Whipping Creams: Advances in Molecular Composition and Nutritional Chemistry
Abstract
1. Introduction
2. Whipping Cream: Emulsion and Foam Characterization
2.1. Main Components of Whipping Cream and Their Key Roles
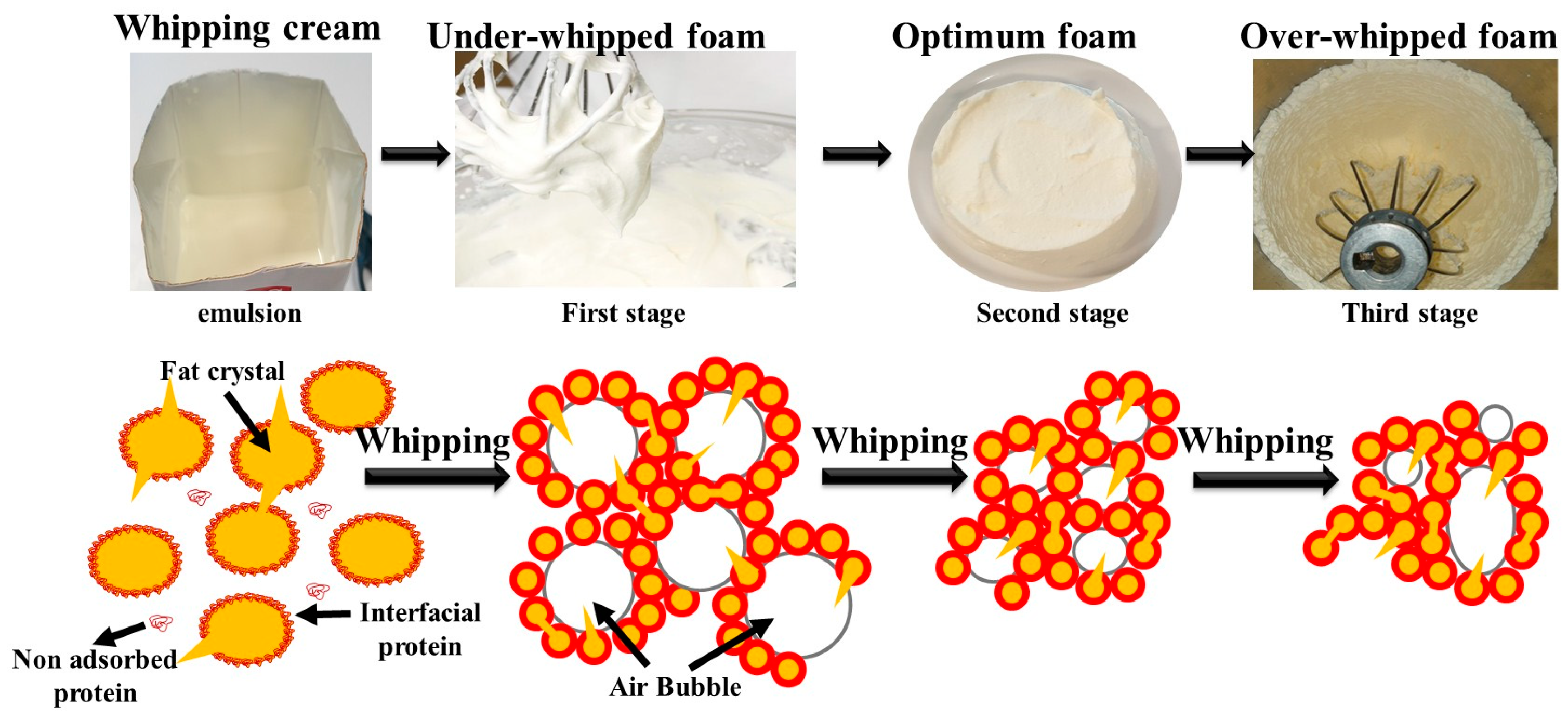
2.2. Whipping Cream: Mechanism of Formation and Stabilization
3. Effect of Emulsifiers at the Interface
3.1. Low-Molecular-Weight Surfactants
3.2. Native or Modified Dairy and Plant-Based Proteins
3.3. Surface-Active Agent Complexation
3.4. Pickering Emulsion Formation
4. Effect of Stabilizers in the Continuous Phase
4.1. Effect of Sugar Addition
4.2. Effect of Polysaccharides Addition
4.3. Effect of Protein–Polysaccharide Complex
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrade, J.; Rousseau, D. Whipping Properties of Recombined, Additive-Free Creams. J. Dairy Sci. 2021, 104, 6487–6495. [Google Scholar] [CrossRef] [PubMed]
- Whipping Cream Market Forecast, Share, Strategies, Scope, Overview. Whipping Cream Market Size, Share and Forecast 2025–2035. Available online: https://www.marketsizeandtrends.com/download-sample/673662/?utm_source=Pulse-2-5visited (accessed on 26 September 2024).
- Gafour, W.; Aly, E. Organoleptic, textural and whipping properties of whipped cream with different stabilizer blends. Acta Sci. Pol. Technol. Aliment. 2020, 19, 425–433. [Google Scholar] [CrossRef]
- Zeng, Y.; Zeng, D.; Liu, T.; Cai, Y.; Li, Y.; Zhao, M.; Zhao, Q. Effects of Glucose and Corn Syrup on the Physical Characteristics and Whipping Properties of Vegetable-Fat Based Whipped Creams. Foods 2022, 11, 1195. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, G.; Chen, W.; Qie, X.; Fu, L.; Li, X.; He, Z.; Zeng, M.; Goff, H.D.; Chen, J. Effects of Soy Proteins and Hydrolysates on Fat Globule Coalescence and Whipping Properties of Recombined Low-Fat Whipped Cream. Food Biophys. 2022, 17, 324–334. [Google Scholar] [CrossRef]
- Han, J.; Zhou, X.; Cao, J.; Wang, Y.; Sun, B.; Li, Y.; Zhang, L. Microstructural Evolution of Whipped Cream in Whipping Process Observed by Confocal Laser Scanning Microscopy. Int. J. Food Prop. 2018, 21, 593–605. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, D.; Li, Y.; Li, M.; Wang, Y.; Li, Y.; Zhang, L. Thermodynamic and Whipping Properties of Milk Fat in Whipped Cream: A Study Based on DSC and TD-NMR. Int. Dairy J. 2019, 97, 149–157. [Google Scholar] [CrossRef]
- Siva Kumar, S.; Balasubramanyam, B.V.; Jayaraj Rao, K.; Heartwin Amala Dhas, P.; Surendra Nath, B. Effect of Flaxseed Oil and Flour on Sensory, Physicochemical and Fatty Acid Profile of the Fruit Yoghurt. J. Food Sci. Technol. 2017, 54, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.Y.; Ahn, J.; Kwak, H.S. Functional Properties of Cholesterol-Removed Whipping Cream Treated by β-Cyclodextrin. J. Dairy Sci. 2003, 86, 2767–2772. [Google Scholar] [CrossRef]
- Anihouvi, P.; Danthine, S.; Karamoko, G.; Blecker, C. Les Crèmes Végétales: Une Alternative Aux Crèmes Laitières (Synthèse Bibliographique); BASE: Tokyo, Japan, 2012; Volume 16. [Google Scholar]
- Carr, N.O.; Hogg, W.F. A Manufacturer’s Perspective on Selected Palm-Based Products. Asia Pac. J. Clin. Nutr. 2005, 14, 381–386. [Google Scholar] [PubMed]
- Riaublanc, A.; Anton, M.; Mariette, F.; Georges, C.; Gravier, E.; Drelon, N.; Omari, A.; Leal-Calderon, F. Impact Des Cristaux de Matière Grasse Sur l’aptitude Au Foisonnement et La Stabilité Des Émulsions Laitières Foisonnées. Sci. Aliment. = Food Sci. Int. J. Food Sci. Technol. 2005, 25, 427–441. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving Emulsion Formation, Stability and Performance Using Mixed Emulsifiers: A Review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, Y.; Di, Z.; Zhao, M.; Zhao, Q. Improving Quality Characteristics of Whipped Cream Based on Novel Additives: A Review of Current Status, Challenges, and Strategies. Food Bioprocess Tech. 2024. [Google Scholar] [CrossRef]
- Goff, H.D. Instability and Partial Coalescence in Whippable Dairy Emulsions. J. Dairy Sci. 1997, 80, 2620–2630. [Google Scholar] [CrossRef]
- Moens, K.; Tavernier, I.; Dewettinck, K. Crystallization Behavior of Emulsified Fats Influences Shear-Induced Partial Coalescence. Food Res. Int. 2018, 113, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Munk, M.B.; Andersen, M.L. Partial Coalescence in Emulsions: The Impact of Solid Fat Content and Fatty Acid Composition. Eur. J. Lipid Sci. Technol. 2015, 117, 1627–1635. [Google Scholar] [CrossRef]
- Fuller, G.T.; Considine, T.; Golding, M.; Matia-Merino, L.; MacGibbon, A. Aggregation Behavior of Partially Crystalline Oil-in-Water Emulsions: Part II—Effect of Solid Fat Content and Interfacial Film Composition on Quiescent and Shear Stability. Food Hydrocoll. 2015, 51, 23–32. [Google Scholar] [CrossRef]
- Ihara, K.; Habara, K.; Ozaki, Y.; Nakamura, K.; Ochi, H.; Saito, H.; Asaoka, H.; Uozumi, M.; Ichihashi, N.; Iwatsuki, K. Influence of Whipping Temperature on the Whipping Properties and Rheological Characteristics of Whipped Cream. J. Dairy. Sci. 2010, 93, 2887–2895. [Google Scholar] [CrossRef]
- Liu, N.; Cui, J.; Li, G.; Li, D.; Chang, D.; Li, C.; Chen, X. The Application of High Purity Diacylglycerol Oil in Whipped Cream: Effect on the Emulsion Properties and Whipping Characteristics. CYTA-J. Food 2019, 17, 60–68. [Google Scholar] [CrossRef]
- Scopus-Document Details-Effects of Sodium Caseinate and Whey Proteins on Whipping Properties and Texture Characteristics of Whipped Cream. Available online: https://www-scopus-com.inc.bib.cnrs.fr/record/display.uri?eid=2-s2.0-51249088328&origin=resultslist&sort=plf-f&src=s&nlo=&nlr=&nls=&sid=d6272db4bba8532cdcf678074490a4cc&sot=a&sdt=cl&cluster=scopubyr%2c%222008%22%2ct&sl=42&s=%28AUTHOR-NAME%28zhao%29%29+AND+%28effect+Caseinate%29&relpos=1&citeCnt=22&searchTerm=&featureToggles=FEATURE_NEW_DOC_DETAILS_EXPORT:1 (accessed on 26 April 2022).
- Srikaeo, K.; Doungjan, S. Hydrocolloids Retard Lipid Digestion in Whipping Cream. Food Res. 2020, 4, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhu, L.; Qi, X.; Zhang, H.; Wu, G. Characteristics of Low-fat Whipped Cream Containing Protein-based Fat Replacers. Int. J. Dairy Technol. 2023, 76, 276–290. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Zouari, M.; Attia, H.; Besbes, S. Study of Protein/k-Carrageenan Mixture’s Effect on Low-Fat Whipping Cream Formulation. LWT 2021, 147, 111647. [Google Scholar] [CrossRef]
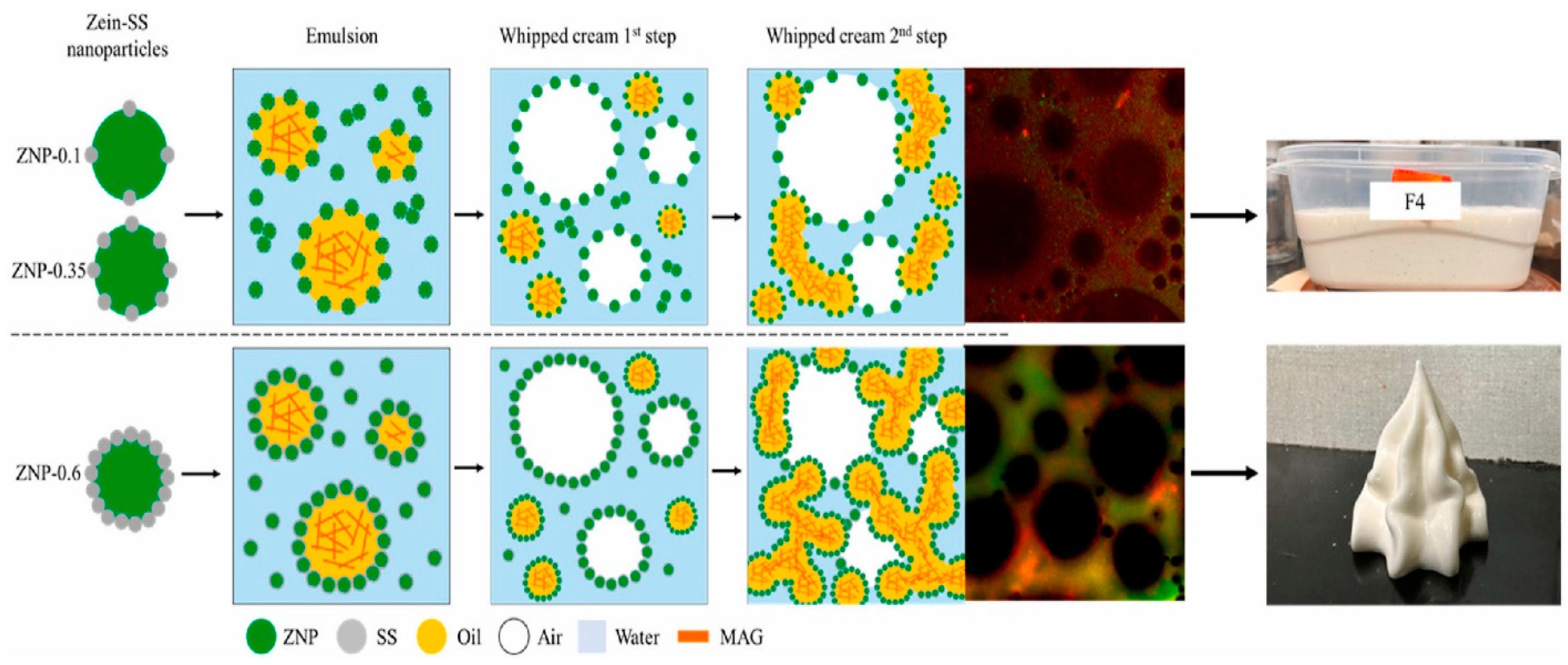
- Grossi, M.; Rao, J.; Chen, B. Tuning the Properties of Plant-Based Whipped Cream through Zein Nanoparticles-Sodium Stearate Complex. Food Hydrocoll. 2024, 155, 110219. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, Z.; Zhang, H.; Wang, J.; Ren, S. Protein Particles Ameliorate the Mechanical Properties of Highly Polyunsaturated Oil-Based Whipped Cream: A Possible Mode of Action. Food Hydrocoll. 2020, 99, 105350. [Google Scholar] [CrossRef]
- Lim, C.W.; Norziah, M.H.; Lu, H.F. Effect of Flaxseed Oil towards Physicochemical and Sensory Characteristic of Reduced Fat Ice Creams and Its Stability in Ice Creams upon Storage. Int. Food Res. J. 2010, 17, 393–403. [Google Scholar]
- Shamsi, K.; Che Man, Y.B.; Yusoff, M.S.A.; Jinap, S. A Comparative Study of Dairy Whipping Cream and Palm Oil-based Whipping Cream in Terms of FA Composition and Foam Stability. J. Am. Oil Chem. Soc. 2002, 79, 583–588. [Google Scholar] [CrossRef]
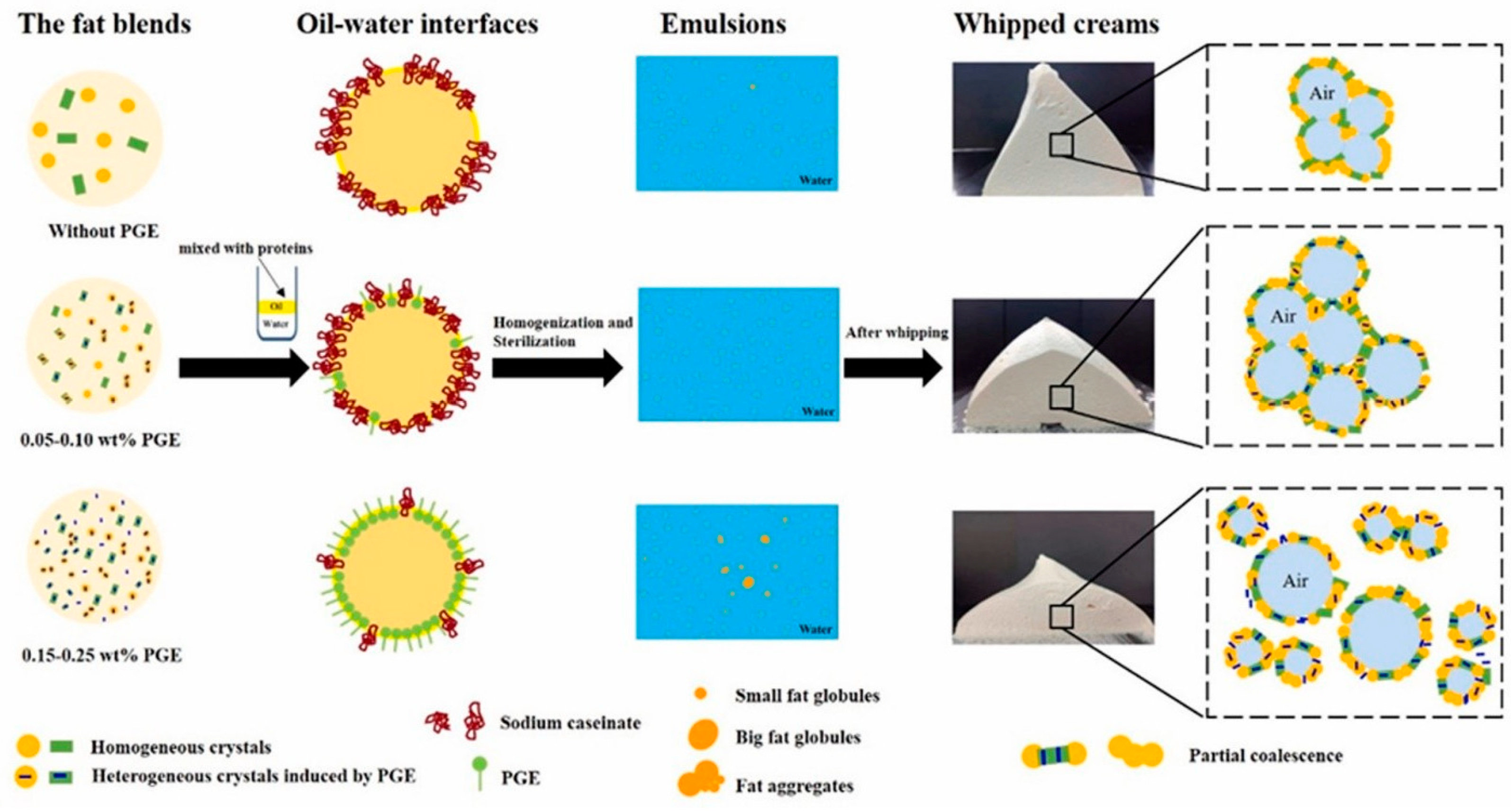
- Li, Y.; Liao, T.; Liu, T.; Yan, R.; Sun, Z.; Zhao, M.; Deng, X.; Zhao, Q. The Quality of Whipped Cream: Effect of Polyglycerol Ester on the Crystallization of Fat Blend and the Properties of Interface. Food Hydrocoll. 2023, 145, 109145. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, M.; Yang, B.; Cui, C. Effect of Xanthan Gum on the Physical Properties and Textural Characteristics of Whipped Cream. Food Chem. 2009, 116, 624–628. [Google Scholar] [CrossRef]
- Lazzaro, F.; Saint-Jalmes, A.; Violleau, F.; Lopez, C.; Gaucher-Delmas, M.; Madec, M.N.; Beaucher, E.; Gaucheron, F. Gradual Disaggregation of the Casein Micelle Improves Its Emulsifying Capacity and Decreases the Stability of Dairy Emulsions. Food Hydrocoll. 2017, 63, 189–200. [Google Scholar] [CrossRef]
- Allen, K.E.; Dickinson, E.; Murray, B. Acidified Sodium Caseinate Emulsion Foams Containing Liquid Fat: A Comparison with Whipped Cream. LWT 2006, 39, 225–234. [Google Scholar] [CrossRef]
- Marinova, K.G.; Basheva, E.S.; Nenova, B.; Temelska, M.; Mirarefi, A.Y.; Campbell, B.; Ivanov, I.B. Physico-Chemical Factors Controlling the Foamability and Foam Stability of Milk Proteins: Sodium Caseinate and Whey Protein Concentrates. Food Hydrocoll. 2009, 23, 1864–1876. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Wang, R.; Wang, Y.; Li, Y.; Zhang, L. Effects of Triglycerol Monostearate on Physical Properties of Recombined Dairy Cream. Int. Dairy J. 2020, 103, 104622. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Pintado, T.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Olive Oil-in-Water Emulsions Stabilized with Caseinate: Elucidation of Protein–Lipid Interactions by Infrared Spectroscopy. Food Hydrocoll. 2011, 25, 12–18. [Google Scholar] [CrossRef]
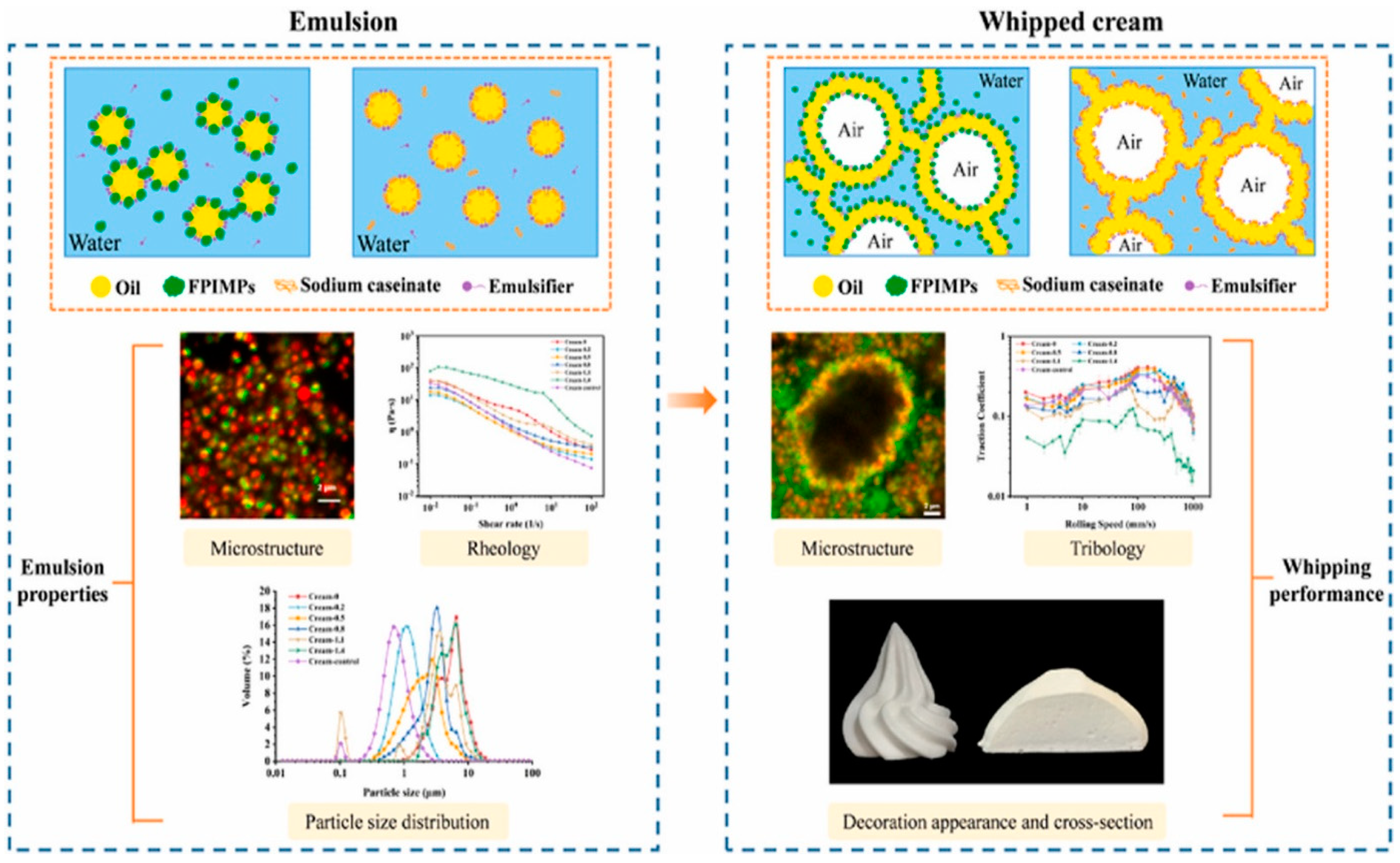
- Wang, J.; Zeng, D.; Yan, R.; Huangfu, J.; Hu, Q.; Cai, Y.; Liu, T.; Zhao, M.; Zhao, Q. Investigating the Impact of Static Destabilization Mechanism on Fat Crystallization Dynamics, Emulsion Rheology, and Whipping Properties of Whipping Cream. Food Chem. 2025, 463, 141272. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Huang, L.; Liu, T.; Cai, Y.; Zeng, D.; Zhou, F.; Zhao, M.; Deng, X.; Zhao, Q. Whipping Properties and Stability of Whipping Cream: The Impact of Fatty Acid Composition and Crystallization Properties. Food Chem. 2021, 347, 128997. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Li, D.; Wang, S.; Zhang, L. Progress in Research on Whipping Cream Quality and the Factors Influencing It. Shipin Kexue/Food Sci. 2022, 43, 327–335. [Google Scholar]
- Nguyen, V.; Rimaux, T.; Truong, V.; Dewettinck, K.; Van Bockstaele, F. Fat Crystallization of Blends of Palm Oil and Anhydrous Milk Fat: A Comparison between Static and Dynamic-Crystallization. Food Res. Int. 2020, 137, 109412. [Google Scholar] [CrossRef]
- Liao, W.; Gharsallaoui, A.; Dumas, E.; Elaïssari, A. Understanding of the Key Factors Influencing the Properties of Emulsions Stabilized by Sodium Caseinate. Food Sci. Food Saf. 2022, 21, 5291–5317. [Google Scholar] [CrossRef]
- Zhan, Y.; Wei, L.; Ge, T.; Chen, D.; Wang, L.; Jiang, J. Enhancing Whipped Cream Anti-Freeze Properties: A Dual Plant System with Aquafaba and Mung Bean Protein. Food Hydrocoll. 2025, 159, 110700. [Google Scholar] [CrossRef]
- Zhao, Q.; Kuang, W.; Long, Z.; Fang, M.; Liu, D.; Yang, B.; Zhao, M. Effect of Sorbitan Monostearate on the Physical Characteristics and Whipping Properties of Whipped Cream. Food Chem. 2013, 141, 1834–1840. [Google Scholar] [CrossRef]
- Li, Y.; Liao, T.; Liu, T.; Wang, J.; Sun, Z.; Zhao, M.; Deng, X.; Zhao, Q. Effect of Stearic and Oleic Acid-Based Lipophilic Emulsifiers on the Crystallization of the Fat Blend and the Stability of Whipped Cream. Food Chem. 2023, 428, 136762. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Cai, Y.; Liu, T.; Huang, L.; Wang, J.; Zhao, M.; Zhu, S.; Zhao, Q. Effect of Hydrophobic Sucrose Esters with Different Fat Acid Composition and Esterification Degree on Whipped Cream Properties. Food Hydrocoll. 2024, 146, 109183. [Google Scholar] [CrossRef]
- Xie, P.; Ji, G.; Jin, J.; Xu, H.; Zhang, L.; Gao, Z.; Fan, Q.; Chen, Y.; Jin, Q.; Wang, X. Partial Coalescence and Whipping Capabilities of Dairy Aerated Emulsions as Affected by Inclusion of Monoglycerides with Different Fatty Acid Species. Int. J. Dairy Technol. 2023, 76, 81–92. [Google Scholar] [CrossRef]
- Denkov, N.; Tcholakova, S.; Cholakova, D. Surface Phase Transitions in Foams and Emulsions. Curr. Opin. Colloid Interface Sci. 2019, 44, 32–47. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Yuan, D.; Wang, Y.; Li, M.; Zhang, L. The Effect of Caseins on the Stability and Whipping Properties of Recombined Dairy Creams. Int. Dairy J. 2020, 105, 104658. [Google Scholar] [CrossRef]
- Fu, L.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Effects of Soy Protein Composition in Recombined Soy-based Cream on the Stability and Physical Properties of Whipping Cream. J. Sci. Food Agric. 2020, 100, 2732–2741. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, R.; Meng, Z. Whipped Cream Stabilized by Faba Bean Protein Isolate Microgel Particles Substituted for Sodium Caseinate: Whipping Performance and Foam Stabilization Analysis. Food Hydrocoll. 2024, 157, 110388. [Google Scholar] [CrossRef]
- Gao, K.; Rao, J.; Chen, B. Plant Protein Solubility: A Challenge or Insurmountable Obstacle. Adv. Colloid. Interface Sci. 2024, 324, 103074. [Google Scholar] [CrossRef] [PubMed]
- Kew, B.; Holmes, M.; Stieger, M.; Sarkar, A. Review on Fat Replacement Using Protein-Based Microparticulated Powders or Microgels: A Textural Perspective. Trends Food Sci. Technol. 2020, 106, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Song, K.; Shao, D.; Dong, C.; Wang, L.; Jiang, J. The Effect of Endogenous Phenol and Gallic Acid on Structure and Functionalities of Mung Bean Protein in Aerated Emulsion System. Food Hydrocoll. 2023, 143, 108942. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Liu, C.; Ma, T. Effect of PH-Shifting and Sonication-Assisted Treatment on Properties and Stability of Vegetable Oil-Based Whipped Cream Stabilized by Kidney Bean Protein Aggregates. Food Hydrocoll. 2023, 141, 108736. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, M.; Zhou, S.; Wang, J.; Huang, Z. Enhancing the Thermal Stability of Zein Particle-Stabilized Aeratable Emulsions Through Genipin-Protein Cross-Linking and Its Possible Mechanism of Action. J. Agric. Food Chem. 2024, 72, 3707–3718. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, Q.; Liu, Y.-C.; Yang, X.-Q.; Wang, J.-M.; Yin, S.-W.; Qi, J.-R. Preparation of Soy Protein-Based Microgel Particles Using a Hydrogel Homogenizing Strategy and Their Interfacial Properties. Food Hydrocoll. 2016, 58, 324–334. [Google Scholar] [CrossRef]
- Shehzad, Q.; Liu, Z.; Zuo, M.; Wang, J. The Role of Polysaccharides in Improving the Functionality of Zein Coated Nanocarriers: Implications for Colloidal Stability under Environmental Stresses. Food Chem. 2024, 431, 136967. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and Zein -Based Nano-Materials for Food and Nutrition Applications: A Review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
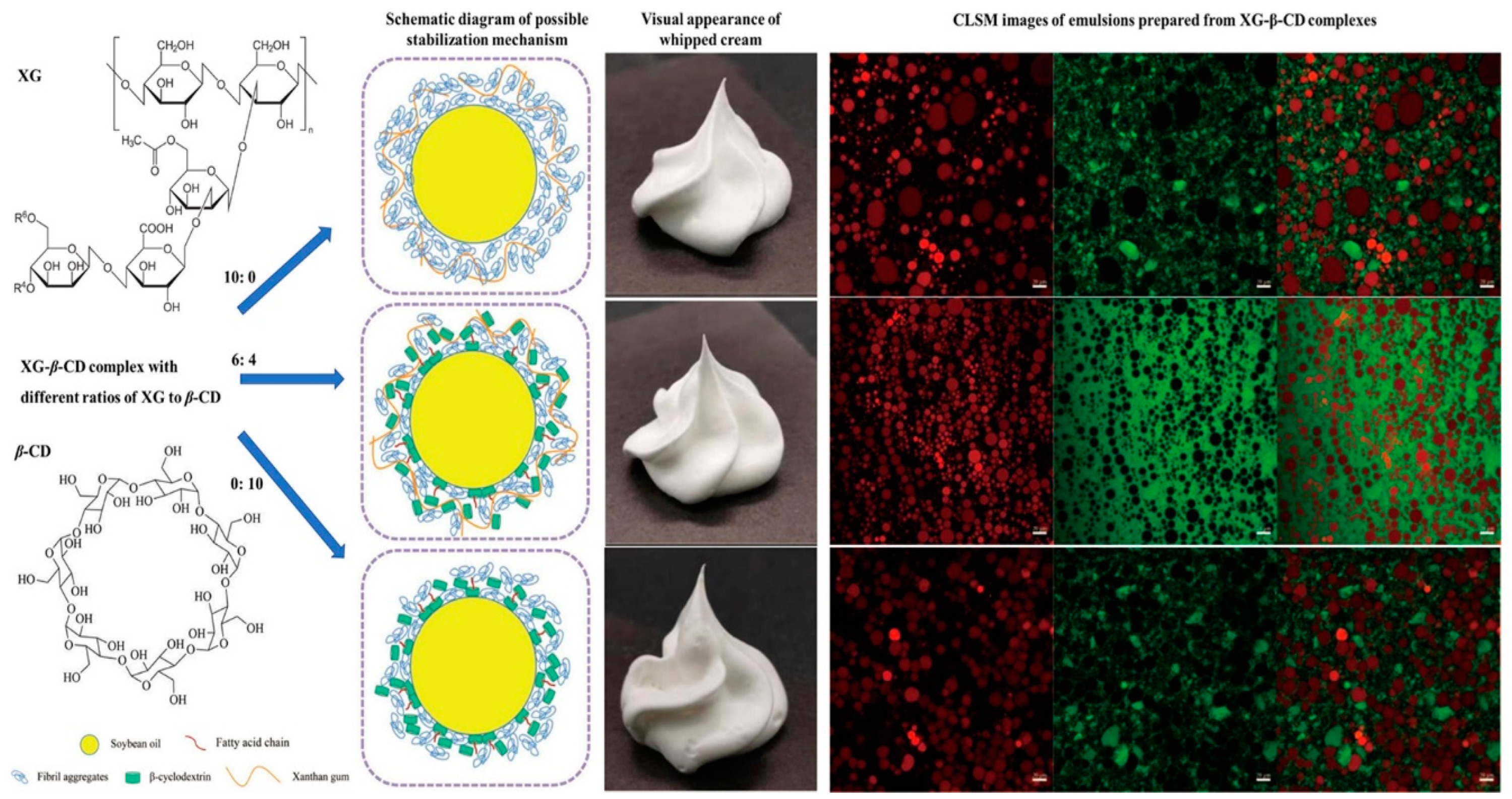
- Wu, S.; Wang, C.; Liu, C.; He, Q.; Zhang, Z.; Ma, T. Synergistic Effects of Xanthan Gum and β-Cyclodextrin on Properties and Stability of Vegetable Oil-Based Whipped Cream. Int. J. Biol. Macromol. 2024, 279, 135379. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
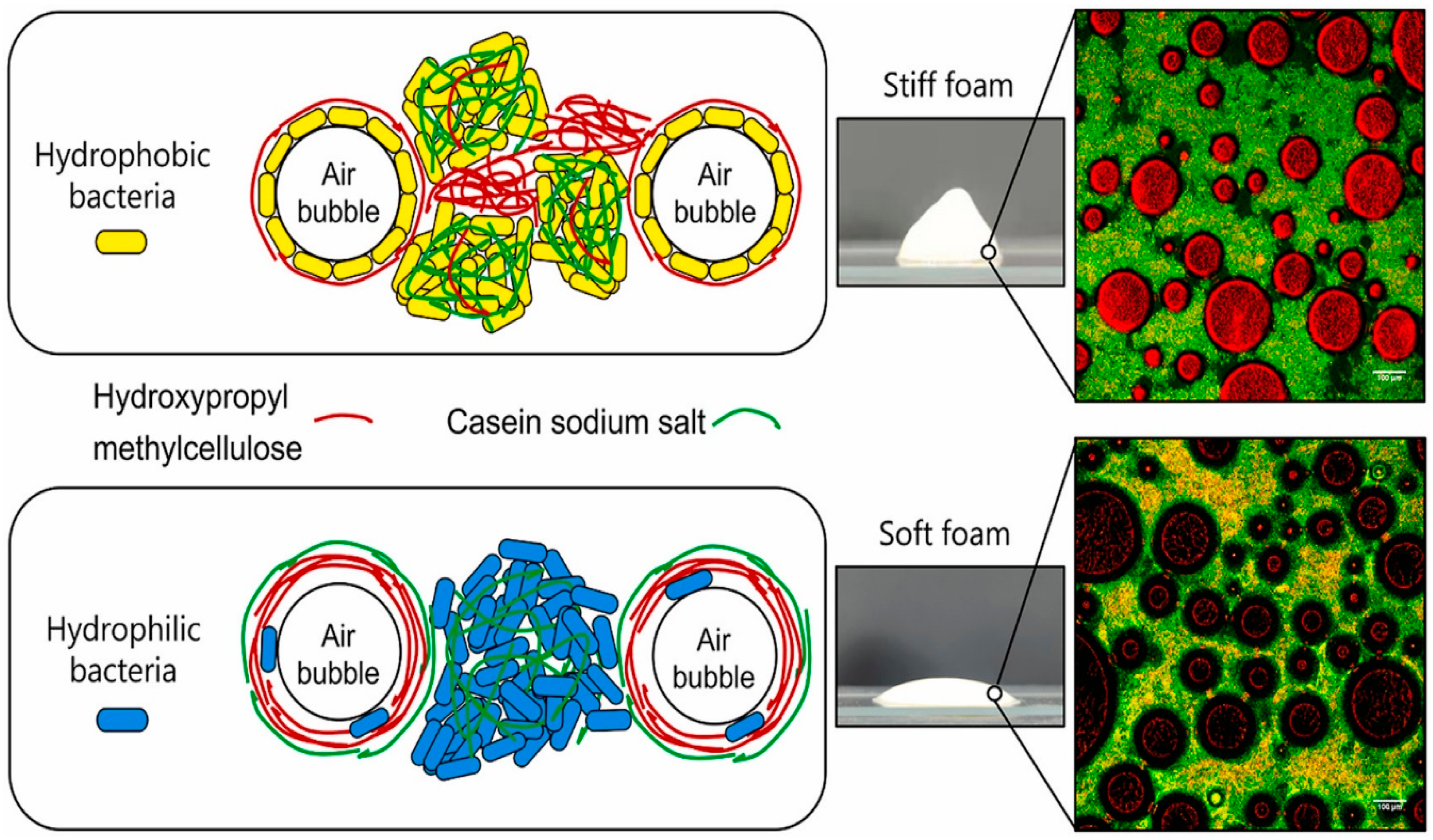
- Jiang, X.; Shekarforoush, E.; Muhammed, M.K.; Whitehead, K.A.; Arneborg, N.; Risbo, J. Lactic Acid Bacteria as Structural Building Blocks in Non-Fat Whipping Cream Analogues. Food Hydrocoll. 2023, 135, 108137. [Google Scholar] [CrossRef]
- Athari, B.; Nasirpour, A.; Saeidy, S.; Esehaghbeygi, A. Physicochemical Properties of Whipped Cream Stabilized with Electrohydrodynamic Modified Cellulose. J. Food Process Preserv. 2021, 45, 15688. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Madhushree, M.; Vairavel, P.; Mahesha, G.; Bhat, K.S. A Comprehensive Review of Cellulose and Cellulose-Based Materials: Extraction, Modification, and Sustainable Applications. J. Nat. Fibers 2024, 21, 2418357. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Electrohydrodynamic Drying of Foods: Principle, Applications, and Prospects. J. Food Eng. 2021, 295, 110449. [Google Scholar] [CrossRef]
- Biglarian, N.; Rafe, A.; Shahidi, S. Effect of Basil Seed Gum and κ-carrageenan on the Rheological, Textural, and Structural Properties of Whipped Cream. J. Sci. Food Agric. 2021, 101, 5851–5860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, M.; Li, J.; Yang, B.; Su, G.; Cui, C.; Jiang, Y. Effect of Hydroxypropyl Methylcellulose on the Textural and Whipping Properties of Whipped Cream. Food Hydrocoll. 2009, 23, 2168–2173. [Google Scholar] [CrossRef]
- Cai, Y.; Zeng, D.; Huang, L.; Zhao, M.; Zhao, Q.; Van der Meeren, P. Emulsifying and Whipping Properties of Mixing Polysaccharide Dispersions: Effect of Ratio between Insoluble Soybean Fiber and Hydroxypropyl Methylcellulose. J. Sci. Food Agric. 2022, 102, 6707–6717. [Google Scholar] [CrossRef]
- Rezvani, F.; Abbasi, H.; Nourani, M. Effects of Protein–Polysaccharide Interactions on the Physical and Textural Characteristics of Low-fat Whipped Cream. J. Food Process Preserv. 2020, 44, 14743. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabo, K.F.; Chèné, C.; Fameau, A.-L.; Karoui, R. Whipping Creams: Advances in Molecular Composition and Nutritional Chemistry. Molecules 2024, 29, 5933. https://doi.org/10.3390/molecules29245933
Dabo KF, Chèné C, Fameau A-L, Karoui R. Whipping Creams: Advances in Molecular Composition and Nutritional Chemistry. Molecules. 2024; 29(24):5933. https://doi.org/10.3390/molecules29245933
Chicago/Turabian StyleDabo, Khadija Florence, Christine Chèné, Anne-Laure Fameau, and Romdhane Karoui. 2024. "Whipping Creams: Advances in Molecular Composition and Nutritional Chemistry" Molecules 29, no. 24: 5933. https://doi.org/10.3390/molecules29245933
APA StyleDabo, K. F., Chèné, C., Fameau, A.-L., & Karoui, R. (2024). Whipping Creams: Advances in Molecular Composition and Nutritional Chemistry. Molecules, 29(24), 5933. https://doi.org/10.3390/molecules29245933






