Predictions of Milk Fatty Acid Contents by Mid-Infrared Spectroscopy in Chinese Holstein Cows
Abstract
1. Introduction
2. Results
2.1. Statistical Description
2.2. Predictions of Milk Fatty Acid Contents
3. Materials and Methods
3.1. Milk Samples and Fatty Acids
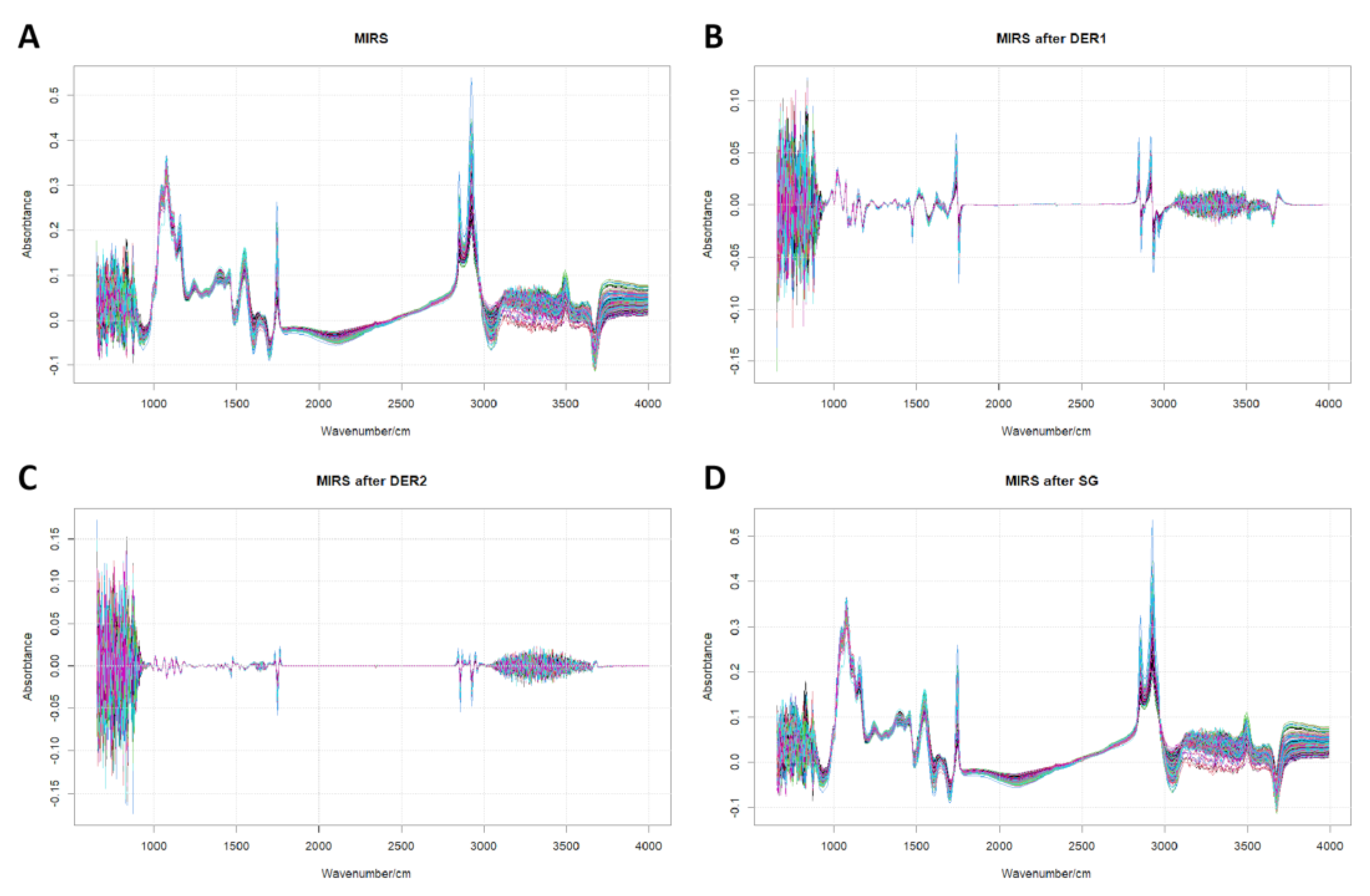
3.2. Predictions of Milk Fatty Acid Contents Using MIRS Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Christie, W. The Composition and Structure of Milk Lipids. In Developments in Dairy Chemistry—2; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar] [CrossRef]
- Balaji, B.; Dehghan, M.; Mente, A.; Rangarajan, S.; Yusuf, S. Association of Dairy Consumption with Metabolic Syndrome, Hypertension and Diabetes in 147,812 Individuals from 21 Countries. SSRN Electron. J. 2019. [Google Scholar] [CrossRef]
- Haug, A.; Stmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Soyeurt, H.; Dehareng, F.; Gengler, N.; McParland, S.; Wall, E.; Berry, D.P.; Coffey, M.; Dardenne, P. Mid-infrared prediction of bovine milk fatty acids across multiple breeds, production systems, and countries. J. Dairy Sci. 2011, 94, 1657–1667. [Google Scholar] [CrossRef]
- Fleming, A.; Schenkel, F.S.; Chen, J.; Malchiodi, F.; Bonfatti, V.; Ali, R.A.; Mallard, B.; Corredig, M.; Miglior, F. Prediction of milk fatty acid content with mid-infrared spectroscopy in Canadian dairy cattle using differently distributed model development sets. J. Dairy Sci. 2017, 100, 5073–5081. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.G. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 2002, 85, 295–350. [Google Scholar] [CrossRef] [PubMed]
- Stoop, W.M.; Bovenhuis, H.; Heck, J.M.L.; van Arendonk, J.A.M. Effect of lactation stage and energy status on milk fat composition of Holstein-Friesian cows. J. Dairy Sci. 2009, 92, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, M.; Luo, R.; Huang, G.; Wu, X.; Zheng, N.; Zhang, Y.; Wang, J. Fatty acid profiles of milk from Holstein cows, Jersey cows, buffalos, yaks, humans, goats, camels, and donkeys based on gas chromatography–mass spectrometry. J. Dairy Sci. 2021, 105, 1687–1700. [Google Scholar] [CrossRef]
- Grelet, C.; Dardenne, P.; Soyeurt, H.; Fernandez, J.A.; Vanlierde, A.; Stevens, F.; Gengler, N.; Dehareng, F. Large-scale phenotyping in dairy sector using milk MIR spectra: Key factors affecting the quality of predictions. Methods 2021, 186, 97–111. [Google Scholar] [CrossRef]
- Rovere, G.; de Los Campos, G.; Lock, A.; Worden, L.; Vazquez, A.I.; Lee, K.; Tempelman, R.J. Prediction of fatty acid composition using milk spectral data and its associations with various mid-infrared spectral regions in Michigan Holsteins. J. Dairy Sci. 2021, 104, 11242–11258. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, Y.; Zhou, Y.; Li, G.H.; Feng, X.S. Progress in Pretreatment and Analysis of Fatty Acids in Foods: An Update since 2012. Sep. Purif. Rev. 2019, 50, 203–222. [Google Scholar] [CrossRef]
- Waktola, H.D.; Zeng, A.X.; Chin, S.-T.; Marriott, P.J. Advanced gas chromatography and mass spectrometry technologies for fatty acids and triacylglycerols analysis. Trends Anal. Chem. 2020, 129, 115957. [Google Scholar] [CrossRef]
- Samková, E.; Špička, J.; Hanuš, O.; Roubal, P.; Pecová, L.; Hasoňová, L.; Smetana, P.; Klimešová, M.; Čítek, J. Comparison of Fatty Acid Proportions Determined by Mid-Infrared Spectroscopy and Gas Chromatography in Bulk and Individual Milk Samples. Animals 2020, 10, 1095. [Google Scholar] [CrossRef]
- Lopez-Villalobos, N.; Spelman, R.; Melis, J.; Davis, S.; Berry, S.; Lehnert, K.; Sneddon, N.; Holroyd, S.; MacGibbon, A.; Snell, R. Genetic correlations of milk fatty acid contents predicted from milk mid-infrared spectra in New Zealand dairy cattle. J. Dairy Sci. 2020, 103, 7238–7248. [Google Scholar] [CrossRef]
- Du, C.; Nan, L.; Yan, L.; Bu, Q.; Ren, X.; Zhang, Z.; Sabek, A.; Zhang, S. Genetic Analysis of Milk Production Traits and Mid-Infrared Spectra in Chinese Holstein Population. Animals 2020, 10, 139. [Google Scholar] [CrossRef]
- Ferrand-Calmelsm, M.; Palhière, I.; Brochard, M.; Leray, O.; Astruc, J.; Aurel, M.; Barbey, S.; Bouvier, F.; Brunschwig, P.; Caillat, H.; et al. Prediction of fatty acid profiles in cow, ewe, and goat milk by mid-infrared spectrometry. J. Dairy Sci. 2014, 97, 17–35. [Google Scholar] [CrossRef]
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Prediction of fatty acid content in sheep milk by Mid-Infrared spectrometry with a selection of wavelengths by Genetic Algorithms. LWT Food Sci. Technol. 2016, 65, 503–510. [Google Scholar] [CrossRef]
- Caredda, M.; Addis, M.; Ibba, I.; Leardi, R.; Scintu, M.F.; Piredda, G.; Sanna, G. Building of prediction models by using Mid-Infrared spectroscopy and fatty acid profile to discriminate the geographical origin of sheep milk. LWT Food Sci. Technol. 2017, 75, 131–136. [Google Scholar] [CrossRef]
- Nakayama, J.Y.; Ho, J.; Cartwright, E.; Simpson, R.; Hertzberg, V.S. Predictors of progression through the cascade of care to a cure for hepatitis C patients using decision trees and random forests. Comput. Biol. Med. 2021, 134, 104461. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, K.; Hu, M.; Weng, Q.; Zhang, H. Spatial heterogeneity modeling of water quality based on random forest regression and model interpretation. Environ. Res. 2021, 202, 111660. [Google Scholar] [CrossRef]
- Soyeurt, H.; Dardenne, P.; Dehareng, F.; Lognay, G.; Veselko, D.; Marlier, M.; Bertozzi, C.; Mayeres, P.; Gengler, N. Estimating Fatty Acid Content in Cow Milk Using Mid-Infrared Spectrometry. J. Dairy Sci. 2006, 89, 3690–3695. [Google Scholar] [CrossRef]
- Soyeurt, H.; Bastin, C.; Colinet, F.G.; Arnould, V.M.R.; Berry, D.P.; Wall, E.; Dehareng, F.; Nguyen, H.N.; Dardenne, P.; Schefers, J.; et al. Mid-infrared prediction of lactoferrin content in bovine milk: Potential indicator of mastitis. Animal 2012, 6, 1830–1838. [Google Scholar] [CrossRef]
- Ho, P.N.; Marett, L.C.; Wales, W.J.; Axford, M.; Oakes, E.M.; Pryce, J.E. Predicting milk fatty acids and energy balance of dairy cows in Australia using milk mid-infrared spectroscopy. Anim. Prod. Sci. 2019, 60, 164. [Google Scholar] [CrossRef]
- Tiplady, K.M.; Lopdell, T.J.; Littlejohn, M.D.; Garrick, D.J. The evolving role of Fourier-transform mid-infrared spectroscopy in genetic improvement of dairy cattle. J. Anim. Sci. Biotechnol. 2020, 11, 39. [Google Scholar] [CrossRef]
- Soyeurt, H.; Dehareng, F.; Mayeres, P.; Bertozzi, C.; Gengler, N. Variation of delta9-desaturase activity in dairy cattle. J. Dairy Sci. 2008, 91, 3211–3224. [Google Scholar] [CrossRef]
- Christie, W. Gas chromatography mass spectrometry methods for structural analysis of fatty acids. Lipids 1998, 33, 343–353. [Google Scholar] [CrossRef]
- Maurice-van Eijndhoven, M.H.T.; Soyeurt, H.; Dehareng, F.; Calus, M. Validation of fatty acid predictions in milk using mid-infrared spectrometry across cattle breeds. Animal 2013, 7, 348–354. [Google Scholar] [CrossRef]
- Coppa, M.; Ferlay, A.; Leroux, C.; Jestin, M.; Chilliard, Y.; Martin, B.; Andueza, D. Prediction of milk fatty acid composition by near infrared reflectance spectroscopy. Int. Dairy J. 2010, 20, 182–189. [Google Scholar] [CrossRef]
- De Marchi, M.; Penasa, M.; Cecchinato, A.; Mele, M.; Secchiari, P.; Bittante, G. Effectiveness of mid-infrared spectroscopy to predict fatty acid composition of Brown Swiss bovine milk. Animal 2011, 5, 1653–1658. [Google Scholar] [CrossRef]
- De Marchi, M.; Toffanin, V.; Cassandro, M.; Penasa, M. Invited review: Mid-infrared spectroscopy as phenotyping tool for milk traits. J. Dairy Sci. 2014, 97, 1171–1186. [Google Scholar] [CrossRef]
- Zaalberg, R.M.; Poulsen, N.A.; Bovenhuis, H.; Sehested, J.; Larsen, L.B.; Buitenhuis, A.J. Genetic analysis on infrared-predicted milk minerals for Danish dairy cattle. J. Dairy Sci. 2021, 104, 8947–8958. [Google Scholar] [CrossRef]
- Cecchinato, A.; Marchi, M.D.; Gallo, L.; Bittante, G.; Carnier, P. Mid-infrared spectroscopy predictions as indicator traits in breeding programs for enhanced coagulation properties of milk. J. Dairy Sci. 2009, 92, 5304–5313. [Google Scholar] [CrossRef] [PubMed]
- Canaza-Cayo, A.W.; Alomar, D.; Quispe, E. Prediction of alpaca fibre quality by near-infrared reflectance spectroscopy. Anim. Int. J. Anim. Biosci. 2013, 7, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Pralle, R.S.; Weigel, K.W.; White, H.M. Predicting blood β-hydroxybutyrate using milk Fourier transform infrared spectrum, milk composition, and producer-reported variables with multiple linear regression, partial least squares regression, and artificial neural network. J. Dairy Sci. 2018, 101, 4378–4387. [Google Scholar] [CrossRef] [PubMed]
- Rutten, M.J.M.; Bovenhuis, H.; Hettinga, K.A.; van Valenberg, H.J.F.; van Arendonk, J.A.M. Predicting bovine milk fat composition using infrared spectroscopy based on milk samples collected in winter and summer. J. Dairy Sci. 2009, 92, 6202–6209. [Google Scholar] [CrossRef]

| Fatty Acid | Milk-Basis (g/100 g of Milk) | Fat-Basis (g/100 g of Fat) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Minimum | Mean | Maximum | Variation Coefficient (%) | Sample Size | Minimum | Mean | Maximum | Variation Coefficient (%) | |
| C8:0 | 325 | 0.007 | 0.016 | 0.028 | 28.784 | 324 | 0.327 | 0.532 | 0.757 | 14.778 |
| C10:0 | 323 | 0.013 | 0.044 | 0.082 | 35.416 | 326 | 0.598 | 1.402 | 2.324 | 21.273 |
| C11:0 | 317 | 0.002 | 0.003 | 0.006 | 25.439 | 319 | 0.053 | 0.110 | 0.175 | 23.125 |
| C12:0 | 321 | 0.019 | 0.062 | 0.115 | 34.871 | 327 | 0.829 | 2.018 | 3.323 | 21.721 |
| C13:0 | 321 | 0.003 | 0.005 | 0.008 | 24.468 | 323 | 0.082 | 0.166 | 0.255 | 22.062 |
| C14:0 | 322 | 0.094 | 0.231 | 0.371 | 27.975 | 321 | 4.058 | 7.546 | 11.358 | 15.075 |
| C15:0 | 320 | 0.012 | 0.029 | 0.052 | 29.138 | 321 | 0.456 | 0.968 | 1.519 | 21.227 |
| C16:0 | 325 | 0.366 | 0.877 | 1.491 | 28.154 | 323 | 17.710 | 28.620 | 42.251 | 13.802 |
| C17:0 | 324 | 0.008 | 0.016 | 0.027 | 26.180 | 322 | 0.285 | 0.528 | 0.797 | 19.289 |
| C18:0 | 320 | 0.098 | 0.313 | 0.600 | 35.024 | 326 | 4.070 | 10.254 | 17.150 | 25.195 |
| C20:0 | 321 | 0.006 | 0.008 | 0.011 | 15.098 | 321 | 0.157 | 0.270 | 0.398 | 19.278 |
| C22:0 | 332 | 0.004 | 0.005 | 0.006 | 9.413 | 329 | 0.074 | 0.172 | 0.286 | 25.529 |
| C24:0 | 324 | 0.004 | 0.005 | 0.005 | 5.837 | 323 | 0.069 | 0.154 | 0.246 | 24.812 |
| C14:1 | 325 | 0.006 | 0.019 | 0.033 | 32.157 | 317 | 0.230 | 0.610 | 1.138 | 31.033 |
| C16:1 | 322 | 0.016 | 0.038 | 0.068 | 30.326 | 320 | 0.602 | 1.258 | 2.202 | 26.094 |
| C18:1n9c | 321 | 0.189 | 0.460 | 0.815 | 28.657 | 323 | 7.953 | 15.282 | 23.746 | 20.201 |
| C20:1 | 321 | 0.003 | 0.003 | 0.005 | 13.134 | 319 | 0.065 | 0.116 | 0.190 | 24.333 |
| C22:1n9 | 322 | 0.007 | 0.015 | 0.028 | 32.090 | 317 | 0.193 | 0.510 | 1.140 | 44.207 |
| C20:3n6 | 322 | 0.003 | 0.006 | 0.009 | 24.212 | 322 | 0.109 | 0.192 | 0.288 | 18.233 |
| C20:4n6 | 323 | 0.004 | 0.007 | 0.010 | 20.368 | 317 | 0.122 | 0.222 | 0.329 | 18.416 |
| C20:5n3 | 332 | 0.002 | 0.003 | 0.004 | 10.163 | 323 | 0.046 | 0.094 | 0.149 | 23.530 |
| C18:2n6c | 323 | 0.030 | 0.070 | 0.120 | 28.437 | 327 | 1.052 | 2.300 | 3.654 | 18.149 |
| C18:3n6 | 321 | 0.003 | 0.003 | 0.004 | 7.621 | 318 | 0.047 | 0.100 | 0.169 | 24.898 |
| C18:3n3 | 324 | 0.004 | 0.008 | 0.013 | 24.352 | 324 | 0.155 | 0.278 | 0.417 | 16.480 |
| SFA | 325 | 0.692 | 1.627 | 2.714 | 28.415 | 322 | 29.494 | 52.710 | 74.351 | 13.287 |
| UFA | 323 | 0.288 | 0.638 | 1.090 | 26.277 | 326 | 13.162 | 21.266 | 31.904 | 18.082 |
| MUFA | 322 | 0.240 | 0.539 | 0.938 | 26.878 | 324 | 9.501 | 17.920 | 27.111 | 19.321 |
| PUFA | 324 | 0.046 | 0.098 | 0.159 | 25.514 | 325 | 1.720 | 3.196 | 4.863 | 16.229 |
| SCFA | 323 | 0.020 | 0.060 | 0.109 | 33.392 | 324 | 0.926 | 1.934 | 2.936 | 18.762 |
| MCFA | 325 | 0.580 | 1.269 | 2.147 | 27.475 | 322 | 25.617 | 41.372 | 61.272 | 12.978 |
| LCFA | 321 | 0.371 | 0.925 | 1.610 | 28.353 | 326 | 17.003 | 30.776 | 46.784 | 19.365 |
| Fatty Acid | Pre-Processing Algorithm | MIRS Range (cm−1) | Model | Basis (g/100 g) | Test Set | |
|---|---|---|---|---|---|---|
| R2 | RPD | |||||
| C8:0 | SNV | 3017~2823/1805~1734 | PLSR | Milk | 0.77 | 2.11 |
| C10:0 | DER1 | 3017~2823/1805~1734 | RFR | Milk | 0.77 | 2.07 |
| C11:0 | DER1 | 3017~2823/1805~1734 | LassoR | Fat | 0.55 | 1.48 |
| C12:0 | DER1 | 3017~2823/1805~1734 | LassoR | Milk | 0.84 | 2.50 |
| C13:0 | SG | 3017~2823/1805~1734 | PLSR | Milk | 0.66 | 1.72 |
| C14:0 | DER1 | 4000~400 | RFR | Milk | 0.78 | 2.05 |
| C15:0 | SG | 3017~2823/1805~1734 | PLSR | Milk | 0.57 | 1.53 |
| C16:0 | SG | 3017~2823/1805~1734 | RFR | Milk | 0.75 | 1.98 |
| C17:0 | SG | 3017~2823/1805~1734 | LassoR | Milk | 0.73 | 1.89 |
| C18:0 | DER1 | 4000~400 | PLSR | Milk | 0.77 | 2.08 |
| C20:0 | SNV | 3017~2823/1805~1734 | PLSR | Fat | 0.82 | 2.35 |
| C22:0 | DER2 | 4000~400 | RFR | Fat | 0.86 | 2.66 |
| C24:0 | SG | 4000~400 | RFR | Fat | 0.80 | 2.20 |
| C14:1 | MSC | 3017~2823/1805~1734 | PLSR | Fat | 0.62 | 1.63 |
| C16:1 | SNV | 3017~2823/1805~1734 | LassoR | Milk | 0.62 | 1.64 |
| C18:1n9c | SG | 3017~2823/1805~1734 | LassoR | Milk | 0.77 | 2.00 |
| C20:1 | DER2 | 4000~400 | RFR | Fat | 0.76 | 2.04 |
| C22:1n9 | DER1 | 4000~400 | RFR | Fat | 0.65 | 1.67 |
| C18:2n6c | MSC | 4000~400 | RFR | Milk | 0.63 | 1.61 |
| C18:3n3 | SG | 4000~400 | RFR | Milk | 0.70 | 1.82 |
| C18:3n6 | DER2 | 3017~2823/1805~1734 | RFR | Fat | 0.76 | 2.00 |
| C20:3n6 | DER1 | 4000~400 | RFR | Milk | 0.62 | 1.61 |
| C20:4n6 | SNV | 4000~400 | RFR | Milk | 0.50 | 1.42 |
| C20:5n3 | DER1 | 4000~400 | RFR | Fat | 0.91 | 3.06 |
| SFA | SG | 3017~2823/1805~1734 | RFR | Milk | 0.76 | 2.01 |
| UFA | DER2 | 3017~2823/1805~1734 | LassoR | Milk | 0.82 | 2.15 |
| MUFA | DER2 | 3017~2823/1805~1734 | LassoR | Milk | 0.79 | 2.06 |
| PUFA | DER2 | 4000~400 | RidgeR | Milk | 0.71 | 1.75 |
| SCFA | DER2 | 4000~400 | RFR | Milk | 0.77 | 2.04 |
| MCFA | DER2 | 3017~2823/1805~1734 | RFR | Milk | 0.75 | 2.00 |
| LCFA | DER2 | 3017~2823/1805~1734 | RidgeR | Milk | 0.83 | 2.29 |
| Fatty Acid | Pre-Processing Algorithm | MIRS Range (cm−1) | Model | Training Set | Test Set | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RPD | R2 | RPD | |||||||||||
| Milk | Fat | Milk | Fat | Milk | Fat | Milk | Fat | Milk | Fat | Milk | Fat | Milk | Fat | |
| C8:0 | SNV | MSC | 3017~2823/1805~1734 | 3017~2823/1805~1734 | PLSR | LassoR | 0.75 | 0.43 | 2.01 | 1.33 | 0.77 | 0.43 | 2.11 | 1.32 |
| C10:0 | DER1 | DER1 | 3017~2823/1805~1734 | 3017~2823/1805~1734 | RFR | LassoR | 0.61 | 0.49 | 1.60 | 1.40 | 0.77 | 0.44 | 2.07 | 1.33 |
| C11:0 | DER2 | DER1 | 3017~2823/1805~1734 | 3017~2823/1805~1734 | LassoR | LassoR | 0.57 | 0.51 | 1.53 | 1.43 | 0.53 | 0.55 | 1.46 | 1.48 |
| C12:0 | DER1 | SNV | 3017~2823/1805~1734 | 3017~2823/1805~1734 | LassoR | LassoR | 0.79 | 0.55 | 2.18 | 1.49 | 0.84 | 0.27 | 2.50 | 1.17 |
| C13:0 | SG | SNV | 3017~2823/1805~1734 | 3017~2823/1805~1734 | PLSR | LassoR | 0.24 | 0.56 | 1.16 | 1.50 | 0.66 | 0.42 | 1.72 | 1.30 |
| C14:0 | DER1 | DER1 | 4000~400 | 3017~2823/1805~1734 | RFR | PLSR | 0.66 | 0.16 | 1.72 | 1.10 | 0.78 | 0.43 | 2.05 | 1.34 |
| C15:0 | SG | MSC | 3017~2823/1805~1734 | 3017~2823/1805~1734 | PLSR | PLSR | 0.45 | 0.25 | 1.37 | 1.17 | 0.57 | 0.32 | 1.53 | 1.22 |
| C16:0 | SG | DER2 | 3017~2823/1805~1734 | 4000~400 | RFR | RidgeR | 0.64 | 0.55 | 1.66 | 1.33 | 0.75 | 0.22 | 1.98 | 1.12 |
| C17:0 | SG | MSC | 3017~2823/1805~1734 | 3017~2823/1805~1734 | LassoR | PLSR | 0.65 | 0.40 | 1.70 | 1.32 | 0.73 | 0.59 | 1.89 | 1.56 |
| C18:0 | DER1 | SNV | 4000~400 | 3017~2823/1805~1734 | PLSR | LassoR | 0.66 | 0.60 | 1.72 | 1.58 | 0.77 | 0.55 | 2.08 | 1.49 |
| C20:0 | SG | SNV | 3017~2823/1805~1734 | 3017~2823/1805~1734 | PLSR | PLSR | 0.52 | 0.76 | 1.46 | 2.04 | 0.71 | 0.82 | 1.88 | 2.35 |
| C22:0 | DER2 | DER2 | 4000~400 | 4000~400 | RidgeR | RFR | 0.70 | 0.83 | 1.76 | 2.42 | 0.52 | 0.86 | 1.44 | 2.66 |
| C24:0 | DER2 | SG | 4000~400 | 4000~400 | RidgeR | RFR | 0.64 | 0.90 | 1.55 | 3.20 | 0.61 | 0.80 | 1.46 | 2.20 |
| C14:1 | SNV | MSC | 3017~2823/1805~1734 | 3017~2823/1805~1734 | LassoR | PLSR | 0.63 | 0.38 | 1.65 | 1.28 | 0.51 | 0.62 | 1.40 | 1.63 |
| C16:1 | SNV | MSC | 3017~2823/1805~1734 | 3017~2823/1805~1734 | LassoR | LassoR | 0.54 | 0.38 | 1.47 | 1.27 | 0.62 | 0.55 | 1.64 | 1.50 |
| C18:1n9c | SG | MSC | 3017~2823/1805~1734 | 3017~2823/1805~1734 | LassoR | LassoR | 0.60 | 0.52 | 1.58 | 1.45 | 0.77 | 0.34 | 2.00 | 1.20 |
| C20:1 | SG | DER2 | 3017~2823/1805~1734 | 4000~400 | PLSR | RFR | 0.54 | 0.77 | 1.48 | 2.06 | 0.49 | 0.76 | 1.41 | 2.04 |
| C22:1n9 | DER2 | DER1 | 4000~400 | 4000~400 | RFR | RFR | 0.51 | 0.53 | 1.43 | 1.45 | 0.45 | 0.65 | 1.36 | 1.67 |
| C18:2n6c | MSC | SG | 4000~400 | 4000~400 | RFR | RidgeR | 0.59 | 0.13 | 1.56 | 1.07 | 0.63 | 0.15 | 1.61 | 1.08 |
| C18:3n3 | SG | DER1 | 4000~400 | 3017~2823/1805~1734 | RFR | RFR | 0.60 | 0.17 | 1.59 | 1.09 | 0.70 | 0.27 | 1.82 | 1.13 |
| C18:3n6 | SG | DER2 | 3017~2823/1805~1734 | 3017~2823/1805~1734 | RFR | RFR | 0.18 | 0.84 | 1.08 | 2.47 | 0.14 | 0.76 | 1.04 | 2.00 |
| C20:3n6 | DER1 | MSC | 4000~400 | 3017~2823/1805~1734 | RFR | PLSR | 0.50 | 0.23 | 1.42 | 1.15 | 0.62 | 0.39 | 1.61 | 1.29 |
| C20:4n6 | SNV | SNV | 4000~400 | 4000~400 | RFR | PLSR | 0.44 | 0.29 | 1.34 | 1.19 | 0.50 | 0.46 | 1.42 | 1.37 |
| C20:5n3 | DER2 | DER1 | 4000~400 | 4000~400 | RFR | RFR | 0.33 | 0.83 | 1.23 | 2.41 | 0.43 | 0.91 | 1.29 | 3.06 |
| LCFA | DER2 | DER1 | 3017~2823/1805~1734 | 4000~400 | RidgeR | RFR | 0.68 | 0.41 | 1.78 | 1.31 | 0.83 | 0.42 | 2.29 | 1.32 |
| MCFA | DER2 | SNV | 3017~2823/1805~1734 | 3017~2823/1805~1734 | RFR | LassoR | 0.64 | 0.23 | 1.67 | 1.14 | 0.75 | 0.28 | 2.00 | 1.18 |
| MUFA | DER2 | DER1 | 3017~2823/1805~1734 | 3017~2823/1805~1734 | LassoR | LassoR | 0.61 | 0.56 | 1.59 | 1.51 | 0.79 | 0.43 | 2.06 | 1.30 |
| PUFA | DER2 | SG | 4000~400 | 3017~2823/1805~1734 | RidgeR | RFR | 0.71 | 0.16 | 1.80 | 1.08 | 0.71 | 0.16 | 1.75 | 1.07 |
| SCFA | DER2 | MSC | 4000~400 | 3017~2823/1805~1734 | RFR | LassoR | 0.66 | 0.51 | 1.71 | 1.43 | 0.77 | 0.48 | 2.04 | 1.37 |
| SFA | SG | SG | 3017~2823/1805~1734 | 3017~2823/1805~1734 | RFR | LassoR | 0.66 | 0.32 | 1.73 | 1.21 | 0.76 | 0.25 | 2.01 | 1.16 |
| UFA | DER2 | MSC | 3017~2823/1805~1734 | 3017~2823/1805~1734 | LassoR | LassoR | 0.62 | 0.42 | 1.62 | 1.31 | 0.82 | 0.48 | 2.15 | 1.38 |
| Fatty Acid Group According to Hydrocarbon Chain Saturation | Fatty Acid Group According to Carbon Chain Length | ||
|---|---|---|---|
| SFA | C8:0, C10:0, C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C17:0, C18:0, C20:0, C22:0, C24:0 | SCFA | C8:0, C10:0 |
| UFA | C14:1, C16:1, C18:1n9c, C18:2n6c, C18:3n6, C18:3n3, C20:1, C20:3n6, C20:4n6, C22:1n9, C20:5n3 | MCFA | C11:0, C12:0, C13:0, C14:0, C15:0, C16:0, C16:1 |
| MUFA | C14:1, C16:1, C18:1n9c, C20:1, C22:1n9 | LCFA | C17:0, C18:0, C18:1n9c, C18:2n6c, C20:0, C18:3n6, C18:3n3, C20:1, C22:0, C20:3n6, C20:4n6, C22:1n9, C20:5n3, C24:0 |
| PUFA | C18:2n6t, C18:2n6c, C18:3n6, C18:3n3, C20:3n6, C20:4n6, C22:2, C20:5n3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Song, Y.; Zhang, Y.; Cai, G.; Xue, G.; Liu, Y.; Chen, K.; Zhang, F.; Wang, K.; Zhang, M.; et al. Predictions of Milk Fatty Acid Contents by Mid-Infrared Spectroscopy in Chinese Holstein Cows. Molecules 2023, 28, 666. https://doi.org/10.3390/molecules28020666
Zhao X, Song Y, Zhang Y, Cai G, Xue G, Liu Y, Chen K, Zhang F, Wang K, Zhang M, et al. Predictions of Milk Fatty Acid Contents by Mid-Infrared Spectroscopy in Chinese Holstein Cows. Molecules. 2023; 28(2):666. https://doi.org/10.3390/molecules28020666
Chicago/Turabian StyleZhao, Xiuxin, Yuetong Song, Yuanpei Zhang, Gaozhan Cai, Guanghui Xue, Yan Liu, Kewei Chen, Fan Zhang, Kun Wang, Miao Zhang, and et al. 2023. "Predictions of Milk Fatty Acid Contents by Mid-Infrared Spectroscopy in Chinese Holstein Cows" Molecules 28, no. 2: 666. https://doi.org/10.3390/molecules28020666
APA StyleZhao, X., Song, Y., Zhang, Y., Cai, G., Xue, G., Liu, Y., Chen, K., Zhang, F., Wang, K., Zhang, M., Gao, Y., Sun, D., Wang, X., & Li, J. (2023). Predictions of Milk Fatty Acid Contents by Mid-Infrared Spectroscopy in Chinese Holstein Cows. Molecules, 28(2), 666. https://doi.org/10.3390/molecules28020666







