Optimization of Naringin Extraction, Synthesis of Dihydrochalcone and Its Effects on Reducing Blood Lipid Levels In Vitro
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Optimum Extraction Conditions of Naringin
2.1.1. Naringin Standard Curve
2.1.2. Single-Factor Experimental Results
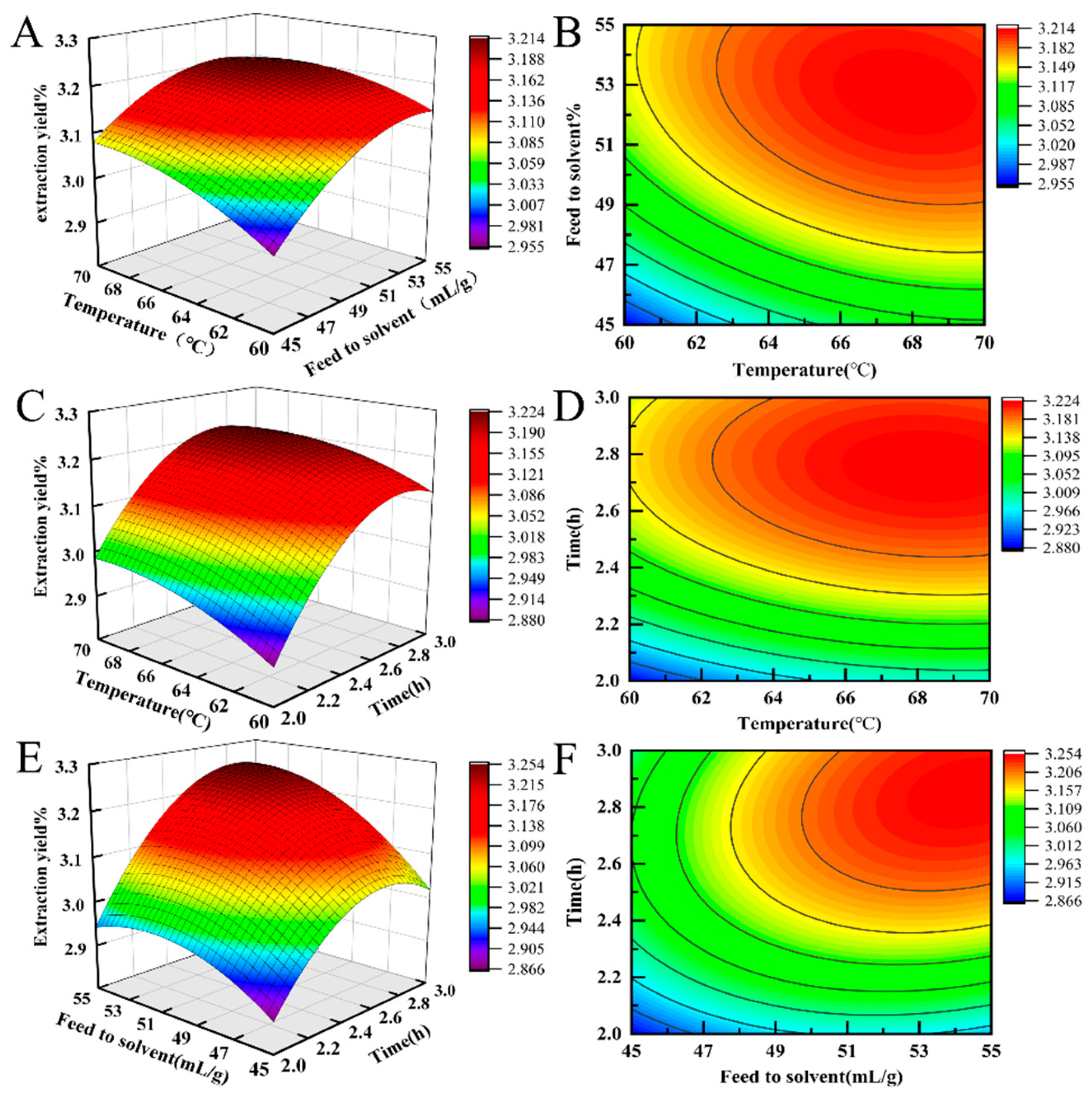
2.1.3. Response Surface Optimization Test Results and Analysis of Variance
2.1.4. Response Surface Graphic Analysis
2.1.5. Determination of Optimal Extraction Conditions
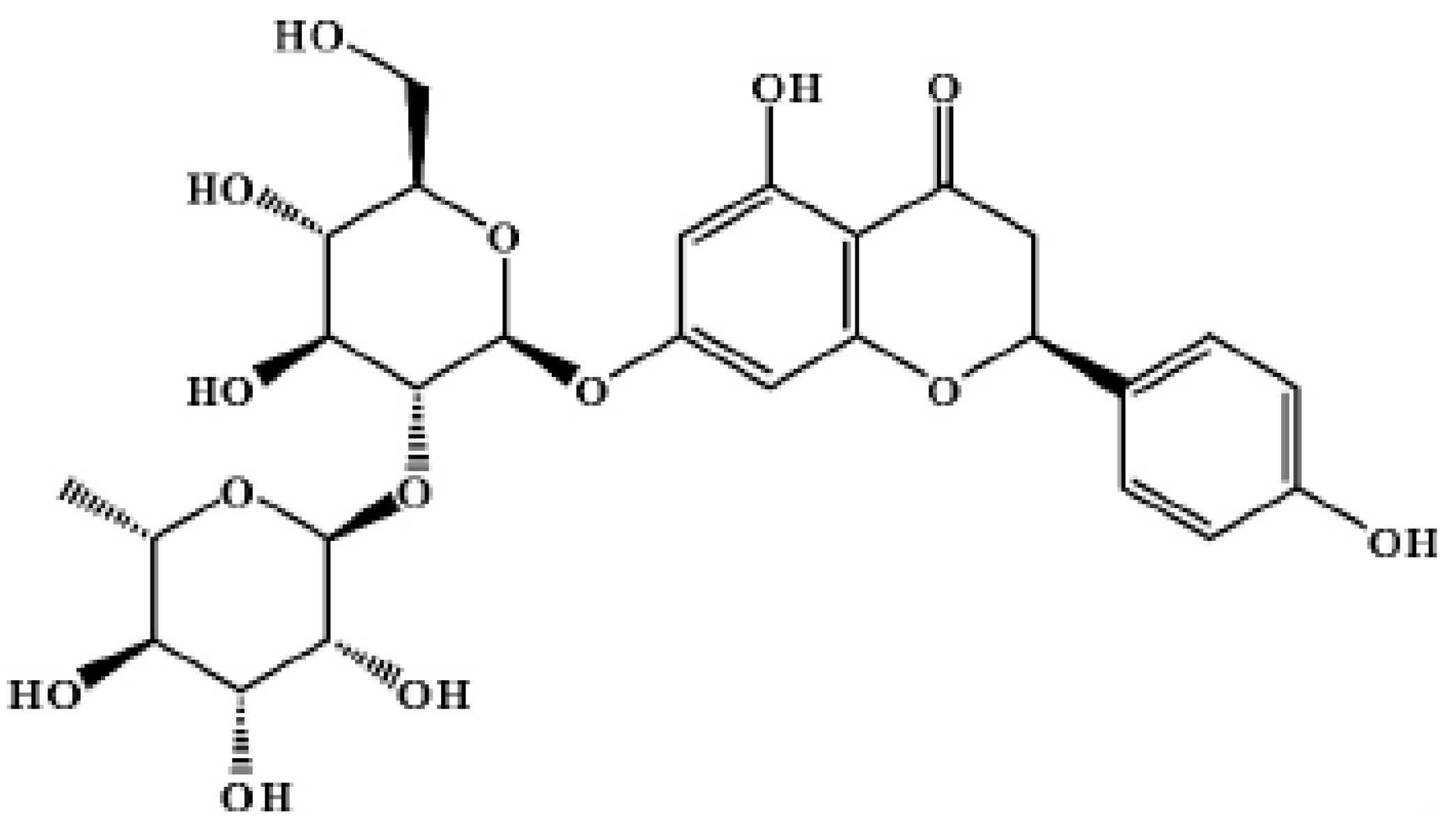
2.2. Structural Analysis of Naringin
2.2.1. IR Spectrum Analysis
2.2.2. NMR Spectrum Analysis
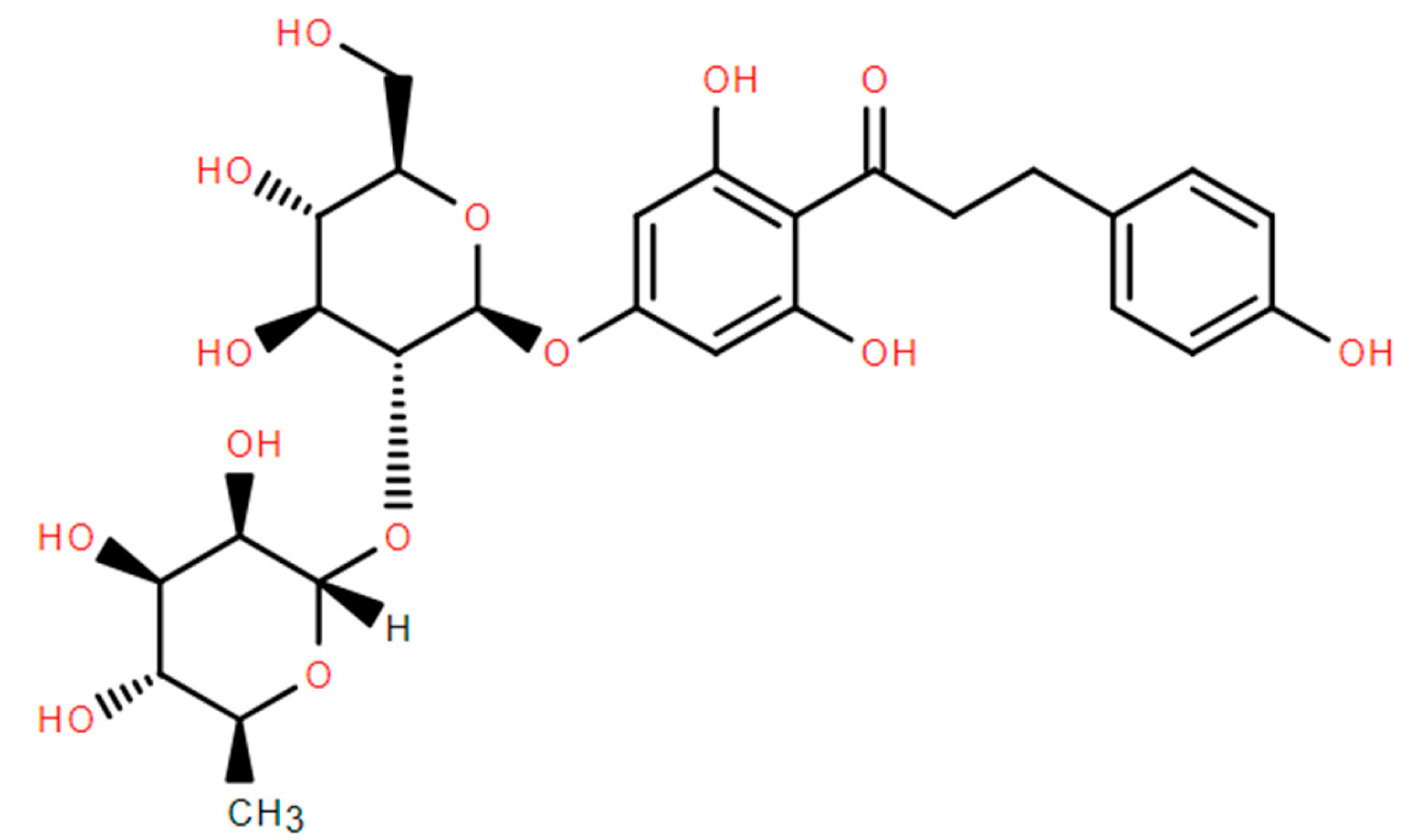
2.3. Structural Analysis of Naringin Dihydrochalcone
2.3.1. IR Spectrum Analysis
2.3.2. NMR Spectrum Analysis
2.4. Cholate Standard Curve
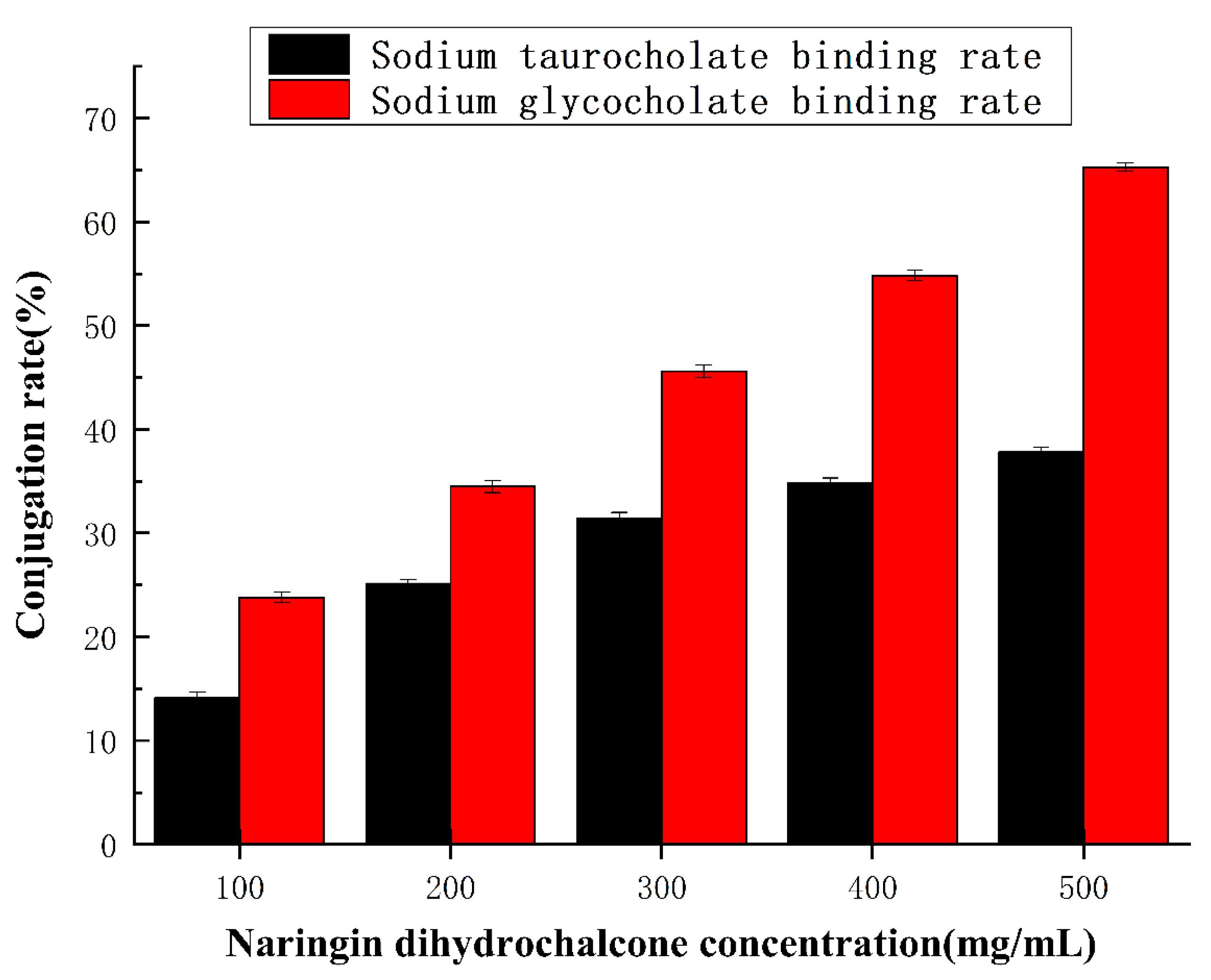
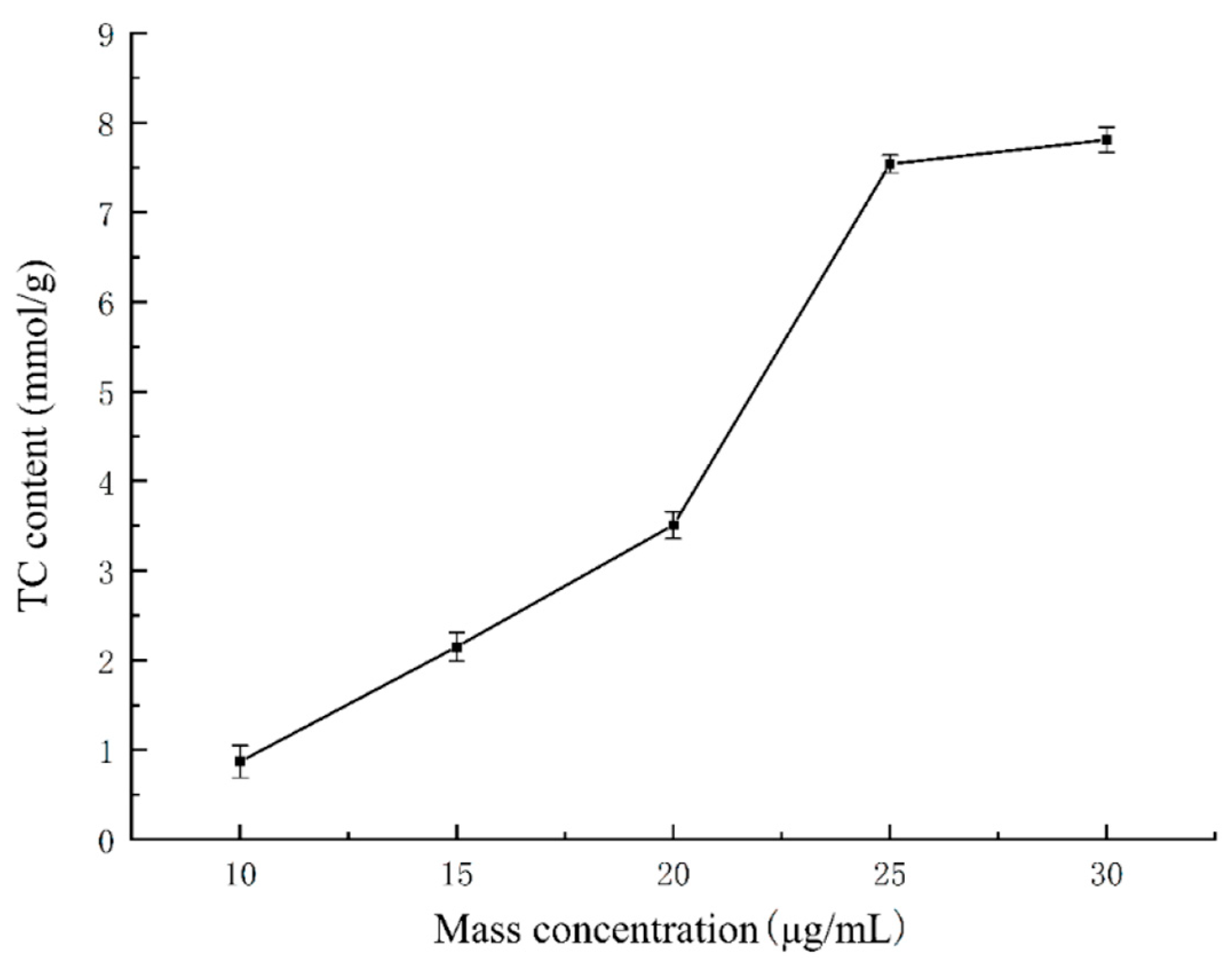
2.5. The Binding Ability of Naringin Dihydrochalcone to Cholate
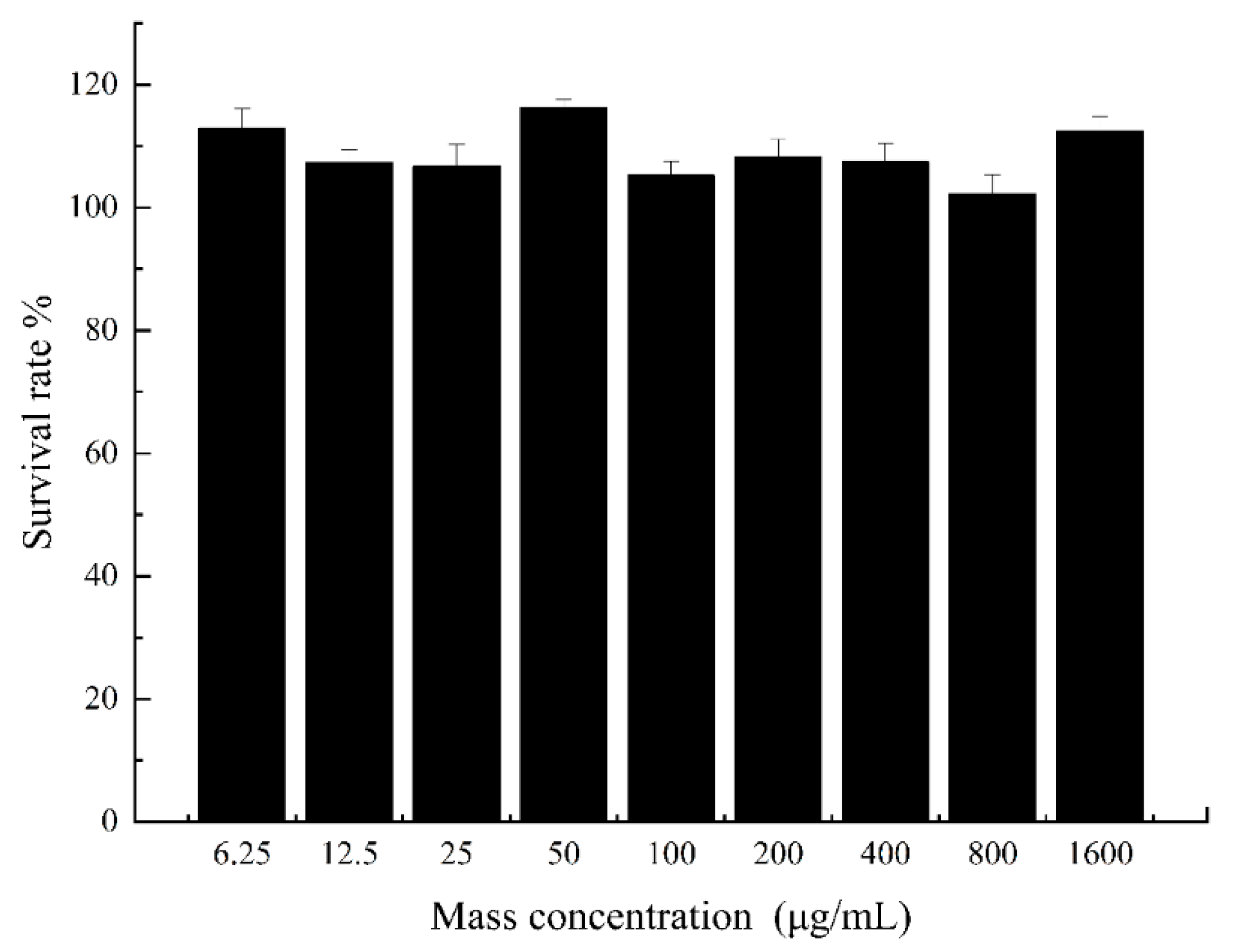
2.6. MTT Experimental Results
2.7. Establishment of the High-Fat Cell Model
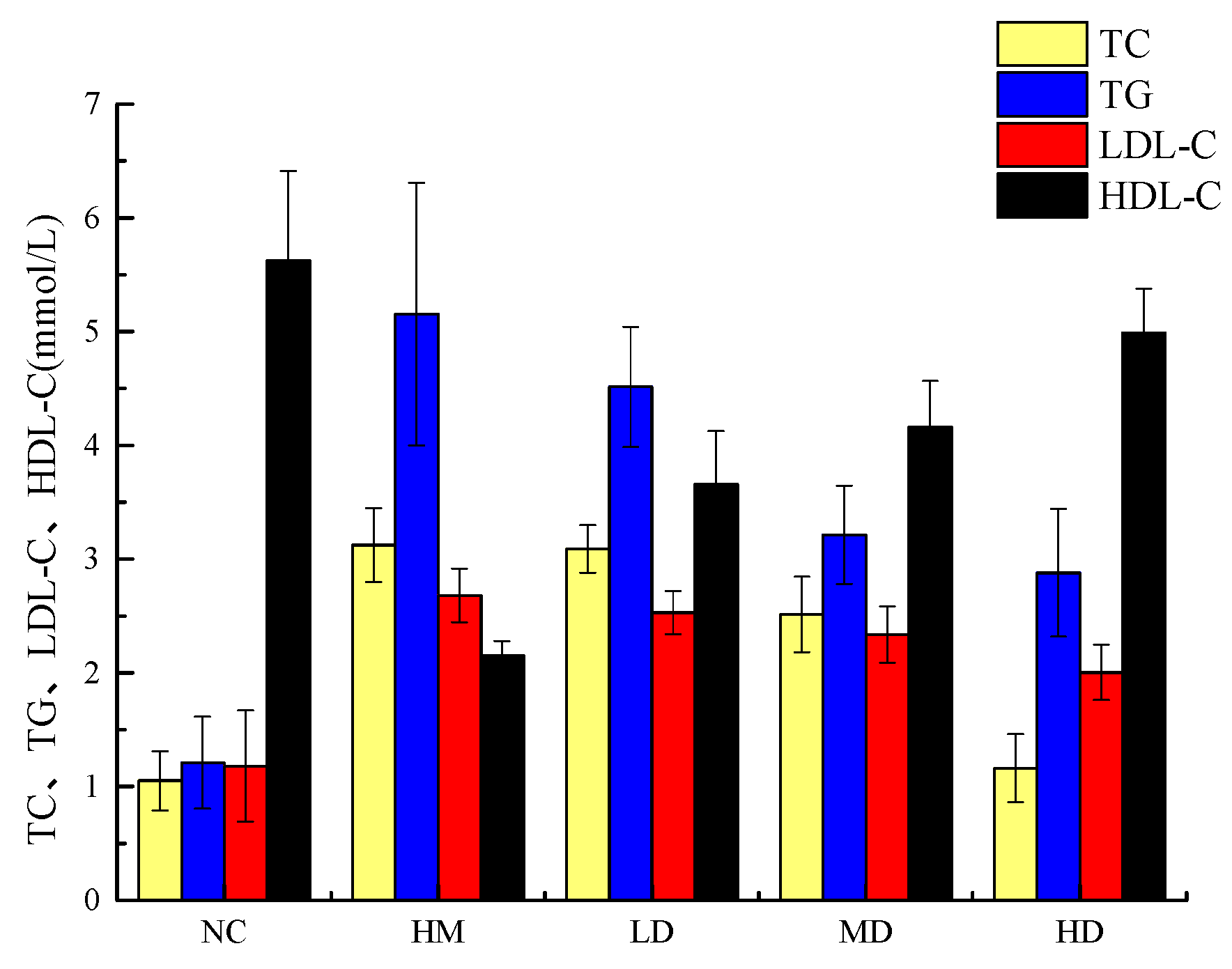
2.8. Effects of Naringin Dihydrochalcone on TC, TG, LDL-C, and HDL-C Levels in HepG2 Cells
3. Materials and Methods
3.1. Materials and Reagents
3.2. Optimization of the Naringin Extraction Process
3.2.1. Pomelo Peel Pretreatment
3.2.2. Establishment of a Naringin Standard Curve
3.2.3. Calculation of Extraction Yield of Naringin
3.2.4. Single-Factor Experimental Design
3.2.5. Response Surface Experiment Design
3.3. Purification of Naringin
3.4. Structural Identification of Naringin
3.5. Preparation of Naringin Dihydrochalcone
3.6. Structural Identification of Naringin Dihydrochalcone
3.7. In Vitro Study of the Effects of Naringin Dihydrochalcone on Lowering Blood Lipid Contents
3.7.1. Drawing the Standard Curve of Cholic Acid Salt
3.7.2. Naringin Dihydrochalcone Binding Cholate Experiment
3.7.3. Determination of Conjugation Rate of Cholic Acid Salts
3.7.4. MTT Cell Proliferation and Toxicity Experiments
3.7.5. High-Cholesterol Cell Model
3.7.6. Effects of Naringin Dihydrochalcone on TG, TC, LDL-C, and HDL-C Levels in HepG2 Cells
3.8. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navar-Boggan, A.M.; Peterson, E.D.; D’Agostino, R.B.; Neely, B.; Sniderman, A.D.; Pencina, M.J. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 2015, 131, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Abudayeh, Z.; Al Khalifa, I.; Mohammed, S.; Ahmad, A. Phytochemical content and antioxidant activities of pomelo peel extract. Pharmacogn. Res. 2019, 11, 244–247. [Google Scholar] [CrossRef]
- Zarzecki, M.S.; Araujo, S.M.; Bortolotto, V.C.; de Paula, M.T.; Jesse, C.R.; Prigol, M. Hypolipidemic action of chrysin on Triton WR-1 339-induced hyperlipidemia in female C57BL/6 mice. Toxicol. Rep. 2014, 1, 200–208. [Google Scholar] [CrossRef]
- Tang, D.M.; Zhu, C.F.; Zhong, S.A.; Zhou, M.D. Extraction of naringin from pomelo peels as dihydrochalcone’s precursor. J. Sep. Sci. 2011, 45, 113–117. [Google Scholar] [CrossRef]
- Peng, Y.; Tong, C.H.; Wang, Y.A. Study on quantitative structure-activity relationship (QSAR) of naringin dihydrochalcone. J. At. Mol. Phys. 2017, 34, 4–37. [Google Scholar] [CrossRef]
- Yu, X.L.; Meng, X.; Yan, Y.D.; Wang, H.; Zhang, L. Extraction of Naringin from Pomelo and Its Therapeutic Potentials Against Hyperlipidemia. Molecules 2022, 27, 9033. [Google Scholar] [CrossRef]
- Dicken, S.J.; Batterham, R.L. The role of diet quality in mediating the association between ultra-processed food intake, obesity and health-related outcomes: A review of prospective cohort studies. Nutrients 2022, 14, 23. [Google Scholar] [CrossRef]
- Lim, S.Y.; Steiner, J.M.; Cridge, H. Lipases: it’s not just pancreatic lipase! Am. J. Vet. Res. 2022, 83, ajvr.22.03.0048. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Lee, S.Y. Long-term efficacy and safety of anti-obesity treatment: Where do we stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Li, R.; Xue, Z.; Jia, Y.; Wang, Y.; Li, S.; Zhou, J.; Liu, J.; Zhang, M.; He, C.; Chen, H. Polysaccharides from mulberry (Morus alba L.) leaf prevents obesity by inhibiting pancreatic lipase in high-fat diet induced mice. Int. J. Biol. Macromol. 2021, 192, 452–460. [Google Scholar] [CrossRef]
- Patel, T.P.; Rawal, K.; Soni, S.; Gupta, S. Swertiamarin ameliorates oleic acid induced lipid accumulation and oxidative stress by attenuating gluconeogenesis and lipogenesis in hepatic steatosis. Biomed. Pharmacother. 2016, 83, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Cossignani, L. An Overview of Natural Extracts with Antioxidant Activity for the Improvement of the Oxidative Stability and Shelf Life of Edible Oils. Processes 2020, 8, 956. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Extraction characterization and biological activity of citrus flavonoids. Rev. Chem. Eng. 2018, 35, 6–17. [Google Scholar] [CrossRef]
- Chen, L.; Long, R.; Huang, G.; Huang, H. Extraction and Antioxidant Activities in Vivo of Pumpkin Polysaccharide. Ind. Crops Prod. 2020, 146, 112199. [Google Scholar] [CrossRef]
- Oziembłowski, M.; Nawirska-Olszańska, A.; Maksimowski, D. Optimization of Chlorogenic Acid in Ethanol Extracts from Elderberry Flowers (Sambucus nigra L.) under Different Conditions: Response Surface Methodology. Appl. Sci. 2023, 13, 3201. [Google Scholar] [CrossRef]
- Ioannou, I.; M’hiri, N.; Chaaban, H.; Boudhrioua, N.M.; Ghoul, M. Effect of the process, temperature, light and oxygen on naringin extraction and the evolution of its antioxidant activity. Int. J. Food Sci. Technol. 2018, 53, 2754–2760. [Google Scholar] [CrossRef]
- Putranto, A.W.; Dewi, S.R.; Puspitasari, Y.; A Nuriah, F. Optimization of free radical scavenging capacity and pH of Hylocereus polyrhizus peel by Response Surface Methodology. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 1–8. [Google Scholar] [CrossRef]
- Naringin (10236-47-2) IR1. Available online: https://www.chemicalbook.com/Spectrum_10236-47-2_IR1.htm (accessed on 1 January 2022).
- Song, S.; Zhou, H.; Lan, X.; Yuan, X.; Li, Y.; Huang, S.; Wang, Z.; Zhang, J. Comprehensive characterization of narirutin metabolites in vitro and in vivo based on Analogous-Core recursion analysis strategy using UHPLC-Q-Exactive Orbitrap MS/MS. Arab. J. Chem. 2023, 16, 104949. [Google Scholar] [CrossRef]
- Krivdin, L.B. Structural applications of the isotropic liquid-phase deuterium nuclear magnetic resonance. Magn. Reson. Chem. 2023, 61, 451–472. [Google Scholar] [CrossRef]
- Naringin Dihydrochalcone (18916-17-1). Available online: http://www.ichemistry.cn/chemistry/18916-17-1.htm (accessed on 1 January 2022).
- Torcello-Gómez, A.; Fraguas, C.F.; Ridout, M.J.; Woodward, N.C.; Wilde, P.J.; Foster, T.J. Effect of substituent pattern and molecular weight of cellulose ethers on interactions with different bile salts. Food Funct. 2015, 6, 730–739. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; Lin, L. Effects of extraction methods on structural characteristics and bile acid-binding capacities of Moringa oleifera leaf polysaccharide fractions. Int. J. Food Sci. Technol. 2020, 55, 1539–1546. [Google Scholar] [CrossRef]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.-X.; Hong, J.-L.; Shi, F.-F.; Liu, G.-L.; Qian, M.; Bai, W.-D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Z.; Song, Y.; Liu, Y.; Zhao, H.; Liu, Y.; Zhang, T.; Yuan, Y.; Cai, X.; Wang, S.; et al. Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin. Chim Acta 2019, 495, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.K.; Madan, P.; Lin, S.S. Elucidation of intestinal absorption mechanism of carvedilol-loaded solid lipid nanoparticles using Caco-2 cell line as an in-vitro model. Pharm. Dev. Technol. 2015, 20, 877–885. [Google Scholar] [CrossRef]
- Kuang, Z.M. Effect of combined antihypertensive and lipid-lowering therapies on cognitive function: A new treatment strategy? Cardiol. Res. Pract. 2020, 2020, 1484357. [Google Scholar] [CrossRef]
- Berger, E.; Géloën, A. Adipocytes as lipid sensors of oleic acid transport through a functional Caco-2/HT29-MTX intestinal barrier. Adipocyte 2019, 8, 83–97. [Google Scholar] [CrossRef]
- Li, T.; Gong, H.; Zhan, B.; Mao, X. Chitosan oligosaccharide attenuates hepatic steatosis in HepG2 cells via the activation of AMP-activated protein kinase. J. Food Biochem. 2022, 46, e14045. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.W.; Liu, Y.F. Effects of epoxy stearic acid on lipid metabolism in HepG2 cells. J. Food Sci. 2020, 85, 3644–3652. [Google Scholar] [CrossRef]
- Song, J.; Qiu, H.; Du, P.; Mou, F.; Nie, Z.; Zheng, Y.; Wang, M. Polyphenols extracted from Shanxi-aged vinegar exert hypolipidemic effects on OA-induced HepG2 cells via the PPARα-LXRα-ABCA1 pathway. J. Food Biochem. 2022, 46, e14029. [Google Scholar] [CrossRef]
- Huang, Y.; Tocmo, R.; Nauman, M.C.; Haughan, M.A.; Johnson, J.J. Defining the cholesterol lowering mechanism of bergamot (Citrus bergamia) extract in HepG2 and Caco-2 cells. Nutrients 2021, 13, 3156. [Google Scholar] [CrossRef]
- Rai, S.; Bhatnagar, S. Hyperlipidemia, disease associ0ations, and top 10 potential drug targets: A network view. Omics 2016, 20, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zeng, J.; Tian, X.; Gao, H.; Sukmanov, V. Effect of ultrafine grinding technology combined with high-pressure, microwave and high-temperature cooking technology on the physicochemical properties of bean dregs. LWT-Food Sci. Technol. 2022, 154, 112810. [Google Scholar] [CrossRef]
- Dong, Y.; Qi, Y.; Liu, M.; Song, X.; Zhang, C.; Jiao, X.; Wang, W.; Zhang, J.; Jia, L. Antioxidant, anti-hyperlipidemia and hepatic protection of enzyme-assisted Morchella esculenta polysaccharide. Int. J. Biol. Macromol. 2018, 120, 1490–1499. [Google Scholar] [CrossRef]
- Norouzian, D.; Hosseinzadeh, A.; Inanlou, D.N.; Moazami, N. Production and partial purification of naringinase by Penicillium decumbens PTCC 5248. World J. Microbiol. Biotechnol. 2000, 16, 471–473. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt fruit attenuates hyperglycemia and hyperlipidemia and regulates colon microbiota in diabetic db/db mice. J. Agric. Food Chem. 2020, 68, 147–159. [Google Scholar] [CrossRef]
- Lee, H.S.; Heo, C.U.; Song, Y.H. Naringin promotes fat browning mediated by UCP1 activation via the AMPK signaling pathway in 3T3-L1 adipocytes. Arch. Pharmacal Res. 2023, 46, 192–205. [Google Scholar] [CrossRef]
- Cameron, R.G.; Chau, H.K.; Manthey, J.A. Continuous process for enhanced release and recovery of pectic hydrocolloids and phenolics from citrus biomass. J. Chem. Technol. Biotechnol. 2016, 91, 2597–2606. [Google Scholar] [CrossRef]
- Cameron, R.G.; Chau, H.K.; Hotchkiss, A.T. Release and recovery of pectic hydrocolloids and phenolics from culled citrus fruits. Food Hydrocoll. 2017, 72, 52–61. [Google Scholar] [CrossRef]
- Kenotron, S.; Surachai, P.; Supason, W. An efficient method for the largescale isolation of naringin from pomelo (Citrus grandis) peel. Int. J. Food Sci. Tecnol. 2009, 44, 1737–1742. [Google Scholar] [CrossRef]
- Wen, L.Z.; Zhang, D.; Rai, D.W.; Sun, B.K. Ultrasound-assisted extraction (UAE) of bioactive compounds from coffee silverskin: Impact on phenolic content, antioxidant activity, and morphological characteristics. J. Food Process. Eng. 2019, 42, 13191. [Google Scholar] [CrossRef]
- Zhou, X.R. The chemical properties of Lycium barbarum polysaccharide extracted by different methods and its effect on exercise fatigue. Genom. Appl. Biol. 2019, 38, 5352–5356. [Google Scholar]
- Yu, X.L.; Meng, X.; Yan, Y.D.; Han, J.C.; Li, J.S.; Wang, H.; Zhang, L. Optimisation of the Extraction Process of Naringin and Its Effect on Reducing Blood Lipid Levels in vitro. Molecules 2023, 28, 1788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, L.; Yuan, L. Conformation-dependent coordination of carboxylic acids with Fe3O4 nanoparticles studied by ATR- FTIR spectral deconvolution. Langmuir 2019, 35, 5770–5778. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Wang, L.B.; Zonderman, J. Automated, high-throughput infrared spectroscopy for secondary structure analysis of protein biopharmaceuticals. J. Pharm. Sci. 2020, 109, 3223–3230. [Google Scholar] [CrossRef]
- Shi, H.; Sun, J.; Han, R. The strategy for correcting interference from water in Fourier transform infrared spectrum based bacterial typing. Talanta 2020, 208, 120347. [Google Scholar] [CrossRef]
- Ackermann, S.M.; Dolsophon, K.; Monakhova, Y.B. Automated multicomponent analysis of soft drinks using 1D1H and 2D1H–1H J-resolved NMR spectroscopy. Food Anal. Methods 2017, 10, 827–836. [Google Scholar] [CrossRef]
- Birgit, L.D.; Daan, W.K.; Katarzyna, J.G. Acombined rheology and time domain NMR approach for determining water dis-tributions in protein blends. Food Hydrocoll. 2016, 60, 525–532. [Google Scholar] [CrossRef]
- Ouyang, Z.Y. Extraction of Some Kind of Effective Ingredients in Pomelo Peel and Synthesis of Dihydrochalcone; Nanchang University: Nanchang, China, 2008. [Google Scholar]
- Chen, L.; Liu, C.B.; Zhang, Q. Research progress of application of neohesperidin dihydrochalcone. Beverage Ind. 2016, 19, 53–56. [Google Scholar] [CrossRef]
- Rjeibi, I.; Feriani, A.; Hentati, F. Structural characterization of water-soluble polysaccharides from Nitraria retusa fruits and their antioxidant and hypolipidemic activities. Int. J. Biol. Macromol. 2019, 129, 422–432. [Google Scholar] [CrossRef]
- Ren, D.; Wang, Q.; Yang, Y. Hypolipidemic effects of fucoidan fractions from Saccharina sculpera. Int. J. Biol. Macromol. 2019, 140, 188–195. [Google Scholar] [CrossRef]
- Gong, S.J.; Teng, C.Q.; Liang, D.Y. In vitro study on hypolipidemic effects of theabrownins in Liupao tea. J. Tea Sci. 2020, 40, 536–543. [Google Scholar] [CrossRef]
- Tim, M. Rapid colorimetric assay for cellular growth and survival application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Lee, M.F.; Lai, C.S.; Cheng, A.C. Krill oil and xanthigen separately inhibit high fat diet induced obesity and hepatic triacylglycerol accumulation in mice. J. Funct. Foods 2015, 19, 913–921. [Google Scholar] [CrossRef]
| Test Number | X1 (Extraction Temperature) | X2 (Feed to Solvent Ratio) | X3 (Extraction Time) | Extraction Efficiency % |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 3.171 |
| 2 | 0 | −1 | 1 | 3.015 |
| 3 | −1 | −1 | 0 | 2.945 |
| 4 | 0 | 0 | 0 | 3.174 |
| 5 | −1 | 0 | 1 | 3.137 |
| 6 | 0 | 0 | 0 | 3.191 |
| 7 | −1 | 1 | 0 | 3.130 |
| 8 | 0 | −1 | −1 | 2.870 |
| 9 | 1 | 0 | 1 | 3.186 |
| 10 | −1 | 0 | −1 | 2.888 |
| 11 | 1 | 1 | 0 | 3.202 |
| 12 | 1 | −1 | 0 | 3.090 |
| 13 | 0 | 0 | 0 | 3.188 |
| 14 | 0 | 0 | 0 | 3.177 |
| 15 | 1 | 0 | −1 | 2.972 |
| 16 | 0 | 1 | −1 | 2.945 |
| 17 | 0 | 1 | 1 | 3.241 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | f Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 23.89 | 9 | 2.65 | 148.02 | <0.0001 | ** |
| X1 | 1.53 | 1 | 1.53 | 85.39 | <0.0001 | ** |
| X2 | 4.47 | 1 | 4.47 | 249.27 | <0.0001 | ** |
| X3 | 10.22 | 1 | 10.22 | 569.64 | <0.0001 | ** |
| X1 X2 | 0.13 | 1 | 0.13 | 7.43 | 0.0295 | * |
| X1 X3 | 0.031 | 1 | 0.031 | 1.71 | 0.2326 | |
| X2 X3 | 0.57 | 1 | 0.57 | 31.79 | 0.0008 | ** |
| X12 | 0.38 | 1 | 0.38 | 21.45 | 0.0024 | ** |
| X22 | 1.43 | 1 | 1.43 | 79.60 | <0.0001 | ** |
| X32 | 4.57 | 1 | 4.57 | 255.05 | <0.0001 | ** |
| residual | 0.13 | 7 | 0.018 | |||
| Spurious term | 0.094 | 3 | 0.031 | 4.05 | 0.1050 | |
| Error term | 0.031 | 4 | 0.00777 | |||
| the sum | 24.02 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Wu, H.; Zhang, L.; Fei, D. Optimization of Naringin Extraction, Synthesis of Dihydrochalcone and Its Effects on Reducing Blood Lipid Levels In Vitro. Molecules 2024, 29, 5778. https://doi.org/10.3390/molecules29235778
Yu X, Wu H, Zhang L, Fei D. Optimization of Naringin Extraction, Synthesis of Dihydrochalcone and Its Effects on Reducing Blood Lipid Levels In Vitro. Molecules. 2024; 29(23):5778. https://doi.org/10.3390/molecules29235778
Chicago/Turabian StyleYu, Xiaolei, Haowei Wu, Lei Zhang, and Dongliang Fei. 2024. "Optimization of Naringin Extraction, Synthesis of Dihydrochalcone and Its Effects on Reducing Blood Lipid Levels In Vitro" Molecules 29, no. 23: 5778. https://doi.org/10.3390/molecules29235778
APA StyleYu, X., Wu, H., Zhang, L., & Fei, D. (2024). Optimization of Naringin Extraction, Synthesis of Dihydrochalcone and Its Effects on Reducing Blood Lipid Levels In Vitro. Molecules, 29(23), 5778. https://doi.org/10.3390/molecules29235778






