- Maidich, L.; Zucca, A.; Clarkson, G.J.; Rourke, J.P. Oxidative Addition of MeI to a Rollover Complex of Platinum(II): Isolation of the Kinetic Product. Organometallics 2013, 32, 3371–3375. [Google Scholar] [CrossRef]
- Byers, P.K.; Canty, A.J. Synthetic routes to methylpalladium(II) and dimethylpalladium(Ii) chemistry and the synthesis of new nitrogen donor ligand systems. Organometallics 1990, 9, 210–220. [Google Scholar] [CrossRef]
- Kaupp, M.; Malkina, O.L.; Malkin, V.G.; Pyykkö, P. How do spin-orbit-induced heavy-atom effects on nmr chemical shifts function? Validation of a simple analogy to spin-spin coupling by density functional theory (DFT) calculations on some iodo compounds. Chem. Eur. J. 1998, 4, 118–126. [Google Scholar] [CrossRef]
- Shaw, P.A.; Rourke, J.P. Selective C–C Coupling at a Pt(IV) Centre: 100% Preference for sp2–sp3 over sp3–sp3. Dalton. Trans. 2017, 46, 4768–4776. [Google Scholar] [CrossRef]
- Madison, B.L.; Thyme, S.B.; Keene, S.; Williams, B.S. Mechanistic Study of Competitive sp3-sp3 and sp2-sp3 Carbon−Carbon Reductive Elimination from a Platinum (IV) Center and the Isolation of a C−C Agostic Complex. J. Am. Chem. Soc. 2007, 129, 9538–9539. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Anderson, C.M.; Kfoury, N.; Font-Bardia, M.; Calvet, T. Reductive Elimination from Cyclometalated Platinum(IV) Complexes To Form Csp2–Csp3 Bonds and Subsequent Competition between Csp2–H and Csp3–H Bond Activation. Organometallics 2012, 31, 4401–4404. [Google Scholar] [CrossRef]
- Anderson, C.M.; Crespo, M.; Kfoury, N.; Weinstein, M.A.; Tanski, J.M. Regioselective C–H Activation Preceded by Csp2–Csp3 Reductive Elimination from Cyclometalated Platinum(IV) Complexes. Organometallics 2013, 32, 4199–4207. [Google Scholar] [CrossRef]
- Wang, T.; Alfonso, B.J.; Love, J.A. Platinum(II)-Catalyzed Cross-Coupling of Polyfluoroaryl Imines. Org. Lett. 2007, 9, 5629–5631. [Google Scholar] [CrossRef]
- Wang, T.; Love, J. Insight into the mechanism of platinum-catalyzed cross-coupling of polyfluoroaryl imines. Organometallics 2008, 27, 3290–3296. [Google Scholar] [CrossRef]
- Buckley, H.L.; Sun, A.D.; Love, J.A. User-Friendly Precatalyst for the Methylation of Polyfluoroaryl Imines. Organometallics 2009, 28, 6622–6624. [Google Scholar] [CrossRef]
- Wang, T.; Keyes, L.; Patrick, B.O.; Love, J. Exploration of the Mechanism of Platinum(II)-Catalyzed C–F Activation: Characterization and Reactivity of Platinum(IV) Fluoroaryl Complexes Relevant to Catalysis. Organometallics 2012, 31, 1397–1407. [Google Scholar] [CrossRef]
- Fang, Y.-Q.; Hanan, G.S. Rapid and Efficient Synthesis of Functionalized Bipyridines. Synlett 2003, 6, 852–854. [Google Scholar] [CrossRef]
- Guetz, C.; Luetzen, A. Synthesis of 2, 2′-Bipyridines via Suzuki-Miyaura Cross-Coupling. Synthesis 2010, 1, 85–90. [Google Scholar]
- Sankio Chemical Co Ltd. Process for the Production of 2-Pyridylpyridine Derivatives. Current Patent Assignee: FUJIFILM HOLDINGS CORP. Patent US6504032 B1, 7 January 2003. [Google Scholar]
- Hogg, A.; Wheatley, M.; Domingo-Legarda, P.; Carral-Menoyo, A.; Cottam, N.; Larrosa, I. Ruthenium-Catalyzed Monoselective C−H Methylation and d3-Methylation of Arenes. J. Am. Chem. Soc. Au 2022, 2, 2529–2538. [Google Scholar] [CrossRef]
- Longman Scientific and Technical. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Scientific & Technical, Longman Group: Harlow, UK, 1989. [Google Scholar]
- Eaborn, C.; Kundu, K.; Pidcock, A.J. Synthesis of platinum(II) alkyl and aryl complexes from K2[PtCl4] and tetraorganotin compounds in dimethyl sulphoxide. Chem. Soc. Dalton Trans. 1981, 4, 933–938. [Google Scholar] [CrossRef]
- Baquero, E.A.; Rodríguez-Zúñiga, A.; Flores, J.C.; Temprado, M.; de Jesús, E. Revisiting the synthesis of trans-Pt(dmso)2ClMe and cis-Pt(dmso)2Me2: Experimental and DFT studies. J. Organomet. Chem. 2019, 896, 108–112. [Google Scholar] [CrossRef]
- Jamali, S.; Nabavizadeh, S.M.; Rashidi, M. Binuclear Cyclometalated Organoplatinum Complexes Containing 1,1′-Bis(diphenylphosphino)ferrocene as Spacer Ligand: Kinetics and Mechanism of MeI Oxidative Addition. Inorg. Chem. 2008, 47, 5441–5452. [Google Scholar] [CrossRef] [PubMed]
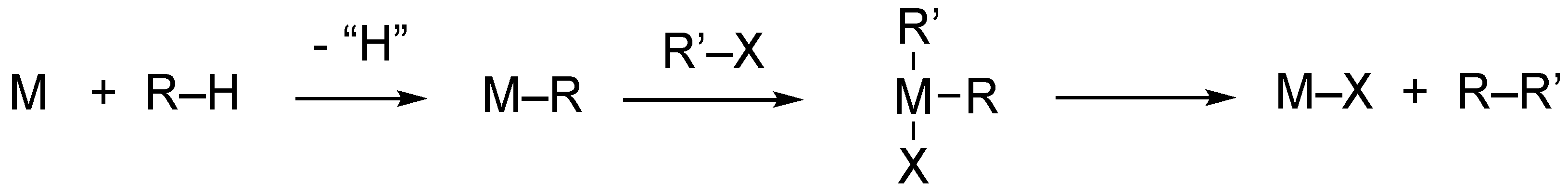


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manca, L.; Senzacqua, G.; Stoccoro, S.; Zucca, A. Regioselective C(sp2)-C(sp3) Coupling Mediated by Classical and Rollover Cyclometalation. Molecules 2024, 29, 707. https://doi.org/10.3390/molecules29030707
Manca L, Senzacqua G, Stoccoro S, Zucca A. Regioselective C(sp2)-C(sp3) Coupling Mediated by Classical and Rollover Cyclometalation. Molecules. 2024; 29(3):707. https://doi.org/10.3390/molecules29030707
Chicago/Turabian StyleManca, Lorenzo, Giacomo Senzacqua, Sergio Stoccoro, and Antonio Zucca. 2024. "Regioselective C(sp2)-C(sp3) Coupling Mediated by Classical and Rollover Cyclometalation" Molecules 29, no. 3: 707. https://doi.org/10.3390/molecules29030707
APA StyleManca, L., Senzacqua, G., Stoccoro, S., & Zucca, A. (2024). Regioselective C(sp2)-C(sp3) Coupling Mediated by Classical and Rollover Cyclometalation. Molecules, 29(3), 707. https://doi.org/10.3390/molecules29030707






