The Potential of Cyclodextrins as Inhibitors for the BM2 Protein: An In Silico Investigation
Abstract
1. Introduction
2. Results and Discussion
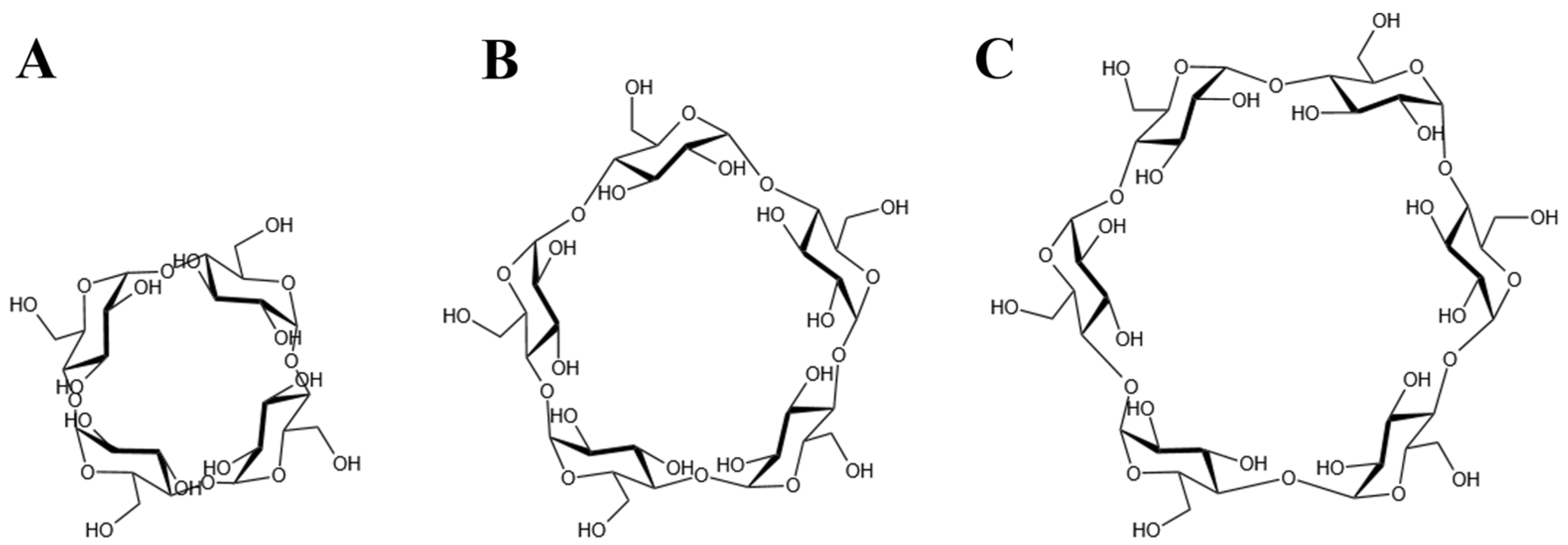
2.1. Selection of the BM2 Protein and the CDs Structures
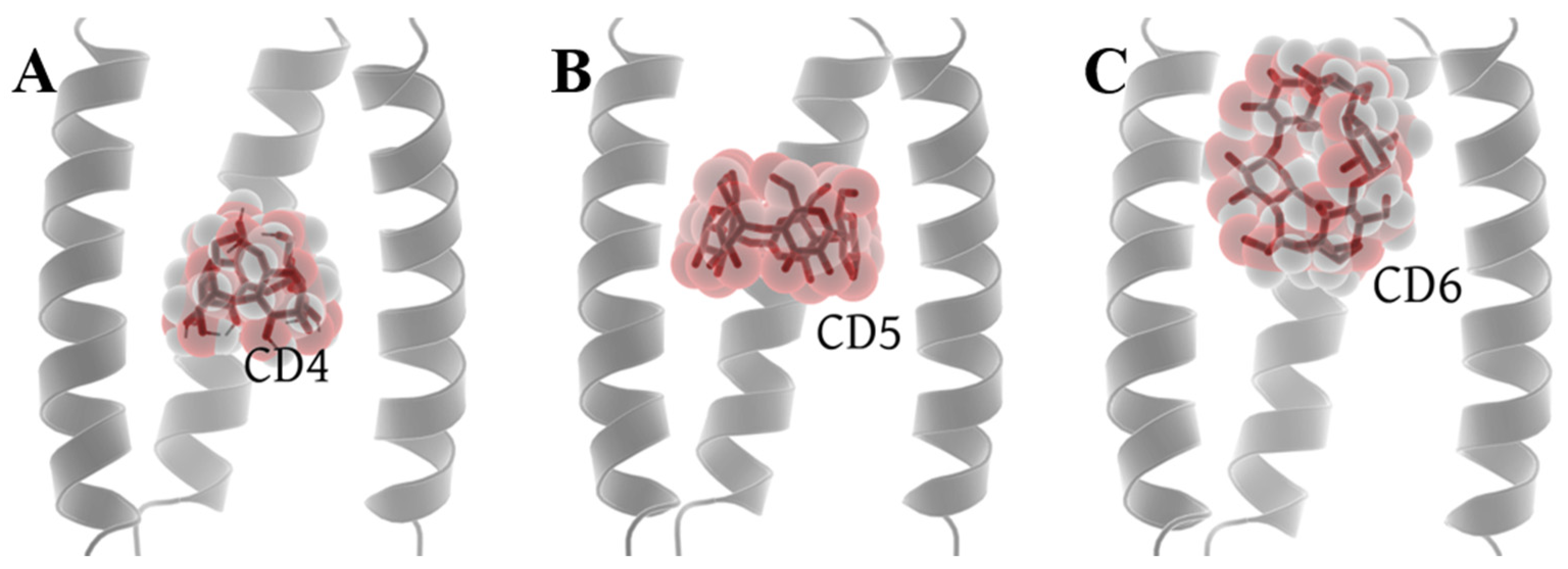
2.2. Molecular Docking Results and System Setup
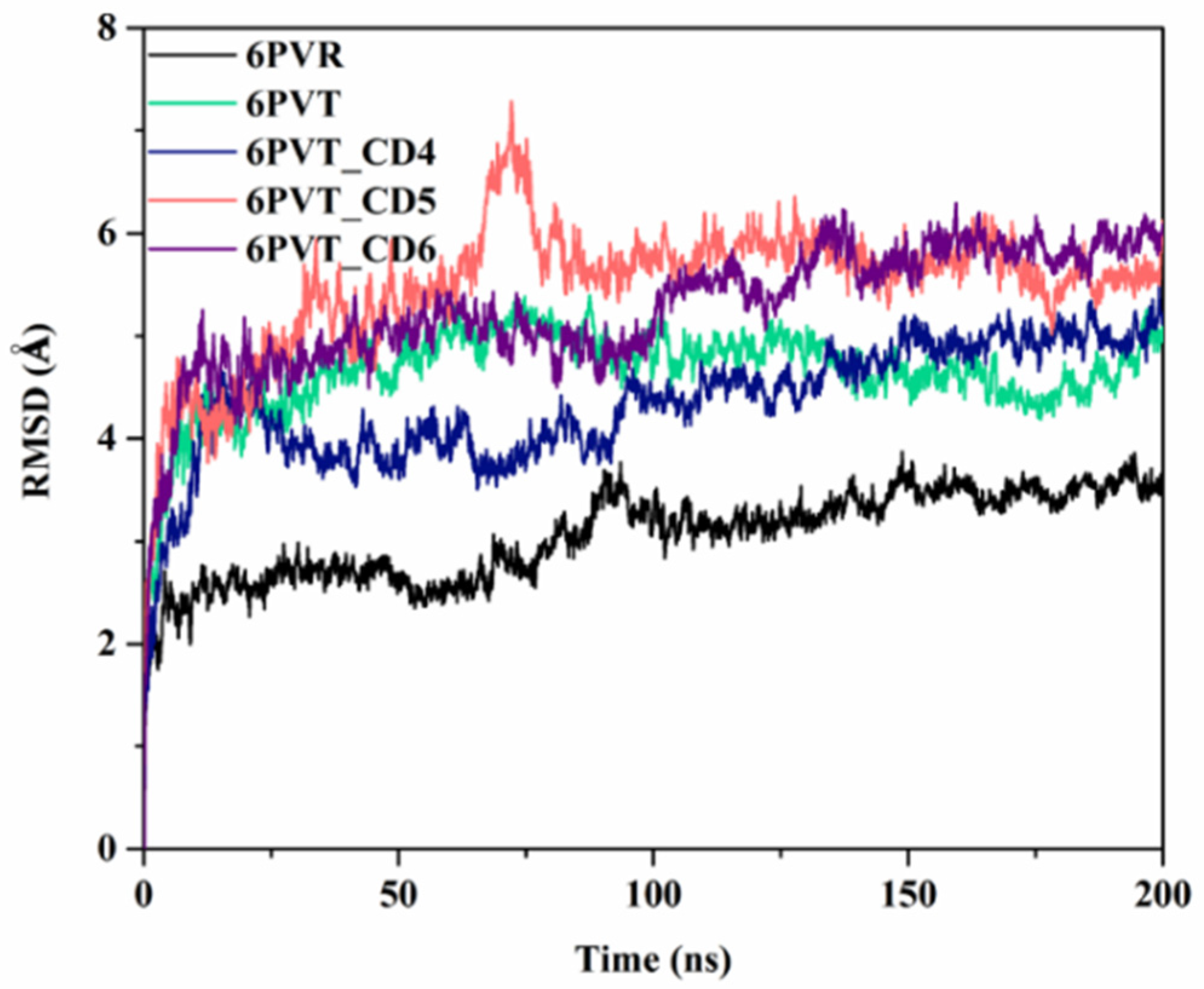
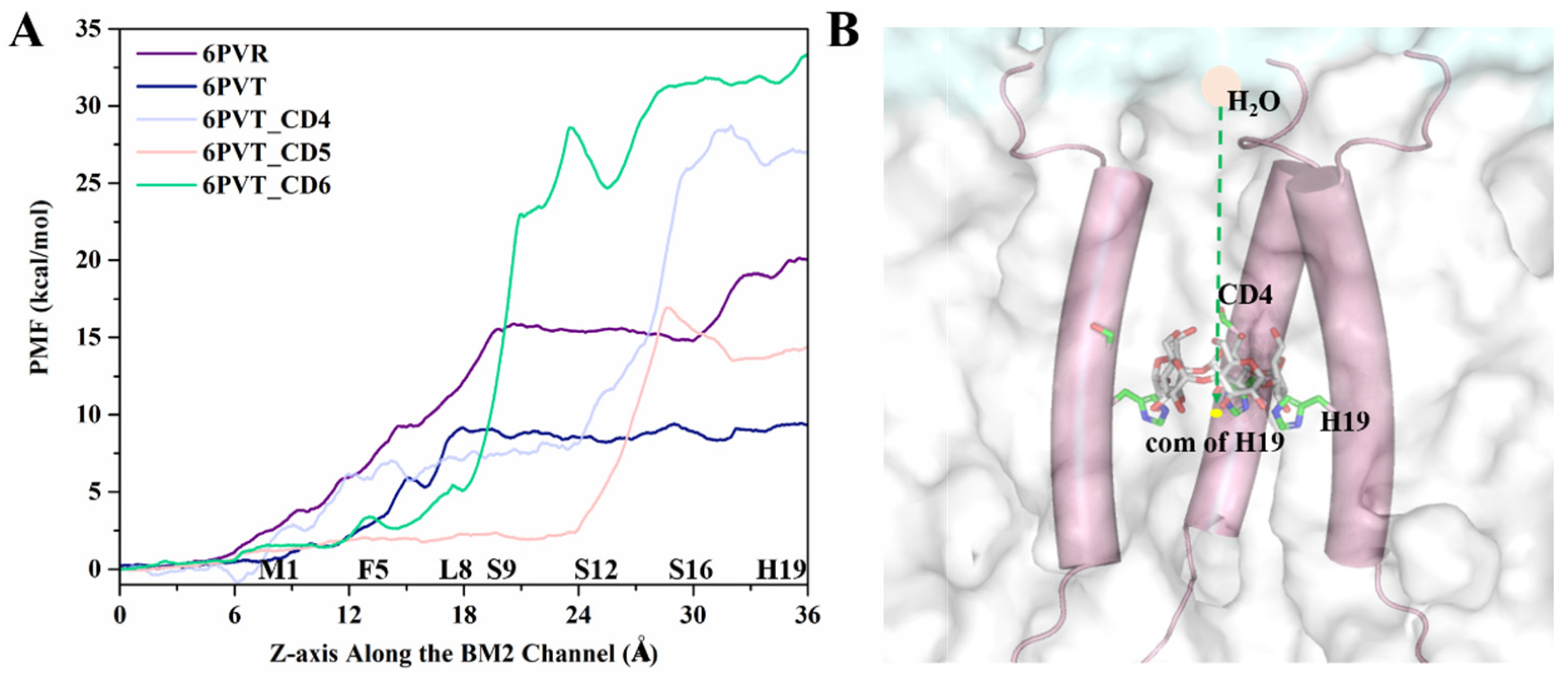
2.3. Molecular Dynamics Simulation and the Stability Assessment of the Results
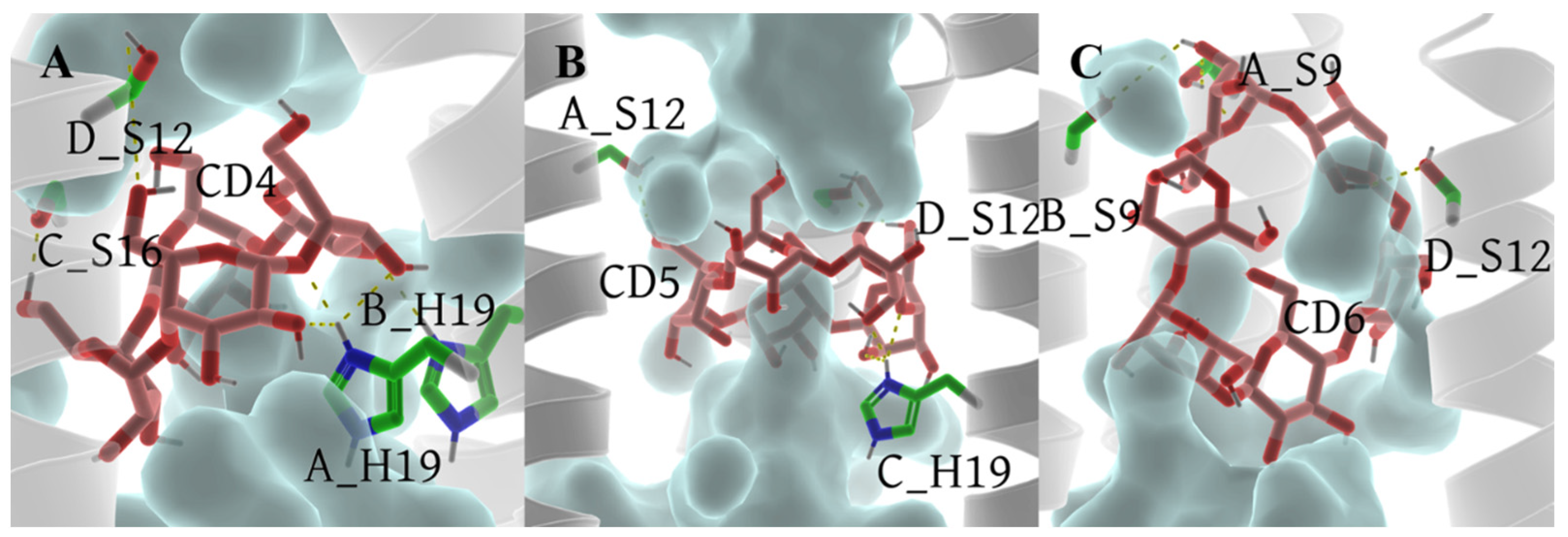
2.4. The Binding Modes between CDs and
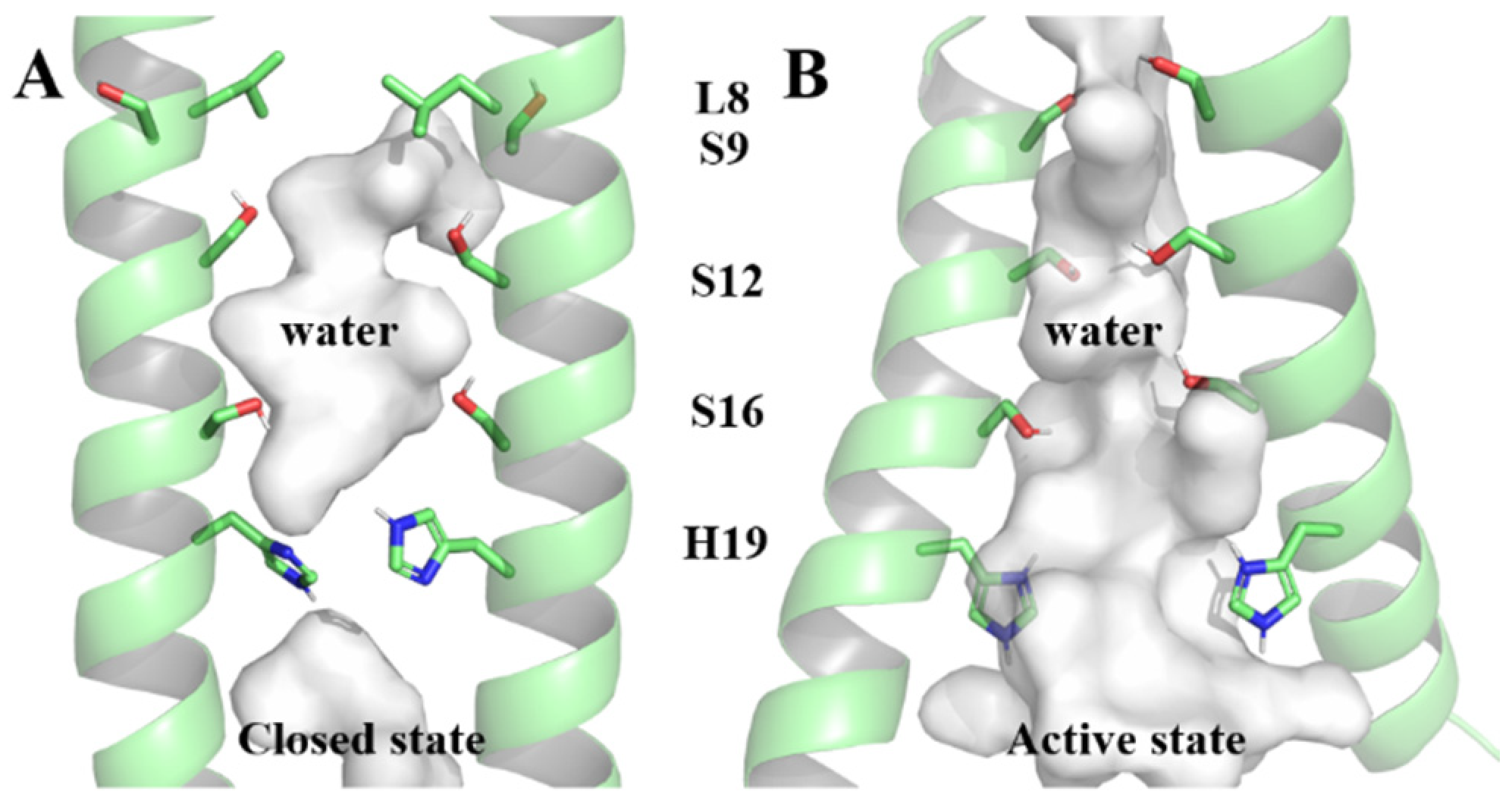
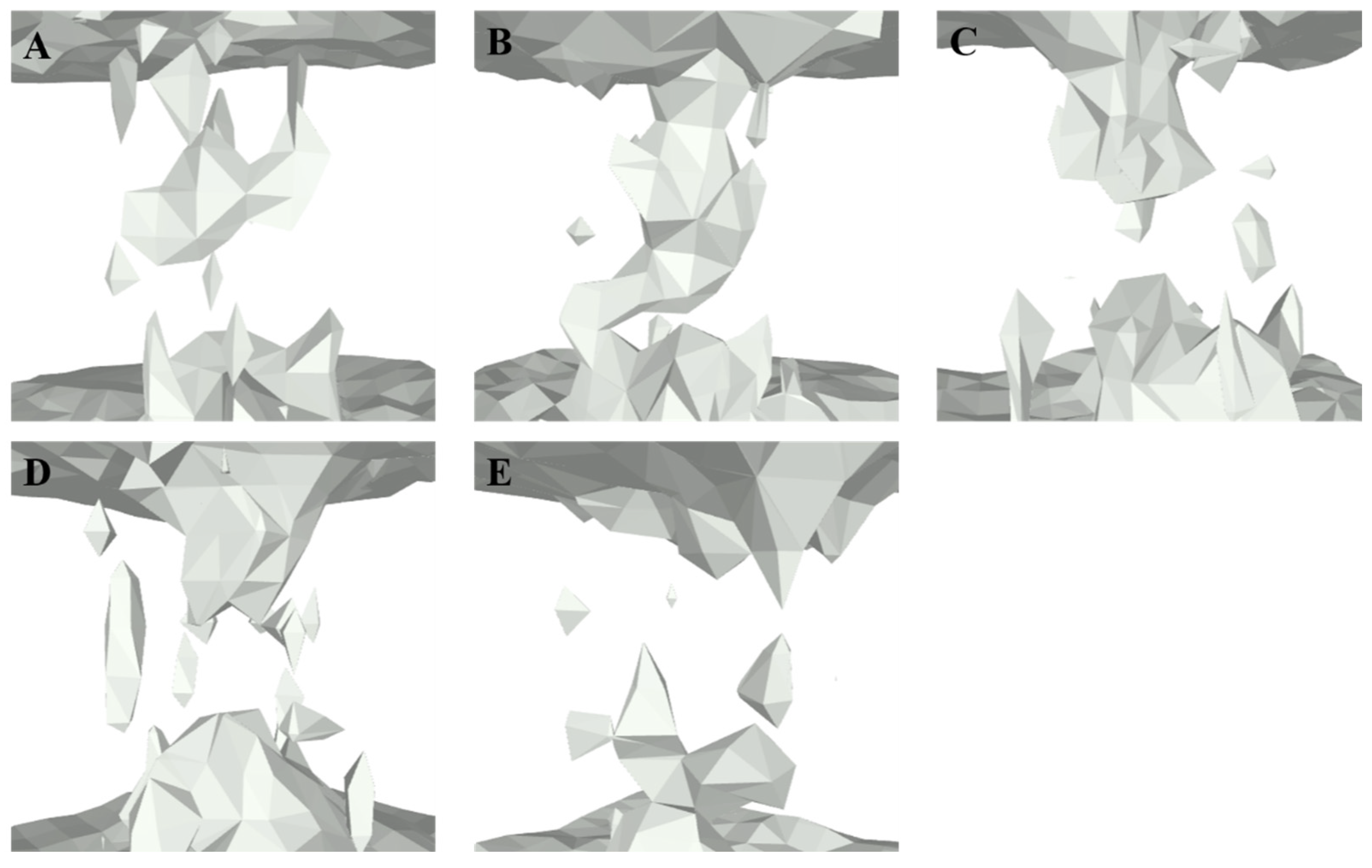
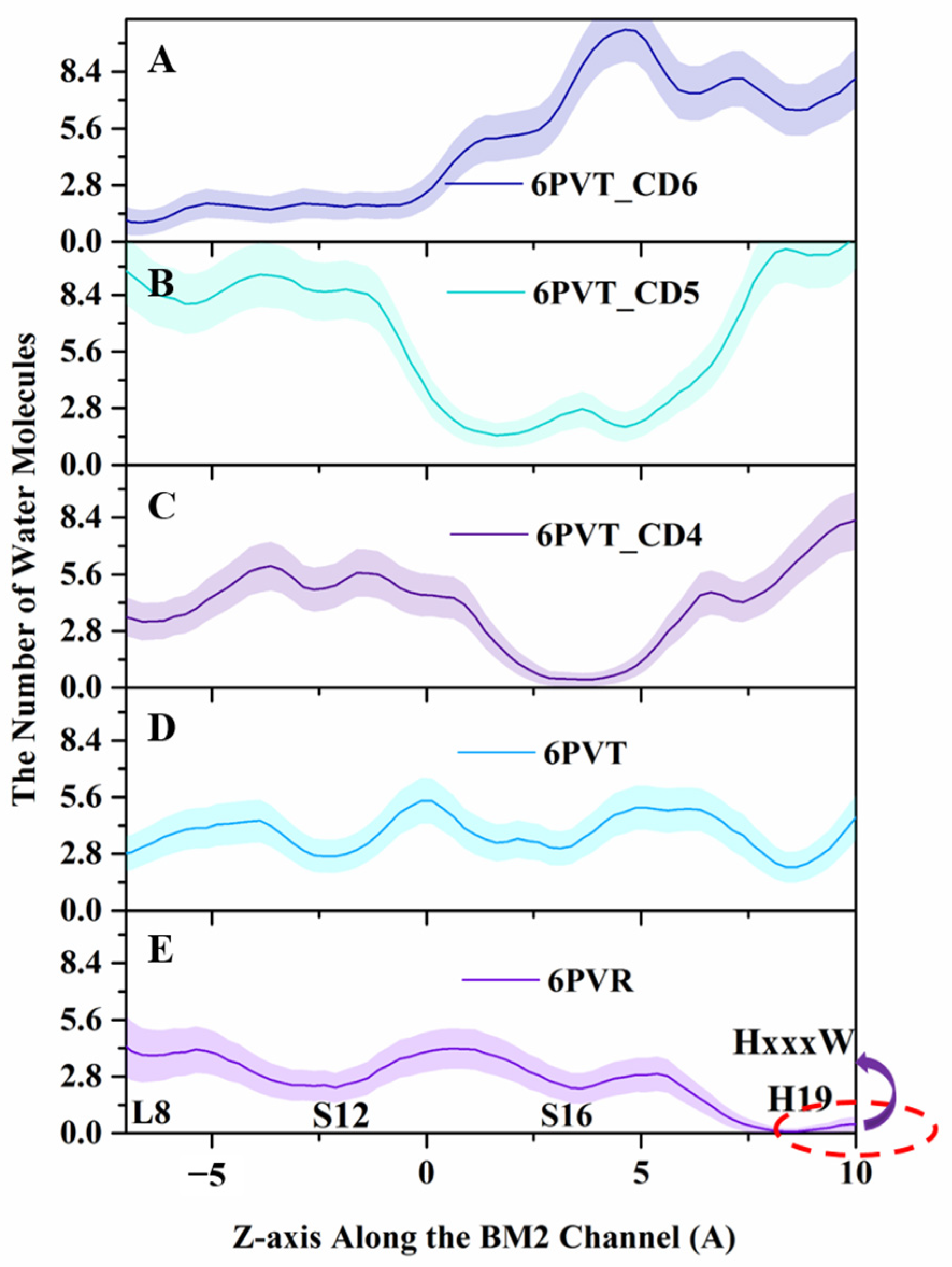
2.5. CD-Containing Systems Have Discrete Water Neworks along the Channel
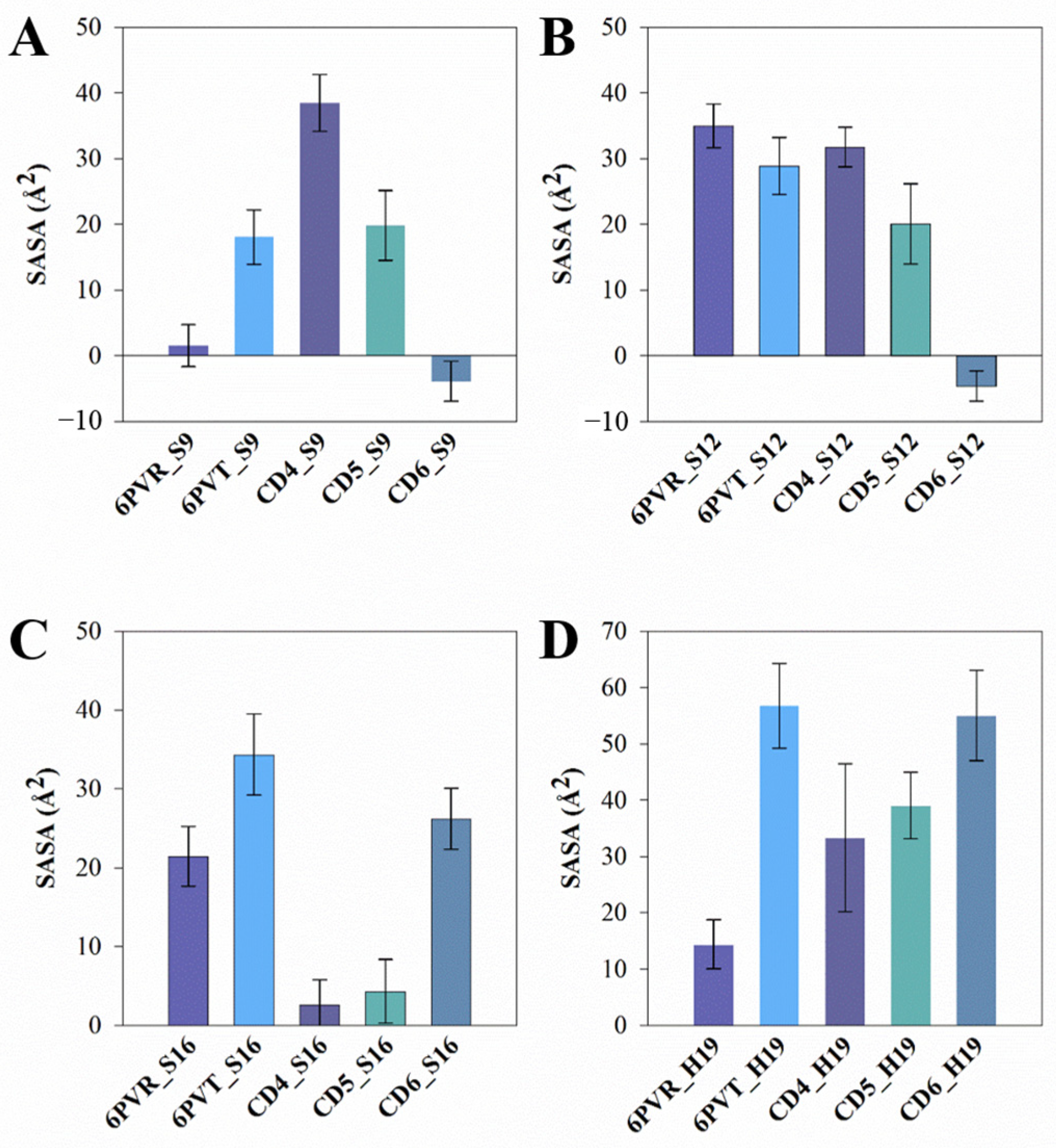
2.6. CD-Containing Systems Have a Narrowed Water Channel and Are Difficult for Water Molecules to Pass Through
2.7. Decreased Pore Hydration and Disrupted Hydrogen Bond
3. Materials and Methods
3.1. Molecular Docking and System Construction
3.2. Molecular Dynamics Simulations
3.3. Binding Free Energy Calculation
3.4. Adaptive Steered Molecular Dynamics (ASMD) Simulations
3.5. Analysis Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caini, S.; Kusznierz, G.; Garate, V.V.; Wangchuk, S.; Thapa, B.; Júnior, F.J.d.P.; de Almeida, W.A.F.; Njouom, R.; Fasce, R.A.; Bustos, P.; et al. The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS ONE 2019, 14, e0222381. [Google Scholar] [CrossRef]
- Glezen, W.P.; Schmier, J.K.; Kuehn, C.M.; Ryan, K.J.; Oxford, J. The Burden of Influenza B: A Structured Literature Review. Am. J. Public Health 2013, 103, e43–e51. [Google Scholar] [CrossRef]
- Shang, M.; Blanton, L.; Brammer, L.; Olsen, S.J.; Fry, A.M. Influenza-Associated Pediatric Deaths in the United States, 2010–2016. Pediatrics 2018, 141, e20172918. [Google Scholar] [CrossRef]
- Virk, R.K.; Jayakumar, J.; Mendenhall, I.H.; Moorthy, M.; Lam, P.; Linster, M.; Lim, J.; Lin, C.; Oon, L.L.E.; Lee, H.K.; et al. Divergent evolutionary trajectories of influenza B viruses underlie their contemporaneous epidemic activity. Proc. Natl. Acad. Sci. USA 2020, 117, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zaraket, H.; Hurt, A.C.; Clinch, B.; Barr, I.; Lee, N. Burden of influenza B virus infection and considerations for clinical management. Antivir. Res. 2021, 185, 104970. [Google Scholar] [CrossRef]
- Shirley, M. Baloxavir Marboxil: A Review in Acute Uncomplicated Influenza. Drugs 2020, 80, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Swierczynska, M.; Mirowska-Guzel, D.M.; Pindelska, E. Antiviral Drugs in Influenza. Int. J. Environ. Res. Public Health 2022, 19, 3018. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.G.; Takeda, M.; Ohigashi, Y.; Pinto, L.H.; A Lamb, R. Influenza B virus BM2 protein is an oligomeric integral membrane protein expressed at the cell surface. Virology 2003, 306, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Odagiri, T.; Kariwa, H.; Ohara, Y. The influenza B virus BM2 protein may be involved in the ribonucleoprotein complexes through the binding with membrane protein M1. Int. Congr. Ser. 2001, 1219, 411–419. [Google Scholar] [CrossRef]
- Imai, M.; Watanabe, S.; Ninomiya, A.; Obuchi, M.; Odagiri, T. Influenza B Virus BM2 Protein Is a Crucial Component for Incorporation of Viral Ribonucleoprotein Complex into Virions during Virus Assembly. J. Virol. 2004, 78, 11007–11015. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.; Goto, H.; Kawaoka, Y. Influenza B Virus Requires BM2 Protein for Replication. J. Virol. 2004, 78, 5576–5583. [Google Scholar] [CrossRef] [PubMed]
- Mandala, V.S.; Liao, S.-Y.; Gelenter, M.D.; Hong, M. The Transmembrane Conformation of the Influenza B Virus M2 Protein in Lipid Bilayers. Sci. Rep. 2019, 9, 3725. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, J. Functional studies reveal the similarities and differences between AM2 and BM2 proton channels from influenza viruses. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1860, 272–280. [Google Scholar] [CrossRef] [PubMed]
- A Mould, J.; Paterson, R.G.; Takeda, M.; Ohigashi, Y.; Venkataraman, P.; A Lamb, R.; Pinto, L.H. Influenza B Virus BM2 Protein Has Ion Channel Activity that Conducts Protons across Membranes. Dev. Cell 2003, 5, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mandala, V.S.; Loftis, A.R.; Shcherbakov, A.A.; Pentelute, B.L.; Hong, M. Atomic structures of closed and open influenza B M2 proton channel reveal the conduction mechanism. Nat. Struct. Mol. Biol. 2020, 27, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Roos, M.; Mandala, V.S.; Shcherbakov, A.A.; Hong, M. Elucidating Relayed Proton Transfer through a His–Trp–His Triad of a Transmembrane Proton Channel by Solid-State NMR. J. Mol. Biol. 2019, 431, 2554–2566. [Google Scholar] [CrossRef]
- Williams, J.K.; Shcherbakov, A.A.; Wang, J.; Hong, M. Protonation equilibria and pore-opening structure of the dual-histidine influenza B virus M2 transmembrane proton channel from solid-state NMR. J. Biol. Chem. 2017, 292, 17876–17884. [Google Scholar] [CrossRef]
- Ma, C.; Soto, C.S.; Ohigashi, Y.; Taylor, A.; Bournas, V.; Glawe, B.; Udo, M.K.; DeGrado, W.F.; Lamb, R.A.; Pinto, L.H. Identification of the Pore-lining Residues of the BM2 Ion Channel Protein of Influenza B Virus. J. Biol. Chem. 2008, 283, 15921–15931. [Google Scholar] [CrossRef]
- Wang, J.; Pielak, R.M.; A McClintock, M.; Chou, J.J. Solution structure and functional analysis of the influenza B proton channel. Nat. Struct. Mol. Biol. 2009, 16, 1267–1271. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Q.-C. What are the effects of the serine triad on proton conduction of an influenza B M2 channel? An investigation by molecular dynamics simulations. Phys. Chem. Chem. Phys. 2019, 21, 8820–8826. [Google Scholar] [CrossRef]
- Williams, J.K.; Tietze, D.; Lee, M.; Wang, J.; Hong, M. Solid-State NMR Investigation of the Conformation, Proton Conduction, and Hydration of the Influenza B Virus M2 Transmembrane Proton Channel. J. Am. Chem. Soc. 2016, 138, 8143–8155. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss Mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Hong, M.; Fritzsching, K.J.; Williams, J.K. Hydrogen-Bonding Partner of the Proton-Conducting Histidine in the Influenza M2 Proton Channel Revealed From 1H Chemical Shifts. J. Am. Chem. Soc. 2012, 134, 14753–14755. [Google Scholar] [CrossRef] [PubMed]
- Thomaston, J.L.; Alfonso-Prieto, M.; Woldeyes, R.A.; Fraser, J.S.; Klein, M.L.; Fiorin, G.; DeGrado, W.F. High-resolution structures of the M2 channel from influenza A virus reveal dynamic pathways for proton stabilization and transduction. Proc. Natl. Acad. Sci. USA 2015, 112, 14260–14265. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.Y.; Yang, Y.; Tietze, D.; Hong, M. The Influenza M2 Cytoplasmic Tail Changes the Proton-Exchange Equilibria and the Backbone Conformation of the Transmembrane Histidine Residue to Facilitate Proton Conduction. J. Am. Chem. Soc. 2015, 137, 6067–6077. [Google Scholar] [CrossRef]
- Acharya, R.; Carnevale, V.; Fiorin, G.; Levine, B.G.; Polishchuk, A.L.; Balannik, V.; Samish, I.; Lamb, R.A.; Pinto, L.H.; DeGrado, W.F.; et al. Structure and mechanism of proton transport through the transmembrane tetrameric M2 protein bundle of the influenza A virus. Proc. Natl. Acad. Sci. USA 2010, 107, 15075–15080. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Cadman, A.; Zurcher, T.; Barclay, W.S. A Reverse Genetics Approach for Recovery of Recombinant Influenza B Viruses Entirely from cDNA. J. Virol. 2002, 76, 11744–11747. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.H.; Holsinger, L.J.; Lamb, R.A. Influenza virus M2 protein has ion channel activity. Cell 1992, 69, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Pekosz, A.; Shuck, K.; Pinto, L.H.; Lamb, R.A. Influenza A Virus M2Ion Channel Activity Is Essential for Efficient Replication in Tissue Culture. J. Virol. 2002, 76, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Helenius, A. Nuclear Transport of Influenza Virus Ribonucleoproteins: The Viral Matrix Protein (M1) Promotes Export and Inhibits Import. Cell 1991, 67, 117–130. [Google Scholar] [CrossRef]
- Davies, W.L.; Grunert, R.R.; Haff, R.F.; McGahen, J.W.; Neumayer, E.M.; Paulshock, M.; Watts, J.C.; Wood, T.R.; Hermann, E.C.; Hoffmann, C.E. Antiviral Activity of 1-Adamantanamine (Amantadine). Science 1964, 144, 862–863. [Google Scholar] [CrossRef]
- Hay, A.; Wolstenholme, A.; Skehel, J.; Smith, M. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985, 4, 3021–3024. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Q.-C. In Silico Analysis Revealed a Unique Binding but Ineffective Mode of Amantadine to Influenza Virus B M2 Channel. J. Phys. Chem. Lett. 2021, 12, 1169–1174. [Google Scholar] [CrossRef]
- Gelenter, M.D.; Mandala, V.S.; Niesen, M.J.M.; Sharon, D.A.; Dregni, A.J.; Willard, A.P.; Hong, M. Water orientation and dynamics in the closed and open influenza B virus M2 proton channels. Commun. Biol. 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Thomaston, J.L.; Polizzi, N.F.; Konstantinidi, A.; Wang, J.; Kolocouris, A.; DeGrado, W.F. Inhibitors of the M2 Proton Channel Engage and Disrupt Transmembrane Networks of Hydrogen-Bonded Waters. J. Am. Chem. Soc. 2018, 140, 15219–15226. [Google Scholar] [CrossRef]
- Li, W.W.; Casey, R.; Gonzalez, E.M.; Folkman, J. Angiostatic steroids potentiated by sulfated cyclodextrins inhibit corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2898–2905. [Google Scholar]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; He, Q.R. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of Staphylococcal Alpha-Hemolysin, a Heptameric Transmembrane Pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Petosa, C.; Collier, R.J.; Klimpel, K.R.; Leppla, S.H.; Liddington, R.C. Crystal structure of the anthrax toxin protective antigen. Nature 1997, 385, 833–838. [Google Scholar] [CrossRef]
- Gu, L.-Q.; Braha, O.; Conlan, S.; Cheley, S.; Bayley, H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature 1999, 398, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Schiffmiller, A.; Anderson, D.; Finkelstein, A. Ion selectivity of the anthrax toxin channel and its effect on protein translocation. J. Gen. Physiol. 2015, 146, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Thelestam, M.; Blomqvist, L. Staphylococcal Alpha Toxin--Recent Advances. Toxicon 1988, 26, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Brossier, F.; Mock, M. Toxins of Bacillus anthracis. Toxicon 2001, 39, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Young, J.A.T. Anthrax Toxin. Annu. Rev. Cell Dev. Biol. 2003, 19, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, M.; Leppla, S.H. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 2004, 7, 19–24. [Google Scholar] [CrossRef]
- Dhule, S.S.; Penfornis, P.; Frazier, T.; Walker, R.; Feldman, J.; Tan, G.; He, J.; Alb, A.; John, V.; Pochampally, R. Curcumin-Loaded Γ-Cyclodextrin Liposomal Nanoparticles as Delivery Vehicles for Osteosarcoma. Nanomed.-Nanotechnol. Biol. Med. 2012, 8, 440–451. [Google Scholar] [CrossRef]
- McCormack, B.; Gregoriadis, G. Entrapment of Cyclodextrin-Drug Complexes into Liposomes: Potential Advantages in Drug Delivery. J. Drug Target. 1994, 2, 449–454. [Google Scholar] [CrossRef]
- Arima, H.; Hagiwara, Y.; Hirayama, F.; Uekama, K. Enhancement of antitumor effect of doxorubicin by its complexation with γ-cyclodextrin in pegylated liposomes. J. Drug Target. 2006, 14, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, S.; Zhang, Y.; Lang, J.; Ding, Y.; Zhao, X.; Zhao, R.; Li, Y.; Shi, J.; Hao, J.; et al. An MMP-2 Responsive Liposome Integrating Antifibrosis and Chemotherapeutic Drugs for Enhanced Drug Perfusion and Efficacy in Pancreatic Cancer. ACS Appl. Mater. Interfaces 2016, 8, 3438–3445. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Nanomedicine–Nanoscale Drugs and Delivery Systems. J. Nanosci. Nanotechnol. 2010, 10, 7906–7918. [Google Scholar] [CrossRef] [PubMed]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2017, 27, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, D.; Hirata, Y.; Wakamori, S.; Shimada, H.; Tomabechi, Y.; Kawasaki, Y.; Ikeuchi, K.; Hagimori, T.; Matsumoto, S.; Yamada, H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ueno, K.; Kashiwa, M.; Watanabe, J. The Stereoselective Synthesis of Cyclomaltopentaose—A Novel Cyclodextrin Homolog with Dp-5. Tetrahedron Lett. 1994, 35, 1921–1924. [Google Scholar] [CrossRef]
- Rimsha, Y.; Abdul, R.F.; Sajid, A.; Muhammad, I.; Ullah, K.I.; Haroon, K.S. Cyclodextrins: An Overview of Fundamentals, Types, and Applications. In Cyclodextrins; Rashid, A., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 2. [Google Scholar]
- Berg, B.v.D.; Bhamidimarri, S.P.; Prajapati, J.D.; Kleinekathöfer, U.; Winterhalter, M. Outer-membrane translocation of bulky small molecules by passive diffusion. Proc. Natl. Acad. Sci. USA 2015, 112, E2991–E2999. [Google Scholar] [CrossRef]
- Gaussview, Version 6; Dennington, R., Keith, T.A., Millam, J.M., Eds.; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. Ix. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 2003, 54, 724–728. [Google Scholar] [CrossRef]
- Raghavachari, K. Perspective on “Density Functional Thermochemistry. Iii. The Role of Exact Exchange”. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Software News and Update Autodock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. Software News and Updates—Charnim-Gui: A Web-Based Grraphical User Interface for Charmm. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. A Density-Based Algorithm for Discovering Clusters in Large Spatial Databases with Noise. In Proceedings of the KDD’96: Proceedings of the Second International Conference on Knowledge Discovery and Data Mining, Portland, OR, USA, 2–4 August 1996. [Google Scholar]
- Shao, J.; Tanner, S.W.; Thompson, N.; Cheatham, T.E. Clustering Molecular Dynamics Trajectories: 1. Characterizing the Performance of Different Clustering Algorithms. J. Chem. Theory Comput. 2007, 3, 2312–2334. [Google Scholar] [CrossRef]
- Jayaram, B.; Sprous, D.; Beveridge, D.L. Solvation Free Energy of Biomacromolecules: Parameters for a Modified Generalized Born Model Consistent with the AMBER Force Field. J. Phys. Chem. B 1998, 102, 9571–9576. [Google Scholar] [CrossRef]
- Bashford, D.; Case, D.A. Generalized Born Models of Macromolecular Solvation Effects. Annu. Rev. Phys. Chem. 2000, 51, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Smart, O.; Goodfellow, J.; Wallace, B. The pore dimensions of gramicidin A. Biophys. J. 1993, 65, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Roux, B. Computational Studies of the Gramicidin Channel. Accounts Chem. Res. 2002, 35, 366–375. [Google Scholar] [CrossRef]
- Pomès, R.; Roux, B. Molecular Mechanism of H+ Conduction in the Single-File Water Chain of the Gramicidin Channel. Biophys. J. 2002, 82, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Pielak, R.M.; Chou, J.J. Flu channel drug resistance: A tale of two sites. Protein Cell 2010, 1, 246–258. [Google Scholar] [CrossRef]
- Thomaston, J.L.; Woldeyes, R.A.; Nakane, T.; Koiwai, K.; Yamashita, A.; Tanaka, T.; Arima, T.; Kobayashi, J.; Masuda, T.; Suzuki, M.; et al. Xfel Structures of the Influenza M2 Proton Channel at 1.4 Angstrom: Room Temperature Water Networks and Insights into Proton Conduction. In Acta Crystallographica A-Foundation and Advances; International Union of Crystallography: Chester, UK, 2017; Volume 73, p. A40. [Google Scholar]
- Pielak, R.M.; Chou, J.J. Influenza M2 Proton Channels. Biochim. Biophys. Acta-Biomembr. 2011, 1808, 522–529. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. Software News and Updates Mdanalysis: A Toolkit for the Analysis of Molecular Dynamics Simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Otomo, K.; Toyama, A.; Miura, T.; Takeuchi, H. Interactions Between Histidine and Tryptophan Residues in the BM2 Proton Channel from Influenza B Virus. J. Biochem. 2009, 145, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Smart, O.S.; Neduvelil, J.G.; Wang, X.; Wallace, B.A.; Sansom, M.S.P. HOLE: A program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 1996, 14, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Khalili-Araghi, F.; Tajkhorshid, E.; Schulten, K. Free energy calculation from steered molecular dynamics simulations using Jarzynski’s equality. J. Chem. Phys. 2003, 119, 3559–3566. [Google Scholar] [CrossRef]
- Schanda, P.; Ernst, M. Studying dynamics by magic-angle spinning solid-state NMR spectroscopy: Principles and applications to biomolecules. Prog. Nucl. Magn. Reson. Spectrosc. 2016, 96, 1–46. [Google Scholar] [CrossRef]
- Smith, A.A.; Ernst, M.; Meier, B.H. Because the Light is Better Here: Correlation-Time Analysis by NMR Spectroscopy. Angew. Chem. Int. Ed. 2017, 56, 13590–13595. [Google Scholar] [CrossRef]
- Ropp, J.; Lawrence, C.; Farrar, T.C.; Skinner, J.L. Rotational Motion in Liquid Water Is Anisotropic: A Nuclear Magnetic Resonance and Molecular Dynamics Simulation Study. J. Am. Chem. Soc. 2001, 123, 8047–8052. [Google Scholar] [CrossRef]
- Hu, F.; Luo, W.; Hong, M. Mechanisms of Proton Conduction and Gating in Influenza M2 Proton Channels from Solid-State NMR. Science 2010, 330, 505–508. [Google Scholar] [CrossRef]
- Hu, F.; Schmidt-Rohr, K.; Hong, M. Nmr Detection of Ph-Dependent Histidine-Water Proton Exchange Reveals the Conduction Mechanism of a Transmembrane Proton Channel. J. Am. Chem. Soc. 2012, 134, 3703–3713. [Google Scholar] [CrossRef]
- Decoursey, T.E. Voltage-Gated Proton Channels and Other Proton Transfer Pathways. Physiol. Rev. 2003, 83, 475–579. [Google Scholar] [CrossRef] [PubMed]
- Biedermannová, L.; Schneider, B. Structure of the ordered hydration of amino acids in proteins: Analysis of crystal structures. Acta Crystallogr. Sect. D Struct. Biol. 2015, 71, 2192–2202. [Google Scholar] [CrossRef]
- Morris, G.M.; Lim-Wilby, M. Molecular Docking. Methods Mol. Biol. 2008, 443, 365–382. [Google Scholar]
- Zhang, Y.; Zhang, H.-X.; Zheng, Q.-C. In Silico Study of Membrane Lipid Composition Regulating Conformation and Hydration of Influenza Virus B M2 Channel. J. Chem. Inf. Model. 2020, 60, 3603–3615. [Google Scholar] [CrossRef]
- Rossman, J.S.; Jing, X.; Leser, G.P.; Lamb, R.A. Influenza Virus M2 Protein Mediates ESCRT-Independent Membrane Scission. Cell 2010, 142, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Dickson, C.J.; Madej, B.D.; Skjevik, A.A.; Betz, R.M.; Teigen, K.; Gould, I.R.; Walker, R.C. Lipid14: The Amber Lipid Force Field. J. Chem. Theory Comput. 2014, 10, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N⋅Log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Anandakrishnan, R.; Drozdetski, A.; Walker, R.C.; Onufriev, A.V. Speed of Conformational Change: Comparing Explicit and Implicit Solvent Molecular Dynamics Simulations. Biophys. J. 2015, 108, 1153–1164. [Google Scholar] [CrossRef]
- Dominy, B.N.; Brooks, C.L. Development of a Generalized Born Model Parametrization for Proteins and Nucleic Acids. J. Phys. Chem. B 1999, 103, 3765–3773. [Google Scholar] [CrossRef]
- Calimet, N.; Schaefer, M.; Simonson, T. Protein molecular dynamics with the generalized born/ACE solvent model. Proteins Struct. Funct. Bioinform. 2001, 45, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.; Shenkin, P.S.; Still, W.C. Approximate Atomic Surfaces from Linear Combinations of Pairwise Overlaps (Lcpo). J. Comput. Chem. 1999, 20, 217–230. [Google Scholar] [CrossRef]
- Ozer, G.; Valeev, E.F.; Quirk, S.; Hernandez, R. Adaptive Steered Molecular Dynamics of the Long-Distance Unfolding of Neuropeptide Y. J. Chem. Theory Comput. 2010, 6, 3026–3038. [Google Scholar] [CrossRef]
- Ozer, G.; Quirk, S.; Hernandez, R. Thermodynamics of Decaalanine Stretching in Water Obtained by Adaptive Steered Molecular Dynamics Simulations. J. Chem. Theory Comput. 2012, 8, 4837–4844. [Google Scholar] [CrossRef]
- Ozer, G.; Quirk, S.; Hernandez, R. Adaptive steered molecular dynamics: Validation of the selection criterion and benchmarking energetics in vacuum. J. Chem. Phys. 2012, 136, 215104. [Google Scholar] [CrossRef]
- Jarzynski, C. Nonequilibrium Equality for Free Energy Differences. Phys. Rev. Lett. 1997, 78, 2690–2693. [Google Scholar] [CrossRef]
- Tajkhorshid, E.; Nollert, P.; Jensen, M.; Miercke, L.J.W.; O’Connell, J.; Stroud, R.M.; Schulten, K. Control of the Selectivity of the Aquaporin Water Channel Family by Global Orientational Tuning. Science 2002, 296, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, U.K.; Fischer, G.; Friemann, R.; Enkavi, G.; Tajkhorshid, E.; Neutze, R. Subangstrom Resolution X-Ray Structure Details Aquaporin-Water Interactions. Science 2013, 340, 1346–1349. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Zhang, H.; Zheng, Q.; Wang, S. The Potential of Cyclodextrins as Inhibitors for the BM2 Protein: An In Silico Investigation. Molecules 2024, 29, 620. https://doi.org/10.3390/molecules29030620
Liu A, Zhang H, Zheng Q, Wang S. The Potential of Cyclodextrins as Inhibitors for the BM2 Protein: An In Silico Investigation. Molecules. 2024; 29(3):620. https://doi.org/10.3390/molecules29030620
Chicago/Turabian StyleLiu, Aijun, Hao Zhang, Qingchuan Zheng, and Song Wang. 2024. "The Potential of Cyclodextrins as Inhibitors for the BM2 Protein: An In Silico Investigation" Molecules 29, no. 3: 620. https://doi.org/10.3390/molecules29030620
APA StyleLiu, A., Zhang, H., Zheng, Q., & Wang, S. (2024). The Potential of Cyclodextrins as Inhibitors for the BM2 Protein: An In Silico Investigation. Molecules, 29(3), 620. https://doi.org/10.3390/molecules29030620




