Low-Toxicity and High-Stability Fluorescence Sensor for the Selective, Rapid, and Visual Detection Tetracycline in Food Samples
Abstract
1. Introduction
2. Results
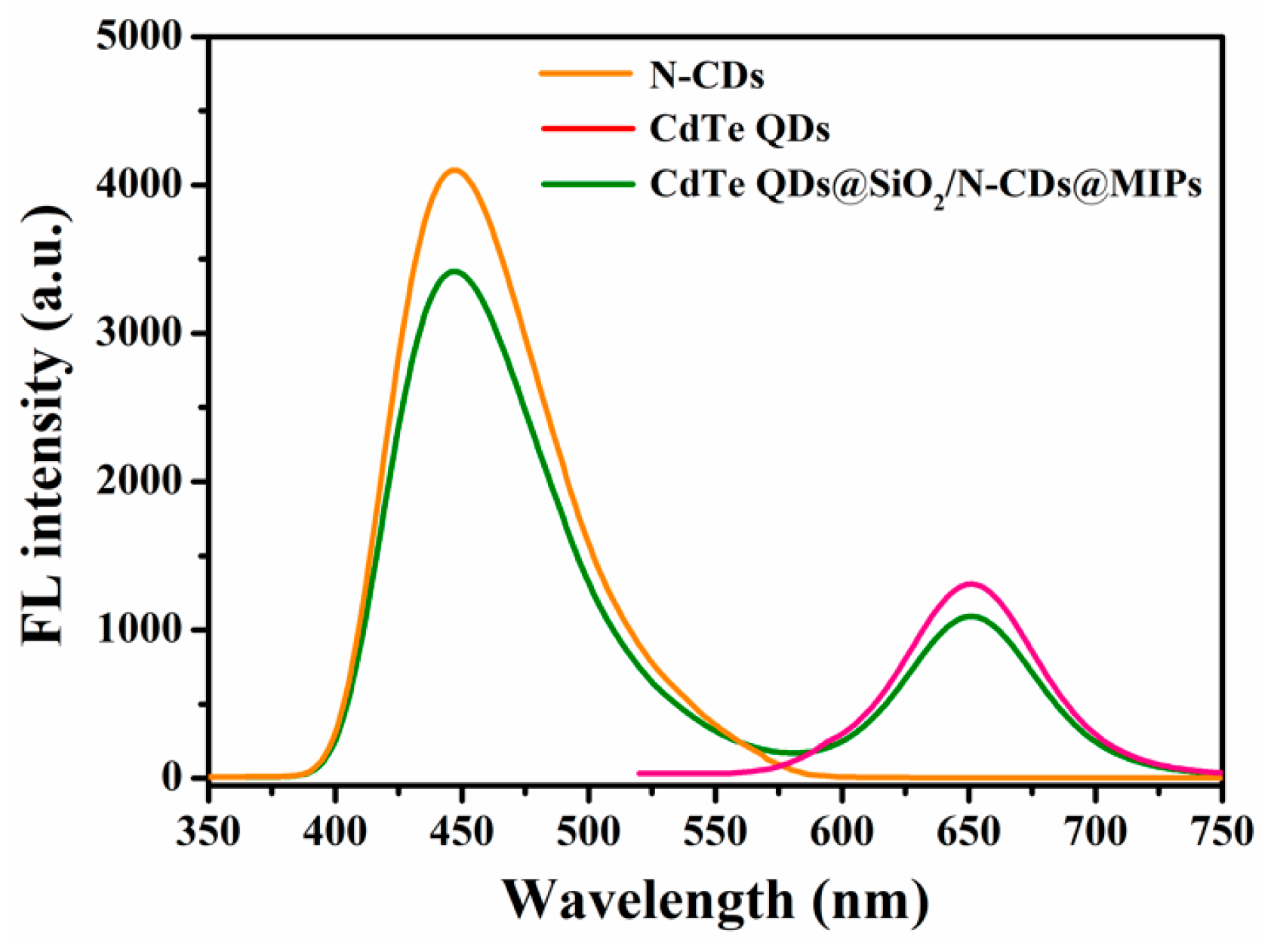
2.1. Characterization of CdTe QDs@SiO2/N-CDs@MIPs
2.2. Optimization of Detection Conditions
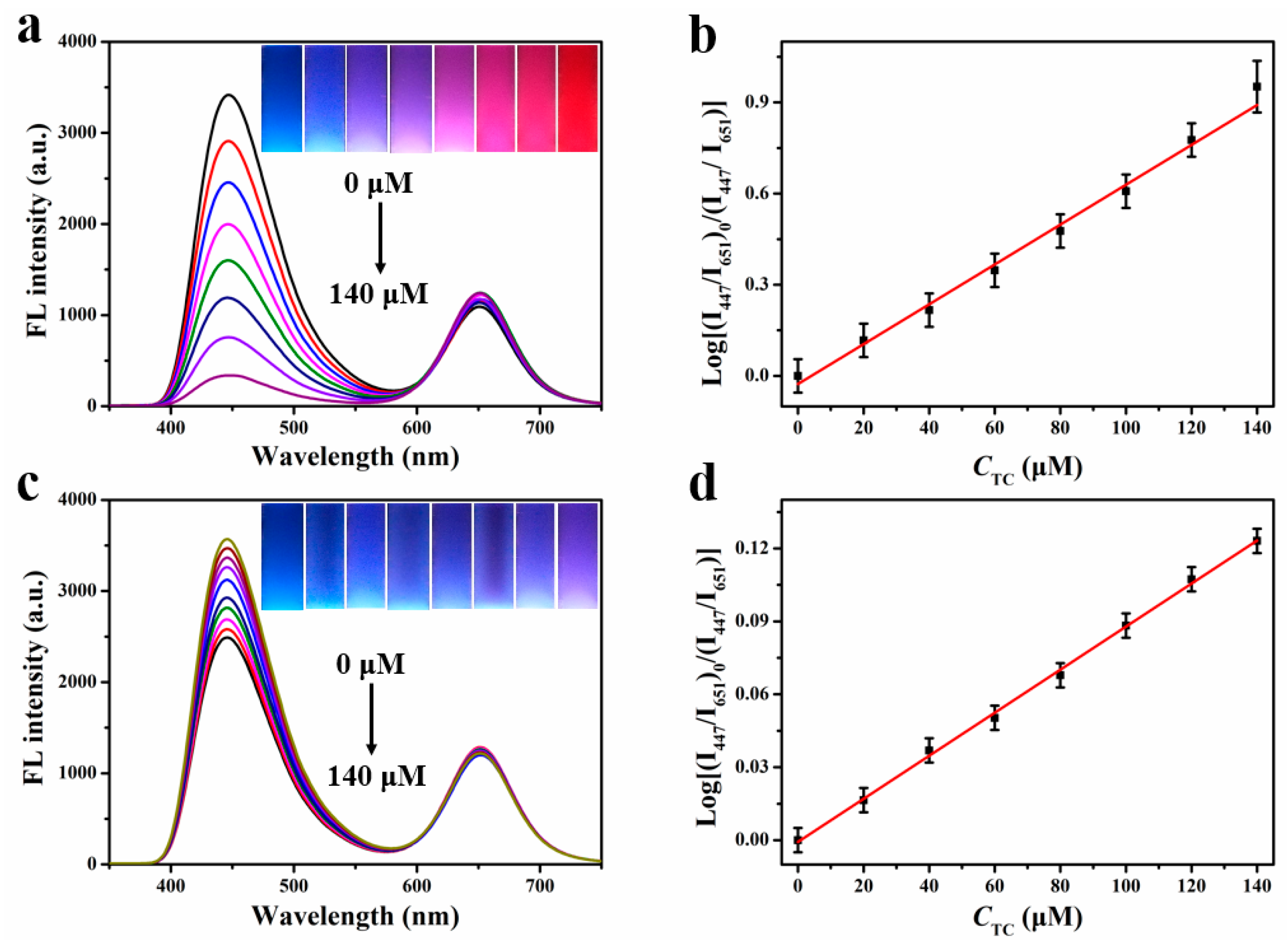
2.3. Fluorescence Determination Capability
2.4. Specific Recognition of TC by CdTe QDs@SiO2/N-CDs@MIPs
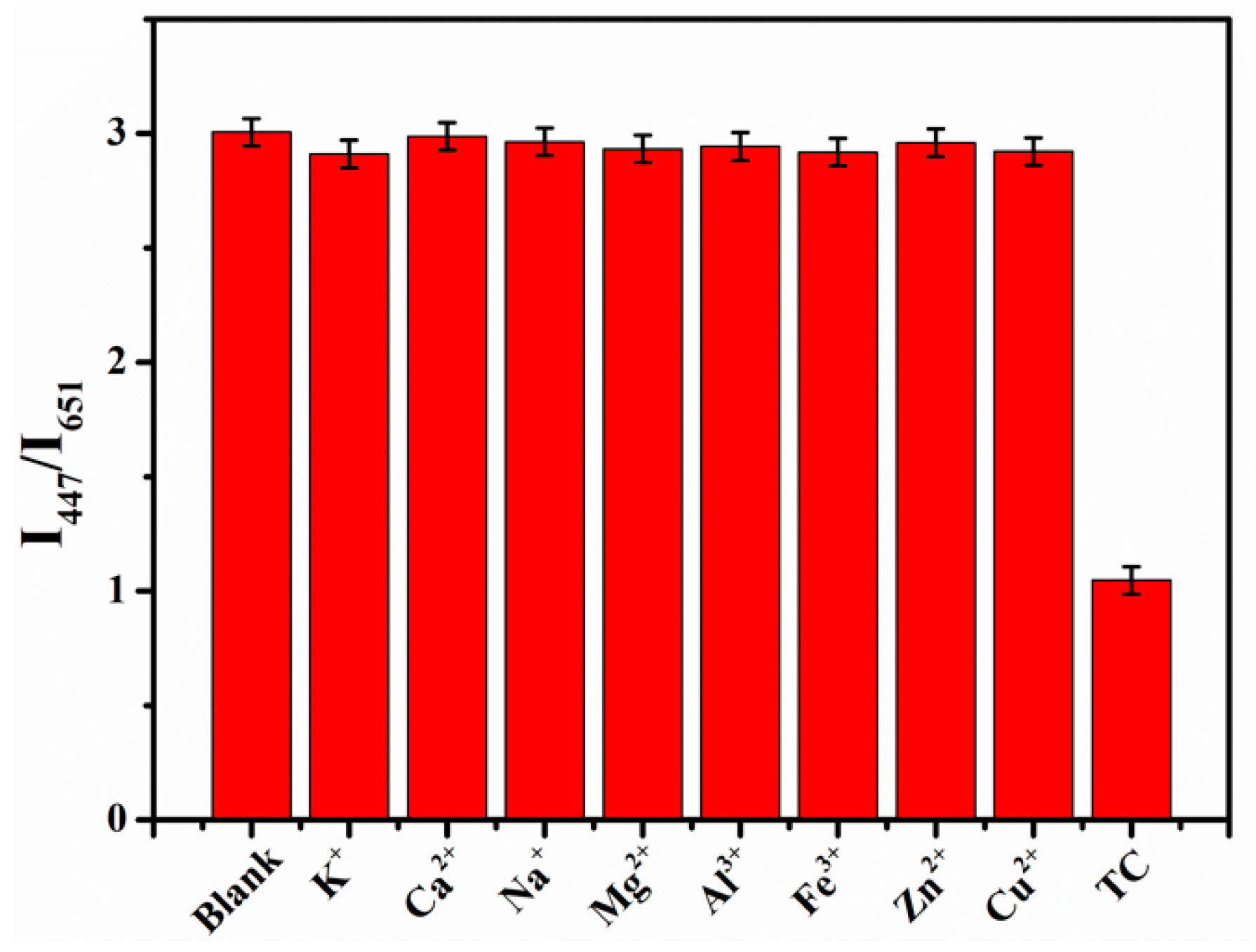
2.5. Ions Inference Study
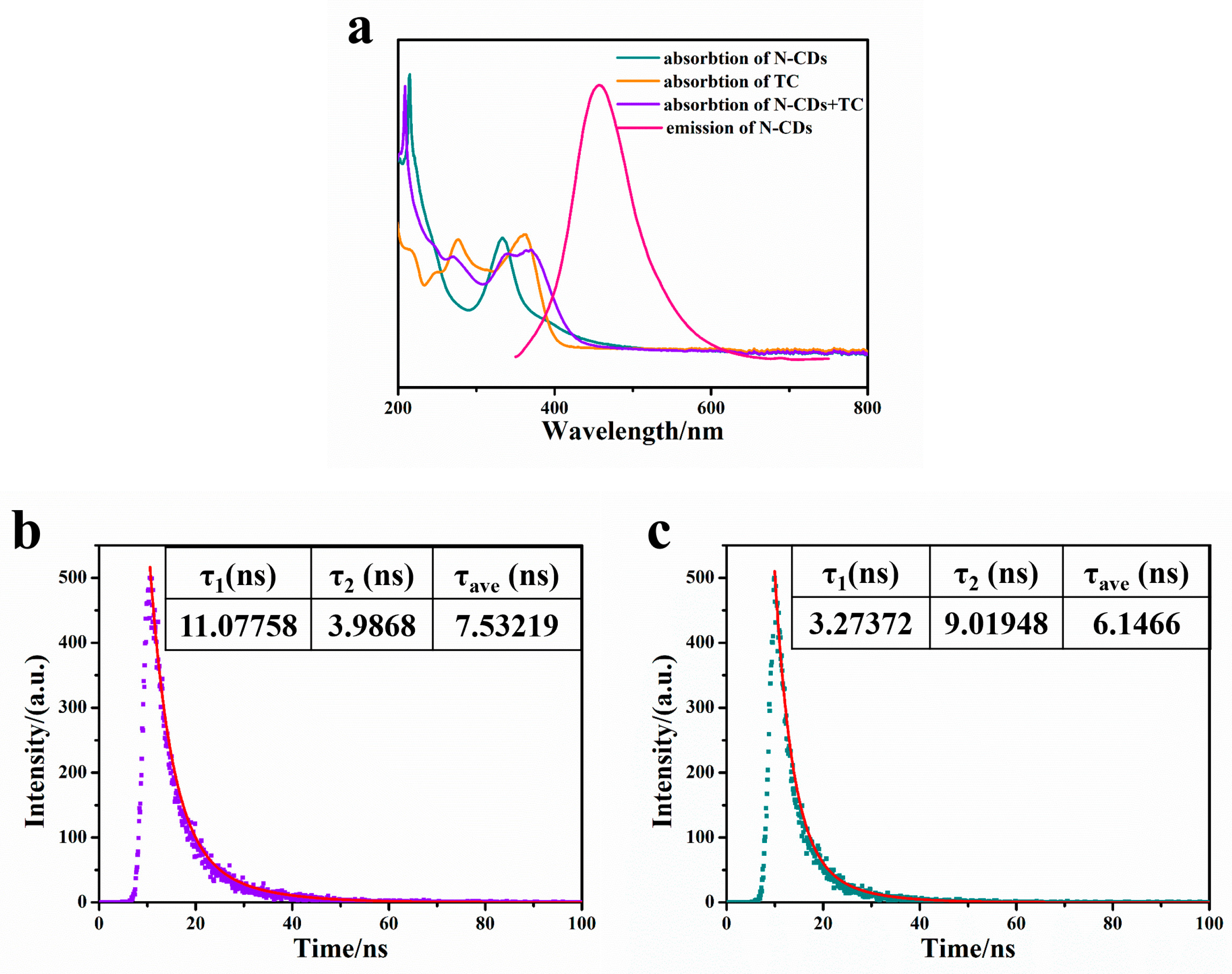
2.6. Fluorescence Quenching Mechanism
2.7. Actual Sample Detection and Recovery Experiment
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.2.1. Synthesis of CdTe QDs and N-CDs
3.2.2. Preparation of CdTe QDs@SiO2
3.2.3. Synthesis of CdTe QDs@SiO2/N-CDs@MIPs
3.3. Fluorescent Detection of TC
3.4. Handling of Actual Samples
3.5. High-Performance Liquid Chromatography (HPLC) Analysis Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, Z.Y.; Hu, X.T.; Xu, X.C.; Zhang, W.; Zou, X.B.; Shi, J.Y.; Zheng, K.Y.; Muhammad, A. A portable test strip based on fluorescent europium-based metal-organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Hu, X.; Zhang, X.; Li, W.; Guo, Z.; Huang, X.; Li, Z.; Zhang, R.; Shen, T.; Zou, X.; et al. Ratiometric Sensing for Ultratrace Tetracycline Using Electrochemically Active Metal-Organic Frameworks as Response Signals. J. Agric. Food Chem. 2023, 71, 7584–7592. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Liu, Y.; Chen, Q.S.; Guo, Z.M.; Zhao, J.W.; Li, H.H.; Hu, W.W. Rapid and specific sensing of tetracycline in food using a novel upconversion aptasensor. Food Control. 2017, 81, 156–163. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Mehedi, H.M.; Rong, Y.W.; Liu, R.; Li, H.H.; Qin, O.Y.; Chen, Q.S. An upconversion nanosensor for rapid and sensitive detection of tetracycline in food based on magnetic-field-assisted separation. Food Chem. 2021, 373, 131497. [Google Scholar] [CrossRef]
- Bai, F.E.; Bu, T.; Zhang, M.; Tian, Y.M.; Sun, X.Y.; Jia, P.; Zhang, Y.L.; Li, R.; Zhao, S.; He, K.Y.; et al. Rhombic-like Al nanosupporter-based fluorescent immunochromatographic assay for the sensitive detection of tetracycline. Sens. Actuators B-Chem. 2020, 324, 128721. [Google Scholar] [CrossRef]
- Bougrini, M.; Florea, A.; Cristea, C.; Sandulescu, R.; Vocanson, F.; Errachid, A.; Bouchikhi, B.; El Bari, N.; Jaffrezic-Renault, N. Development of a novel sensitive molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework for tetracycline detection in honey. Food Control 2016, 59, 424–429. [Google Scholar] [CrossRef]
- Bu, T.; Jia, P.; Sun, X.Y.; Liu, Y.N.; Wang, Q.Z.; Wang, L. Hierarchical molybdenum disulfide nanosheets based lateral flow immunoassay for highly sensitive detection of tetracycline in food samples. Sens. Actuators B-Chem. 2020, 320, 128440. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.L.; Li, S.Y.; Xu, F.H.; Zhang, Q. Ratio fluorescence detection of tetracycline by a Eu3+/NH2-MIL-53(Al) composite. RSC Adv. 2021, 11, 2397–2404. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Lyu, T.; Wang, Y.; Yang, X.; Wu, X. A facile strategy for the synthesis of water-soluble fluorescent nonconjugated polymer dots and their application in tetracycline detection. J. Mater. Chem. C 2019, 7, 241–9247. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Z.Z.; Nur, A.Z.; Lu, Y.; Wu, M.H.; Zeng, J.; Chen, X.M.; Huang, Z.Y. Detection of trace tetracycline in fish via synchronous fluorescence quenching with carbon quantum dots coated with molecularly imprinted silica. Spectrochim. Acta A 2018, 190, 450–456. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, M.K.; Su, X.G. Ratiometric fluorescent detection of azodicarbonamide based on silicon nanoparticles and quantum dots, Sens. Actuators B-Chem. 2019, 296, 126643. [Google Scholar] [CrossRef]
- Li, W.T.; Zhang, X.N.; Hu, X.T.; Shi, Y.Q.; Li, Z.H.; Huang, X.W.; Zhang, W.; Zhang, D.; Zou, X.B.; Shi, J.Y. A smartphone-integrated ratiometric fluorescence sensor for visual detection of cadmium ions. J. Hazard. Mater. 2021, 408, 124872. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Huang, T.; Wang, S.A.; Yan, Y.S. Mesoporous silica-based molecularly imprinted fluorescence sensor for the ultrafast and sensitive recognition of oxytetracycline. J. Food Compos. Anal. 2022, 108, 104427. [Google Scholar] [CrossRef]
- Bi, X.Y.; Li, L.B.; Luo, L.J.; Liu, X.H.; Li, J.M.; You, T.Y. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef]
- Wang, L.; Gong, C.C.; Shen, Y.; Ye, W.H.; Xu, M.L.; Song, Y.H. A novel ratiometric electrochemical biosensor for sensitive detection of ascorbic acid. Sens. Actuators B-Chem. 2017, 242, 625–631. [Google Scholar] [CrossRef]
- Han, C.; Xiao, Y.; Liu, Z.; Du, D.; Li, M. Cascade amplifying aptasensor for positively correlated detecting OTA: Based on DNase I-assisted cyclic enzyme digestion and AgNPs@gel-enhanced fluorescence. Food Control 2023, 153, 109970. [Google Scholar] [CrossRef]
- Zhan, Y.J.; Zeng, Y.B.; Li, L.; Luo, F.; Qiu, B.; Lin, Z.Y.; Guo, L.H. Ratiometric Fluorescent Hydrogel Test Kit for On-Spot Visual Detection of Nitrite. ACS Sens. 2019, 4, 1252–1260. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, N.; Lee, J.H.; Yoon, J.; Shin, I. Synthetic ratiometric fluorescent probes for detection of ions. Chem. Soc. Rev. 2020, 49, 143–179. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, H.; Pan, S.; Liu, H.; Hu, X. Fluorescence-Scattering Dual-Signal Response of Carbon Dots@ZIF-90 for Phosphate Ratiometric Detection. ACS Sens. 2020, 5, 2211–2220. [Google Scholar] [CrossRef]
- Wei, J.R.; Chen, H.Y.; Zhang, W.; Pan, J.X.; Dang, F.Q.; Zhang, Z.Q.; Zhang, J. Ratiometric fluorescence for sensitive and selective detection of mitoxantrone using a MIP@rQDs@SiO2 fluorescence probe. Sens. Actuators B-Chem. 2017, 244, 31–37. [Google Scholar] [CrossRef]
- Luan, K.; Meng, R.Q.; Shan, C.F.; Cao, J.; Jia, J.G.; Liu, W.S.; Tang, Y. Terbium Functionalized Micelle Nanoprobe for Ratiometric Fluorescence Detection of Anthrax Spore Biomarker. Anal. Chem. 2018, 90, 3600–3607. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Hu, X.; Li, W.; Mwakosya, A.W.; Guo, Z.; Xu, Y.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; et al. Fluorescence and colorimetric dual-mode sensor for visual detection of malathion in cabbage based on carbon quantum dots and gold nanoparticles. Food Chem. 2021, 343, 128494. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Park, S.Y. Carbon-dot-based ratiometric fluorescence glucose biosensor. Sens. Actuators B-Chem. 2019, 282, 719–729. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Hu, X.; Zhang, X.; Huang, X.; Li, Z.; Li, M.; Zou, X.; Shi, J. Efficient preparation of dual-emission ratiometric fluorescence sensor system based on aptamer-composite and detection of bis(2-ethylhexyl) phthalate in pork. Food Chem. 2021, 352, 129352. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Wang, J.X.; Lu, Y.; Dai, X.H.; Yan, Y.S. Preparation of functionalized double ratio fluorescent imprinted sensors for visual determination and recognition of dopamine in human serum. Spectrochim. Acta A 2019, 219, 225–231. [Google Scholar] [CrossRef]
- Ali, H.R.H.; Hassan, A.I.; Hassan, Y.F.; El-Wekil, M.M. Colorimetric and fluorometric nanoprobe for selective and sensitive recognition of hazardous colorant indigo carmine in beverages based on ion pairing with nitrogen doped carbon dots. Food Chem. 2021, 349, 129160. [Google Scholar] [CrossRef]
- Bai, J.P.; Xiao, N.; Wang, Y.W.; Li, H.Q.; Liu, C.; Xiao, J.; Wei, Y.B.; Guo, Z.; Qiu, J.S. Coal tar pitch derived nitrogen-doped carbon dots with adjustable particle size for photocatalytic hydrogen generation. Carbon 2021, 174, 750–756. [Google Scholar] [CrossRef]
- Babusenan, A.; Pandey, B.; Roy, S.C.; Bhattacharyya, J. Charge transfer mediated photoluminescence enhancement in carbon dots embedded in TiO2 nanotube matrix. Carbon 2020, 161, 535–541. [Google Scholar] [CrossRef]
- Bhatt, M.; Bhatt, S.; Vyas, G.; Raval, I.H.; Haldar, S.; Paul, P. Water-Dispersible Fluorescent Carbon Dots as Bioimaging Agents and Probes for Hg2+ and Cu2+ Ions. ACS Appl. Nano Mater. 2020, 3, 7096–7104. [Google Scholar] [CrossRef]
- Chang, Q.; Shen, Z.H.; Guo, Z.Y.; Xue, C.R.; Li, N.; Yang, J.L.; Hu, S.L. Hydroxypropylmethyl Cellulose Modified with Carbon Dots Exhibits Light-Responsive and Reversible Optical Switching. ACS Appl. Mater. Interfaces 2021, 13, 12375–12382. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Dong, J.; Liu, C.; Qi, Y.; Fang, G.; Wang, S. A strong blue fluorescent nanoprobe based on Mg/N co-doped carbon dots coupled with molecularly imprinted polymer for ultrasensitive and highly selective detection of tetracycline in animal-derived foods. Sens. Actuators B-Chem. 2021, 338, 129809. [Google Scholar] [CrossRef]
- Wang, S.L.; Liang, N.N.; Hu, X.T.; Li, W.T.; Guo, Z.A.; Zhang, X.A.; Huang, X.W.; Li, Z.H.; Zou, X.B.; Shi, J.Y. Carbon dots and covalent organic frameworks based FRET immunosensor for sensitive detection of Escherichia coli O157:H7. Food Chem. 2024, 447, 138663. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, T.; Sun, C.; Xia, J.; Jiao, Y.; Xu, H. Selenium-Doped Carbon Quantum Dots for Free-Radical Scavenging. Angew. Chem. Int. Ed. 2017, 56, 9910–9914. [Google Scholar] [CrossRef]
- Wang, J.X.; Cheng, R.J.; Wang, Y.Y.; Sun, L.; Chen, L.; Dai, X.H.; Pan, J.M.; Pan, G.Q.; Yan, Y.S. Surface-imprinted fluorescence microspheres as ultrasensitive sensor for rapid and effective detection of tetracycline in real biological samples. Sens. Actuators B-Chem. 2018, 263, 533–542. [Google Scholar] [CrossRef]
- Wang, J.X.; Qiu, H.; Shen, H.Q.; Pan, J.M.; Dai, X.H.; Yan, Y.S.; Pan, G.Q.; Sellergren, B. Molecularly imprinted fluorescent hollow nanoparticles as sensors for rapid and efficient detection λ-cyhalothrin in environmental water. Biosens. Bioelectron. 2016, 85, 387–394. [Google Scholar] [CrossRef]
- Chen, X.Z.; Xu, J.; Li, Y.X.; Zhang, L.N.; Bi, N.; Guo, J.; Zhu, T.F.; Jia, L. A novel intelligently integrated MOF-based ratio fluorescence sensor for ultra-sensitive monitoring of TC in water and food samples. Food Chem. 2022, 405, 134899. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Bai, L.; Jiang, Y.H.; Qiu, J.; Meng, M.J.; Liu, Z.C.; Ni, L. A molecularly imprinted polymer placed on the surface of graphene oxide and doped with Mn(II)-doped ZnS quantum dots for selective fluorometric determination of acrylamide. Microchim. Acta 2018, 185, 48. [Google Scholar] [CrossRef]
- Chaiendoo, K.; Ittisanronnachai, S.; Promarak, V.; Ngeontae, W. Polydopamine-coated carbon nanodots are a highly selective turn-on fluorescent probe for dopamine. Carbon 2019, 146, 728–735. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Huang, T.; Hu, B.; Meng, M.J.; Yan, Y.S. Molecularly imprinted polydopamine coated CdTe@SiO2 as a ratiometric fluorescent probe for ultrafast and visual p-nitrophenol monitoring. Microchem. J. 2022, 172, 106899. [Google Scholar] [CrossRef]
- Ma, Y.S.; Cen, Y.; Sohail, M.; Xu, G.H.; Wei, F.D.; Shi, M.L.; Xu, X.M.; Song, Y.Y.; Ma, Y.J.; Hu, Q. A Ratiometric Fluorescence Universal Platform Based on N, Cu Codoped Carbon Dots to Detect Metabolites Participating in H2O2-Generation Reactions. ACS Appl. Mater. Interfaces 2017, 9, 33011–33019. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Huang, T.; Hu, B.; Meng, M.J.; Yan, Y.S. An ultrasensitive PVDF-based molecularly imprinted fluorescent test strip for the rapid and off-line detection of 4-NP with improved anti-coffee ring effect. J. Mater. Chem. C 2021, 9, 16587–16601. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, T.; Wang, S.; Meng, M.; Yan, Y. SiO2-coated molecularly imprinted sensor based on Si quantum dots for selective detection of catechol in river water. J. Environ. Chem. Eng. 2021, 10, 106850. [Google Scholar] [CrossRef]
- Yang, C.Y.; Bie, J.X.; Zhang, X.M.; Yan, C.Y.; Li, H.J.; Zhang, M.H.; Su, R.F.; Zhang, X.G.; Sun, C.Y. A label-free aptasensor for the detection of tetracycline based on the luminescence of SYBR Green I. Spectrochim. Acta A 2018, 202, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, J.H.; Li, R.S.; Li, Y.F.; Huang, C.Z.; Zhen, S.J. Carbon dots synthesized at room temperature for detection of tetracycline hydrochloride. Anal. Chim. Acta 2019, 1063, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, W.S.; Zaccarin, L.F.; Oliveira, G.G.; de Oliveira, P.R.; Kalinke, C.; Bonacin, J.A.; Prakash, J.; Janegitz, B.C. Electrochemical Sensor Based on Nanodiamonds and Manioc Starch for Detection of Tetracycline. J. Sens. 2021, 2021, 6622612. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Huang, C.Z. Investigations on the amalgamation of gold nanorods by iodine and the detection of tetracycline. Sci. China Ser. B 2009, 52, 188–195. [Google Scholar] [CrossRef]
- CCalixto, M.F.; Cavalheiro, E.T.G. Determination of Tetracycline in Bovine and Breast Milk Using a Graphite–Polyurethane Composite Electrode. Anal. Lett. 2017, 50, 2323–2334. [Google Scholar] [CrossRef]
- Wang, H.-H.; Zhang, Y.; Yang, D.-B.; Hou, L.; Li, Z.-Y.; Wang, Y.-Y. Fluorine-Substituted Regulation in Two Comparable Isostructural Cd(II) Coordination Polymers: Enhanced Fluorescence Detection for Tetracyclines in Water. Cryst. Growth Des. 2021, 21, 2488–2497. [Google Scholar] [CrossRef]
- Zhao, J.H.; Liu, P.; Yuan, H.C.; Peng, Y.J.; Hong, Q.; Liu, M.H. Rapid Detection of Tetracycline Residues in Duck Meat Using Surface Enhanced Raman Spectroscopy. J. Spectrosc. 2016, 2016, 1845237. [Google Scholar] [CrossRef]
- Zhou, Z.P.; Lu, K.; Wei, X.; Hao, T.F.; Xu, Y.Q.; Lv, X.D.; Zhang, Y.F. A mesoporous fluorescent sensor based on ZnO nanorods for the fluorescent detection and selective recognition of tetracycline. RSC Adv. 2016, 6, 71061–71069. [Google Scholar] [CrossRef]
- Dong, Z.Q.; Lu, J.; Wu, Y.L.; Meng, M.J.; Yu, C.; Sun, C.; Chen, M.; Da, Z.L.; Yan, Y.S. Antifouling molecularly imprinted membranes for pretreatment of milk samples: Selective separation and detection of lincomycin. Food Chem. 2020, 333, 127477. [Google Scholar] [CrossRef]




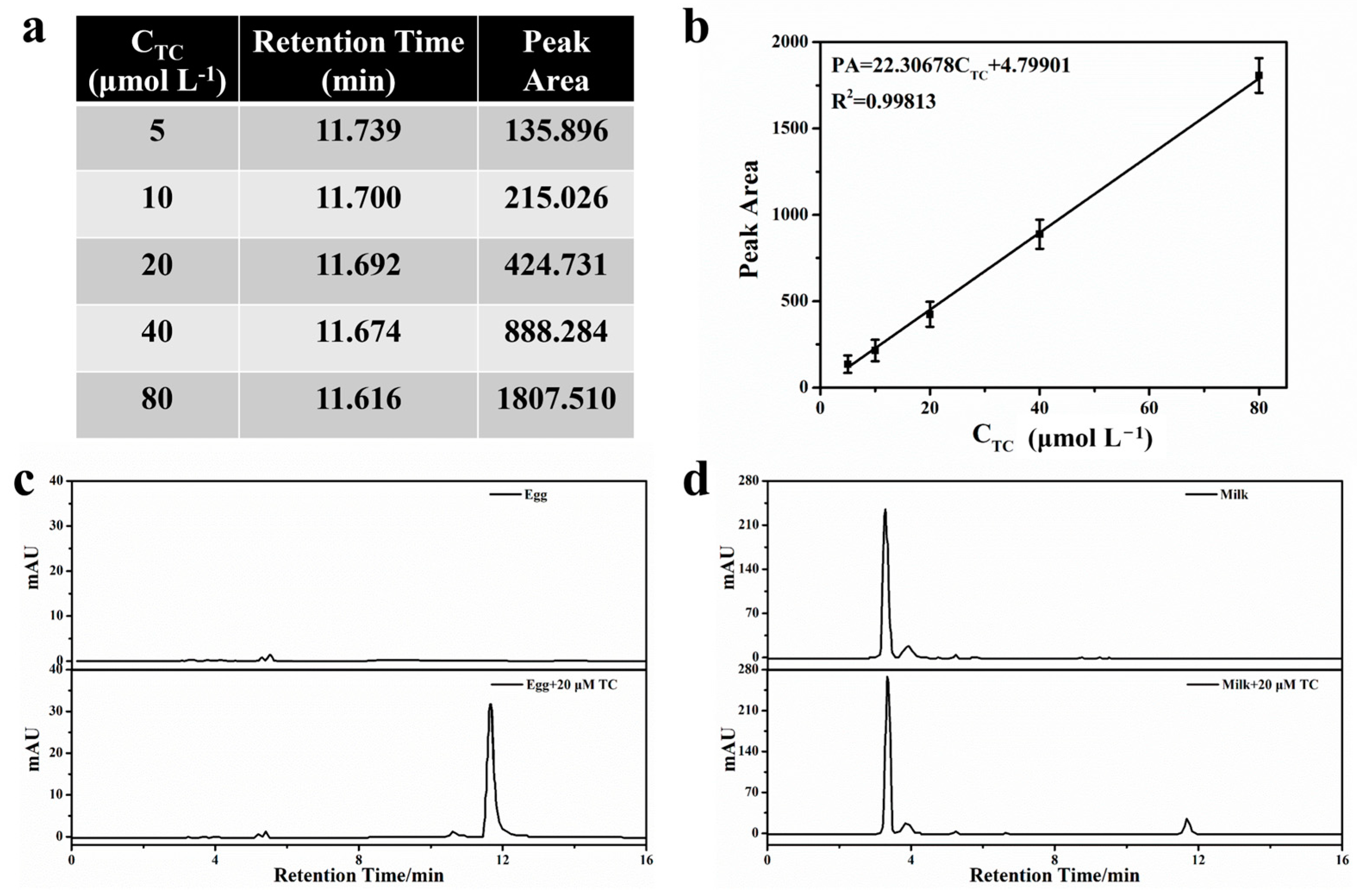

| CdTe QDs@SiO2/N-CDs@MIPs | HPLC | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Add (μM) | Detected (μM) | Recovery Rate (%) | RSD (%) | Add (μM) | Detected (μM) | Recovery Rate (%) | RSD (%) |
| Milk | 0 | — | — | — | 0 | — | — | — |
| 10.00 | 9.96 | 99.60 | 1.86 | 10.00 | 10.18 | 101.80 | 1.24 | |
| 20.00 | 20.17 | 100.85 | 1.57 | 20.00 | 20.28 | 101.40 | 1.32 | |
| 40.00 | 40.18 | 100.45 | 1.16 | 40.00 | 40.15 | 100.38 | 1.09 | |
| Egg | 0 | — | — | — | 0 | — | — | — |
| 10.00 | 10.15 | 100.15 | 1.71 | 10.00 | 9.50 | 95.00 | 1.87 | |
| 20.00 | 19.98 | 99.90 | 1.38 | 20.00 | 19.68 | 98.40 | 1.56 | |
| 40.00 | 40.54 | 101.35 | 1.12 | 40.00 | 40.15 | 100.38 | 1.24 | |
| Method | Linear Range | Detection Time | Detection Limit | References |
|---|---|---|---|---|
| Aptamer-based method | 0.011–0.056 mM | 10 min | 0.0225 mM | [44] |
| Fluorescence analysis | 10–400 μM | 10 min | 6.0 μM | [45] |
| Electrochemical analysis | 5.0–180 μM | — | 2.0 μM | [46] |
| Spectrophotometric method | 50–500 μM | 40 min | 2.4 μM | [47] |
| Voltammetric analysis method | 0–95 μM | — | 2.6 μM | [48] |
| Fluorescence analysis | 0–33.23 μM | 60 min | 8.97–18.39 μM | [49] |
| Surface-enhanced Raman spectroscopy | 9.0–56 μM | 1.0 min | 2.52 μM | [50] |
| Molecular imprinting method | 2.0–120 μM | 5.0 min | 1.54 μM | [51] |
| CdTe QDs@SiO2/N-CDs@MIPs | 0–140 μM | 1.0 min | 0.846 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qin, Y.; Ma, Y.; Meng, M.; Xu, Y. Low-Toxicity and High-Stability Fluorescence Sensor for the Selective, Rapid, and Visual Detection Tetracycline in Food Samples. Molecules 2024, 29, 5888. https://doi.org/10.3390/molecules29245888
Wang J, Qin Y, Ma Y, Meng M, Xu Y. Low-Toxicity and High-Stability Fluorescence Sensor for the Selective, Rapid, and Visual Detection Tetracycline in Food Samples. Molecules. 2024; 29(24):5888. https://doi.org/10.3390/molecules29245888
Chicago/Turabian StyleWang, Jixiang, Yaowei Qin, Yue Ma, Minjia Meng, and Yeqing Xu. 2024. "Low-Toxicity and High-Stability Fluorescence Sensor for the Selective, Rapid, and Visual Detection Tetracycline in Food Samples" Molecules 29, no. 24: 5888. https://doi.org/10.3390/molecules29245888
APA StyleWang, J., Qin, Y., Ma, Y., Meng, M., & Xu, Y. (2024). Low-Toxicity and High-Stability Fluorescence Sensor for the Selective, Rapid, and Visual Detection Tetracycline in Food Samples. Molecules, 29(24), 5888. https://doi.org/10.3390/molecules29245888







