Study on the Catalytic Oxidation of Toluene Using CeO2@S-AZMB Prepared from Spent Zn-Mn Batteries
Abstract
1. Introduction
2. Results and Discussion
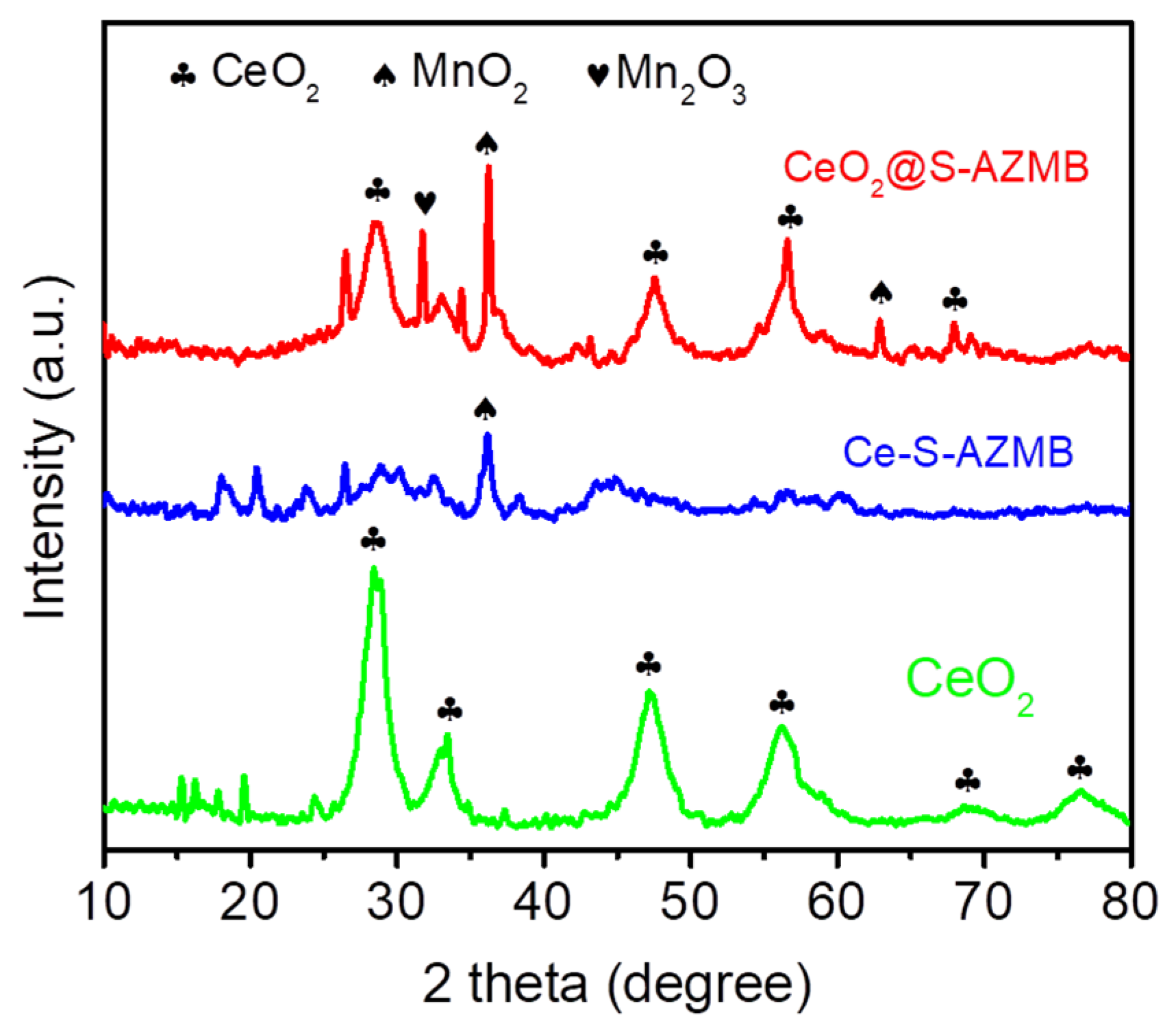
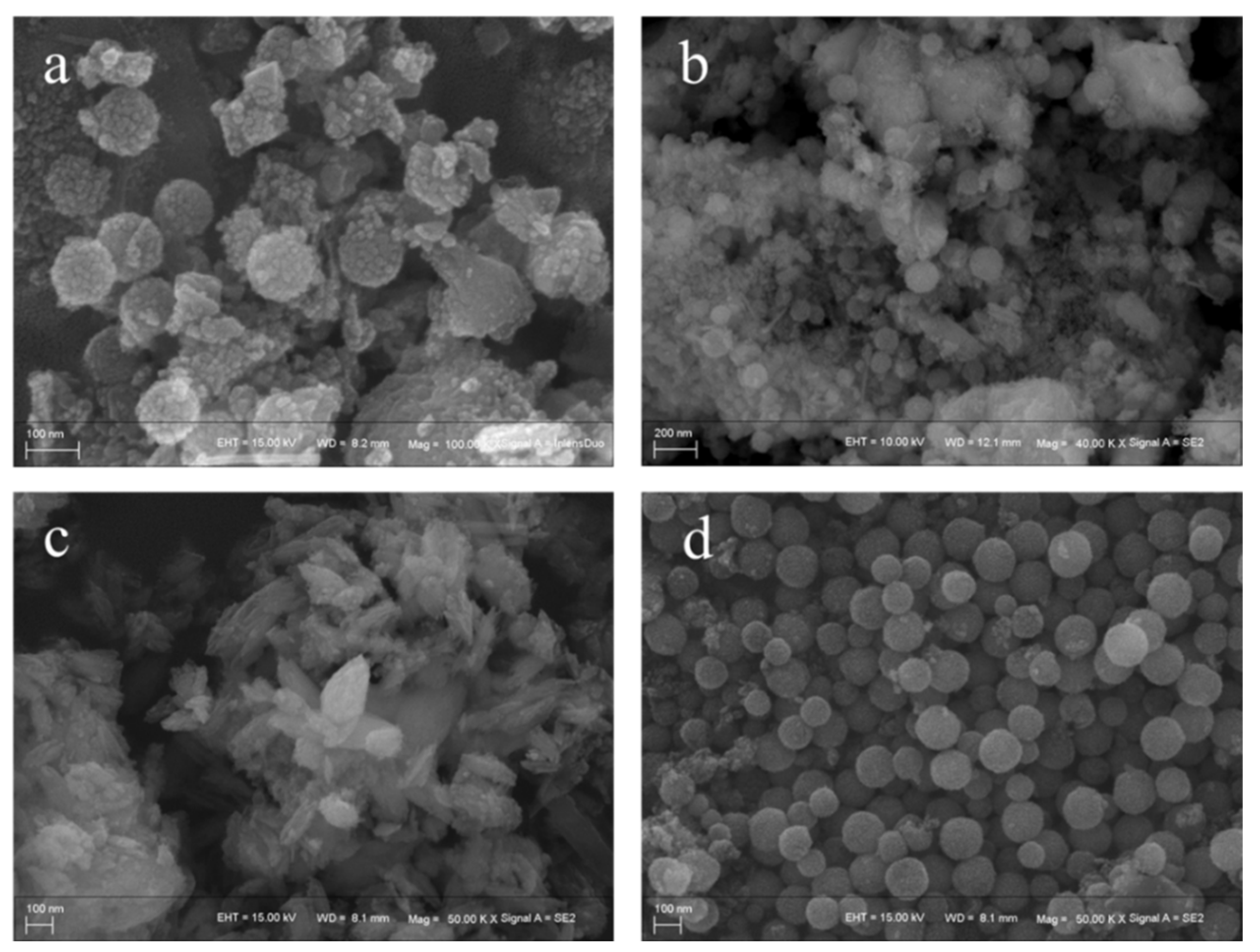
2.1. Phase and Microstructure
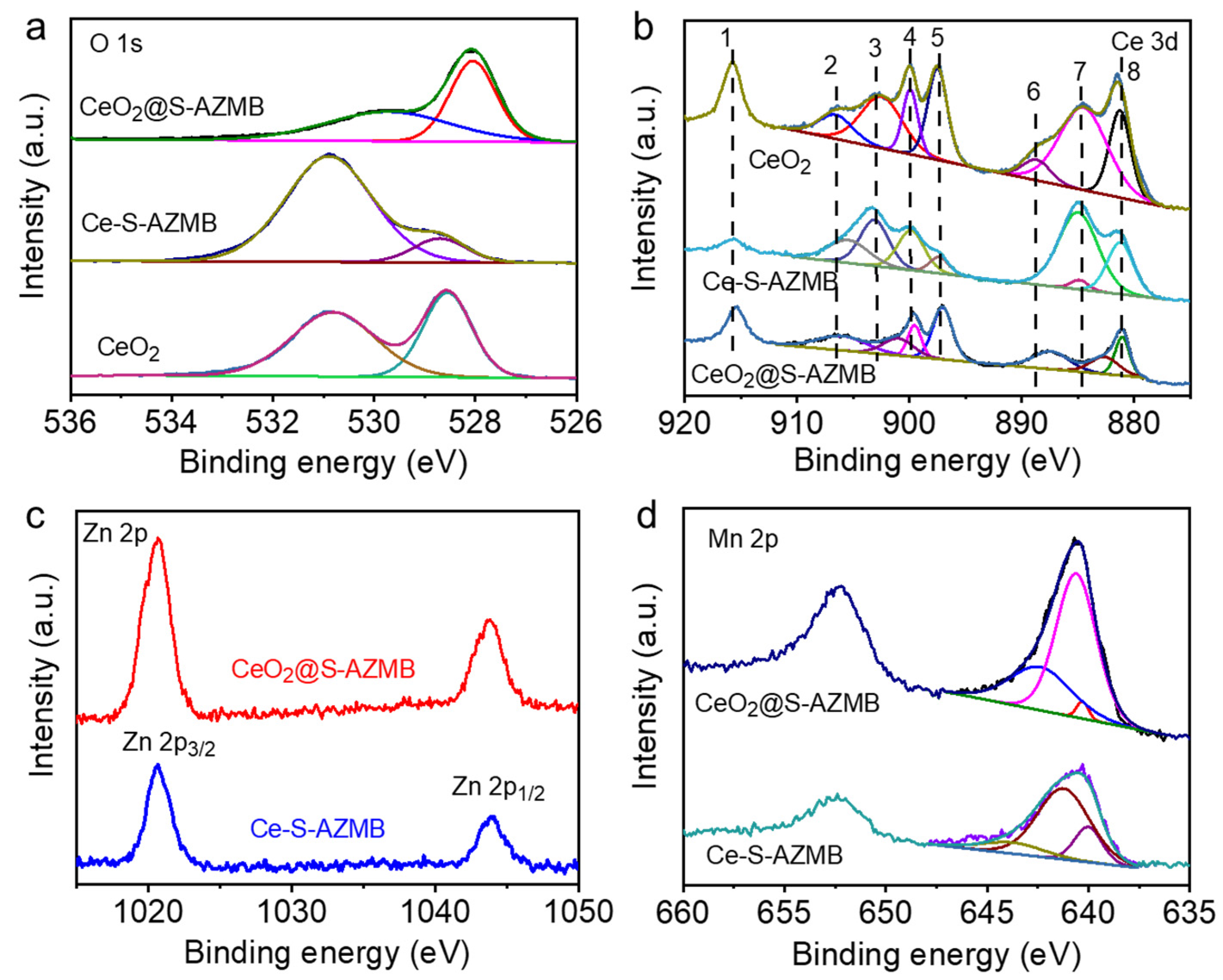
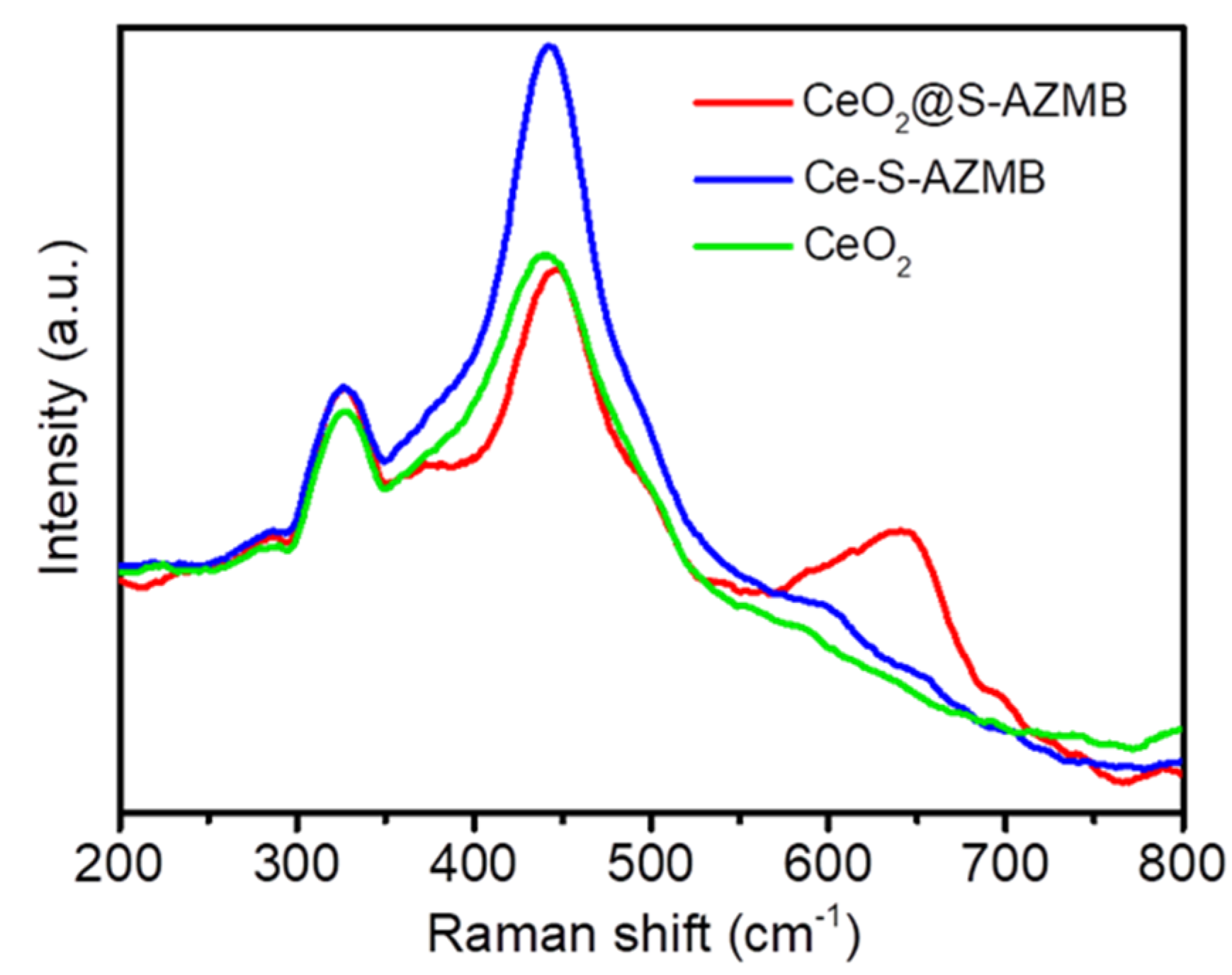
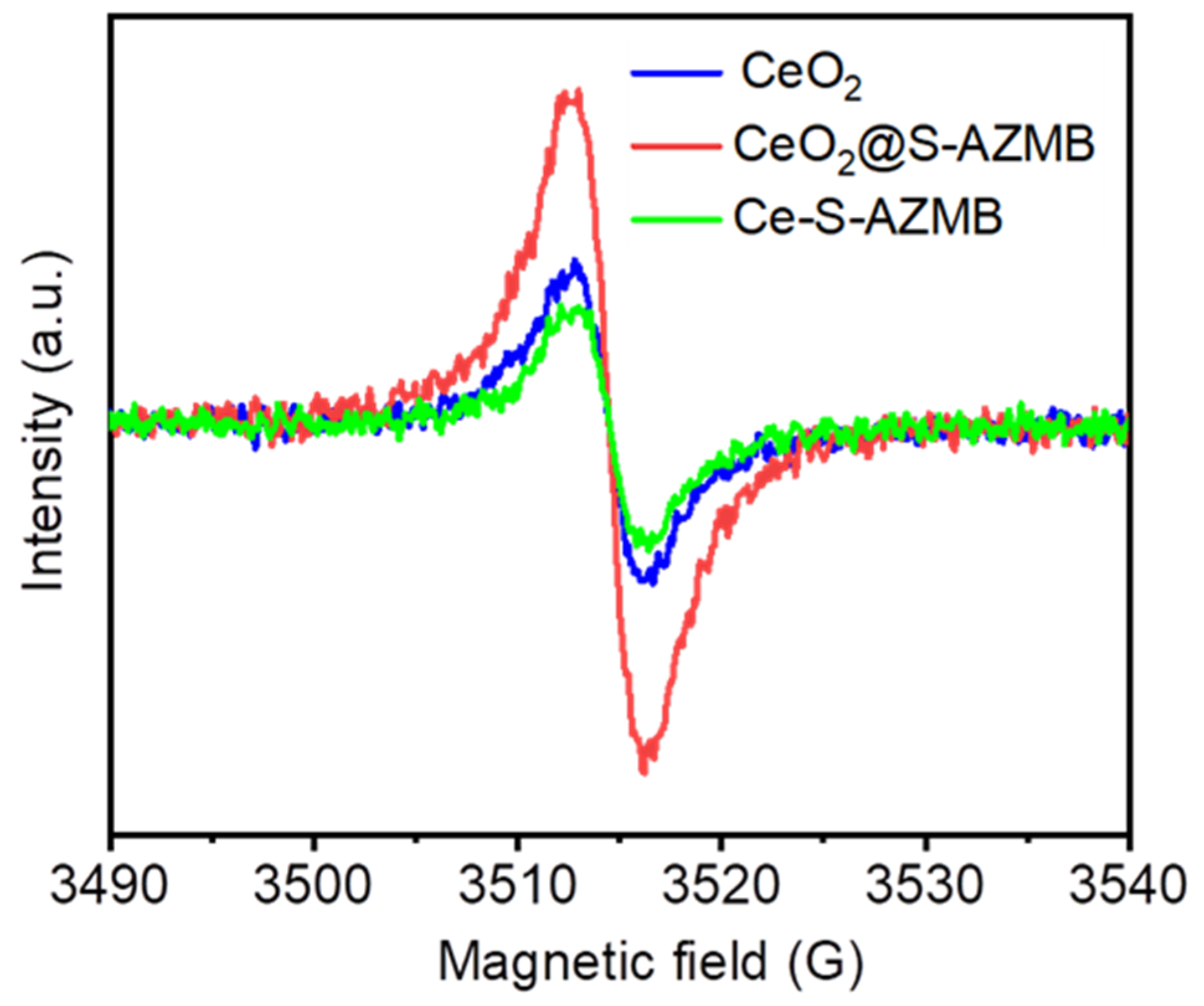
2.2. Oxidation-Reduction Capacity
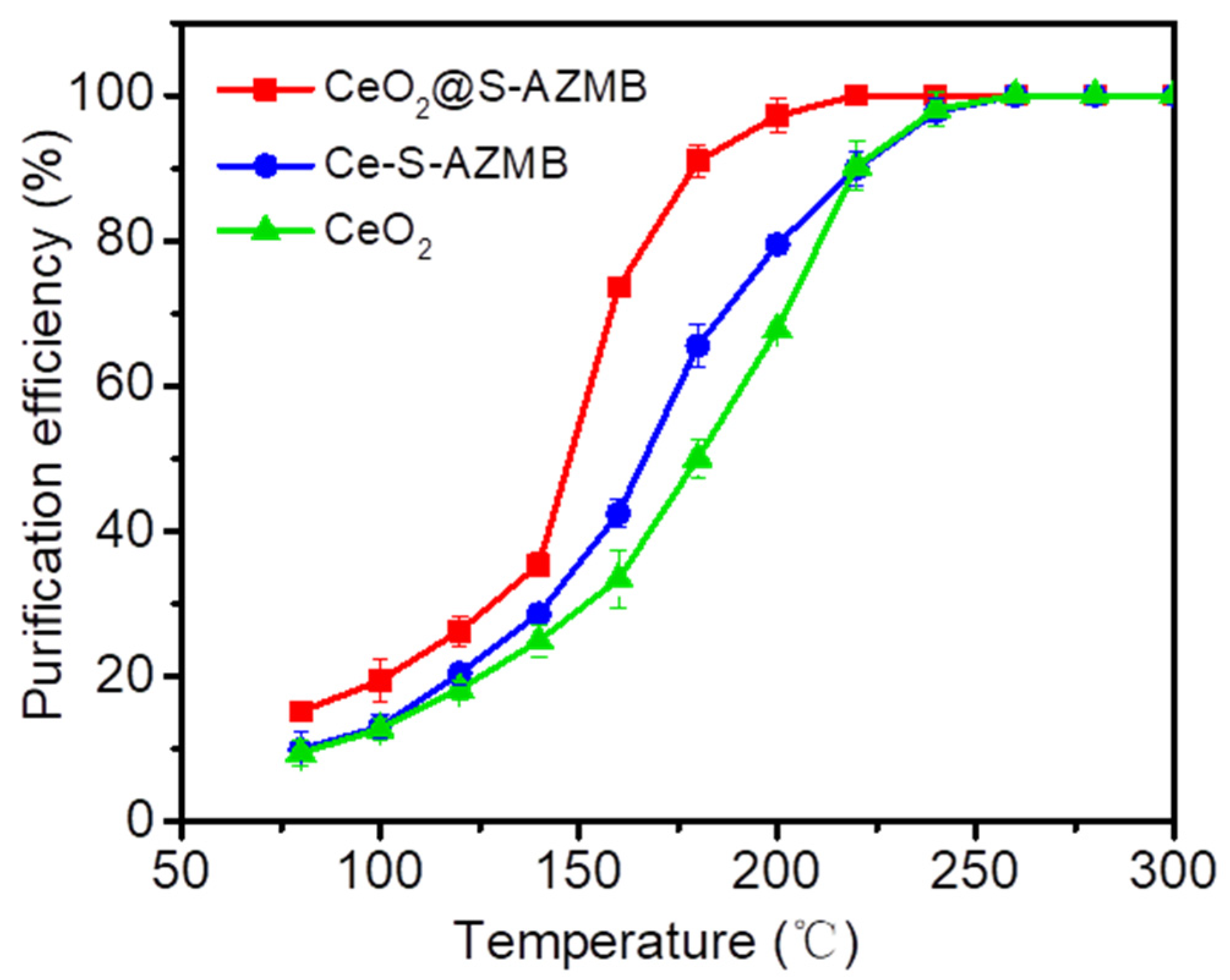
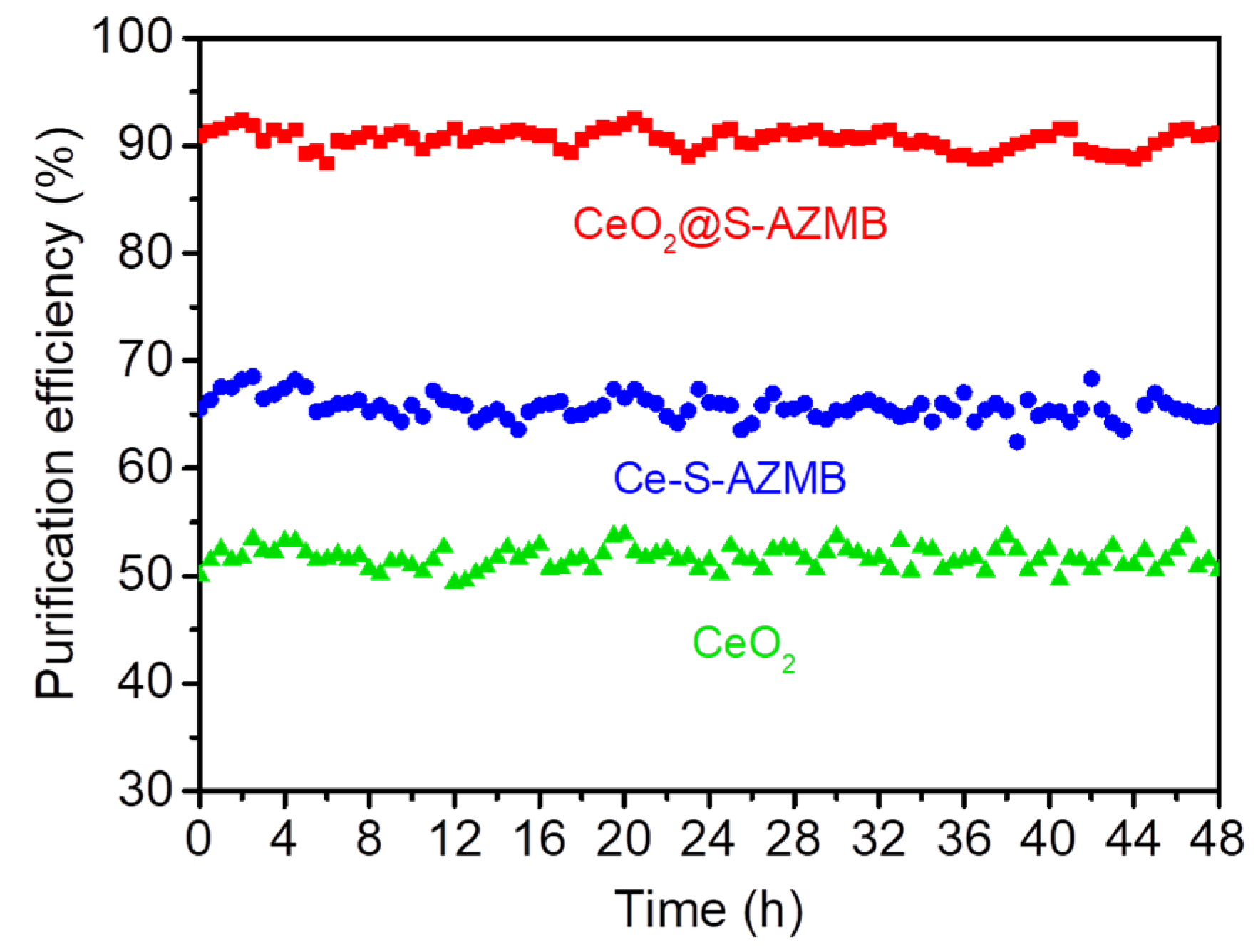
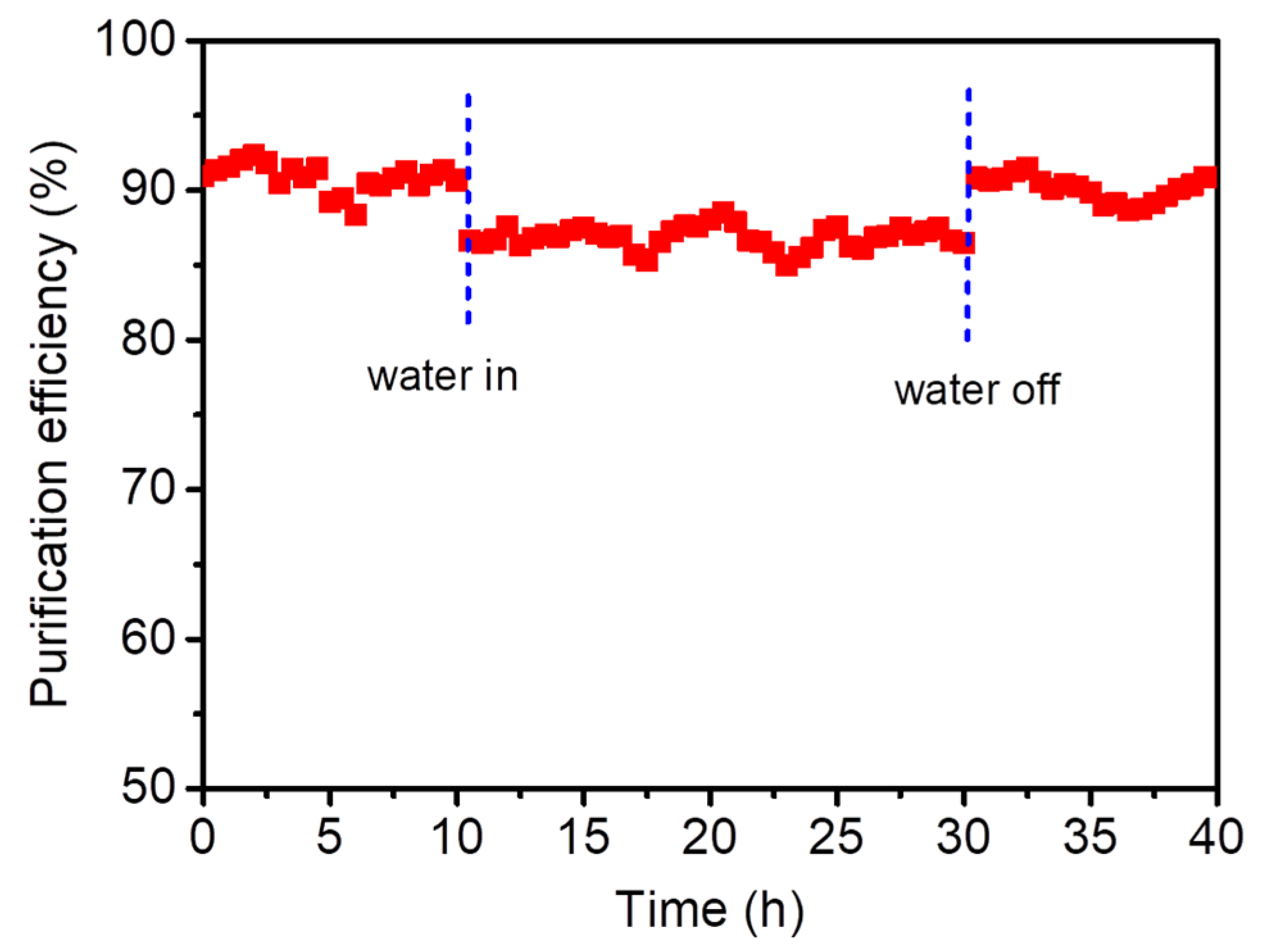
2.3. Thermal Catalytic Activity and Stability
3. Materials and Methods
3.1. Materials and Fabrication
3.2. Characterization
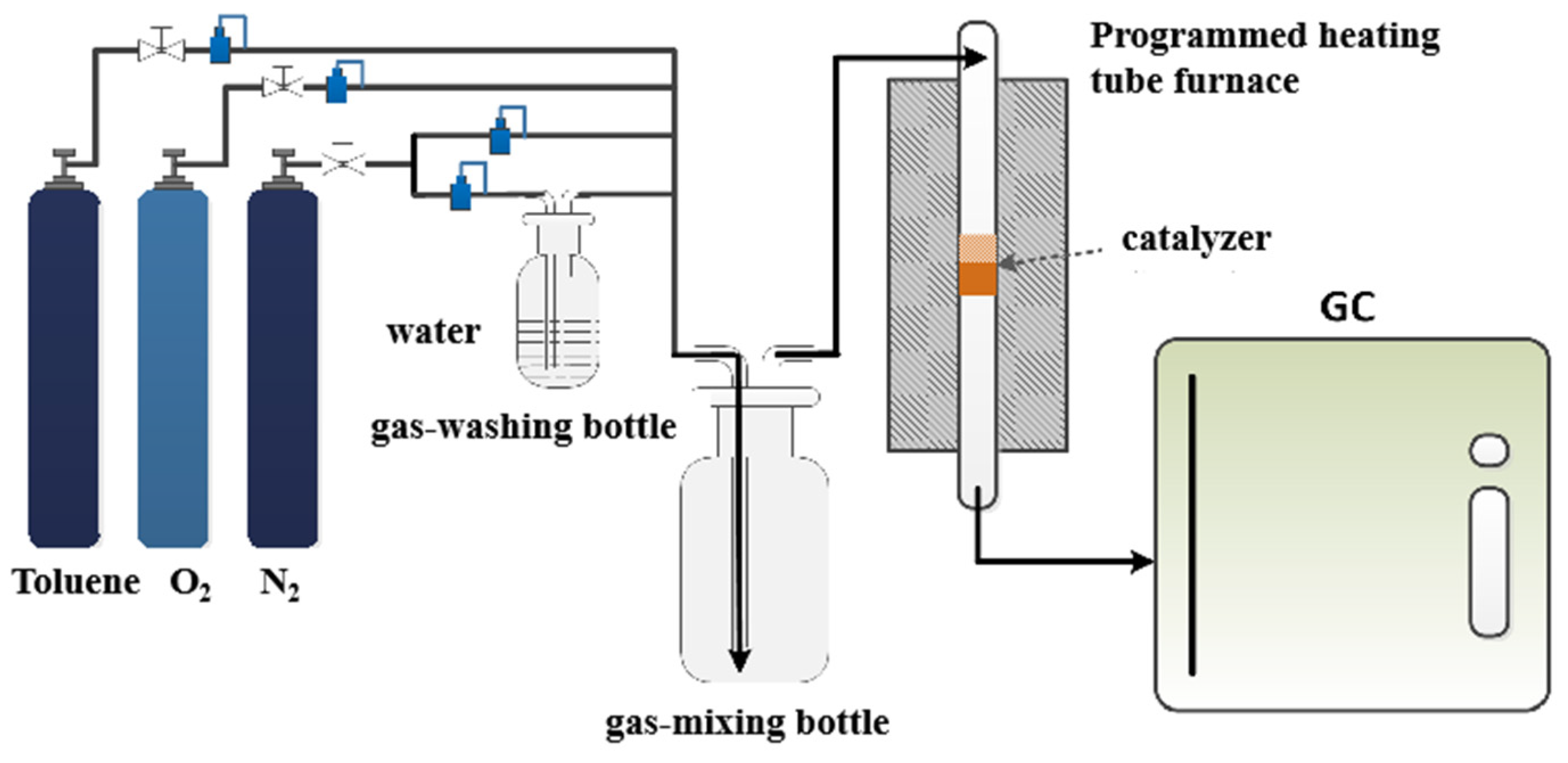
3.3. Thermal Catalytic Degradation of Toluene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, H.; Qu, Z. Synergistic effects in Mn-Co mixed oxide supported on cordierite honeycomb for catalytic deep oxidation of VOCs. J. Environ. Sci. 2022, 112, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Wang, Z.; Ma, J.; Kong, W.; Yuan, P.; Sun, R.; Shen, B. Analysis of functionality distribution and microstructural characteristics of upgraded rice husk after undergoing non-oxidative and oxidative torrefaction. Fuel 2022, 310, 122477. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, B.; Liu, L.; Zhang, Y.; Chen, Y.; Zhang, Q.; Yang, M.; Hu, L.; Wang, M.; Tang, Y. Enhanced catalytic oxidation of VOCs over porous Mn-based mullite synthesized by in-situ dismutation. J. Colloid Interface Sci. 2021, 585, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Tian, X.; Ding, X.; Hou, Z.; Li, Z.; Yu, X.; Wang, J.; Wu, L.; Jing, L.; Deng, J.; et al. Regulating catalytic stability of PtSnM/CeO2 (M = Mn, W, Nb) catalysts via the closely coupled multi-active sites to promote multicomponent VOCs oxidation. Chem. Eng. J. 2023, 471, 144456. [Google Scholar] [CrossRef]
- Ji, W.; Qu, G.; Zhou, J.; Ning, P.; Li, J.; Tang, H.; Pan, K.; Xie, R. Cracking of toluene by corona plasma combined with MnO2/CeO2 catalyst loaded on corona anode surface. Sep. Purif. Technol. 2023, 320, 124185. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Lv, L.; Zhang, T.; Chen, Y.; Tang, S.; Wang, Y.; Tang, W. Confinement effect and Hetero-interface enable High-Performing MnOx/CeO2 oxidation catalysts with exceptional sintering resistance: Morphology effect of ceria support. Chem. Eng. J. 2023, 462, 142257. [Google Scholar] [CrossRef]
- Pan, T.; Deng, H.; Kang, S.; He, H. A simple strategy to tune α-MnO2 and enhance VOC oxidation via precipitation rate control. Appl. Surf. Sci. 2022, 576, 151823. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, S.; Jin, X.; Chong, Y.; Li, Y.; Li, A.; Lin, J.; Qiu, Y.; Ye, D. Acid-activated layered δ-MnO2 promotes VOCs combustion. Appl. Surf. Sci. 2022, 574, 151707. [Google Scholar] [CrossRef]
- Yan, D.; Mo, S.; Sun, Y.; Ren, Q.; Feng, Z.; Chen, P.; Wu, J.; Fu, M.; Ye, D. Morphology-activity correlation of electrospun CeO2 for toluene catalytic combustion. Chemosphere 2020, 247, 125860. [Google Scholar] [CrossRef]
- Soltan, W.B.; Sun, J.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Zhang, Z. Discovering the key role of MnO2 and CeO2 particles in the Fe2O3 catalysts for enhancing the catalytic oxidation of VOC: Synergistic effect of the lattice oxygen species and surface-adsorbed oxygen. Sci. Total Environ. 2022, 819, 152844. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, S.; Zhao, Y.; Wang, Z.; Ma, J.; Xu, L.; Yang, J.; Shen, B. Investigation on the fuel quality and hydrophobicity of upgraded rice husk derived from various inert and oxidative torrefaction conditions. Renew. Energy 2022, 189, 1234–1248. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, H.; Liu, H.; Shi, P.; Fan, J.; Min, Y.; Xu, Q. Recycling application of waste Li–MnO2 batteries as efficient catalysts based on electrochemical lithiation to improve catalytic activity. Green Chem. 2018, 20, 4901–4910. [Google Scholar] [CrossRef]
- Zhan, L.; Li, O.; Wang, Z.; Xie, B. Recycling Zinc and Preparing High-Value-Added Nanozinc Oxide from Waste Zinc–Manganese Batteries by High-Temperature Evaporation-Separation and Oxygen Control Oxidation. ACS Sustain. Chem. Eng. 2018, 6, 12104–12109. [Google Scholar] [CrossRef]
- Liu, P. Recycling Waste Batteries: Recovery of Valuable Resources or Reutilization as Functional Materials. ACS Sustain. Chem. Eng. 2018, 6, 11176–11185. [Google Scholar] [CrossRef]
- Gallegos, M.V.; Falco, L.R.; Peluso, M.A.; Sambeth, J.E.; Thomas, H.J. Recovery of manganese oxides from spent alkaline and zinc-carbon batteries. An application as catalysts for VOCs elimination. Waste Manag. 2013, 33, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, M.V.; Peluso, M.A.; Finocchio, E.; Thomas, H.J.; Busca, G.; Sambeth, J.E. Removal of VOCs by catalytic process. A study of MnZnO composites synthesized from waste alkaline and Zn/C batteries. Chem. Eng. J. 2017, 313, 1099–1111. [Google Scholar] [CrossRef]
- Hoseini, S.; Rahemi, N.; Allahyari, S.; Tasbihi, M.; Ghareshabani, E. Effect of hydrometallurgical process parameters on the Mn2O3 nano catalysts derived from spent batteries used in the plasma catalytic oxidation of BTX. Adv. Powder Technol. 2020, 31, 4187–4196. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, M.K.; Jung, S.C.; Jung, H.Y.; Kim, H.; Park, Y.K. Effect of palladium on the black mass-based catalyst prepared from spent Zn/Mn alkaline batteries for catalytic combustion of volatile organic compounds. Chemosphere 2021, 276, 130209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, M.; Wang, Y.; Zhao, X.; Leung, D.Y. Low-cost and efficient Mn/CeO2 catalyst for photocatalytic VOCs degradation via scalable colloidal solution combustion synthesis method. J. Mater. Sci. Technol. 2022, 116, 169–179. [Google Scholar] [CrossRef]
- Sun, Q.; Ke, M.; Zhao, Y.; Wang, B.; Zhang, J.; Sheng, J. Embellishing {001} surface of Bi2MoO6 nanobelts with enhanced photocatalytic performance and mechanisms exploration. Appl. Surf. Sci. 2021, 563, 150104. [Google Scholar] [CrossRef]
- Feng, S.; Liu, J.; Gao, B. Synergistic mechanism of Cu-Mn-Ce oxides in mesoporous ceramic base catalyst for VOCs microwave catalytic combustion. Chem. Eng. J. 2022, 429, 132302. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Shi, N.; Yang, J.; Serageldin, M.A.; Pan, W.P. Enhancing the interaction between Mn and Ce oxides supported on fly ash with organic acid ligands interface modification for effective VOC removal: A combined experimental and DFT + U study. Fuel 2022, 313, 123043. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Liu, G.; Li, S.; Li, D.; Li, W.; Chen, Y. Preparation of hierarchical layer-stacking Mn-Ce composite oxide for catalytic total oxidation of VOCs. J. Rare Earths 2015, 33, 62–69. [Google Scholar] [CrossRef]
- Wan, J.; Tao, F.; Shi, Y.; Shi, Z.; Liu, Y.; Wu, G.; Kan, J.; Zhou, R. Designed preparation of nano rod shaped CeO2-MnO catalysts with different Ce/Mn ratios and its highly efficient catalytic performance for chlorobenzene complete oxidation: New insights into structure–activity correlations. Chem. Eng. J. 2022, 433, 133788. [Google Scholar] [CrossRef]
- Dong, F.; Han, W.; Guo, Y.; Han, W.; Tang, Z. CeCoOx-MNS catalyst derived from three-dimensional mesh nanosheet Co-based metal–organic frameworks for highly efficient catalytic combustion of VOCs. Chem. Eng. J. 2021, 405, 126948. [Google Scholar] [CrossRef]
- Geng, L.; Chen, B.; Yang, J.; Shui, C.; Ye, S.; Fu, J.; Zhang, N.; Xie, J.; Chen, B. Synergistic effect between Mn and Ce for active and stable catalytic wet air oxidation of phenol over MnCeOx. Appl. Catal. A Gen. 2020, 604, 117774. [Google Scholar] [CrossRef]
- Wu, P.; Dai, S.; Chen, G.; Zhao, S.; Xu, Z.; Fu, M.; Chen, P.; Chen, Q.; Jin, X.; Qiu, Y.; et al. Interfacial effects in hierarchically porous α-MnO2/Mn3O4 heterostructures promote photocatalytic oxidation activity. Appl. Catal. B Environ. 2020, 268, 118418. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L.; Pan, Y.; Wang, M. Adsorptive-photocatalytic performance and mechanism of Me (Mn,Fe)-N co-doped TiO2/SiO2 in cyanide wastewater. J. Alloys Compd. 2021, 867, 159020. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Song, Z.; Liu, W.; Zhao, H.; Zhao, M.; Xing, Y.; Du, H. The catalytic oxidation performance of toluene over the Ce-Mn-Ox catalysts: Effect of synthetic routes. J. Colloid Interface Sci. 2020, 562, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lin, D.; Zheng, Y.; Feng, X.; Chen, Q.; Zhang, K.; Wang, X.; Jiang, L. MnO2 nanoparticles encapsuled in spheres of Ce-Mn solid solution: Efficient catalyst and good water tolerance for low-temperature toluene oxidation. Appl. Surf. Sci. 2020, 504, 144481. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, J.; Liang, X.; Long, C. Niobium doping enhanced catalytic performance of Mn/MCM-41 for toluene degradation in the NTP-catalysis system. Chemosphere 2019, 230, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Dong, F.; Feng, H.; Tang, Z. Construction of superhydrophobic layer for enhancing the water-resistant performance of VOCs catalytic combustion. Fuel 2022, 314, 123139. [Google Scholar] [CrossRef]




| Samples | Specific Surface Area (m2g−1) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| CeO2@S-AZMB | 44.04 | 0.12 | 13.63 |
| Ce-S-AZMB | 92.93 | 0.26 | 11.02 |
| CeO2 | 143.99 | 0.29 | 8.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Du, H.; Zhao, Z.; Wang, Z. Study on the Catalytic Oxidation of Toluene Using CeO2@S-AZMB Prepared from Spent Zn-Mn Batteries. Molecules 2024, 29, 616. https://doi.org/10.3390/molecules29030616
Zou Y, Du H, Zhao Z, Wang Z. Study on the Catalytic Oxidation of Toluene Using CeO2@S-AZMB Prepared from Spent Zn-Mn Batteries. Molecules. 2024; 29(3):616. https://doi.org/10.3390/molecules29030616
Chicago/Turabian StyleZou, Yu, Huan Du, Zhong Zhao, and Zhuozhi Wang. 2024. "Study on the Catalytic Oxidation of Toluene Using CeO2@S-AZMB Prepared from Spent Zn-Mn Batteries" Molecules 29, no. 3: 616. https://doi.org/10.3390/molecules29030616
APA StyleZou, Y., Du, H., Zhao, Z., & Wang, Z. (2024). Study on the Catalytic Oxidation of Toluene Using CeO2@S-AZMB Prepared from Spent Zn-Mn Batteries. Molecules, 29(3), 616. https://doi.org/10.3390/molecules29030616






