Enhanced Circularly Polarized Green Luminescence Metrics from New Enantiopure Binary Tris-Pyrazolonate-Tb3+ Complexes
Abstract
1. Introduction
2. Results and Discussion
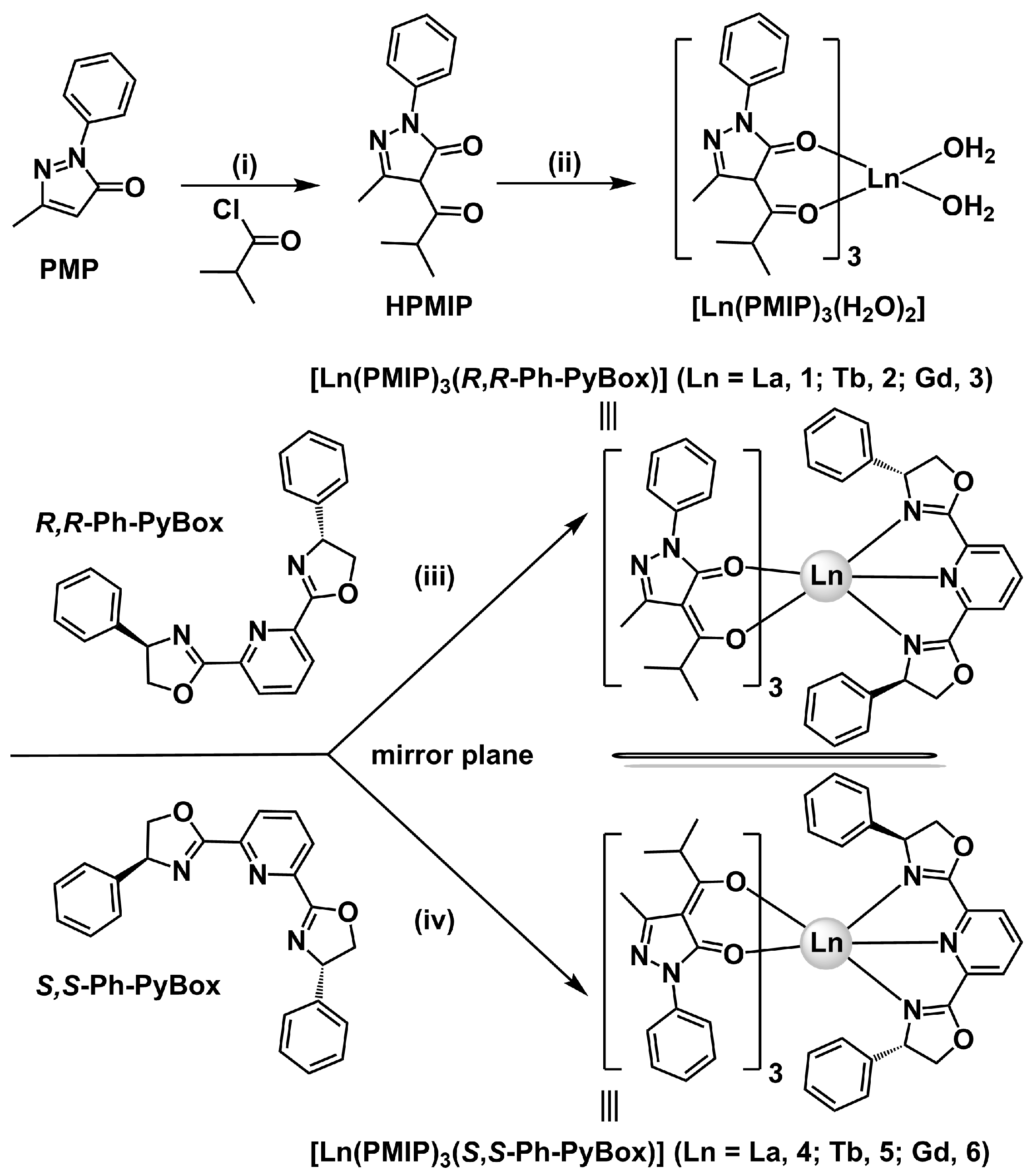
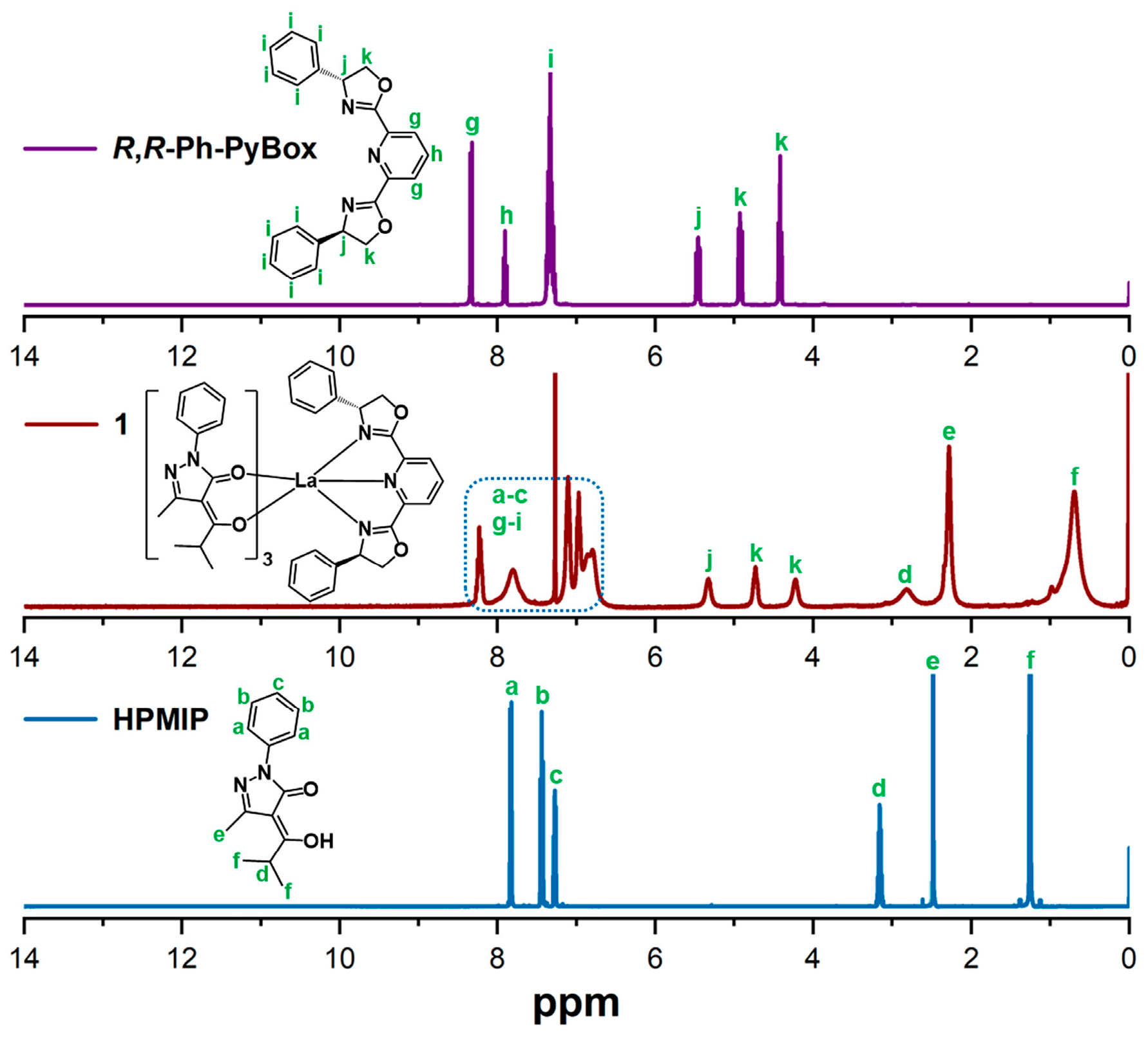
2.1. Synthesis and Characterization
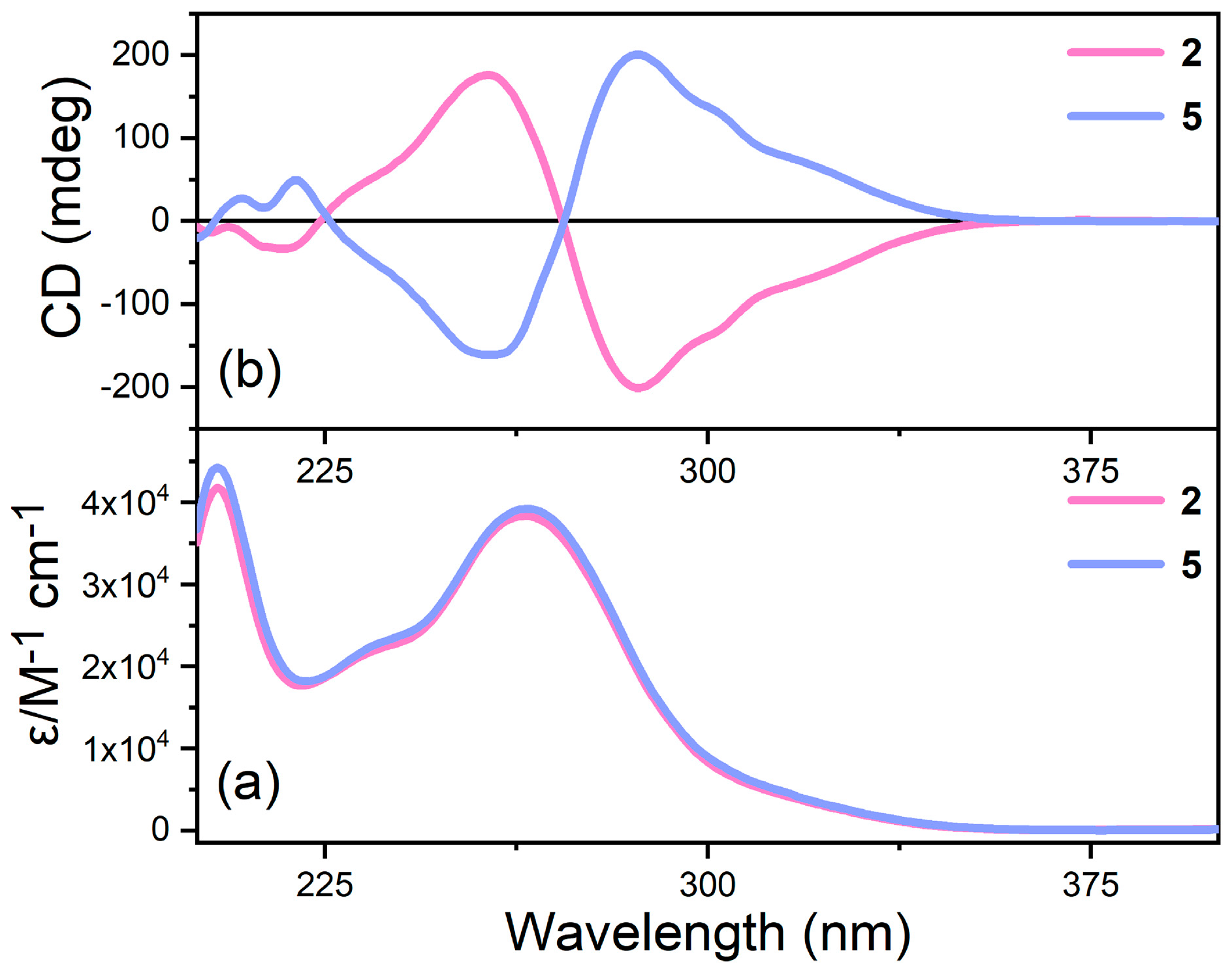
2.2. Ground-State Optical Properties
2.3. Excited-State Optical Properties
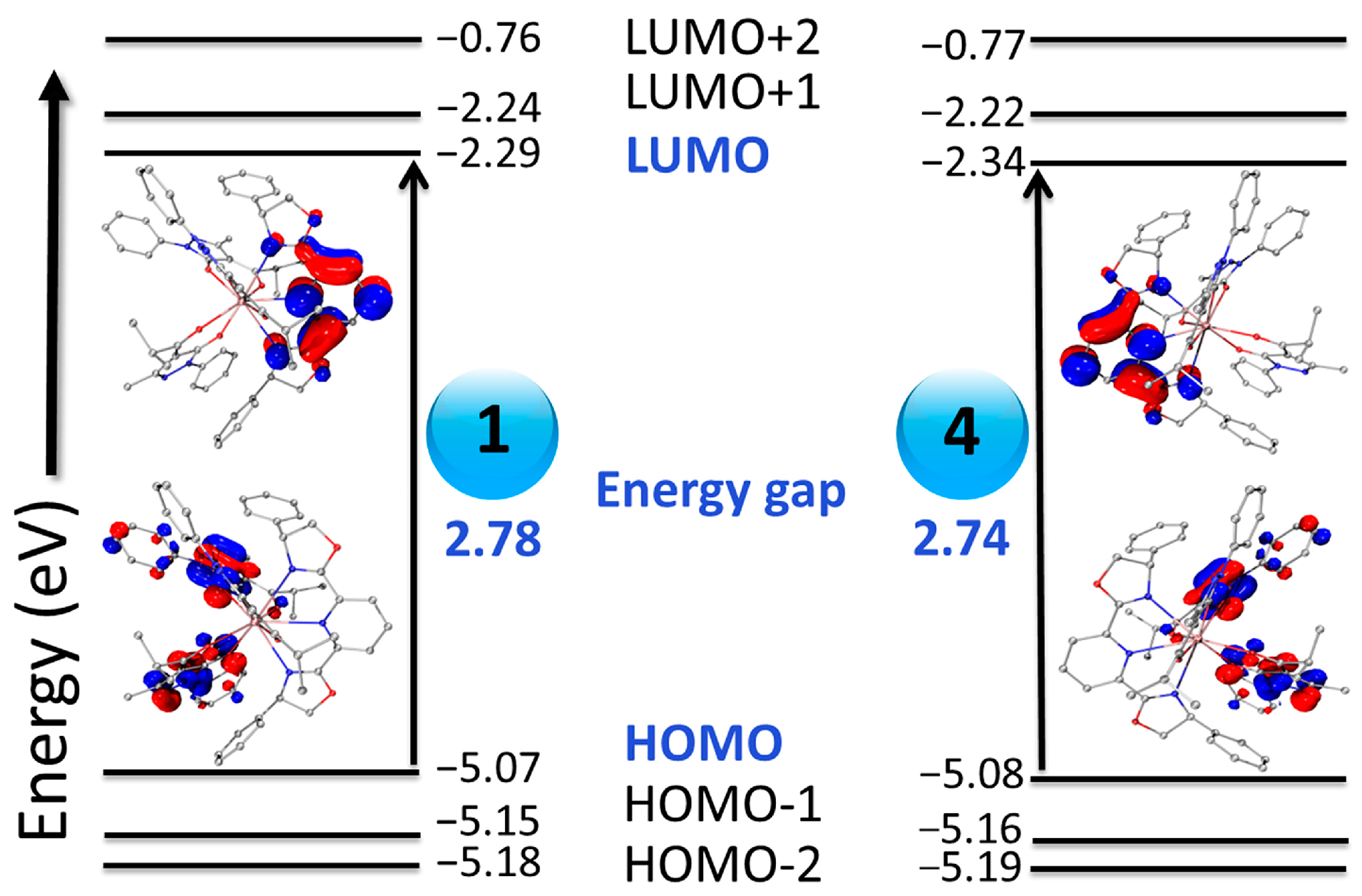
2.4. Theoretical Calculations of Electronic Structure
3. Materials and Methods
3.1. General Information and Synthesis of Intermediates
3.2. Synthetic Procedures and Characterizations of the Chiral Organo-Ln3+ Complexes 1–6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arrico, L.; De Rosa, C.; Di Bari, L.; Melchior, A.; Piccinelli, F. Effect of the Counterion on Circularly Polarized Luminescence of Europium(III) and Samarium(III) Complexes. Inorg. Chem. 2020, 59, 5050–5062. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, L.E.; Pal, R. Circularly Polarized Lanthanide Luminescence for Advanced Security Inks. Nat. Rev. Chem. 2021, 5, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Guan, Y.; Huang, J.; Luo, Z.W.; Huang, A.; Wang, P.; Xie, H.L. Circularly Polarized Luminescent Behavior of Delayed Fluorescence Liquid Crystals Based on Carbonized Polymer Dots. Adv. Opt. Mater. 2023, 11, 2300816. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Li, M.; Chen, C.-F. Recent Advances in Circularly Polarized Electroluminescence Based on Organic Light-Emitting Diodes. Chem. Soc. Rev. 2020, 49, 1331–1343. [Google Scholar] [CrossRef]
- Zinna, F.; Di Bari, L. Lanthanide-Based Multifunctional Materials from OLEDs to SIMs; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 171–194. [Google Scholar]
- Wong, H.-Y.; Lo, W.-S.; Yim, K.-H.; Law, G.-L. Chirality and Chiroptics of Lanthanide Molecular and Supramolecular Assemblies. Chem 2019, 5, 3058–3095. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Zhao, B.; Deng, J. Frontiers in Circularly Polarized Phosphorescent Materials. Adv. Funct. Mater. 2023, 33, 2214364. [Google Scholar] [CrossRef]
- Willis, O.G.; Zinna, F.; Di Bari, L. NIR-Circularly Polarized Luminescence from Chiral Complexes of Lanthanides and d-Metals. Angew. Chem. Int. Ed. 2023, 62, e202302358. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Zhang, Z.; Yu, J.; Lü, X.; Fu, G.; Li, W.; Wong, W.-Y. A Circularly Polarized (CP) White Organic Light-Emitting Diode (WOLED) Based on a Chiral Organo-Sm3+ Complex. J. Mater. Chem. C 2023, 11, 1265–1270. [Google Scholar] [CrossRef]
- Li, Y.-L.; Wang, H.-L.; Zhu, Z.-H.; Wang, Y.-F.; Liang, F.-P.; Zou, H.-H. Aggregation Induced Emission Dynamic Chiral Europium(III) Complexes with Excellent Circularly Polarized Luminescence and Smart Sensors. Nat. Commun. 2024, 15, 2896. [Google Scholar] [CrossRef]
- Wang, Q.; Lucas, F.; Quinton, C.; Qu, Y.-K.; Rault-Berthelot, J.; Jeannin, O.; Yang, S.-Y.; Kong, F.-C.; Kumar, S.; Liao, L.-S. Evolution of Pure Hydrocarbon Hosts: Simpler Structure, Higher Performance and Universal Application in RGB Phosphorescent Organic Light-Emitting Diodes. Chem. Sci. 2020, 11, 4887–4894. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, F.; Yu, M.; Li, S.; Chen, L.; Hong, M. Achieving Gas Pressure-Dependent Luminescence from an AIEgen-Based Metal-Organic Framework. Nat. Commun. 2022, 13, 2142. [Google Scholar] [CrossRef]
- Arrico, L.; Di Bari, L.; Zinna, F. Quantifying the Overall Efficiency of Circularly Polarized Emitters. Chem. Eur. J. 2021, 27, 2920–2934. [Google Scholar] [CrossRef]
- Górecki, M.; Carpita, L.; Arrico, L.; Zinna, F.; Di Bari, L. Chiroptical Methods in a Wide Wavelength Range for Obtaining Ln3+ Complexes with Circularly Polarized Luminescence of Practical Interest. Dalton Trans. 2018, 47, 7166–7177. [Google Scholar] [CrossRef] [PubMed]
- Zinna, F.; Arrico, L.; Funaioli, T.; Di Bari, L.; Pasini, M.; Botta, C.; Giovanella, U. Modular Chiral Eu(III) Complexes for Efficient Circularly Polarized OLEDs. J. Mater. Chem. C 2022, 10, 463–468. [Google Scholar] [CrossRef]
- Zinna, F.; Pasini, M.; Cabras, M.; Scavia, G.; Botta, C.; Di Bari, L.; Giovanella, U. Impact of Chiral Ligands on Photophysical and Electro-Optical Properties of β-Diketonate Europium Complexes in Circularly Polarized OLEDs. Chirality 2023, 35, 270–280. [Google Scholar] [CrossRef]
- Diogenis, I.M.S.; Bispo-Jr, A.G.; Pirovani, R.V.; Saraiva, L.F.; Gozzo, F.C.; Correia, C.R.D.; Mazali, I.O.; Nome, R.A.; Sigoli, F.A. Towards Opto-Structural Parameters to Enhance the Circularly Polarized Luminescence Brightness of EuIII β-Diketone Complexes with Chiral Auxiliary Ligands. J. Mater. Chem. C 2024, 12, 5097–5107. [Google Scholar] [CrossRef]
- Yuasa, J.; Ohno, T.; Miyata, K.; Tsumatori, H.; Hasegawa, Y.; Kawai, T. Noncovalent Ligand-to-Ligand Interactions Alter Sense of Optical Chirality in Luminescent Tris(β-diketonate) Lanthanide(III) Complexes Containing a Chiral Bis(oxazolinyl) Pyridine Ligand. J. Am. Chem. Soc. 2011, 133, 9892–9902. [Google Scholar] [CrossRef]
- Tan, Y.B.; Okayasu, Y.; Katao, S.; Nishikawa, Y.; Asanoma, F.; Yamada, M.; Yuasa, J.; Kawai, T. Visible Circularly Polarized Luminescence of Octanuclear Circular Eu(III) Helicate. J. Am. Chem. Soc. 2020, 142, 17653–17661. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Hara, N.; Shizuma, M.; Tajima, N.; Fujiki, M.; Imai, Y. Circularly Polarised Luminescence from Planar-Chiral Phanephos/Tb(III)(hfa)3 Hybrid Luminophores. Photochem. Photobiol. Sci. 2019, 18, 2859–2864. [Google Scholar] [CrossRef]
- Tanase, T.; Nakamae, K.; Okawa, Y.; Hamada, M.; Matsumoto, A.; Nakajima, T.; Nakashima, T.; Kawai, T. Chiral Dinuclear EuIII, TbIII, and YIII Complexes Supported by P-Stereogenic Linear Tetraphosphine Tetraoxide. Chem. Eur. J. 2022, 28, e202104060. [Google Scholar] [CrossRef]
- Ruggieri, S.; Mizzoni, S.; Nardon, C.; Cavalli, E.; Sissa, C.; Anselmi, M.; Cozzi, P.G.; Gualandi, A.; Sanadar, M.; Melchior, A. Circularly Polarized Luminescence from New Heteroleptic Eu(III) and Tb(III) Complexes. Inorg. Chem. 2023, 62, 8812–8822. [Google Scholar] [CrossRef]
- Zhao, Z.; Bian, M.; Lin, C.; Fu, X.; Yu, G.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Efficient Green OLEDs Achieved by a Terbium(III) Complex with Photoluminescent Quantum Yield Close to 100%. Sci. China Chem. 2021, 64, 1504–1509. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Z.; Zhou, L.; Zhu, Q.; Liu, Z.; Bian, Z.; Huang, C.; Zhang, H. Highly Efficient Green Organic Light-Emitting Devices Based on Terbium Complex by Employing Hole Block Material as Host. Sci. China Technol. Sci. 2018, 61, 1334–1339. [Google Scholar] [CrossRef]
- Yu, G.; Ding, F.; Wei, H.; Zhao, Z.; Liu, Z.; Bian, Z.; Xiao, L.; Huang, C. Highly Efficient Terbium(III)-Based Organic Light-Emitting Diodes Obtained by Exciton Confinement. J. Mater. Chem. C 2016, 4, 121–125. [Google Scholar] [CrossRef]
- Sun, S.; Ou, C.; Lin, D.; Zuo, Z.; Yan, Y.; Wei, Y.; Liu, Y.; Xie, L.; Huang, W. Organic Nanosynthesis of Diarylfluorene-Based Ladder-Type Gridarene Isomers Via Intramolecular A1-B1 Type Friedel-Crafts Gridization. Tetrahedron 2018, 74, 5833–5838. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, M.; Liu, Z.; Li, F.; Yi, T.; Huang, C. Luminescence Modulation of a Terbium Complex with Anions and Its Application as a Reagent. Eur. J. Inorg. Chem. 2006, 11, 2277–2284. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, Y.; Cheng, Z.; Gao, T.; Li, H.; Yan, P. Point Chirality Controlled Diastereoselective Self-Assembly and Circularly Polarized Luminescence in Quadruple-Stranded Europium(III) Helicates. Inorg. Chem. 2020, 59, 12850–12857. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, Y.; Zhang, Y.; Li, H.; Chen, P.; Sun, W.; Gao, T.; Yan, P. Chiral BINAPO-Controlled Diastereoselective Self-Assembly and Circularly Polarized Luminescence in Triple-Stranded Europium(III) Podates. Inorg. Chem. 2018, 57, 8332–8337. [Google Scholar] [CrossRef]
- Bala, M.; Kumar, S.; Devi, R.; Khatkar, A.; Taxak, V.; Boora, P.; Khatkar, S. Synthesis, Photoluminescence Behavior of Green Light Emitting Tb(III) Complexes and Mechanistic Investigation of Energy Transfer Process. J. Fluoresc. 2018, 28, 775–784. [Google Scholar] [CrossRef]
- Ilmi, R.; Khan, M.S.; Sun, W.; Zhou, L.; Wong, W.-Y.; Raithby, P.R. A Single Component White Electroluminescent Device Fabricated from a Metallo-Organic Terbium Complex. J. Mater. Chem. C 2019, 7, 13966–13975. [Google Scholar] [CrossRef]
- Ahmed, Z.; Iftikhar, K. Efficient Layers of Emitting Ternary Lanthanide Complexes for Fabricating Red, Green, and Yellow OLEDs. Inorg. Chem. 2015, 54, 11209–11225. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pang, M.; Chen, H.; Fu, G.; Li, B.; Lü, X.; Wang, L. Efficient and High Colour-Purity Green-Light Polymer Light-Emitting Diodes (PLEDs) Based on a PVK-Supported Tb3+-Containing Metallopolymer. J. Mater. Chem. C 2017, 5, 9021–9027. [Google Scholar] [CrossRef]
- Barry, D.E.; Kitchen, J.A.; Mercs, L.; Peacock, R.D.; Albrecht, M.; Gunnlaugsson, T. Chiral Luminescent Lanthanide Complexes Possessing Strong (samarium, SmIII) Circularly Polarised Luminescence (CPL), and Their Self-Assembly into Langmuir-Blodgett Films. Dalton Trans. 2019, 48, 11317–11325. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.-A.N.; Schnable, D.; Schley, N.D.; Ung, G. Spinolate Lanthanide Complexes for High Circularly Polarized Luminescence Metrics in the Visible and Near-Infrared. J. Am. Chem. Soc. 2022, 144, 22421–22425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-P.; Chang, V.Y.; Liu, J.; Yang, X.-L.; Huang, W.; Li, Y.; Li, C.-H.; Muller, G.; You, X.-Z. Potential Switchable Circularly Polarized Luminescence from Chiral Cyclometalated Platinum(II) Complexes. Inorg. Chem. 2015, 54, 143–152. [Google Scholar] [CrossRef]
- Okayasu, Y.; Yuasa, J. Evaluation of Circularly Polarized Luminescence in a Chiral Lanthanide Ensemble. Mol. Syst. Des. Eng. 2018, 3, 66–72. [Google Scholar] [CrossRef]
- Zinna, F.; Arrico, L.; Di Bari, L. Near-Infrared Circularly Polarized Luminescence from Chiral Yb(III)-Diketonates. Chem. Commun. 2019, 55, 6607–6609. [Google Scholar] [CrossRef]
- Tsurui, M.; Kitagawa, Y.; Fushimi, K.; Gon, M.; Tanaka, K.; Hasegawa, Y. Electronic Strain Effect on Eu(III) Complexes for Enhanced Circularly Polarized Luminescence. Dalton Trans. 2020, 49, 5352–5361. [Google Scholar] [CrossRef]
- Abherve, A.; Mastropasqua Talamo, M.; Vanthuyne, N.; Zinna, F.; Di Bari, L.; Grasser, M.; Le Guennic, B.; Avarvari, N. Chiral Emissive Lanthanide Complexes from Enantiopure [6]Helicene-Bis(Pyrazolyl)-Pyridine Ligands. Eur. J. Inorg. Chem. 2022, 2022, e202200010. [Google Scholar] [CrossRef]
- Liu, D.; El-Zohry, A.M.; Taddei, M.; Matt, C.; Bussotti, L.; Wang, Z.; Zhao, J.; Mohammed, O.F.; Di Donato, M.; Weber, S. Long-Lived Charge-Transfer State Induced by Spin-Orbit Charge Transfer Intersystem Crossing (SOCT-ISC) in a Compact Spiro Electron Donor/Acceptor Dyad. Angew. Chem. 2020, 132, 11688–11696. [Google Scholar] [CrossRef]
- Sanders, S.N.; Kumarasamy, E.; Fallon, K.J.; Sfeir, M.Y.; Campos, L.M. Singlet Fission in a Hexacene Dimer: Energetics Dictate Dynamics. Chem. Sci. 2020, 11, 1079–1084. [Google Scholar] [CrossRef]
- Steemers, F.J.; Verboom, W.; Reinhoudt, D.N.; van der Tol, E.B.; Verhoeven, J.W. New Sensitizer-Modified Calix[4]arenes Enabling Near-UV Excitation of Complexed Luminescent Lanthanide Ions. J. Am. Chem. Soc. 1995, 117, 9408–9414. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.-M.; Matachescu, C.; Rodríguez-Ubis, J.C.; Kankare, J. Correlation Between the Lowest Triplet State Energy Level of the Ligand and Lanthanide(III) Luminescence Quantum Yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Beeke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Liu, J.; Shi, Q.; He, Y.; Fu, G.; Li, W.; Miao, T.; Lü, X. Single-Molecule White-Light of Tris-Pyrazolonate-Dy3+ Complexes. Inorg. Chem. Commun. 2019, 109, 107573. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Zhang, Z.; Li, M.; Song, W.; Li, W.; Miao, Z. Circularly Polarized Blue Fluorescence Based on Chiral Heteroleptic Six-Coordinate Bis-Pyrazolonate-Zn2+ Complexes. Dalton Trans. 2024, 53, 6625–6630. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Liu, L.; Li, W.; He, Y.; Miao, T.; Lü, X.; He, H. Efficient White Polymer Light-Emitting Diode (WPLED) Based on Single-Component Eu3+-Tb3+-Containing Metallopolymer. Adv. Opt. Mater. 2019, 7, 1900776. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Wang, B.; Hou, S.; Zhang, Y.; Fu, G.; Lü, X. Straightforward and High-Performance White-Light of an Isostructurally Organo-(Gd3+x, Tb3+y, Sm3+1-x-y)-Ternary Blending Through Typical Trichromatic Integration. Inorg. Chem. Commun. 2022, 145, 110021. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Gong, Y.-J.; Zhang, X.-B.; Mao, G.-J.; Su, L.; Meng, H.-M.; Tan, W.; Feng, S.; Zhang, G. A Unique Approach Toward Near-Infrared Fluorescent Probes for Bioimaging with Remarkably Enhanced Contrast. Chem. Sci. 2016, 7, 2275–2285. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Hay, P.; Wadt, W. An Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
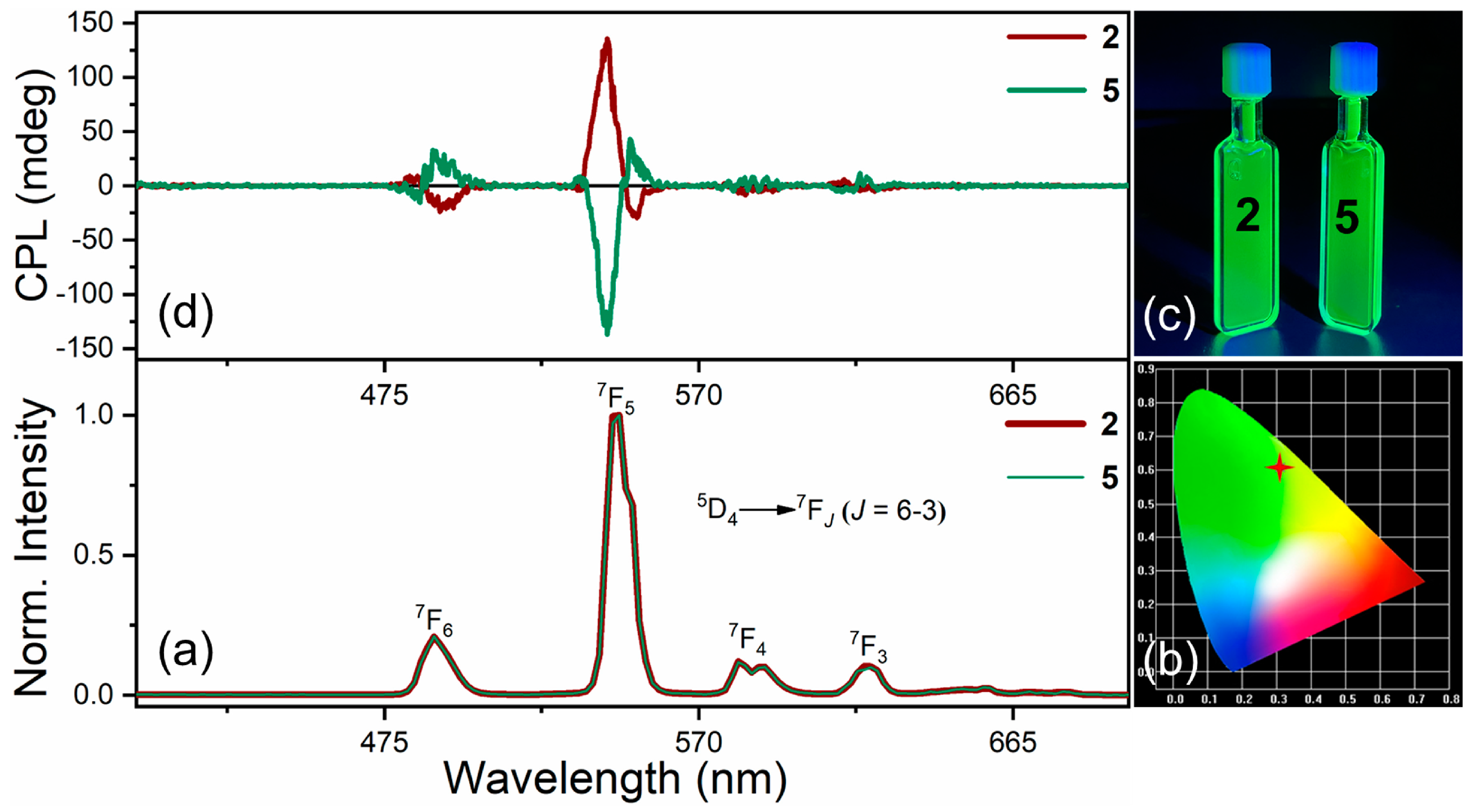

| Compound | λem (nm) | ε263 nm (M−1 cm−1) | ΦPL | Transition | |glum| | βi | BCPL (M−1 cm−1) a |
|---|---|---|---|---|---|---|---|
| 2 | 490 | 38,400 | 47% | 5D4→7F6 | 0.013 | 0.15 | 18 |
| 545 | 5D4→7F5 | 0.103 | 0.67 | 623 | |||
| 5 | 490 | 39,500 | 48% | 5D4→7F6 | 0.015 | 0.15 | 21 |
| 545 | 5D4→7F5 | 0.096 | 0.67 | 610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhang, Y.; Yao, R.; Ren, H.; Wang, W.; Feng, H.; Li, W.; Miao, Z. Enhanced Circularly Polarized Green Luminescence Metrics from New Enantiopure Binary Tris-Pyrazolonate-Tb3+ Complexes. Molecules 2024, 29, 5887. https://doi.org/10.3390/molecules29245887
Liu J, Zhang Y, Yao R, Ren H, Wang W, Feng H, Li W, Miao Z. Enhanced Circularly Polarized Green Luminescence Metrics from New Enantiopure Binary Tris-Pyrazolonate-Tb3+ Complexes. Molecules. 2024; 29(24):5887. https://doi.org/10.3390/molecules29245887
Chicago/Turabian StyleLiu, Jiaxiang, Yongwen Zhang, Ruijuan Yao, Haitao Ren, Weijie Wang, Haohao Feng, Wentao Li, and Zongcheng Miao. 2024. "Enhanced Circularly Polarized Green Luminescence Metrics from New Enantiopure Binary Tris-Pyrazolonate-Tb3+ Complexes" Molecules 29, no. 24: 5887. https://doi.org/10.3390/molecules29245887
APA StyleLiu, J., Zhang, Y., Yao, R., Ren, H., Wang, W., Feng, H., Li, W., & Miao, Z. (2024). Enhanced Circularly Polarized Green Luminescence Metrics from New Enantiopure Binary Tris-Pyrazolonate-Tb3+ Complexes. Molecules, 29(24), 5887. https://doi.org/10.3390/molecules29245887







