Abstract
The coupling between bis(2-oxazolines) and two equivalents of aromatic aldehydes in the presence of catalytic amounts of NiCl2 affords an ester-imine product in synthetically useful yields. This virtually unknown, 100% atom-economic transformation involves the formal metathesis between the C=N double bond of the bis(2-oxazoline) moiety, which undergoes ring-opening, and the C=O double bond of the aldehyde. The scope of this transformation is studied, and a mechanism is proposed based on DFT calculations.
1. Introduction
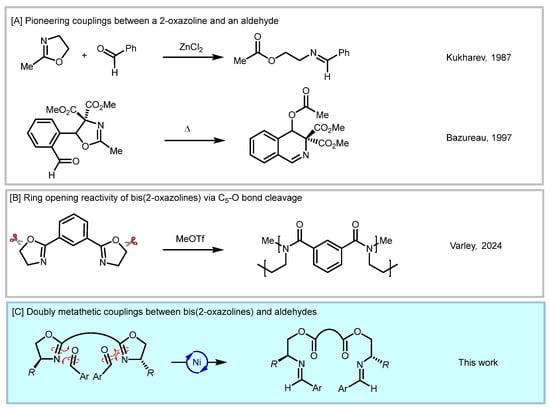
The oxazolinyl scaffold is present in several compounds that find important applications in both pharmaceuticals and innovative materials [1,2,3]. 2-Oxazolines are also efficient protecting groups and versatile ligands in transition metal asymmetric catalysis, providing a valuable alternative to the easily oxidizable phosphine ligands [4,5,6], especially as bis(2-oxazolines) [7]. Additionally, the 2-oxazolyl moiety is the precursor of several functional groups, such as carboxamides, N-(2-aminoethyl)acylamides, 2-aminoethyl acetates, β-aminoaldehydes and isoquinolone derivatives [8,9,10,11,12,13,14,15].
In particular, the coupling between a bis(2-oxazoline) and an aldehyde has been briefly reported only in two articles dating back about 30 years (Scheme 1A) [16,17,18,19]. In these papers, the reaction conditions were only superficially detailed, with no systematic investigation. This 100% atom-economic transformation involves the formal metathesis between the C=N double bond of the oxazoline moiety (which undergoes ring-opening) and the C=O double bond of the aldehyde, generating an ester-imine product. Given the interest of our group in the chemistry of nitrogen-containing five-membered heterocycles [20,21,22], we decided to further investigate this intriguing coupling, focusing in particular on bis(2-oxazolines), whose ring cleavage chemistry has been mainly addressed at O–C5 bond cleavage (Scheme 1B) [23,24,25]. Accordingly, we present our results on the metathetic coupling between bis(2-oxazolines) and aldehydes in this paper (Scheme 1C).
Scheme 1.
Overview of the reactivity of bis- and mono-oxazolines [16,17,23].
2. Results and Discussion
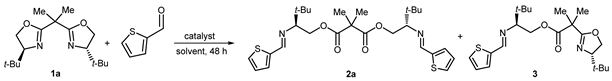
We started our study by selecting the coupling between bis(2-oxazoline) 1a and 2-thiophenecarboxaldehyde as the model reaction (Table 1). Reacting bis(2-oxazoline) 1a (1.0 mmol) with the aldehyde (2.0 mmol) in the presence of NiCl2 (10 mol%) in acetonitrile gave the bis-imino malonate ester 2a in 53% isolated yield after 48 h at room temperature (r.t.) (Entry 1). The use of THF instead of acetonitrile resulted in a significant improvement in the isolated yield of 2a (Entry 2). Not too unexpectedly, when the reaction was performed using equimolar amounts of 1a and aldehyde, a low yield of 2a was obtained, along with the mono-metathesis product 3 (Entry 3). Returning to the original 1:2 stoichiometric ratio, the use of CH2Cl2 or toluene as solvents gave again a mixture of 2a and 3 (Entries 4 and 5). Heating the reaction at the reflux of THF resulted in a reduced yield of product 2a (Entry 6). The reaction failed when NiCl2 was replaced by Ni(acac)2 and was less efficient when Ni(OTf)2 was used (Entries 7 and 8). After examining the behavior of nickel salts, other Lewis acids were evaluated as catalysts. Using PdCl2 or ZnCl2 instead of NiCl2 gave no reaction at room temperature and only intractable products when the reaction was heated (Entries 9–12). Cu(OTf)2 was also tested as a possible catalyst, given its ability to smoothly release triflic acid into the reaction medium [26,27,28,29,30]. However, its use only included a mixture of tarry products (Entry 13). Conversely, the reaction proceeded in the presence of TMSOTf, providing the ester-imine 2a in 45% yield (Entry 14). Finally, a control experiment was carried out without any catalyst and only the unreacted substrates were restituted, thus confirming the need for a catalyst (Entry 15).

Table 1.
Optimization of the reaction conditions a.
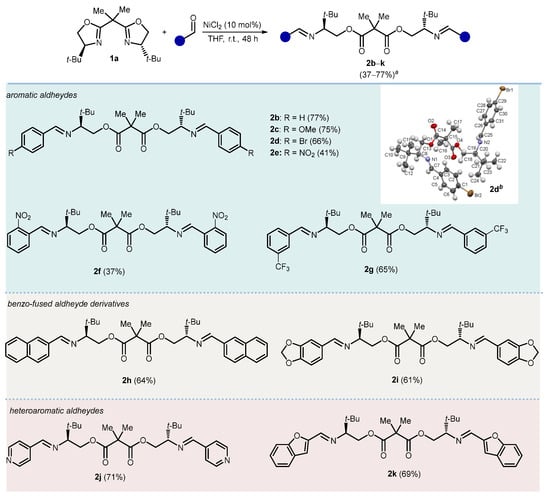
With the optimal conditions in hand, the substrate scope was investigated in couplings between bis(2-oxazoline) 1a and various aldehydes (Scheme 2). Under the reaction conditions from Table 1, Entry 2 (i.e., NiCl2 10 mol% in THF at r.t.), benzaldehydes bearing electron-withdrawing and electron-donating groups, as well as 2-naphthyl, piperonal, 4-pyridyl, and 2-benzofuryl-aldehydes, successfully triggered the opening of both oxazolinyl rings, leading to the formation of the corresponding ester-imines 2b–k, which were isolated in moderate to satisfactory yields. The structure of the products was further confirmed by single-crystal X-ray diffraction analysis of the brominated product 2d. The reactions carried out with cinnamaldehyde and 2-pyridinecarboxaldehyde also resulted in the complete conversion of the substrate to the ester-imines, as evidenced by the NMR spectra of the crude reaction mixtures. Unfortunately, in these two cases, it was not possible to isolate and fully characterize the products due to their instability.
Scheme 2.
Synthesis of ester-imines from bis(2-oxazoline) 1a. Reaction conditions: 1a (1.0 mmol), aldehyde (2.0 mmol), NiCl2 (10 mol%), THF (0.1 M), r.t., 48 h. a Isolated yieldsIsolated yields. b CCDC 2385941 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
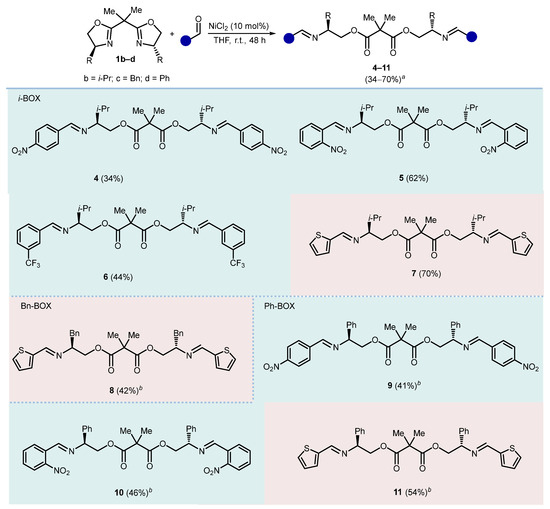
Differently substituted bis(2-oxazolines) were then examined in reactions with representative electron-poor or electron-rich aromatic aldehydes (Scheme 3). Accordingly, treatment of isopropyl, benzyl, and phenyl bis(2-oxazoline) (1b–d) with 3-nitro-, 4-nitro-, 3-trifluoromethyl benzaldehydes or 2-thiophenecarboxaldehyde under the standard conditions gave the corresponding bis(ester-imines) 4–11 with comparable efficiency. The bis(ester-imines) derived from benzyl bis(2-oxazoline) 1c were found quite difficult to purify. In fact, compound 8 was isolated in 42% yield, whereas the products arising from reactions with 2-nitro and 3-trifluoromethyl benzaldehydes could not be isolated in a pure state for characterization (probably for the hydrolysis of the imine).
Scheme 3.
Reactivity of different bis(2-oxazolines) with different aldehydes. Reaction conditions: 1b or 1c (1.0 mmol), aldehyde (2.0 mmol), NiCl2 (10 mol%), THF (0.1 M), and r.t., 48 h. a Isolated yields. b Reaction time 72 h.
After studying bis(2-oxazolines) joined through a propane-2,2-diyl moiety, we evaluated the behavior of 2,2′-bis(2-oxazolines) and of those bearing the two 2-oxazoline moieties either 1,3-linked to a phenyl ring or 2,6-linked to a pyridine ring (Scheme 4). While the former substrates (1e,f) were prone to degradation, 1g and 1h underwent the expected metathetic ring-opening to give the corresponding ester-imines 12 and 13 in acceptable yields.
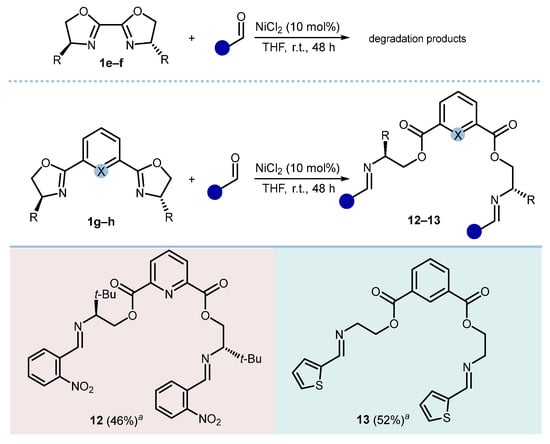
Scheme 4.
Reactivity of different bis(2-oxazolines) with different aldehydes. Reaction conditions: 1g or 1h (1.0 mmol), aldehyde (2.0 mmol), NiCl2 (10 mol%), THF (0.1 M), and r.t., 48 h. X = C, N. a Isolated yields.
The investigation continued with the evaluation of some simple 2-oxazolines under the standard conditions. These compounds exhibited different behavior compared to bis(2-oxazolines). In fact, treatment of (S)-4-isopropyl-2-phenyl-oxazoline 14 with 2-thiophenecarboxaldehyde under the standard reaction conditions produced a complex mixture of products after 48 h. From the mixture, amide 15 was isolated in 22% yield along with traces of the expected ester-imine 16 (Scheme 5). Since amide 15 is most likely the result of a C5-O cleavage of the 2-oxazolinyl moiety by the nucleophile water, it appears that passing from 2-oxazolines to bis(2-oxazolines) is associated with a reactivity shift from substitution at C5 (C5-O cleavage) to addition at C2 (metathesis). The unsuitability of mono-oxazolines to undergo the metathetic ring-opening was also observed with 2-phenyl or thienyl, 4-benzyl or isobutyl substituted substrates. Interestingly, treatment of 15 with 2-thiophenecarboxaldehyde in the presence of TMSOTf (1.0 equiv.) gave the ester-imine 16, plausibly via an N→O benzoyl transfer followed by imine formation (Scheme 5).
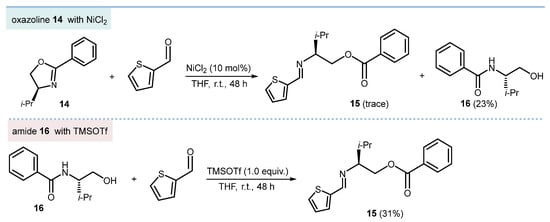
Scheme 5.
Insight into the behavior of mono-oxazolines.
To gain insights into the mechanism of this transformation, the model reaction between bis(2-oxazoline) 1a and benzaldehyde was studied via DFT calculations. The geometries of reactants, products, and intermediates were optimized at the WB97XD/6-31+G(d,p) level, followed by single-point energy calculations using the WB97XD functional with the Def2TZVP basis set and CPCM(THF) solvation model (see Figures S1 and S2 and Tables S1–S3, Supplementary Materials, for details). The focus was the geometries of the intermediates—particularly the Ni chelation sites—as well as the energies. As this preliminary study was not focused on the kinetics, the transition structures were not modeled. To start the modeling with reliable assumptions, the following points were considered: (a) the presence of NiCl2 is crucial for the success of the reaction; (b) although water is not involved in the global transformation, its presence is essential for the success of the reaction; (c) in contrast to bis(2-oxazolines), simple 2-oxazolines do not undergo equally well this transformation. Therefore, a chelating effect of the metal by the bis(2-oxazoline) is expected; (d) NiCl2·(4H2O) I is assumed to be the most likely starting catalyst.
Both singlet and triplet configurations were considered for optimizing each Ni-containing complex. However, singlet configurations consistently resulted in high-energy geometries or convergence failures. Starting from the reactants, put at ΔH = 0 kcal/mol (Figure 1), the exothermic (δΔH = −10 kcal/mol) interaction between NiCl2·(4H2O) I and bis(2-oxazoline) 1a generates complex II with the expulsion of two molecules of water. The metal in II maintains hexacoordination, adopting a square bipyramidal configuration. The two chlorine atoms are apically coordinated, while the equatorial sites host the four dative ligands: two oxazolyl nitrogen atoms and two water molecules. At this point, an external water molecule adds to the C2 of one oxazolyl unit, generating the 2-hydroxy oxazolidine complex III. Unexpectedly, this complex adopts a distorted trigonal bipyramidal geometry. This switch from hexa- to penta-coordination is likely caused by steric hindrance of the tert-butyl groups, which prevent the coordination of a second molecule of water (a hexacoordinated geometry of III was found but was 7.5 kcal/mol less stable; see Figure S2A,B and Table S2, Supplementary Materials). Then, in a slightly endothermic step (+3 kcal/mol), the 2-hydroxy oxazolidine III opens up, generating the ester-primary amine IV. In this step, the externally appended molecule of water can reenter the nickel coordination sphere, thereby restoring the hexavalent coordination at the metal. At this point, the primary amine and benzaldehyde each coordinate with a Ni site, with the expulsion of two water molecules. This step is exothermic (δΔH = −3.6 kcal/mol), and the resulting primary amine V is the most stable intermediate along the pathway. After a likely equilibration with the decoordinated free amine (δΔH = +2.5 kcal/mol; see Figure S2C, Supplementary Materials), intramolecular attack of the amine to the coordinated benzaldehyde leads to the hemiaminal VI, with an enthalpy increase of 8.1 kcal/mol respect to V. This step liberates a coordination site at the metal, which can be occupied by an incoming water molecule. Interestingly, one H atom of the coordinated water is involved in an intramolecular H–bond with the hemiaminal oxygen atom (O···HO distance = 1.98 Å; O···H-O angle = 122.9 deg). Subsequent exothermal dehydration of VI generates the imine VII (δΔH = −2.7 kcal/mol). In this intermediate, the coordinated hemiaminal hydroxyl oxygen atom becomes part of the newly formed coordinated water molecule, which is also involved in an intramolecular H–bond with the imino nitrogen (N···HO distance = 1.74 Å; N···H-O angle = 161.2 deg). The same sequence of steps as before is then expected to transform the second oxazolinyl unit, generating Ni-coordinated product VIII (not computed). Finally, the entrance of two molecules of water generates the bis(imine-ester) product 2b, closing the catalytic cycle. The enthalpy change from the starting materials to the product is significant (ΔH = −30.7 kcal/mol) and well supports the product formation.
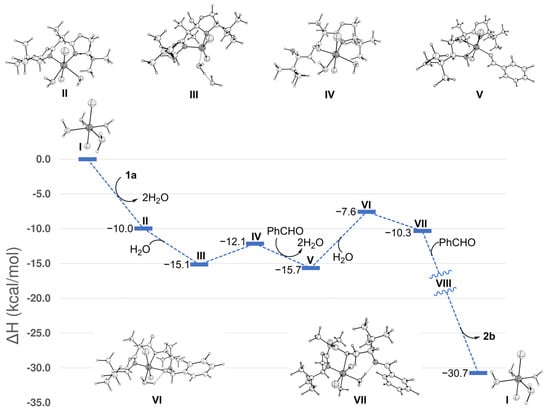
Figure 1.
Enthalpy profile and optimized geometries of reactants, intermediates, and products along the reaction coordinate, modeled using DFT calculations at the WB97XD/Def2TZVP/CPCM(THF)//WB97XD/6-31+G(d,p) level. The reference energy corresponds to the sum of the energies of bis(2-oxazoline) 1a, NiCl2·4H2O I, and two equivalents of benzaldehyde. The energies of intermediates and products were obtained by summing the energy of the main structure with that of water or benzaldehyde, ensuring the correct stoichiometry at each step.
Scheme 6 summarizes the overall mechanism of this doubly metathetic NiCl2-catalyzed coupling for the model reaction, as deduced from the above computations.
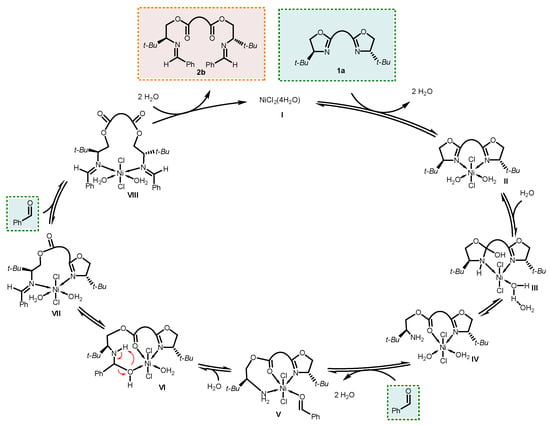
Scheme 6.
Proposed mechanism for the doubly metathetic NiCl2-catalyzed coupling between bis(2-oxazoline) 1a and benzaldehyde.
3. Materials and Methods
3.1. General Information
All available chemicals and solvents were purchased from commercial sources and were used without further purification. Thin-layer chromatography (TLC) was performed using 0.25 mm silica gel precoated plates Si 60-F254 from Merck (Darmstadt, Germany), visualized by UV-254 light and CAM staining. Purification by flash column chromatography (FCC) was conducted by using silica gel Si 60, 230–400 mesh, 0.040–0.063 mm from Merck (Darmstadt, Germany). Melting points were determined on a Stuart Scientific SMP3 from Barloworld Scientific Ltd. (Stone, UK) and are corrected. 1H and 13C NMR spectra were recorded on a Bruker Avance 400 (Billerica, MA, USA, 400 and 101 MHz, respectively); chemical shifts are given in parts per million downfield from SiMe4, using the residual proton (CHCl3 = 7.26 ppm) and carbon (CDCl3 = 77.0 ppm) solvent resonances as an internal reference. Coupling constant values J are given in Hz. High-resolution mass spectra (HRMS) were recorded using a mass spectrometer MicroTOF from Bruker (Billerica, MA, USA) with an electron spray ion source (ESI) and a TOF detector or using a mass spectrometer from Thermo Fisher Scientific (Waltham, MA, USA) with an electron spray ion source (ESI) and a LTQ Orbitrap as detector at Università degli Studi dell’Insubria (Como, Italy). FT-IR spectra were recorded on a Tensor 27 (ATR Diamond) from Bruker (Billerica, MA, USA) infrared spectrophotometer and are reported in the frequency of absorption (cm–1). Optical rotations were measured on a JASCO P-2000 polarimeter (Hachioji, Japan) using a 100 mm path-length cell at 589 nm and reported as follows: (c mg/10 mL, solvent). Elemental analyses were executed on a Perkin-Elmer CHN Analyzer Series II 2400 (Waltham, MA, USA).
3.2. General Experimental Procedures
Starting material 14 was prepared according to the literature procedure [31]. The experimental procedure for 14 is detailed in Supplementary Materials.
3.2.1. Experimental Procedure for Compounds 2a–k, 4–13, and 16
To a solution of the appropriate bis(2-oxazoline) (1.0 mmol, 1 equiv.) in THF (0.1 M, 10 mL), NiCl2 (10 mol%, 0.1 mmol) and the aldehyde (2.0 mmol, 2 equiv.) were added and the mixture was stirred at room temperature for 24–48 h.
The mixture was concentrated, and the residue was dissolved in ethyl acetate (10 mL) and washed with water (10 mL). The organic phase was dried over Na2SO4 and the solvent was evaporated. Starting from bis(2-oxazoline) and aldehydes, the yields and physical, spectroscopic and analytical data of compounds 2a–k, 4–13, and 16 are as follows.
Details of the X-ray structural determination of 2d are reported in Supplementary Materials.
3.2.2. Experimental Procedure for Compound 15
To a solution of the amide 16 (190 mg, 1.0 mmol, 1 equiv.) in THF (0.1 M, 10 mL), TMSOTf (180 μL, 1 equiv., 1 mmol) and the aldehyde (100 μL, 1.0 mmol, 1 equiv.) were added and the mixture was stirred at room temperature for 48 h.
The mixture was concentrated, and the residue was dissolved in ethyl acetate (10 mL) and washed with water (10 mL). The organic phase was dried over Na2SO4, and the solvent was evaporated to make compound 15 a yellow oil.
3.3. Characterization Data of Synthesized Compounds
The 1H NMR spectra of known compound 14 is reported in Supplementary Materials.
Characterization Data of Final Compounds 2a–k, 3–13 and 15–16
Bis((S)-3,3-dimethyl-2-((thiophen-2-ylmethylene)amino)tert-butyl) 2,2-dimethylmalonate (2a). Starting from 1a (294 mg) and 2-thiophenecarboxaldehyde (224 mg); FCC-AcOEt/hexane (2:3). 2a (543 mg, 81%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.09 (s, 2H), 7.29 (d, 2H, J = 5.1 Hz), 7.20 (d, 2H, J = 4.6 Hz), 6.97 (t, 2H, J = 5.0 Hz), 4.29 (dd, 2H, J = 2.7, 10.9 Hz), 3.90 (t, 2H, J = 9.9 Hz), 2.85 (dd, 2H, J = 2.8, 9.8 Hz), 1.8 (s, 6H), 0.85 (s, 18 H); 13C NMR (CDCl3, 101 MHz) δ 172.5, 154.3, 142.7, 130.1, 128.8, 127.3, 77.8, 65.3, 49.9, 33.7, 269, 22.8. HRMS (ESI) calcd. for C27H38N2O4S2 [M + H+]: 519.2346, found: 519.2330. = +38.0 (c 0.55 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((benzylidene)amino)tert-butyl) 2,2-dimethylmalonate (2b). Starting from 1a (294 mg) and benzaldehyde (212 mg); FCC-AcOEt/hexane (2:3). 2b (390 mg, 77%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.08 (s, 2H), 7.71–7.60 (m, 4H), 7.41–7.35 (m, 6H), 4.37 (dd, 2H, J = 2.7, 10.8 Hz), 3.97 (t, 2H, J = 9.8 Hz), 2.95 (dd, 2H, J = 2.8, 9.8 Hz), 1.23 (s, 6H), 0.92 (s, 18H); 13C NMR (CDCl3, 101 MHz) δ 172.5, 161.1, 136.3, 130.5, 128.5, 128.2, 78.2, 65.4, 49.9, 33.6, 26.9, 22.8. HRMS (ESI) calcd. for C31H42N2O4 [M + H+]: 507.3218, found: 507.3194. = −23.5 (c 0.55 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((4-methoxybenzylidene)amino)tert-butyl) 2,2-dimethylmalonate (2c). Starting from 1a (294 mg) and 4-methoxybenzaldehyde (272 mg); FCC-AcOEt/hexane (2:3). 2a (425 mg, 75%); 1H NMR (CDCl3, 400 MHz) δ 8.10 (s, 2H), 7.67 (d, 2H, J = 8.8 Hz), 6.90 (d, 2H, J = 8.8 Hz), 4.33 (dd, 2H, J = 2.7, 10.8 Hz), 3.94 (t, 2H, J = 9.9 Hz), 3.83 (s, 6H), 2.90 (dd, 2H, J = 2.8, 9.8 Hz), 1.22 (s, 6H), 0.91 (s, 18 H); 13C NMR (CDCl3, 101 MHz) δ 172.5, 161.5, 1603, 129.9, 129.3, 113.9, 78.1, 65.5, 55.3, 49.9, 33.6, 27.0, 22.8. HRMS (ESI) calcd. for C33H46N2O6 [M + H+]: 567.3429, found: 567.3413. = −43.3 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((4-bromobenzylidene)amino)tert-butyl) 2,2-dimethylmalonate (2d). Starting from 1a (294 mg) and 4-bromobenzaldehyde (370 mg); FCC-AcOEt/hexane (2:3). 2d (438 mg, 66%); white wax. 1H NMR (CDCl3, 400 MHz) δ 7.76 (s, 2H), 7.33 (d, 4H, J = 8.5 Hz), 7.27 (d, 2H, J = 8.4 Hz), 4.05 (dd, 2H, J = 2.7, 10.9 Hz), 3.62 (t, 2H, J = 9.9 Hz), 2.67 (dd, 2H, J = 2.7, 9.8 Hz), 0.96 (s, 6H), 0.65 (s, 18H); 13C NMR (CDCl3, 101 MHz) δ 172.5, 161.5, 160.3, 129.9, 129.3, 113.9, 78.1, 65.5, 55.3, 33.6, 27.0, 22.8. Anal. calcd. for C31H40Br2N2O4: C, 56.04; H, 6.07; Br, 24.05; N, 4.22; O, 9.63. Found: C, 55.98; H, 6.15; Br, 24.32; N, 4.20. = −77.5 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((4-nitrobenzylidene)amino)tert-butyl) 2,2-dimethylmalonate (2e). Starting from 1a (294 mg) and 4-nitrobenzaldehyde (302 mg); FCC-AcOEt/hexane (2:3). 2e (244 mg, 41%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.27 (d, 4H, J = 8.7 Hz), 8.16 (s, 2H), 7.89 (d, 4H, J = 8.7 Hz), 4.32 (dd, 2H, J = 2.7, 10.9 Hz), 3.84 (t, 2H, J = 10 Hz), 3.00 (dd, 2H, J = 2.7, 9.8 Hz), 1.22 (s, 6H), 0.90 (s, 18H); 13C NMR (CDCl3, 101 MHz) δ 172.2, 159.1, 149.1, 141.4, 128.9, 123.9, 78.3, 65.0, 49.8, 33.6, 26.8, 22.7. Anal. calcd. for C31H40N4O8: C, 62.40; H, 6.76; N, 9.39. Found: C, 62.42; H, 6.77; N, 9.39; O, 21.44. = +20.7 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((2-nitrobenzylidene)amino)tert-butyl) 2,2-dimethylmalonate (2f). Starting from 1a (294 mg) and 2-nitrobenzaldehyde (302 mg); FCC-AcOEt/hexane (2:3). 2f (220 mg, 37%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.54 (s, 2H), 8.04 (d, 2H, J = 9.4 Hz), 7.97 (d, 2H, J = 9.4 Hz), 7.67–7.63 (m, 2H), 7.57–7.50 (m, 2H), 4.41 (dd, 2H, J = 2.6, 10.9 Hz), 4.11 (t, 2H, J = 10.9 Hz), 3.10 (dd, 2H, J = 2.6, 9.6 Hz), 1.27 (s, 6H), 0.96 (s, 18H); 13C NMR (CDCl3, 101 MHz) δ 172.5, 157.1, 148.9, 133.3, 130.9, 130.6, 130.1, 124.2, 78.5, 65.3, 50.1, 33.8, 26.9, 22.7. Anal. calcd. for C31H40N4O8: C, 62.40; H, 6.76; N, 9.39; O, 21.45. Found: C, 62.44; H, 6.78; N, 9.42; O, 21.48. = −37.2 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((3-trifluoromethylbenzylidene)amino)tert-butyl) 2,2-dimethylmalonate (2g). Starting from 1a (294 mg) and 3-trifluoromethylbenzaldehyde (348 mg); FCC-AcOEt/hexane (2:3). 2g (417 mg, 65%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.12 (s, 2H), 7.98 (s, 2H), 7.90 (d, 2H, J = 7.7 Hz), 7.66 (d, 2H, J = 7.9 Hz), 7.52 (t, 2H, J = 7.7 Hz), 4.35 (dd, 2H, J = 2.7, 10.9 Hz), 3.89 (t, 2H, J = 9.6 Hz), 2.97 (dd, 2H, J = 2.7, 9.7 Hz), 1.23 (s, 6H), 0.90 (s, 18 H); 13C NMR (CDCl3, 101 MHz) δ 172.4, 159.6, 136.9, 131.4 (q, J = 1.54 Hz), 131.1 (q, J = 32 Hz), 129.1, 127.0 (q, J = 3.8 Hz), 124.8 (q, J = 4.2 Hz), 124.0 (q, J = 273 Hz), 78.1, 65.2, 49.9, 33.6, 26.8, 22.7. Anal. calcd. for C33H40F6N2O4: C, 61.67; H, 6.27; N, 4.36; O, 9.96. Found: C, 61.70; H, 6.30; N, 4.40; O, 9.98. = −12.3 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((naphthalen-2-ylmethylene)amino)tert-butyl) 2,2-dimethylmalonate (2h). Starting from 1a (294 mg) and 2-naphtaldehyde (312 mg); FCC-AcOEt/hexane (2:3). 2h (388 mg, 64%); white wax. 1H NMR (CDCl3, 400 MHz) δ 9.02 (d, 2H, J = 8.4 Hz), 8.66 (s, 2H), 7.88 (t, 4H, J = 4.0 Hz), 7.80 (d, 2H, J = 7.2 Hz), 7.60–7.48 (m, 6H), 4.41 (dd, 2H, J = 2.7, 10.7 Hz), 4.07 (t, 2H, J = 9.8 Hz), 3.00 (dd, 2H, J = 2.7, 9.7 Hz), 1.23 (s, 6H), 0.94 (s, 18 H); 13C NMR (CDCl3, 101 MHz) δ 172.6, 161.3, 133.9, 131.5, 131.3, 131.0, 129.6, 128.5, 127.2, 126.1, 125.2, 124.9, 79.5, 65.6, 50.0, 33.6, 27.0, 22.8. Anal. calcd. for C39H46N2O4: C, 77.20; H, 7.64; N, 4.62; O, 10.55. Found: C, 77.31; H, 7.69; N, 4.71; O, 10.60. = −37.1 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((benzo[d][1,3]dioxol-5-ylmethylene)amino)tert-butyl) 2,2-dimethylmalonate (2i). Starting from 1a (294 mg) and piperonal (300 mg); FCC-AcOEt/hexane (2:3). 2i (362 mg, 61%); white wax. 1H NMR (CDCl3, 400 MHz) δ 7.95 (s, 2H), 7.45–7.35 (m, 2H), 7.09–7.07 (d, 2H, J = 8.0 Hz), 6.80 (d, 2H, J = 7.9 Hz), 5.98 (s, 4H), 4.34 (dd, 2H, J = 2.7, 10.8 Hz), 3.92 (t, 2H, J = 10.3 Hz), 2.90 (dd, 2H, J = 2.6, 9.8 Hz), 1.24 (s, 6H), 0.91 (s, 18H); 13C NMR (CDCl3, 101 MHz) δ 172.5, 160.1, 149.7, 148.2, 131.2, 124.3, 107.9, 106.8, 101.4, 77.8, 65.4, 49.9, 33.6, 26.9, 22.8. Anal. calcd. for C33H42N2O8: C, 66.65; H, 7.12; N, 4.71; O, 21.52. Found: C, 66.70; H, 7.25; N, 4.76; O, 21.61. = −20.3 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((pyridin-4-ylmethylene)amino)tert-butyl) 2,2-dimethylmalonate (2j). Starting from 1a (294 mg) and 4-pyridinecarboxaldehyde (214 mg); FCC-AcOEt/hexane (2:3). 2j (361 mg, 71%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.70 (s, 4H), 8.06 (br s, 2H), 7.58 (br s, 4H), 4.34 (dd, 2H, J = 2.8, 10.9 Hz), 3.89 (t, 2H, J = 10.0 Hz), 3.00 (dd, 2H, J = 2.7, 9.8 Hz), 1.22 (s, 6H), 0.91 (s, 18H); 13C NMR (CDCl3, 101 MHz) δ 172.3, 159.4, 150.4, 142.8, 122.1, 78.3, 65.0, 49.9, 33.6, 26.9, 22.7. Anal. calcd. for C29H40N4O4: C, 68.48; H, 7.93; N, 11.01; O, 12.58. Found: C, 68.52; H, 7.98; N, 11.07; O, 12.70. = −29.5 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((benzofuran-2-ylmethylene)amino)tert-butyl) 2,2-dimethylmalonate (2k). Starting from 1a (294 mg) and 2-benzofurancarboxaldehyde (292 mg); FCC-AcOEt/hexane (2:3). 2k (404 mg, 69%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.06 (s, 2H), 7.59 (d, 2H, J = 7.7 Hz), 7.56 (d, 2H, J = 8.1 Hz), 7.39–7.33 (m, 2H), 7.28–7.23 (m, 2H), 7.06 (s, 2H), 4.48 (dd, 2H, J = 2.8, 11.0 Hz), 4.11 (t, 2H, J = 10.2 Hz), 2.99 (dd, 2H, J = 2.8, 9.8 Hz), 1.24 (s, 6H), 0.92 (s, 18H); 13C NMR (CDCl3, 101 MHz) δ 172.4, 155.6, 152.5, 151.0, 127.7, 126.4, 123.3, 122.0, 112.2, 110.9, 79.3, 65.3, 50.0, 33.7, 26.9, 22.8. Anal. calcd. for C35H42N2O6: C, 71.65; H, 7.22; N, 4.77; O, 16.36. Found: C, 71.73; H, 7.36; N, 4.79; O, 16.42. = +12.2 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((4-nitrobenzylidene)amino)iso-propyl) 2,2-dimethylmalonate (3). 3, white wax. 1H NMR (CDCl3, 400 MHz) δ 8.25 (s, 1H), 7.37 (d, 1H, J = 5.0 Hz), 7.29 (d, 1H, J = 4.9 Hz), 7.05 (dd, 1H, J = 3.6, 5.1 Hz), 4.45 (dd, 1H, J = 2.6, 10.8 Hz), 4.14 (t, 1H, J = 9.9 Hz), 3.91–3.83 (m, 2H), 3.60 (dd, 1H, J = 7.6, 9.5 Hz), 2.99 (dd, 1H, J = 2.7, 9.8 Hz), 1.42 (s, 3H), 1.36 (s, 3H), 0.96 (s, 9H), 0.80 (s, 9H); 13C NMR (CDCl3, 101 MHz) δ 1736, 167.9, 154.2, 142.9, 129.9, 128.7, 127.2, 77.9, 75.3, 68.9, 65.7, 44.2, 33.7, 26.9, 25.7, 23.7. HRMS (ESI) calcd. for C22H34N2O3S [M + H+]: 407.2363, found: 407.2350. = −236.6 (c 0.55 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((4-nitrobenzylidene)amino)iso-propyl) 2,2-dimethylmalonate (4). Starting from 1b (266 mg) and 4-nitrobenzaldehyde (302 mg); FCC-AcOEt/hexane (2:3). 4 (193 mg, 34%); white wax. 1H NMR (CDCl3, 400 MHz) δ 7.26 (d, 4H, J = 8.8 Hz), 8.20 (s, 2H), 7.89 (d, 4H, J = 8.8 Hz), 4.27 (dd, 2H, J = 3.6, 10.9 Hz), 3.98 (dd, 2H, J = 8.9, 11.0 Hz), 3.18–3.13 (m, 2H), 1.93–1.83 (m, 2H), 1.25 (s, 6H), 0.91 (d, 6 H, J = 6.8 Hz), 0.89 (d, 6H, J = 6.8 Hz); 13C NMR (CDCl3, 101 MHz) δ 172.2, 159.2, 149.1, 141.4, 128.9, 123.9, 75.0, 66.3, 49.9, 30.5, 22.7, 19.5, 18.5. Anal. calcd. for C29H36N4O8: C, 61.26; H, 6.38; N, 9.85; O, 22.51. Found: C, 61.43; H, 6.61; N, 9.94; O, 22.72. = +17.5 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((2-nitrobenzylidene)amino)iso-propyl) 2,2-dimethylmalonate (5). Starting from 1b (266 mg) and 2-nitrobenzaldehyde (302 mg); FCC-AcOEt/hexane (2:3). 5 (352 mg, 62%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.58 (s, 2H), 8.01 (dd, 4H, J = 4.6, 9.2 Hz), 7.65 (t, 2H, J = 7.6 Hz), 7.56 (t, 2H, J = 6.5 Hz), 4.35 (dd, 2H, J = 3.5, 11.0 Hz), 4.16 (dd, 2H, J = 8.8, 11.1 Hz), 3.25–3.20 (m, 2H), 1.97–1.89 (m, 2H), 1.31 (s, 6H), 0.95 (d, 6H, J = 7.9 Hz), 0.93 (d, 6H, J = 7.0 Hz); 13C NMR (CDCl3, 101 MHz) δ 172.4, 157.5, 133.5, 131.1, 130.7, 130.1, 124.3, 75.2, 66.6, 50.1, 30.5, 22.8, 19.6, 18.6. Anal. calcd. for C29H36N4O8: C, 61.26; H, 6.38; N, 9.85; O, 22.51. Found: C, 61.35; H, 6.51; N, 9.92; O, 22.63. = −6.4 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((trifluoromethyl)benzylidene)amino)iso-propyl) 2,2-dimethylmalonate (6). Starting from 1b (266 mg) and 3-trifluoromethylbenzaldehyde (348 mg); FCC-AcOEt/hexane (2:3). 6 (270 mg, 44%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.16 (s, 2H), 7.99 (s, 2H), 7.89 (d, 2H, J = 7.8 Hz), 7.65 (d, 2H, J = 7.7 Hz), 7.52 (t, 2H, J = 7.7 Hz), 4.28 (dd, 2H, J = 3.6, 10.9 Hz), 3.98 (dd, 2H, J = 8.9, 10.9 Hz), 3.13–3.08 (m, 2H), 1.91–1.83 (m, 2H), 1.27 (s, 6H), 0.90 (d, 6H, J = 6.1 Hz), 0.89 (d, 6H, J = 6.1 Hz); 13C NMR (CDCl3, 101 MHz) δ 172.3, 159.8, 136.8, 131.4, 131.2 (q, J = 32.7 Hz), 129.1, 127.1 (q, J = 3.8 Hz), 124.8 (q, J = 3.9 Hz), 123.9 (q, J = 273.6 Hz) 74.9, 66.5, 49.9, 30.4, 22.7, 19.6, 18.5. Anal. calcd. for C31H36F6N2O4: C, 60.58; H, 5.90; F, 18.55; N, 4.56; O, 10.41. Found: C, 60.71; H, 5.96; F, 18.76; N, 4.64; O, 10.49. = +44.8 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-(thiophen-2-ylmethylene)amino)iso-propyl) 2,2-dimethylmalonate (7). Starting from 1b (266 mg) and 2-thiophenecarboxaldehyde (224 mg); FCC-AcOEt/hexane (2:3). 7 (343 mg, 70%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.21 (s, 2H), 7.36 (d, 2H, J = 5.0 Hz), 7.28 (d, 2H, J = 4.8 Hz), 7.04 (t, 2H, J = 5.0 Hz), 4.30 (dd, 2H, J = 3.8, 10.9 Hz), 4.03 (dd, 2H, J = 8.8, 10.9 Hz), 3.07–3.02 (m, 2H), 1.92–1.83 (m, 2H), 1.29 (s, 6H), 0.92 (d, 6H, J = 6.7 Hz), 0.89 (d, 6H, J = 6.7 Hz); 13C NMR (CDCl3, 101 MHz) δ 172.5, 154.6, 142.4, 130.3, 128.8, 127.3, 74.7, 66.6, 49.9, 30.4, 22.8, 19.7, 18.9. HRMS (ESI) calcd. for C25H34N2O4S2 [M + H+]: 491.2033, found: 491.2013. = +8.7 (c 0.5 CH2Cl2).
Bis((S)-3-phenyl-2-((thiophen-2-ylmethylene)amino)propyl) 2,2-dimethylmalonate (8). Starting from 1c (362 mg) and 2-thiophenecarboxaldehyde (224 mg); FCC-AcOEt/hexane (2:3). 8 (246 mg, 42%); white wax. 1H NMR (CDCl3, 400 MHz) δ 7.95 (s, 2H), 7.35 (d, 2H, J = 5.2 Hz), 7.22–7.20 (m, 4H), 7.18–7.10 (m, 8H), 7.01 (t, 2H, J = 4.8 Hz), 4.23 (dd, 2H, J = 4.6, 10.8 Hz), 4.13 (t, 2H, J = 8.1 Hz), 3.63–3.52 (m, 2H), 2.92–2.81 (m, 4H), 1.33 (s, 6H); 13C NMR (CDCl3, 101 MHz) δ 172.3, 155.1, 142.1, 137.9, 130.7, 129.7, 129.0, 128.3, 127.3, 126.4, 70.4, 67.4, 49.9, 39.0, 22.8. Anal. calcd. for C33H34N2O4S2: C, 67.55; H, 5.84; N, 4.77; O, 10.91; S, 10.93. Found: C, 67.59; H, 5.92; N, 4.83; O, 10.98; S, 10.94. = −37.1 (c 0.5 CH2Cl2).
Bis((S)-2-((4-nitrobenzylidene)amino)-2-phenylethyl) 2,2-dimethylmalonate (9). Starting from 1d (334 mg) and 4-nitrobenzaldehyde (302 mg); FCC-AcOEt/hexane (2:3). 9 (261 mg, 41%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.33 (s, 2H), 8.23 (d, 4H, J = 8.8 Hz), 7.93 (d, 4H, J = 8.8 Hz), 7.39–7.29 (m, 10H), 4.56 (dd, 2H, J = 4.2, 9.3 Hz), 4.26 (dd, 2H, J = 4.2, 11.0 Hz), 4.11 (dd, 2H, J = 9.3, 11.0 Hz), 1.28 (s, 6H); 13C NMR (CDCl3, 101 MHz) δ 172.0, 159.8, 149.2, 138.7, 130.5, 129.1, 128.8, 128.2, 127.2, 124.3, 123.9, 73.1, 68.5, 49.8, 22.7. Anal. calcd. for C35H32N4O8: C, 66.03; H, 5.07; N, 8.80; O, 20.10. Found: C, 66.05; H, 5.21; N, 8.96; O, 20.27. = −46.8 (c 0.5 CH2Cl2).
Bis((S)-2-((2-nitrobenzylidene)amino)-2-phenylethyl) 2,2-dimethylmalonate (10). Starting from 1d (334 mg) and 2-nitrobenzaldehyde (302 mg); FCC-AcOEt/hexane (2:3). 10 (292 mg, 46%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.74 (s, 2H), 8.12 (d, 2H, J = 7.8 Hz), 7.95 (d, 2H, J = 8.3 Hz), 7.64 (t, 2H, J = 7.6 Hz), 7.56–7.50 (m, 2H), 7.43–7.34 (m, 10 H), 4.64 (dd, 2H, J = 4.6, 8.7 Hz), 4.35 (dd, 2H, J = 4.6, 11.0 Hz), 3.74 (t, 2H, J = 6.6 Hz), 1.31 (s, 6H); 13C NMR (CDCl3, 101 MHz) δ 172.2, 157.9, 139.0, 133.4, 130.9, 130.0, 128.7, 127.9, 127.3, 124.3, 73.1, 68.4, 53.4, 22.7. Anal. calcd. for C35H32N4O8: C, 66.03; H, 5.07; N, 8.80; O, 20.10. Found: C, 66.25; H, 5.18; N, 8.99; O, 20.33. = +15.1 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-(thiophen-2-ylmethylene)amino)iso-propyl) 2,2-dimethylmalonate (11). Starting from 1d (334 mg) and 2-thiophenecarboxaldehyde (224 mg); FCC-AcOEt/hexane (2:3). 11 (301 mg, 54%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.26 (s, 2H), 7.35–7.26 (m, 14 H), 6.94 (t, 2H, J = 5.0 Hz), 4.42 (dd, 2H, J = 4.5, 8.8 Hz), 4.25 (dd, 2H, J = 4.6, 10.9 Hz), 4.13 (dd, 2H, J = 8.9, 11.1 Hz), 1.22 (s, 6H); 13C NMR (CDCl3, 101 MHz) δ 172.3, 155.2, 139.7, 130.9, 129.3, 128.6, 127.7, 127.5, 127.3, 72.4, 68.7, 22.7. Anal. calcd. for C31H30N2O4S2: C, 66.64; H, 5.41; N, 5.01; O, 11.45; S, 11.48. Found: C, 66.75; H, 5.54; N, 5.22; O, 11.61; S, 11.57. = −8.7 (c 0.5 CH2Cl2).
Bis((S)-3,3-dimethyl-2-((2-nitrobenzylidene)amino)butyl) pyridine-2,6-dicarboxylate (12). Starting from 1g (329 mg) and 2-nitrobenzaldehyde (302 mg); FCC-AcOEt/hexane (2:3). 12 (290 mg, 46%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.65 (s, 2H), 8.14 (d, 2H, J = 7.8 Hz), 8.05 (d, 2H, J = 6.3 Hz), 7.94 (d, 2H, J = 6.9 Hz), 7.88 (t, 2H, J = 7.8 Hz), 7.63 (t, 2H, J = 6.4 Hz), 7.52 (t, 2H, J = 6.6 Hz), 4.75 (dd, 2H, J = 2.6, 10.9 Hz), 4.55 (t, 2H, J = 9.6 Hz), 3.36 (dd, 2H, J = 2.7, 9.6 Hz), 1.06 (s, 18H); 13C NMR (CDCl3, 101 MHz) δ 164.1, 157.6, 148.3, 138.1, 133.4, 131.2, 130.6, 130.2, 129.6, 124.5, 124.1, 78.5, 65.9, 33.9, 27.1. Anal. calcd. for C33H37N5O8: C, 62.75; H, 5.90; N, 11.09; O, 20.26. Found: C, 62.80; H, 5.97; N, 11.22; O, 20.37. = +27.1 (c 0.5 CH2Cl2).
Bis(2-((thiophen-2-ylmethylene)amino)ethyl) isophthalate (13). Starting from 1h (216 mg) and 2-thiophenecarboxaldehyde (224 mg); FCC-AcOEt/hexane (2:3). 13 (229 mg, 52%); white wax. 1H NMR (CDCl3, 400 MHz) δ 8.55 (s, 1H), 8.36 (s, 2H), 8.09 (d, 2H, J = 1.8, 7.8 Hz), 7.40 (t, 1H, J = 7.7 Hz), 7.34 (d, 2H, J = 5.0 Hz), 7.28 (d, 2H, J = 3.7 Hz), 7.00 (dd, 2H, J = 3.5, 5.0 Hz), 4.55 (t, 4H, J = 5.6 Hz), 3.86 (t, 4H, J = 4.9 Hz); 13C NMR (CDCl3, 101 MHz) δ 165.6, 156.6, 133.8, 131.0, 130.7, 130.6, 129.3, 128.6, 128.3, 127.4, 64.5, 59.4. Anal. calcd. for C22H20N2O4S2: C, 59.98; H, 4.58; N, 6.36; O, 14.53; S, 14.56. Found: C, 60.04; H, 4.71; N, 6.42; O, 14.62; S, 14.78.
(S)-3-Methyl-2-((thiophen-2-ylmethylene)amino)butyl benzoate (15). Starting from 14 (189 mg) and 2-thiophenecarboxaldehyde (112 mg, 1 equiv.); FCC-AcOEt/hexane (2:3). 15 (93 mg, 31%); yellow oil. 1H NMR (CDCl3, 400 MHz) δ 8.37 (s, 1H), 7.97 (d, 2H, J = 7.0 Hz), 7.52 (t, 1H, J = 7.3 Hz), 7.40–7.36 (m, 4H), 7.07 (t, 1H, J = 5.0 Hz), 4.68 (dd, 1H, J = 3.9, 11.0 Hz), 4.36 (dd, 1H, J = 8.7, 11.0 Hz), 3.29–3.24 (m, 1H), 2.13–2.01 (m, 1H), 1.02 (d, 3H, J = 6.7 Hz), 0.99 (d, 3H, J = 6.7 Hz); 13C NMR (CDCl3, 101 MHz) δ 166.4, 154.5, 142.5, 133.1, 132.9, 130.3, 129.5, 128.9, 128.3, 127.3, 75.2, 66.5, 30.6, 19.7, 18.8. Anal. calcd. for C17H19NO2S: C, 67.75; H, 6.35; N, 4.65; O, 10.62; S, 10.64. Found: C, 67.89; H, 6.47; N, 4.69; O, 10.68; S, 10.72. = +63.4 (c 0.5 CH2Cl2).
(S)-N-(1-Hydroxy-3-methylbutan-2-yl)benzamide (16). 1H NMR (CDCl3, 400 MHz) δ 7.78 (d, 2H, J = 7.0 Hz), 7.53–7.49 (m, 1H), 7.45–7.41 (m, 2H), 6.35 (d, 1H, J = 5.7 Hz), 3.98–3.91 (m, 1H), 3.84–3.71 (m, 2H), 2.07−1.98 (m, 1H), 1.04 (d, 3H, J = 6.8 Hz), 1.02 (d, 3H, J = 6.8 Hz). The characterization of 16 is consistent with that reported in the literature [32].
The detailed 1H, 13C NMR spectra for all compounds are provided in the Supplementary Materials.
3.4. Computational Methods
The structure of product 2b was initially constructed in MOE [33] based on the X-ray structure of compound 2d, followed by energy minimization using the MMFF94x force field [34] in the gas phase. A conformational search using the LowModeMD method in MOE was performed at the same level of theory with Hydrogen Mass Repartitioning (HMR), a Rejection Limit of 1000, and an Iteration Limit of 100,000, with default settings for other parameters. The 21 resulting geometries were reoptimized by density functional theory (DFT) with the ωB97X-D functional, which includes empirical dispersion corrections [35] and the 6-31+G(d,p) basis set, shown effective in similar studies [36]. Single-point energy (SP) calculations were then performed using the same function with the Def2TZVP basis set [37] and the CPCM solvation model for THF [38]. The geometry resembling the X-ray structure of compound 2d (product2b_C0 in Table S3, Supplementary Materials) was the most stable, supporting the chosen methodology. NiCl2·4H2O was constructed and optimized in both singlet (multiplicity = 1) and triplet (multiplicity = 3) states. The singlet state converged to a square planar geometry with two water dissociating, while the triplet state converged to a square bipyramidal geometry, using all six coordination sites on Ni. This triplet configuration was 16.1 kcal/mol more stable and was thus used for all Ni chelates. Geometries involving Ni were manually constructed based on chemical insight, as reliable conformational searches for metal chelates could not be performed. Several conformations and coordination patterns were evaluated for each intermediate, and the most stable were selected for discussion. All geometry optimizations and SP energy calculations were performed using Gaussian16 [39].
4. Conclusions
In summary, we have studied the coupling between bis(2-oxazolines) and aromatic aldehydes, which, in the presence of catalytic amounts of NiCl2, affords ester-imine products in synthetically useful yields. This almost unknown transformation features 100% atom economy and involves the formal metathesis between the C=N double bond of the bis(2-oxazoline) moiety and the C=O double bond of the aldehyde. Future synthetic applications of this intriguing reaction are planned in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29235756/s1, Figure S1: Enlarged picture of optimized geometries of reactants, intermediates and products discussed in Figure 1. A: catalyst NiCl2·4H2O, reactants 1a, benzaldehyde and water. B–G: intermediates II–VII, respectively. J: product 2b; Table S1: Average distances and angles involving Ni atom. Angles involving Ni with O or N are generally measured between adjacent atoms unless otherwise specified; Figure S2: Alternative configurations for IntIII (A: pentavalent, and B: hexavalent; ΔHpenta-hexa= 7.5 kcal/mol), and alternative structure for IntV (C) where the amine is free and replaced by a water molecule in coordinating Ni; Table S2: Energies and corrections of each compound considered in this study; Table S3: Energies and corrections of all the conformations of product 2b obtained by a conformational search using molecular mechanics and the MMFF94x force field in the gas phase, successively reoptimized at the WB97XD/Def2TZVP/CPCM(THF)//WB97XD/6-31+G(d,p) level; Figure S3: X-ray quality specimen of 2d. The crystal has needle habit and is transparent and colorless with dimensions of 0.105 × 0.015 × 0.015 mm3; Figure S4: Up: Asymmetric unit of 2d at RT, with the atom-numbering scheme. Thermal ellipsoids of non-H atoms were drawn at the 25% probability level. The usual color code was employed for atoms (grey: C; white: H; blue: N; red: O; dark yellow: Br). Down: Molecular structure of 2d, with the Cahn–Ingold–Prelog descriptors highlighted; Table S4: CH···O contacts in 2d; Figure S5: Crystal packing of 2d at RT, as seen (a) along the a-cell axis; (b) the c-cell axis; (c) the b-cell axis. Color code as in Figure S4. The crystallographic reference system is also shown. Hydrogen atoms were omitted for clarity. References [31,40,41] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, methodology, and organic synthesis work supervision, C.L. and M.P.; supervision and project administration, G.B.; conceptualization and writing—original draft preparation, J.O., G.P. and G.B.; investigation, analysis, and writing the experimental section, S.C., M.P. and C.L.; X-ray structure determination L.L.P. and G.M.; mechanistic investigation, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article and Supplementary Materials.
Acknowledgments
The authors gratefully acknowledge the support from Università dell’Insubria, Sorbonne Université, CNRS and Università degli Studi di Milano. The Unitech COSPECT platform at Università degli Studi di Milano is acknowledged for providing X-ray beamtime.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dargaville, T.R.; Park, J.R.; Hoodenboom, R. Poly(2-oxazoline) hydrogels: State-of-the-art and emerging applications. Macromol. Biosci. 2018, 18, 1800070. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Onda, Y.; Masuda, Y.; Doi, T. Potent oxazoline analog of Apratoxin C; synthesis, biological evaluation, and conformational analysis. PeptideScience 2016, 106, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.N.; Beveridge, R.E.; Blay, J.; Boyd, A.R.; Chojnacka, M.W.; Decken, A.; Deshpande, A.A.; Gardiner, M.G.; Hambley, T.W.; Hughes, M.J.; et al. Platinum-oxazole complexes as anti-cancer agents: Syntheses, characterization and initial biological studies. Med. Chem. Commun. 2011, 2, 274–277. [Google Scholar] [CrossRef]
- Connon, R.; Roche, B.; Rokadem, B.V.; Guiry, P.J. Further developments and application of oxazoline-containing ligands in asymmetric catalysis. Chem. Rev. 2021, 121, 6373–6521. [Google Scholar] [CrossRef]
- McManus, A.H.; Guiry, P.J. Recent developments in the application of oxazoline-containing ligands in asymmetric catalysis. Chem. Rev. 2004, 104, 4151–4202. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; Muller, G.; Rocamora, M. Coordination chemistry of oxazoline ligands. Coord. Chem. Rev. 1999, 193–195, 769–835. [Google Scholar] [CrossRef]
- Desimoni, G.; Faita, G.; Jørgensen, K.A. C2-Symmetric Chiral Bis (Oxazoline) Ligands in Asymmetric Catalysis. Chem. Rev. 2006, 106, 3561–3651. [Google Scholar] [CrossRef]
- Deng, S.K.; Chen, H.C.; Liu, Y.-F.; Ji, H.T.; Shen, J. Rin-opening phosphanylation of oxazolines. Tetrahedron Lett. 2024, 134, 154866. [Google Scholar] [CrossRef]
- Hess, A.; Guelen, H.C.; Alandini, N.; Mourati, A.; Guersoy, Y.C.; Knochel, P. Preparation of polyfunctionalized aromatic nitriles from aryl oxazolines. Chem. Eur. J. 2022, 28, e202103700. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, S.; Li, B. KOt-Bu-Promoted selective ring-opening N-alkylation of 2-oxazolines to access 2-aminoethyl acetates and N-substituted thiazolidinones. Beilstein J. Org. Chem. 2020, 16, 492–501. [Google Scholar] [CrossRef]
- Yang, Z.; Jie, L.; Yao, Z.; Yang, Z.; Cui, X. Rhodium (III)-catalyzed synthesis of N-(2-acetoxyalkyl) isoquinolones from oxazolines and alkynes through C-N bond formation and ring-opening. Adv. Synth. Catal. 2019, 361, 214–218. [Google Scholar] [CrossRef]
- Guan, D.; Luan, H.X.; Patiguli, M.; Jiao, Q.J.; Yun, Q.Q.; Chen, Q.S.; Xu, C.J.; Nie, X.B.; Hu, F.P.; Huang, G.S. Metal–free Efficient Method for the Synthesis of N-(2-haloethyl)benzamides through the Ring-opening of 2-oxazolines. ChemistrySelect 2019, 4, 6668–6671. [Google Scholar] [CrossRef]
- Qiao, K.; Yuan, X.; Wan, L.; Zheng, M.-W.; Zhang, D.; Fan, B.B.; Di, Z.C.; Fang, Z.; Guo, K. Highly efficient synthesis of β-nitrate ester carboxamides through the ring-opening of 2-oxazolines. Green Chem. 2017, 19, 5789–5793. [Google Scholar] [CrossRef]
- Gutmann, B.; Roduit, J.P.; Roberge, D.; Kappe, C.O. A two-step continuous-flow synthesis of N-(2-aminoethyl) acylamides through ring-opening/hydrogenation of oxazolines. Chem. Eur. J. 2011, 17, 13146–13150. [Google Scholar] [CrossRef]
- Laitar, D.S.; Kramer, J.W.; Whiting, B.T.; Lobkovsky, E.B.; Coates, G.W. β-Amidoaldehydes via oxazoline hydroformylation. Chem. Commun. 2009, 14, 5704–5706. [Google Scholar] [CrossRef]
- Kukharev, B.F.; Stankevich, V.K.; Terent’eva, V.P.; Kukhareva, V.A. Reaction of oxazolines with aldehydes. Zh. Org. Khim. 1987, 23, 1336–1337. [Google Scholar]
- Lerestif, J.M.; Toupet, L.; Sindandhit, S.; Tonnard, F.; Bazureau, J.P.; Hamelin, J.A. New route to 2-oxazolines, bis-oxazolines, and 2-imidazoline-5-ones from imidates using solvent-free cycloadditions: Synthesis, chemical properties, and PM3 MO calculations. Tetrahedron 1997, 53, 6351–6364. [Google Scholar] [CrossRef]
- Maguet, M.; Poirier, Y.; Guglielmetti, R. Spiropyrannes et mérocyanines en séries azahétérocycliques saturées. Bull. Soc. Chem. Fr. 1978, 11–12. [Google Scholar]
- Lerestif, J.M.; Feuillet, S.; Bazureau, J.P.; Hamelin, J. Novel Synthesis of Protected Methyl 4-Hydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylate via Cleavage of Functionalized Dihydrooxazoles (Oxazolines). J. Chem. Res. 1999, 1, 32–33. [Google Scholar] [CrossRef]
- Molteni, L.; Beccalli, E.M.; Castoldi, L.; Broggini, G.; Loro, C. Methanol as a C1 Source for the Synthesis of 1,3-Polyheterocyclic Systems. Eur. J. Org. Chem. 2023, 26, e202301106. [Google Scholar] [CrossRef]
- Loro, C.; Molteni, L.; Lo Presti, L.; Foschi, F.; Beccalli, E.M.; Broggini, G. Non-decarboxylative ruthenium-catalyzed rearrangement of 4-alkylidene-isoxazol-5-ones to pyrazole- and isoxazole-4-carboxylic acids. Org. Lett. 2022, 24, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Molteni, L.; Loro, C.; Christodoulou, M.S.; Papis, M.; Foschi, F.; Beccalli, E.M.; Broggini, G. Ruthenium-catalyzed decarboxylative rearrangement of 4-alkenyl-isoxazol-5-ones to pyrrole derivatives. Eur. J. Org. Chem. 2022, 2022, e202200496. [Google Scholar] [CrossRef]
- Issazadeh, S.; Khan, M.J.; Gan, H.; Zhang, J.; Henderson, L.C.; Varley, R.J. Synthesis and cure kinetics of bisoxazoline monomers during cationic ring-opening polymerization. J. Appl. Polym. Sci. 2024, 141, e54900. [Google Scholar] [CrossRef]
- Taylan, E.; Küsefoğlu, S.H. Chain Extension Reactions of Unsaturated Polyesters with Bis(2-Oxazoline)s. J. Appl. Polym. Sci. 2012, 124, 3229. [Google Scholar] [CrossRef]
- Fry, E.M. Oxazolidine ring-opening. J. Org. Chem. 1950, 15, 802–806. [Google Scholar] [CrossRef]
- Tschan, M.; Thomas, C.M.; Strub, H.; Carpentier, J.F. Copper(II) triflate as a source of triflic acid: Effective, green catalyst of hydroalkoxylation reactions. Adv. Synth. Catal. 2009, 351, 2496–2504. [Google Scholar] [CrossRef]
- Kang, Y.B.; Gade, L.H. The nature of the catalytically active species in olefin deoxygenation with PhI(OAc)2: Metal or proton? J. Am. Chem. Soc. 2011, 133, 3658–3667. [Google Scholar] [CrossRef]
- Dang, T.; Boeck, F.; Hinterman, L. Hidden Brønsted acid catalysis: Pathways of accidental or delicerate generation of triflic acid from metal triflates. J. Org. Chem. 2011, 76, 9353–9361. [Google Scholar] [CrossRef]
- Loro, C.; Oble, J.; Foschi, F.; Papis, M.; Beccalli, E.M.; Giofrè, S.; Poli, G.; Broggini, G. Acid-mediated decarboxylative C–H coupling between arenes and O-allyl carbamates. Org. Chem. Front. 2022, 9, 1711–1718. [Google Scholar] [CrossRef]
- Loro, C.; Papis, M.; Foschi, F.; Broggini, G.; Poli, G.; Oble, J. Copper(II)-Catalyzed Three-Component Arylation/Hydroamination Cascade from Allyl Alcohol: Access to 1-Aryl-2-sulfonylamino-propanes. J. Org. Chem. 2023, 88, 13995–14003. [Google Scholar] [CrossRef]
- Schwekendiek, K.; Glorius, F. Efficient Oxidative Synthesis of 2-Oxazolines. Synthesis 2006, 18, 2996–3002. [Google Scholar] [CrossRef]
- Soleymani Movahed, F.; Foo, S.W.; Mori, S.; Ogawa, S.; Saito, S. Phosphorus-Based Organocatalysis for the Dehydrative Cyclization of N-(2-Hydroxyethyl)amides into 2-Oxazolines. J. Org. Chem. 2022, 87, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Chemical Computing Group ULC. Molecular Operating Environment (MOE), Version 2022.02; Chemical Computing Group ULC: Montreal, QC, Canada, 2023.
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Papis, M.; Bucci, R.; Contini, A.; Gelmi, M.L.; Lo Presti, L.; Poli, G.; Broggini, G.; Loro, C. Phosphine-Catalyzed Domino Regio- and Stereo-Selective Hexamerization of 2-(Bromomethyl)acrylates to 1,2-Bis(cyclohexenyl)ethenyl Derivatives. Org. Lett. 2023, 25, 7380–7384. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadrupole zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A. 03; Gaussian. Inc.: Wallingford, UK, 2016. [Google Scholar]
- Sheldrick, G.M. A short history of Shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H.D.; Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. Sect. B 2013, 69, 249–259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).