A Review of Synthesis and Applications of Al2O3 for Organic Dye Degradation/Adsorption
Abstract
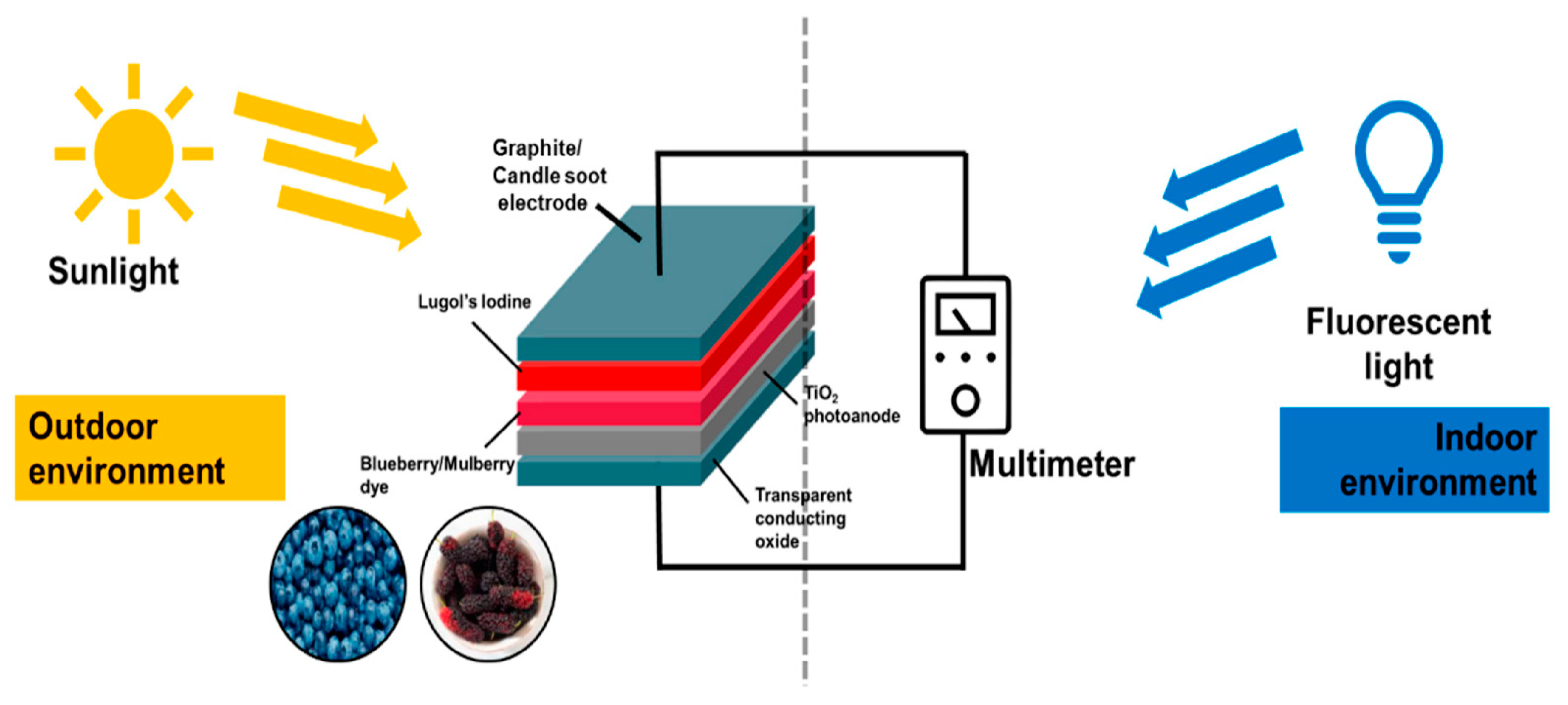
:1. Introduction
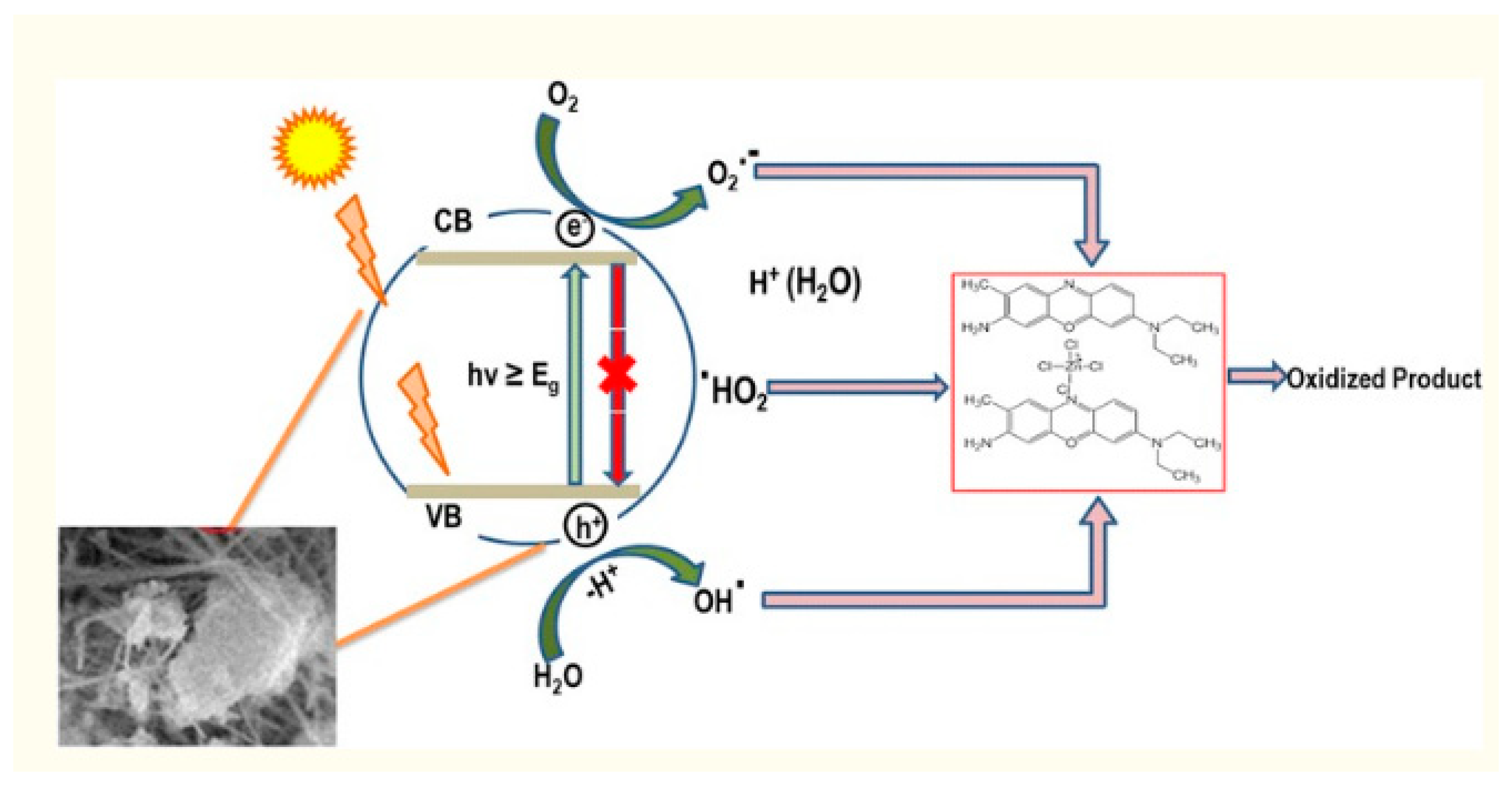
2. Photocatalytic Activity of AL2O3 NMs
- H2O2 ------Al2O3----→2OH
- Dye + *OH --------------→ Dye Mineralization Products
- H2O2 + *OH -------------→ HO*2 + H2O
- H2O2 + HO*2 -------------→*OH+ H2O+ O2
- HO2+ *OH ------------→ H2O + O2
3. Photocatalytic Activity of RhB
4. Adsorption Followed by Photocatalysis
5. Photocatalytic Effect of Implemented Oxides
6. Photo-Degradation of Malachite Green
7. Green Synthesis of Al2O3 Nanoparticles
8. Photo-Degradation of Dyes
9. Photocatalytic Test
10. Recycling Methods
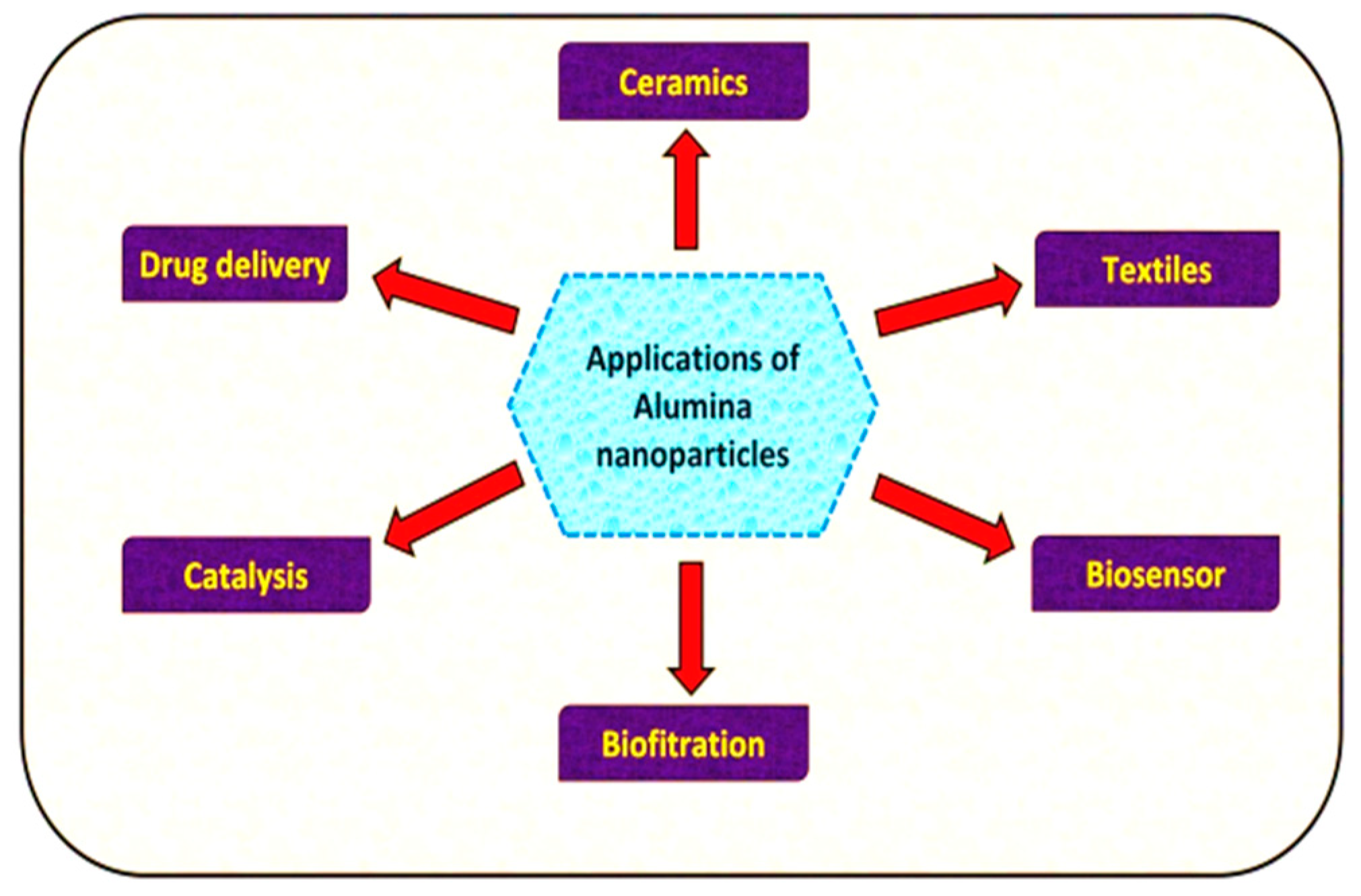
11. Applications
11.1. Organic Pollutant Elimination
11.2. Other Applications
11.3. Environmental Impact
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spadaro, D.; Barichello, J.; Citro, I.; Calogero, G. Environmentally Friendly Water-Based Electrolyte for Dye-Sensitized Solar Cells: Future Prospective and Outlook. Solar 2023, 3, 229–252. [Google Scholar] [CrossRef]
- Barichello, J.; Vesce, L.; Mariani, P.; Leonardi, E.; Braglia, R.; Di Carlo, A.; Canini, A.; Reale, A. Stable Semi-Transparent Dye-Sensitized Solar Modules and Panels for Greenhouse Application. Energies 2021, 14, 6393. [Google Scholar] [CrossRef]
- Schoden, F.; Dotter, M.; Knefelkamp, D.; Blachowicz, T.; Schwenzfeier Hellkamp, E. Review of State of the Art Recycling Methods in the Context of Dye Sensitized Solar Cells. Energies 2021, 14, 3741. [Google Scholar] [CrossRef]
- Schoden, F.; Schnatmann, A.K.; Davies, E.; Diederich, D.; Storck, J.L.; Knefelkamp, D.; Blachowicz, T.; Schwenzfeier-Hellkamp, E. Investigating the Recycling Potential of Glass Based Dye-Sensitized Solar Cells—Melting Experiment. Materials 2021, 14, 6622. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Haleem, A.; Siddiq, M.; Hussain, M.K.; Qamar, S.; Hameed, S.; Waris, M. Research on dye sensitized solar cells: Recent advancement toward the various constituents of dye sensitized solar cells for efficiency enhancement and future prospects. RSC Adv. 2023, 13, 19508–19529. [Google Scholar] [CrossRef] [PubMed]
- Miettunen, K.; Santasalo-Aarnio, A. Eco-design for dye solar cells: From hazardous waste to profitable recovery. J. Clean. Prod. 2021, 320, 128743. [Google Scholar] [CrossRef]
- Schoden, F.; Schnatmann, A.K.; Blachowicz, T.; Manz-Schumacher, H.; Schwenzfeier-Hellkamp, E. Circular Design Principles Applied on Dye-Sensitized Solar Cells. Sustainability 2022, 14, 15280. [Google Scholar] [CrossRef]
- Sánchez-García, M.A.; Bokhimi, X.; Velázquez Martínez, S.; Jiménez-González, A.E. Dye-Sensitized Solar Cells Prepared with Mexican Pre-Hispanic Dyes. J. Nanotechnol. 2018, 2018, 1236878. [Google Scholar] [CrossRef]
- Pawlus, K.; Jarosz, T. Transition Metal Coordination Compounds as Novel Materials for Dye-Sensitized Solar Cells. Appl. Sci. 2022, 12, 3442. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Suriyaprabha, R.; Satheesh, S.K.; Sivarama Krishna, L. Emergent nanomaterials and their composite fabrication for multifunctional applications. In Nanomaterials in Bionanotechnology, 1st ed.; Pratap Singh, R., Singh, K.R.B., Eds.; CRC Press: Boca Raton, FL, USA, 2021; p. 19. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K.M. Zn–Cr layered double hydroxide: Visible light responsive photocatalyst for photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2012, 91, 73–80. [Google Scholar] [CrossRef]
- Majhi, A.; Monash, P.; Pugazhenthi, G. Fabrication and Characterization of γ-Al2O3-Clay Composite Ultrafiltration Membrane for the Separation of Electrolytes from Its Aqueous Solution. J. Membr. Sci. 2009, 340, 181–191. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Prakash Shukla, S.; Kumar, K.; Shree Bharti, V. Acute toxicity of textile dye Methylene blue on growth and metabolism of selected freshwater microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef] [PubMed]
- Vattikuti, S.V.P.; Byon, C. Synthesis and Structural Characterization of Al2O3-Coated MoS2 Spheres for Photocatalysis Applications. J. Nanomater. 2015, 2015, 978409. [Google Scholar] [CrossRef]
- Karunakaran, C.; Magesan, P.; Gomathisankar, P.; Vinayagamoorthy, P. Photocatalytic degradation of dyes by Al2O3-TiO2 and ZrO2-TiO2 nanocomposites. Mater. Sci. Forum 2013, 734, 325–333. [Google Scholar] [CrossRef]
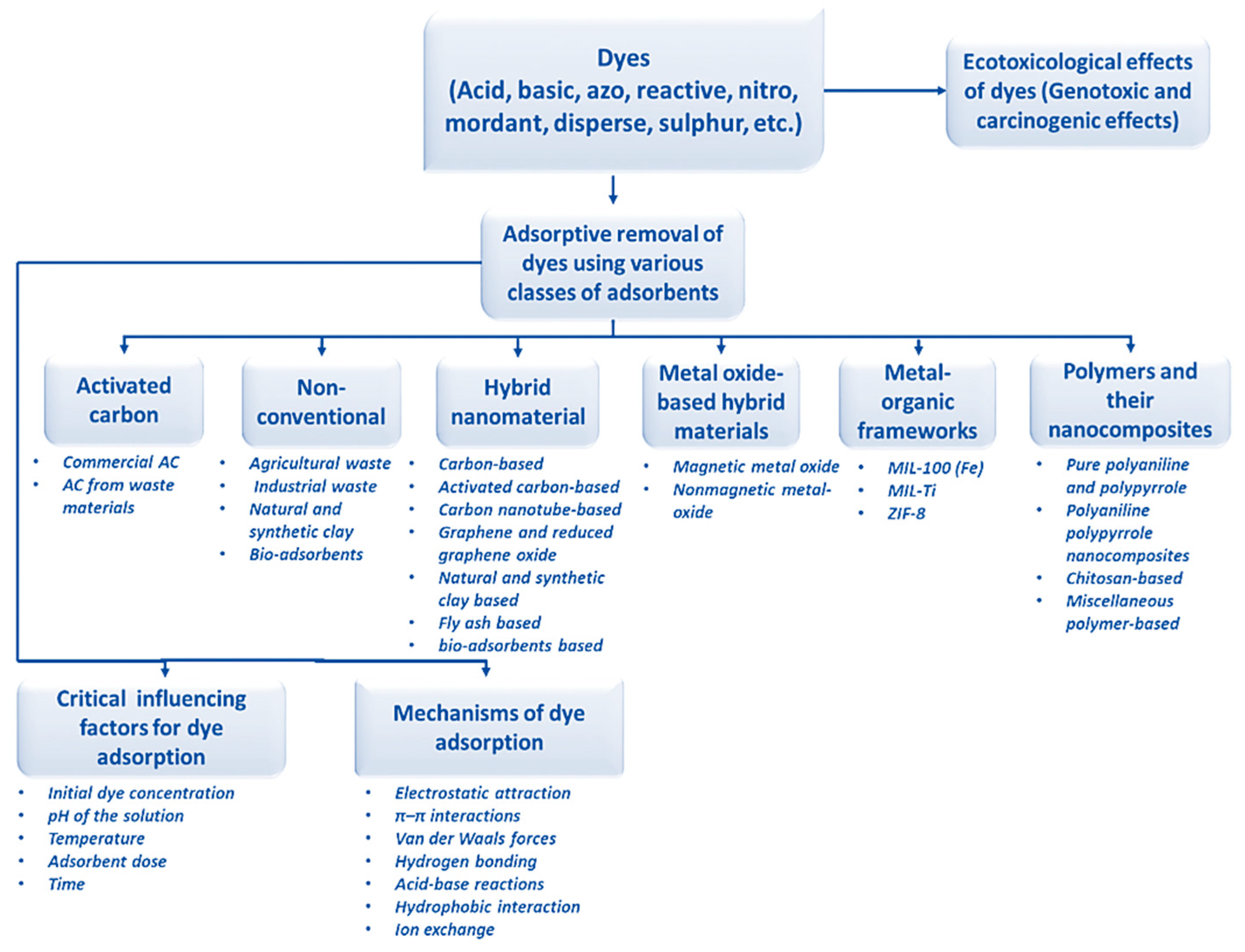
- Dutta, S.; Gupta, B.; Kumar Srivastava, S.; Kumar Gupta, A. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497. [Google Scholar] [CrossRef]
- Salama, A.; Mohamed, A.; Aboamera, N.M.; Osman, T.A.; Khattab, A. Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Appl. Nanosci. 2018, 8, 155–161. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Palani, G.; Arputhalatha, A.; Kannan, K.; Lakkaboyana, S.K.; Hanafiah, M.M.; Kumar, V.; Marella, R.K. Current Trends in the Application of Nanomaterials for the Removal of Pollutants from Industrial Wastewater Treatment—A Review. Molecules 2021, 26, 2799. [Google Scholar] [CrossRef]
- Brame, J.; Li, Q.; Alvarez, P.J.J. Nanotechnology-enabled water treatment and reuse: Emerging opportunities and challenges for developing countries. Trends Food Sci. Technol. 2011, 22, 618–624. [Google Scholar] [CrossRef]
- Pathania, D.; Rishu, K.; Harpreet, K. Enhanced photocatalytic activity of electrochemically synthesized aluminum oxide nanoparticles. Int. J. Miner. Metall. Mater. 2016, 23, 358–371. [Google Scholar] [CrossRef]
- Rani, A.; Singh, K.; Patel, A.S.; Chakraborti, A.; Kumar, S.; Ghosh, K.; Sharma, P. Visible light driven photocatalysis of organic dyes using SnO2 decorated MoS2 nanocomposites. Chem. Phys. Lett. 2020, 738, 136874. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Parida, K.M. Facile synthesis of mesoporous composite Fe/Al2O3–MCM-41: An efficient adsorbent/catalyst for swift removal of methylene blue and mixed dyes. J. Mater. Chem. 2012, 22, 7567–7579. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, V.C.; Mandal, T.K.; Mall, I.D. Synthesis of Different Crystallographic Al2O3 Nanomaterials from Solid Waste for Application in Dye Degradation. RSC Adv. 2014, 4, 50801–50810. [Google Scholar] [CrossRef]
- Isfahani, T.D.; Javadpour, J.; Khavandi, A.; Dinnebier, R.; Goodarzi, M.; Rezaie, H.R. Mechanochemical synthesis of alumina nanoparticles: Formation mechanism and phase transformation. Powder Technol. 2012, 229, 17. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Dai, H.; He, H. Facile synthesis and unique physicochemical properties of three-dimensionally ordered macroporous magnesium oxide, gamma-alumina, and ceria-zirconia solid solutions with crystalline mesoporous wall. Inorg. Chem. 2009, 48, 4421. [Google Scholar] [CrossRef]
- Shelke, P.D.; Rajbhoj, A.S. Electrochemical Synthesis and their Photocatalytic Application of Mesoporous γ-Al2O3 Nanoparticles. Der Chemica Sinica 2017, 8, 482–486. [Google Scholar]
- Renuka, N.K.; Shijina, A.V.; Praveen, A.K. Mesoporous γ-alumina nanoparticles: Synthesis, characterization and dye removal efficiency. Mater. Lett. 2012, 82, 42–44. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Srivastava, V.; Mukherjee, A.K. Synthesis and application of nano-Al2O3 powder for the reclamation of hexavalent chromium from aqueous solutions. J. Chem. Eng. Data 2010, 55, 2390–2398. [Google Scholar] [CrossRef]
- Palani, G.; Apsari, R.; Hanafiah, M.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Kannan, K.; Shivanna, A.T.; Idris, A.M.; Yadav, C.H. Metal-Doped Graphitic Carbon Nitride Nanomaterials for Photocatalytic Environmental Applications—A Review. Nanomaterials 2022, 12, 1754. [Google Scholar] [CrossRef]
- Tok, A.I.Y.; Boey, F.Y.C.; Zhao, X.L. Novel synthesis of Al2O3 nano-particles by flame spray pyrolysis. J. Mater. Process. Technol. 2006, 178, 270–273. [Google Scholar] [CrossRef]
- Ali, J.; Bibi, S.; Bux Jatoi, W.; Tuzen, M.; Ahmed Jakhrani, M.; Feng, X.; Saleh, T.A. Green synthesized zinc oxide nanostructures and their applications in dye-sensitized solar cells and photocatalysis: A review. Mater. Today Commun. 2023, 36, 106840. [Google Scholar] [CrossRef]
- Tuan Cuong, D.L.; Cuong, L.V.; Trung Nghia, L.T.; Trinh, D.N.; Thuy Linh, N.T.; Hai, N.D.; Liem Chau, P.T.; Hoang, N.T.; Phong, M.T.; Hieu, N.H. Synthesis of titanium dioxide/reduced graphene oxide nanocomposite by ultrasound-assisted mechanical mixing method for fabricating photoanode to upgrade the performance and stability of dye-sensitized solar cell. Mater. Res. Bull. 2022, 156, 112000. [Google Scholar] [CrossRef]
- Logita, H.H.; Tadesse, A.; Kebede, T. Synthesis, characterization and photocatalytic activity of MnO2/Al2O3/Fe2O3 nanocomposite for degradation of malachite green. Afr. J. Pure Appl. Chem. 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Asif, S.A.B.; Khan, S.B.; Asiri, A.M. Visible light functioning photocatalyst based on Al2O3 doped Mn3O4 nanomaterial for the degradation of organic toxin. Nanoscale Res Lett. 2015, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, Y.J.; Khanna, P.K.; Jun, K.W.; Bae, J.W.; Kim, Y.H. Alumina-supported iron oxide nanoparticles as Fischer-Tropsch catalysts: Effect of particle size of iron oxide. J. Mol. Catal. A Chem. 2010, 323, 84–90. [Google Scholar] [CrossRef]
- Dai, K.; Chen, H.; Peng, T.; Ke, D.; Yi, H. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere 2007, 69, 1361–1367. [Google Scholar] [CrossRef]
- Ramos-Fernandez, M.; Normand, L.; Sorbier, L. Structural and Morphological Characterization of Alumina Supported Pd Nanoparticles Obtained by Colloidal Synthesis. Oil Gas Sci. Technol. 2007, 62, 101–113. [Google Scholar] [CrossRef]
- Praveen, S.C.; Timon, V.; Valant, M. Electronic band gaps of ternary corundum solid solutions from Fe2O3–Cr2O3–Al2O3 system for photocatalytic applications: A theoretical study. Comput. Mater. Sci. 2012, 55, 192–198. [Google Scholar] [CrossRef]
- Dharmalingam, P.; Palani, G.; Apsari, R.; Kannan, K.; Krishna Lakkaboyana, S.; Venkateswarlu, K.; Kumar, V.; Ali, Y. Synthesis of metal oxides/sulfides-based nanocomposites and their environmental applications: A review. Mater. Today Sustain. 2022, 20, 100232. [Google Scholar] [CrossRef]
- Wang, J.A.; Bokhimi, X.; Morales, A.; Novaro, O.; Lopez, T.; Gomez, R. Aluminum Local Environment and Defects in the Crystalline Structure of Sol-Gel Alumina Catalyst. J. Phys. Chem. B 1999, 103, 299–303. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. Gamma-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. Eur. J. Inorg. Chem. 2005, 17, 3393–3403. [Google Scholar] [CrossRef]
- Almasi, A.; Mohammadi, M.; Baniamerian, F.; Berizi, Z.; Almasi, M.H.; Pariz, Z. Modeling of antibiotic degradation in sonophotocatalytic process, increasing biodegradability and process optimization by response surface methodology (RSM). Int. J. Environ. Sci. Technol. 2019, 16, 8437–8448. [Google Scholar] [CrossRef]
- Li, F.T.; Zhao, Y.; Wang, Q.; Wang, X.J.; Hao, Y.J.; Liu, R.H.; Zhao, D. Enhanced visible-light photocatalytic activity of active Al2O3/g-C3N4 heterojunctions synthesized via surface hydroxyl modification. J. Hazard. Mater. 2015, 283, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Dharamalingam, K.; Kumar, B.A.; Ramalingam, G.; Sasi Florence, S.; Raju, K.; Senthil Kumar, P.; Govindaraju, S.; Thangavel, E. The role of sodium dodecyl sulfate mediated hydrothermal synthesis of MoS2 nanosheets for photocatalytic dye degradation and dye-sensitized solar cell application. Chemosphere 2022, 294, 133725. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Li, Y.; Sheng, P.; Tian, Z.; Liu, W.; Wu, H.; Bao, Y.; Wu, S. Fabrication of Al2O3/AlN composite ceramics with enhanced performance via a heterogeneous precipitation coating process. Ceram. Int. 2020, 46, 21156–21165. [Google Scholar] [CrossRef]
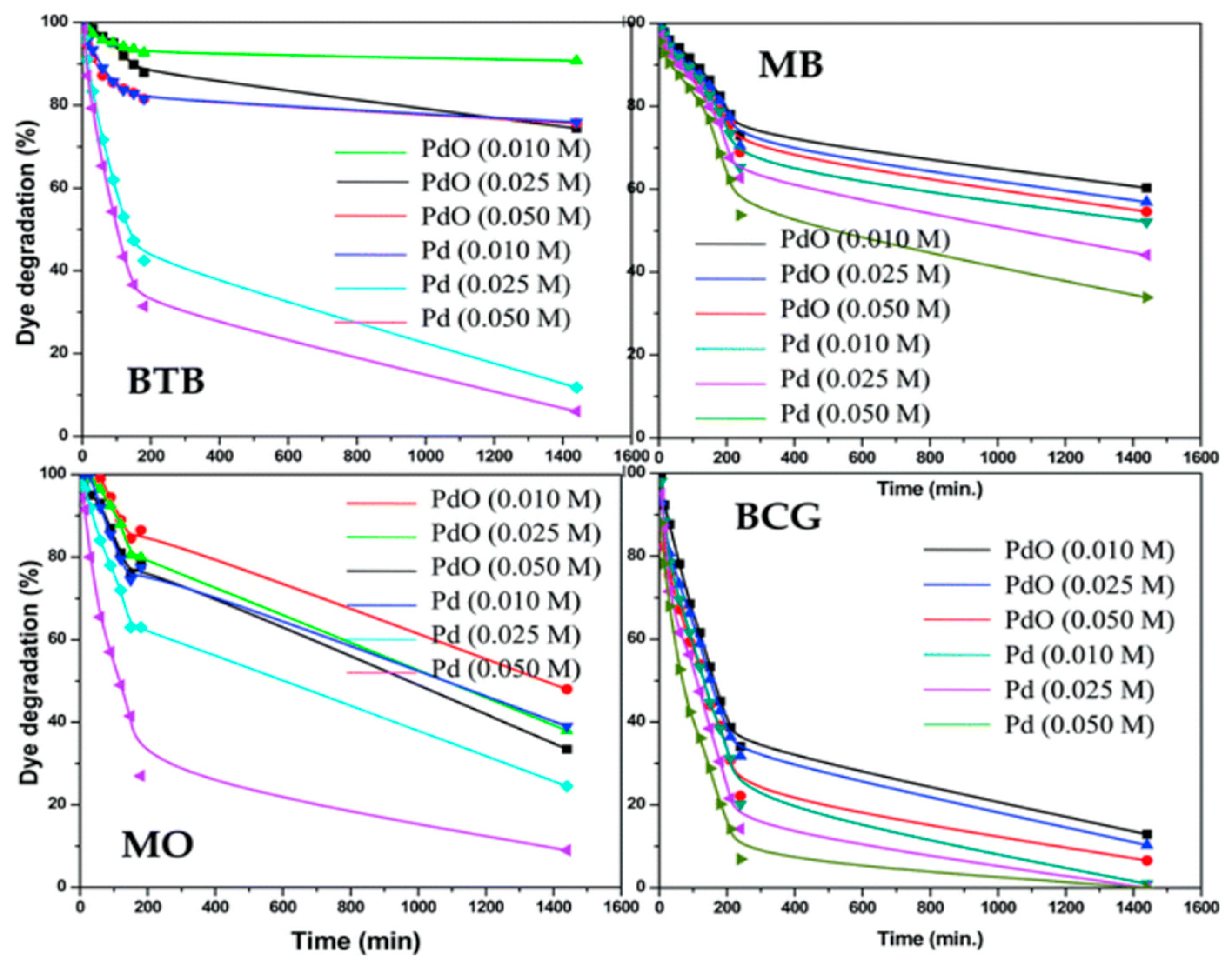
- Kumar, A.P.; Bilehal, D.; Tadesse, A.; Kumar, D. Photocatalytic degradation of organic dyes: Pd-γ-Al2O3 and PdO-γ-Al2O3 as potential photocatalysts. RSC Adv. 2021, 11, 6396. [Google Scholar] [CrossRef]
- Andualem, A.; Demiss, S. Review on dye-sensitized solar cells (DSSCs). Edelweiss Appl. Sci. Technol. 2018, 2, 145–150. [Google Scholar] [CrossRef]
- Alam, M.; Rahman, M.M.; Uddin, M.; Asiri, A.M.; Inamuddin; Chani, M.T.S.; Islam, M. Development of l-glutamic acid biosensor with ternary ZnO/NiO/Al2O3 nanoparticles. J. Lumin. 2020, 227, 117528. [Google Scholar] [CrossRef]
- Casillas, J.E.; Campa-Molina, J.; Tzompantzi, F.; Carbajal Arízaga, G.G.; López-Gaona, A.; Ulloa-Godínez, S.; Cano, M.E.; Barrera, A. Photocatalytic Degradation of Diclofenac Using Al2O3-Nd2O3 Binary Oxides Prepared by the Sol-Gel Method. Materials 2020, 13, 1345. [Google Scholar] [CrossRef]
- Shih, T.S.; Wei, P.S.; Huang, Y.S. Optical properties of anodic aluminum oxide films on Al1050 alloys. Surf. Coat. Technol. 2008, 202, 3298–3305. [Google Scholar] [CrossRef]
- Ateş, S.; Baranb, E.; Yazıcı, B. The nanoporous anodic alumina oxide formed by two-step anodization. Thin Solid Films 2018, 648, 94–102. [Google Scholar] [CrossRef]
- Piot, A.; Earl, S.K.; Ng, C.; Dligatch, S.; Roberts, A.; Davis, T.J.; Gómez, D.E. Collective excitation of plasmonic hot-spots for enhanced hot charge carrier transfer in metal/semiconductor contacts. Nanoscale 2015, 7, 8294–8298. [Google Scholar] [CrossRef] [PubMed]
- Hassena, H. Photocatalytic degradation of methylene blue by using Al2O3/Fe2O3 nano composite under visible light. Mod. Chem. Applic. 2016, 4, 176. [Google Scholar] [CrossRef]
- Ikpesu, J.E.; Iyuke, S.E.; Daramola, M.; Okewale, A.O. Synthesis of improved dye-sensitized solar cell for renewable energy power generation. Sol. Energy 2020, 206, 918–934. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, M.Y.; Cao, Y.; Lin, Y. Decolorization of Reactive Black 5 by Mesoporous Al2O3 @TiO2 Nanocomposites. Environ. Prog. Sustain. Energy 2019, 38, S230–S242. [Google Scholar] [CrossRef]
- Liu, D.; Robertson, J. Oxygen vacancy levels and interfaces of Al2O3. Microelectron. Eng. 2009, 86, 1668–1671. [Google Scholar] [CrossRef]
- Ramadurgam, S.; Lin, T.G.; Yang, C. Aluminum Plasmonics for Enhanced Visible Light Absorption and High Efficiency Water Splitting in Core–Multishell Nanowire Photoelectrodes with Ultrathin Hematite Shells. Nano Lett. 2014, 14, 4517–4522. [Google Scholar] [CrossRef]
- Soylu, A.M.; Polat, M.; Erdogan, D.A.; Say, Z.; Yıldırım, C.; Birer, Ö.; Ozensoy, E. TiO2–Al2O3 binary mixed oxide surfaces for photocatalytic NOx abatement. Appl. Surf. Sci. 2014, 318, 142–149. [Google Scholar] [CrossRef]
- Jwad, K.H.; Saleh, T.H.; Abd-Alhamza, B. Preparation of Aluminum Oxide Nanoparticles by Laser Ablation and a Study of Their Applications as Antibacterial and Wounds Healing Agent. Nano Biomed. Eng. 2019, 11, 313–319. [Google Scholar] [CrossRef]
- Shukor, N.I.A.; Chan, K.-Y.; Thien, G.S.H.; Yeoh, M.-E.; Low, P.-L.; Devaraj, N.K.; Ng, Z.-N.; Yap, B.K. A Green Approach to Natural Dyes in Dye-Sensitized Solar Cells. Sensors 2023, 23, 8412. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendran, S.; Palani, G.; Shanmugam, V.; Trilaksanna, H.; Kannan, K.; Nykiel, M.; Korniejenko, K.; Marimuthu, U. A Review of Synthesis and Applications of Al2O3 for Organic Dye Degradation/Adsorption. Molecules 2023, 28, 7922. https://doi.org/10.3390/molecules28237922
Rajendran S, Palani G, Shanmugam V, Trilaksanna H, Kannan K, Nykiel M, Korniejenko K, Marimuthu U. A Review of Synthesis and Applications of Al2O3 for Organic Dye Degradation/Adsorption. Molecules. 2023; 28(23):7922. https://doi.org/10.3390/molecules28237922
Chicago/Turabian StyleRajendran, Sundarakannan, Geetha Palani, Vigneshwaran Shanmugam, Herri Trilaksanna, Karthik Kannan, Marek Nykiel, Kinga Korniejenko, and Uthayakumar Marimuthu. 2023. "A Review of Synthesis and Applications of Al2O3 for Organic Dye Degradation/Adsorption" Molecules 28, no. 23: 7922. https://doi.org/10.3390/molecules28237922
APA StyleRajendran, S., Palani, G., Shanmugam, V., Trilaksanna, H., Kannan, K., Nykiel, M., Korniejenko, K., & Marimuthu, U. (2023). A Review of Synthesis and Applications of Al2O3 for Organic Dye Degradation/Adsorption. Molecules, 28(23), 7922. https://doi.org/10.3390/molecules28237922









