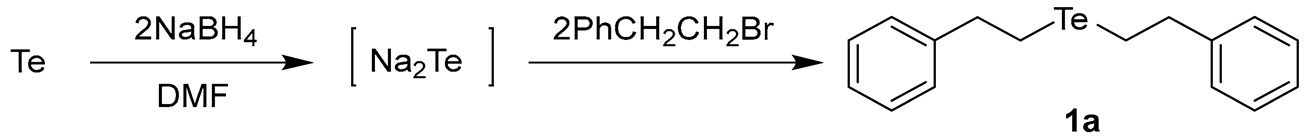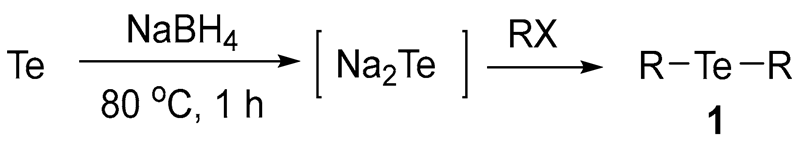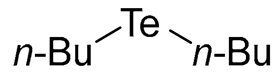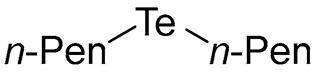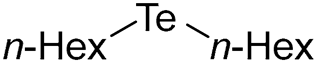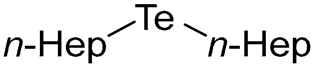Abstract
Studies on organotellurium compounds have not been extensively conducted due to a lack of tolerable synthetic methods, difficult isolation processes, and their chemical instabilities. Overcoming these hurdles, we developed an efficient and mild method for the selective synthesis of symmetrical diorganyl tellurides 1, a representative class of organotellurium compounds, using a proper reducing reagent. The reaction condition was optimized for the selective formation of 1 by forming the telluride dianion (Te2−) using a reducing reagent, sodium borohydride (NaBH4), and then followed by the addition of organyl halides. The optimized reaction condition was as follows: (1) Te (1.0 eq), NaBH4 (2.5 eq) in DMF for 1 h at 80 °C; (2) organyl halides (2.0 eq) for 3–5 h at 25–153 °C. Using this condition, 18 various diorganyl tellurides 1 were selectively and efficiently synthesized in reasonable yields (37–93%). The reaction pathways for the formation of diorganyl tellurides 1 were also investigated. Consequently, we established a practical and efficient method for the selective synthesis of diorganyl tellurides 1 as a representative class of organotellurium compounds.
1. Introduction
Tellurium (Te) is one of the chalcogen group of elements, together with oxygen (O), sulfur (S), and selenium (Se). Tellurium is often compared to sulfur or selenium based on its similar but different physicochemical properties. While sulfur and selenium compounds have shown excellent pharmacological effects [1], tellurium compounds have rarely been used in in vivo studies due to their toxicity, particularly their toxicity on the nervous system [2,3]. However, recent studies have reported that tellurium compounds have meaningful biological effects [4,5,6], and some of them revealed that organotellurium compounds show significantly higher efficacy compared to organoselenium compounds [7]. These are known to exhibit antitumor [5,6], antimicrobial [8,9,10], and antioxidant [11,12,13] activities. As a result, organotellurium compounds have been emerging as significant substances in the area of organic synthesis and medicinal chemistry over the years [4].
The important classes of organotellurium compounds are presented in Figure 1. Among these, we are particularly interested in organyl telluride compounds based on their biological importance. Previously, we reported a study on the selective synthesis of diorganyl ditellurides (R-Te-Te-R) [14]. In addition, we were also interested in organoselenium compounds and reported studies on the selective synthesis of diorganyl diselenides (R-Se-Se-R) [15] and diorganyl selenides (R-Se-R) [16]. As an extension of our interest in chalcogen compounds, we aimed to develop efficient and tolerable methods for the selective synthesis of diorganyl tellurides 1. As an example, we attempted to establish reaction conditions that facilitate the selective synthesis of diorganyl tellurides 1. According to our preliminary studies, we chose NaBH4 as an appropriate reducing reagent to synthesize sodium telluride (Na2Te) because it can reduce elemental tellurium to Na2Te under milder conditions compared to other reducing reagents such as sodium (Na) or sodium hydride [17,18,19,20]. There have also been several studies on synthesizing organotellurium compounds using various reagents [21,22,23,24,25,26]. In this report, we describe a mild synthetic method for the selective synthesis of Na2Te and the corresponding diorganyl tellurides 1.
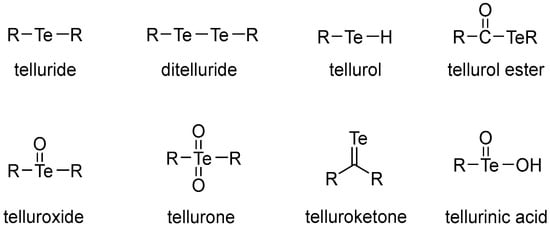
Figure 1.
Types of organotellurium compounds.
2. Results and Discussion
2.1. Optimization for the Reaction Conditions
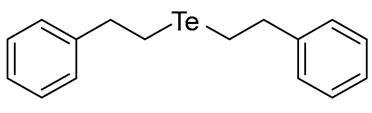
To conduct selective syntheses of diorganyl tellurides 1, we first investigated the reaction conditions for the selective formation of sodium telluride (Na2Te). Based on the previous procedures for ditellurides [14], we applied NaBH4 as a suitable reducing reagent for the formation of Na2Te with minor modifications. This choice was made to avoid the use of hazardous reducing reagents such as sodium (Na), sodium hydride, or hydrazine, after the consideration of previous studies and our preliminary experiments. As depicted in Scheme 1, we chose 2-phenethyl bromide (PhCH2CH2Br) as a template organyl halide to capture the Na2Te in situ.
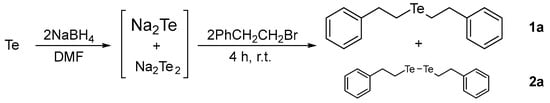
Scheme 1.
Formation of sodium telluride and bis(2-phenylethyl) telluride (1a).
One-pot reactions were performed using tellurium and NaBH4 in N,N-dimethylformamide (DMF) solvent. Given the importance of reducing reagents, we attempted to optimize the reaction conditions by adjusting the amount of NaBH4, temperature, and reaction time. The generated Na2Te was then directly reacted with PhCH2CH2Br to provide 1a (Table 1). To ensure the reliability of the optimized conditions, we applied consistent conditions for the subsequent alkylation step (2.0 eq of PhCH2CH2Br, 25 °C, and 4 h).

Table 1.
Optimized reaction method using the reducing reagent NaBH4 a.
In view of the theoretical amount, 2.0 eq of NaBH4 (compared to Te), we performed reactions using 2.0–3.0 eq of NaBH4 to ensure the full reduction of tellurium to telluride (oxidation state: −2), at temperatures ranging from 60 to 100 °C. As expected, we found that the amount of NaBH4 had a significant impact on the reaction. Considering the required theoretical amount (2.0 eq) of the reducing reagent, the use of 2.5 eq seemed excessive. However, we believed that the excess reducing reagents could be needed due to the additional consumption by oxygen or its incomplete working. Therefore, we concluded that 2.5 eq of reducing reagents would be a practically relevant amount for telluride formation at our reaction scale. Although the reactions at high temperatures (e.g., 100 °C) gave reasonable yields, the temperature was too high to be applied to the reactions with the organyl halide substrates with low boiling points.
Regarding the yields of the products and the selectivity of 1a over 2a, we have adopted the optimal conditions as follows: Te (1.0 eq), NaBH4 (2.5 eq), and a reaction time of 1 h at 80 °C; PhCH2CH2Br (2.0 eq) and a reaction time of 4 h at 25 °C.
2.2. Synthesis of Dioramic Tellurides 1
Under the optimized conditions, we attempted to selectively synthesize diorganyl tellurides 1 by using various organyl halides (Table 2). We adopted primary, secondary, and tertiary alkyl halides, and aryl halides. At first, 2-phenethyl bromide afforded good yields (79%, Table 2, entry 1) as shown in Table 2. Other primary alkyl bromides such as n-butyl, n-pentyl, n-hexyl, n-heptyl, and n-octyl bromides also gave good to excellent yields (75–93%, entries 2 to 6). Then, secondary alkyl bromides gave moderate to good yields (40–76%, entries 7 to 11). Among these, c-hexyl bromide seemed to have lower reactivity than the others, requiring a longer reaction time (5 h) to provide 1j (entry 10) in a relatively low yield (40%). Unfortunately, tertiary alkyl halides such as t-butyl bromide and even iodide did not give the product. We believed that tertiary alkyl halide could hardly react with Na2Te due to steric effects. When we used benzyl bromide, it did not provide good results either. To improve the results, we tried to elevate the reaction temperature (25–153 °C) and elongate reaction times (2–24 h). However, these reactions resulted in the gradual formation of a black solid during the reaction and the work-up process. It appeared that the generated product (dibenzyl telluride) might be decomposed and oxidized to elemental tellurium, which is also observed in our previous study [14]. This phenomenon is quite surprising and further investigation is underway.

Table 2.
Synthesis of diorganyl tellurides 1 a.
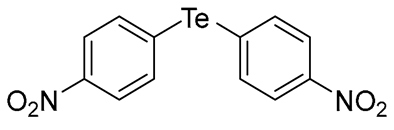
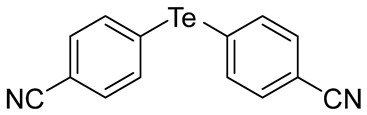
When we conducted the reaction with aryl halides, we chose iodide instead of bromide due to iodide’s higher reactivity. Upon using unsubstituted phenyl iodide at 153 °C for 4 h, the resulting product was obtained in a good yield (67%, entry 12). We also conducted the reactions using phenyl iodides with electron-withdrawing groups (EWG), such as 3-(trifluoromethyl)phenyl, 4-(trifluoromethyl)phenyl, 4-nitrophenyl, and 4-cyanophenyl groups (entries 13 to 16). These reactions generally provided reasonable yields (37–45%). Additionally, 2-(trifluoromethyl)phenyl iodide, 2-cyanophenyl iodide, and 3-nitrophenyl iodide were also tested to examine the steric effect of the substituents on the phenyl ring, but they gave poor yields (<10%). We then conducted the reactions using phenyl iodides with electron-donating groups (EDG), such as 4-methylphenyl and 4-methoxyphenyl iodides (entries 17 and 18). 4-Methylphenyl iodide gave a relatively good yield (61%), while 4-methoxyphenyl iodide gave a low yield (43%). We believed that the aryl halides react with Na2Te through a nucleophilic aromatic substitution mechanism (SNAr). Considering this mechanism, it was surprising that the reaction of phenyl iodide with EDG (e.g., 4-methylphenyl iodide) gave better yields than those of phenyl iodides with EWG. Notably, the results of aryl halides did not seem to consistently match the results expected by the SNAr mechanism. The reactions of telluride species still seemed complex and unclear, and further studies are needed to clarify the reaction mechanism. Taken together, we have successfully synthesized eighteen diorganyl tellurides 1 with the minimal formation of diorganyl ditellurides 2, among which four compounds (1g, 1h, 1k, and 1m) are new.
2.3. Investigation of Reaction Pathways
Based on these results, we have conducted studies on the reaction pathways. Here, we suggest the reaction pathways for diorganyl tellurides 1 and diorganyl ditellurides 2, as shown in Scheme 2. First, for the synthesis of tellurides 1 (path A), NaBH4 (2.0 eq) reacts with Te (1.0 eq) in the presence of H2O, resulting in the formation of Na2Te (1.0 eq), H3BO3 (2.0 eq), and H2 gas (7.0 eq). Subsequently, the generated Na2Te (1.0 eq) is reacted with organyl halides (RX) (2.0 eq) to form the desired diorganyl tellurides 1. According to the proposed reaction pathway, the theoretical amounts of reagents required for the formation of tellurides 1 (1.0 eq) are as follows: (1) Te (1.0 eq), NaBH4 (2.0 eq), and H2O (6.0 eq); (2) RX (2.0 eq). For the synthesis of ditellurides 2 (Path B), NaBH4 (1.0 eq) reacts with Te (1.0 eq) in the presence of H2O, giving Na2Te2 (0.5 eq), H3BO3 (1.0 eq), and H2 gas (3.5 eq). Subsequently, the generated Na2Te2 (0.5 eq) is reacted with organyl halides (RX) (1.0 eq) to form the diorganyl ditellurides 2. Based on these proposed reaction pathways, the amount of reducing reagent (NaBH4) must be a significant factor in deciding the reaction pathway, Path A or Path B. Thus, according to the amount of reducing reagent, the equilibrium of the two pathways could shift between each other, resulting in the formation of a mixture of tellurides 1 and ditellurides 2. A total of 2.0 eq of NaBH4 (compared to Te) reduces Te to Te2− (oxidation state: −2), and 1.0 eq of NaBH4 reduces Te to Te22− (oxidation state: −1). In order to verify the proposed reaction pathways, we conducted the reactions by regulating the amount of NaBH4. For example, when we used 1.5 eq of NaBH4, the reactions followed not only Path A but also Path B [telluride 1 (Path A): ditellurides 2 (Path B)~1:0.8]. When we used 2.0 and 2.5 eq of NaBH4, the reactions selectively followed Path A. In reality, it was observed that a slightly higher quantity (2.5 eq) of NaBH4 than the theoretical amount (2.0 eq) gave the best result as shown in Table 1. In addition, it is believed that other reaction conditions such as reaction temperature and time should be carefully controlled to selectively synthesize product compounds 1 through Path A.
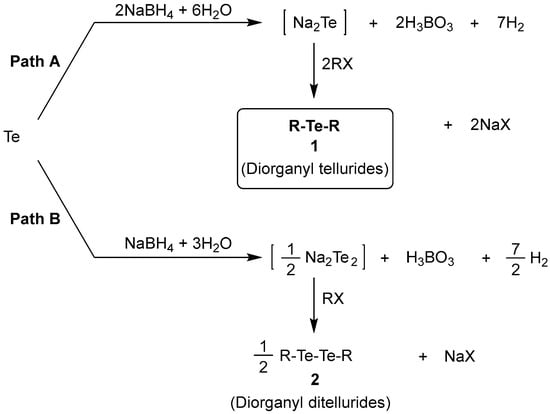
Scheme 2.
The proposed reaction pathways for diorgnyl tellurides 1 and diorganyl ditellurides 2.
3. Experimental Procedure
3.1. General Methods
All reagents were purchased from commercial suppliers and used without further purification unless otherwise noted. Reaction progress was routinely monitored using thin-layer chromatography (TLC). Purification of the crude products was conducted via column chromatography, utilizing Merck silica gel (230–400 mesh). Melting and boiling points (uncorrected) were determined on round glass coverslips using a Fisher-Johns melting point apparatus (Waltham, MA, USA). FT-IR spectra were recorded using a PerkinElmer Spectrum GX spectrometer (Waltham, MA, USA) and frequencies (ν) are expressed in reciprocal centimeters (cm−1). 1H (400 MHz) and 13C (100 MHz) NMR spectra were recorded on an Agilent Technologies 400/54 spectrometer (Santa Clara, CA, USA), with chemical shifts (δ) reported relative to tetramethylsilane (TMS). Mass spectra were recorded in electron ionization (EI) mode. HPLC analyses were conducted with a Waters 1525 binary HPLC pump (Milford, MA, USA), a dual λ absorbance 2998 detector, and a COSMOSIL 5C18-AR-II Packed Column (Kyoto, Japan, 4.6 × 250 mm). Reaction products were analyzed under gradient conditions. Condition 1: from 50% A (aqueous) and 50% B (MeCN) in 3 min (isocratic) to 10% A and 90% B for 17 min (gradient), then 10% A and 90% B in 40 min (isocratic), and finally, from 50% A and 50% B over 5 min (gradient), then keep 50% A and 50% B for 5 min (isocratic). Condition 2: from 50% A (aqueous) and 50% B (MeCN) in 3 min (isocratic) to 10% A and 90% B in 15 min (gradient), then 10% A and 90% B for 25 min (isocratic), and finally, from 50% A and 50% B over 5 min (gradient). The flow rate was 1 mL/min with eluent monitoring at 254 nm. HPLC solvents were filtered (aqueous solution with Millipore HVLP, 0.45 mm; MeCN with Millipore HV, 0.45 mm, Burlington, MA, USA) and degassed before utilization.
3.2. General Procedure for the Synthesis of Diorganyl Tellurides 1
Under a nitrogen atmosphere, Te (200 mg, 1.57 mmol, 1.0 eq) was added to a stirred solution of NaBH4 (149 mg, 3.92 mmol, 2.5 eq) in DMF solvent (2.4 mL). After stirring at 80 °C for 1 h, the reaction mixture turned a deep purple color (formation of Na2Te). Subsequently, the organyl halide was added, and the resulting mixture was stirred at 25–153 °C for 3–5 h (Table 2). After completion, the mixture was diluted with H2O (50 mL) and extracted twice with n-hexane or ethyl acetate (2 × 50 mL). The combined organic layers were washed with brine (50 mL), dried over anhydrous MgSO4, and concentrated under reduced pressure to give a sticky residue. This residue was purified via column chromatography, yielding diorganyl tellurides 1. Spectra data of some compounds were in keeping with the literature information.
- Bis(2-phenylethyl) Telluride (1a) [27]. The use of 2-phenethyl bromide (429 μL, 3.1 mmol, 2.0 eq) and a 4 h reaction time at 25 °C in the general procedure afforded the title compound 1a (418 mg, 79%) as a bright yellow oil. Bp 140–142 °C; Rf 0.51 (1:2 Dichloromethane/n-hexane); HPLC tR 22.04 min (condition 1); λmax = 348 nm; IR (ZnSe) 3024, 2925, 1493, 1148, 694 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.31–7.16 (m, 10 H, Ar), 3.05 (t, J = 8.0 Hz, 4 H, TeCH2), 2.83 (t, J = 8.0 Hz, 4 H, CH2); 13C NMR (100 MHz, CDCl3): δ = 142.73 (Ar), 128.50 (Ar), 128.12 (Ar), 126.30 (Ar), 38.74 (TeCH2), 3.47 (CH2); MS m/z 340 [M]+; HRMS (+EI) calcd for C16H18Te [M]+ 340.0471, found 340.0471.
- Di-n-butyl Telluride (1b) [28]. The use of 1-bromobutane (339 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1b (292 mg, 77%) as a yellow oil. Bp 106–108 °C; Rf 0.44 (n-hexane); HPLC tR 24.67 min (condition 1); λmax = 348, 240 nm; IR (ZnSe) 2956, 2924, 2870, 1459, 1160 cm−1; 1H NMR (400 MHz, CDCl3): δ = 2.63 (t, J = 7.5 Hz, 4 H, TeCH2), 1.72 (quintet, J = 7.4 Hz, 4 H, CH2), 1.38 (sextet, J = 7.4 Hz, 4 H, CH2), 0.92 (t, J = 7.5 Hz, 6 H, CH3); 13C NMR (100 MHz, CDCl3): δ = 34.42 (TeCH2), 25.13 (CH2), 13.43 (CH2), 2.37 (CH2); MS m/z 244 [M]+; HRMS (+EI) calcd for C8H18Te [M]+ 244.0471, found 244.0473.
- Di-n-pentyl Telluride (1c) [29]. The use of 1-bromopentane (390 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1c (317 mg, 75%) as a bright yellow oil. Bp 122–124 °C; Rf 0.42 (n-hexane); HPLC tR 30.93 min (condition 1); λmax = 345, 240 nm; IR (ZnSe) 2956, 2922, 2856, 1461, 1157 cm−1; 1H NMR (400 MHz, CDCl3): δ = 2.63 (t, J = 7.6 Hz, 4 H, TeCH2), 1.78–1.71 (m, 4 H, CH2), 1.39–1.31 (m, 8 H, CH2), 0.90 (t, J = 6.6 Hz, 6 H, CH3); 13C NMR (100 MHz, CDCl3): δ = 34.25 (TeCH2), 31.99 (CH2), 22.06 (CH2), 14.00 (CH2), 2.71 (CH3); MS m/z 272 [M]+; HRMS (+EI) calcd for C10H22Te [M]+ 272.0784, found 272.0781.
- Di-n-hexyl Telluride (1d) [30]. The use of 1-bromohexane (457 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1d (380 mg, 81%) as a bright yellow oil. Bp 146–148 °C; Rf 0.47 (n-hexane); HPLC tR 41.21 min (condition 1); λmax = 347, 240 nm; IR (ZnSe) 2956, 2922, 2853, 1461, 1155 cm−1; 1H NMR (400 MHz, CDCl3): δ = 2.63 (t, J = 7.6 Hz, 4 H, TeCH2), 1.77–1.70 (m, 4 H, CH2), 1.40–1.27 (m, 12 H, CH2), 0.89 (t, J = 6.5 Hz, 6 H, CH3); 13C NMR (100 MHz, CDCl3): δ = 32.27 (TeCH2), 31.75 (CH2), 31.20 (CH2), 22.58 (CH2), 14.07 (CH2), 2.76 (CH3); MS m/z 300 [M]+; HRMS (+EI) calcd for C12H26Te [M]+ 300.1097, found 300.1096.
- Di-n-heptyl Telluride (1e) [31]. The use of 1-bromoheptane (493 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1e (470 mg, 92%) as an orange oil. Bp 168–170 °C; Rf 0.49 (n-hexane); HPLC tR 17.25 min (condition 2); λmax = 347, 289, 240 nm; IR (ZnSe) 2956, 2921, 2852, 1459, 1154 cm−1; 1H NMR (400 MHz, CDCl3): δ = 2.62 (t, J = 7.6 Hz, 4 H, TeCH2), 1.77–1.70 (m, 4 H, CH2), 1.39–1.27 (m, 16 H, CH2), 0.88 (t, J = 6.9 Hz, 6 H, CH3); 13C NMR (100 MHz, CDCl3): δ = 32.31 (TeCH2), 32.04 (CH2), 31.99 (CH2), 28.67 (CH2), 22.65 (CH2), 14.09 (CH2), 2.77 (CH3); MS m/z 328 [M]+; HRMS (+EI) calcd for C14H30Te [M]+ 328.1410, found 328.1414.
- Di-n-octyl Telluride (1f) [32]. The use of 1-bromooctane (541 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1f (515 mg, 93%) as a yellow oil. Bp 194–196 °C; Rf 0.49 (n-hexane); HPLC tR 29.68 min (condition 2); λmax = 350, 240 nm; IR (ZnSe) 2956, 2921, 2852, 1460, 1153 cm−1; 1H NMR (400 MHz, CDCl3): δ = 2.62 (t, J = 7.6 Hz, 4H, TeCH2), 1.77–1.70 (m, 4 H, CH2), 1.39–1.26 (m, 20 H, CH2), 0.88 (t, J = 6.9 Hz, 6 H, CH3); 13C NMR (100 MHz, CDCl3): δ = 32.30 (TeCH2), 32.08 (CH2), 31.85 (CH2), 29.22 (CH2), 28.96 (CH2), 22.67 (CH2), 14.12 (CH2), 2.78 (CH3); MS m/z 356 [M]+; HRMS (+EI) calcd for C16H34Te [M]+ 356.1723, found 356.1719.
- Bis(3-pentyl) Telluride (1g). The use of 3-bromopentane (390 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1g (259 mg, 61%) as an orange oil. Bp 91–92 °C (dec); Rf 0.45 (n-hexane); HPLC tR 28.80 min (condition 1); λmax = 350, 293, 238 nm; IR (ZnSe) 2959, 2927, 2871, 2845, 1455, 1130 cm−1; 1H NMR (400 MHz, CDCl3): δ = 2.95 (quintet, J = 6.5 Hz, 2 H, TeCH), 1.82–1.71 (m, 8 H, CH2), 0.99 (t, J = 7.3 Hz, 12 H, CH3); 13C NMR (100 MHz, CDCl3): δ = 31.74 (TeCH), 30.54 (CH2), 13.91 (CH3); MS m/z 272 [M]+; HRMS (+EI) calcd for C10H22Te [M]+ 272.0784, found 272.0784.
- Bis(4-heptyl) Telluride (1h). The use of 4-bromoheptane (493 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1h (389 mg, 76%) as an orange oil. Bp 100–102 °C (dec); Rf 0.45 (n-hexane); HPLC tR 12.40 min (condition 2); λmax = 344 nm; IR (ZnSe) 2956, 2926, 2871, 1459, 1133 cm−1; 1H NMR (400 MHz, CDCl3): δ = 3.07 (quintet, J = 6.6 Hz, 2 H, TeCH), 1.75–1.63 (m, 8 H, CH2), 1.56–1.34 (m, 8 H, CH2), 0.92 (t, J = 7.3 Hz, 12H, CH3); 13C NMR (100 MHz, CDCl3): δ = 40.47 (TeCH), 27.09 (CH2), 22.57 (CH2), 13.87 (CH3); MS m/z 328 [M]+; HRMS (+EI) calcd for C14H30Te [M]+ 328.1410, found 328.1409.
- Di-c-pentyl Telluride (1i) [33]. The use of bromocyclopentane (318 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1i (262 mg, 63%) as a yellow oil. Bp 117–119 °C; Rf 0.39 (n-hexane); HPLC tR 8.18 min (condition 1); λmax = 338 nm; IR (ZnSe) 2949, 2863, 1447, 1197 cm−1; 1H NMR (400 MHz, CDCl3): δ = 3.37 (quintet, J = 7.5 Hz, 2 H, TeCH), 2.18–2.12 (m, 4 H, CH2), 1.78–1.68 (m, 8 H, CH2), 1.61–1.54 (m, 4 H, CH2); 13C NMR (100 MHz, CDCl3): δ = 36.95 (TeCH), 25.52 (CH2), 18.39 (CH2); MS m/z 268 [M]+; HRMS (+EI) calcd for C10H18Te [M]+ 268.0471, found 268.0473.
- Di-c-hexyl Telluride (1j) [19]. The use of bromocyclohexane (388 μL, 3.1 mmol, 2.0 eq) and a 5 h reaction time at 25 °C in the general procedure afforded the title compound 1j (182 mg, 40%) as an orange oil. Bp 161–162 °C; Rf 0.38 (n-hexane); HPLC tR 9.94 min (condition 1); λmax = 337 nm; IR (ZnSe) 2920, 2848, 1444, 1166 cm−1; 1H NMR (400 MHz, CDCl3): δ = 3.33–3.26 (m, 2 H, TeCH), 2.13–2.08 (m, 4 H, CH2), 1.78–1.59 (m, 10 H, CH2), 1.42–1.32 (m, 6 H, CH2); 13C NMR (100 MHz, CDCl3): δ = 37.25 (TeCH), 28.18 (CH2), 25.95 (CH2), 22.67 (CH2); MS m/z 296 [M]+; HRMS (+EI) calcd for C12H22Te [M]+ 296.0784, found 296.0785.
- Di-c-heptyl Telluride (1k). The use of bromocycloheptane (431 μL, 3.1 mmol, 2.0 eq) and a 3 h reaction time at 25 °C in the general procedure afforded the title compound 1k (249 mg, 49%) as an orange oil. Bp 156–158 °C; Rf 0.47 (n-hexane); HPLC tR 12.06 min (condition 1); λmax = 347 nm; IR (ZnSe) 2917, 2850, 1454, 1182 cm−1; 1H NMR (400 MHz, CDCl3): δ = 3.44–3.38 (m, 2 H, TeCH), 2.20–2.14 (m, 4 H, CH2), 1.92–1.83 (m, 4 H, CH2), 1.68–1.43 (m, 16 H, CH2); 13C NMR (100 MHz, CDCl3): δ = 37.28 (TeCH), 28.20 (CH2), 25.97 (CH2), 22.68 (CH2); MS m/z 324 [M]+; HRMS (+EI) calcd for C14H26Te [M]+ 324.1097, found 324.1099.
- Diphenyl Telluride (1l) [34]. The use of iodobenzene (350 μL, 3.1 mmol, 2.0 eq) and a 4 h reaction time at 153 °C in the general procedure afforded the title compound 1l (295 mg, 67%) as a red oil. Bp 194–196 °C; Rf 0.50 (1:3 Dichloromethane/n-hexane); HPLC tR 19.36 min (condition 1); λmax = 328, 283, 253 nm; IR (ZnSe) 3051, 1571, 1472, 1015, 725 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.70 (d, J = 7.2 Hz, 4 H, Ar), 7.30–7.18 (m, 6 H, Ar); 13C NMR (100 MHz, CDCl3): δ = 137.99 (Ar), 129.50 (Ar), 127.83 (Ar), 114.66 (Ar); MS m/z 284 [M]+; HRMS (+EI) calcd for C12H10Te [M]+ 283.9845, found 283.9842.
- Bis(3-(trifluoromethyl)phenyl) Telluride (1m). The use of 3-iodobenzotrifluoride (452 μL, 3.1 mmol, 2.0 eq) and a 4 h reaction time at 153 °C in the general procedure afforded the title compound 1m (251 mg, 38%) as a brown oil. Bp 165–167 °C; Rf 0.44 (1:10 Dichloromethane/n-hexane); HPLC tR 21.96 min (condition 1); λmax = 290, 260 nm; IR (ZnSe) 2919, 1486, 1318, 1121, 791 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.96 (s, 2 H, Ar), 7.85 (d, J = 7.8 Hz, 2 H, Ar), 7.57 (d, J = 7.8 Hz, 2 H, Ar), 7.35 (t, J = 7.8 Hz, 2 H, Ar); 13C NMR (100 MHz, CDCl3): δ = 141.28 (Ar), 134.51 (q, 3JCF = 3.8 Hz, Ar), 131.81 (q, 2JCF = 32.4 Hz, Ar), 129.92 (Ar), 125.17 (q, 3JCF = 3.8 Hz, Ar), 123.44 (q, 1JCF = 273 Hz, CF3), 114.70 (Ar); MS m/z 420 [M]+; HRMS (+EI) calcd for C14H8F6Te [M]+ 419.9592, found 419.9595.
- Bis(4-(trifluoromethyl)phenyl) Telluride (1n) [35]. The use of 4-iodobenzotrifluoride (462 μL, 3.1 mmol, 2.0 eq) and a 4 h reaction time at 153 °C in the general procedure afforded the title compound 1n (295 mg, 45%) as an orange solid. Mp 52–54 °C (lit [35]. 52–53 °C); Rf 0.56 (1:10 Dichloromethane/n-hexane); HPLC tR 38.14 min (condition 1); λmax = 225, 268, 299 nm; IR (ZnSe) 2930, 1596, 1393, 1320, 1161, 822 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.79 (d, J = 8.1 Hz, 4 H, Ar), 7.48 (d, J = 8.1 Hz, 4 H, Ar); 13C NMR (100 MHz, CDCl3): δ = 138.03 (Ar), 137.31 (Ar), 130.46 (q, 2JCF = 32.8 Hz, Ar), 126.28 (q, 3JCF = 3.8 Hz, Ar), 123.94 (q, 1JCF = 272 Hz, CF3); MS m/z 420 [M]+; HRMS (+EI) calcd for C14H8F6Te [M]+ 419.9592, found 419.9594.
- Bis(4-nitrophenyl) Telluride (1o) [36]. The use of 4-iodonitrobenzene (782 mg, 3.1 mmol, 2.0 eq) and a 4 h reaction time at 153 °C in the general procedure afforded the title compound 1o (235 mg, 40%) as an orange solid. Mp 170–172 °C (lit [36]. 170–172 °C); Rf 0.18 (1:1 Dichloromethane/n-hexane); HPLC tR 12.74 min (condition 1); λmax = 318, 238 nm; IR (ZnSe) 3056, 1590, 1499, 1335, 836 cm−1; 1H NMR (400 MHz, CDCl3): δ = 8.08 (d, J = 8.3 Hz, 4 H, Ar), 7.83 (d, J = 8.2 Hz, 4 H, Ar); 13C NMR (100 MHz, CDCl3): δ = 138.25 (Ar), 137.32 (Ar), 124.40 (Ar), 123.94 (Ar); MS m/z 374 [M]+; HRMS (+EI) calcd for C12H8N2O4Te [M]+ 373.9546, found 373.9547.
- Bis(4-cyanophenyl) Telluride (1p) [37]. The use of 4-iodobenzonitrle (719 mg, 3.1 mmol, 2.0 eq) and a 4 h reaction time at 153 °C in the general procedure afforded the title compound 1p (192 mg, 37%) as an orange solid. Mp 140–141 °C (lit [37]. 141 °C); Rf 0.28 (1:1 Dichloromethane/n-hexane); HPLC tR 12.83 min (condition 1); λmax = 318, 238 nm; IR (ZnSe) 3081, 2222, 1580, 1477, 812 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.76 (d, J = 7.5 Hz, 4 H, Ar), 7.50 (d, J = 7.5 Hz, 4 H, Ar); 13C NMR (100 MHz, CDCl3): δ = 138.13 (Ar), 137.35 (Ar), 132.81 (Ar), 132.44 (Ar), 118.27 (CN); MS m/z 334 [M]+; HRMS (+EI) calcd for C14H8N2Te [M]+ 333.9750, found 333.9749.
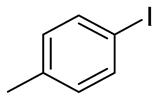
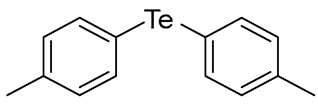
- Bis(4-methylphenyl) Telluride (1q) [36]. The use of 4-iodotoluene (685 mg, 3.1 mmol, 2.0 eq) and a 4 h reaction time at 153 °C in the general procedure afforded the title compound 1q (294 mg, 61%) as an orange solid. Mp 61–62 °C (lit [36]. 67 °C); Rf 0.30 (1:20 Dichloromethane/n-hexane); HPLC tR 23.24 min (condition 1); λmax = 328, 281, 234, 211 nm; IR (ZnSe) 3019, 1484, 1008, 797 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.58 (d, J = 7.8 Hz, 4 H, Ar), 7.02 (d, J = 8.0 Hz, 4 H, Ar), 2.32 (s, 6 H, CH3); 13C NMR (100 MHz, CDCl3): δ = 138.08 (Ar), 137.75 (Ar), 130.37 (Ar), 130.13 (Ar), 21.20 (CH3); MS m/z 312 [M]+; HRMS (+EI) calcd for C14H14Te [M]+ 312.0158, found 312.0161.
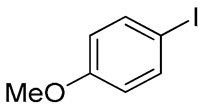
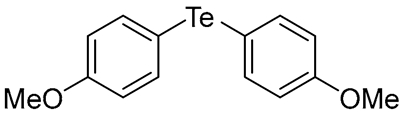
- Bis(4-methoxyphenyl) Telluride (1r) [36]. The use of 4-iodoanisole (735 mg, 3.1 mmol, 2.0 eq) and a 4 h reaction time at 153 °C in the general procedure afforded the title compound 1r (231 mg, 43%) as a brown solid. Mp 51–53 °C (lit [36]. 53–54 °C); Rf 0.40 (1:1 Dichloromethane/n-hexane); HPLC tR 18.24 min (condition 1); λmax = 245 nm; IR (ZnSe) 3006, 2942, 2840, 1487, 1280, 1026, 832 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.62 (d, J = 8.0 Hz, 4 H, Ar), 6.76 (d, J = 8.0 Hz, 4 H, Ar), 3.78 (s, 6 H, CH3); 13C NMR (100 MHz, CDCl3): δ = 159.68 (Ar), 139.72 (Ar), 115.39 (Ar), 104.30 (Ar), 55.16 (CH3); MS m/z 344 [M]+; HRMS (+EI) calcd for C14H14O2Te [M]+ 344.0056, found 344.0058.
4. Conclusions
We report an efficient and tolerable method for the selective synthesis of Na2Te and the corresponding diorganyl tellurides 1 instead of diorganyl ditellurides 2. We aimed to optimize the reactions using NaBH4 under mild and convenient conditions, avoiding the use of hazardous reagents such as sodium (Na), sodium hydride, or hydrazine. The optimal conditions were as follows: (1) Te (1.0 eq), NaBH4 (2.5 eq) in DMF for 1 h at 80 °C; (2) organyl halides (2.0 eq) for 3–5 h at 25–153 °C. Using these optimized conditions, we successfully synthesized eighteen diorganyl tellurides 1, among which four compounds (1g, 1h, 1k, and 1m) are new. The primary alkyl bromides gave 1a–1f in good to excellent yields (75–93%), while the secondary alkyl bromides provided 1g–1k in modest to good yields (40–76%). The aryl iodides gave 1l–1r in modest to good yields (37–67%). Additionally, we conducted mechanistic studies to understand the reaction pathways for the formation of Na2Te and diorganyl tellurides 1. Consequently, we have achieved a systematic study on the selective syntheses of Na2Te and the corresponding diorganyl telluride compounds 1 using NaBH4 as a convenient reducing reagent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29225398/s1. The charts for 1H- and 13C-NMR spectroscopies.
Author Contributions
Conceptualization, H.C. and S.H.L.; data curation, C.K., Y.J.L. and Y.E.K.; methodology, C.K., Y.J.L. and Y.E.K.; writing—original draft, C.K.; writing—review and editing, A.S.N.M., H.C., H.L., M.-S.P. and S.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program (2021R1F1A1062059) of the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (MSIT), and by the Priority Research Centers Program (2016R1A6A1A03007648) of the NRF, funded by the Ministry of Education, Science and Technology (MEST). It was also supported by the Technology Development Project for Safety Management of Household Chemical Products (2022002980001) of the Korea Environmental Industry & Technology Institute (KEITI), funded by the Korea Ministry of Environment (MOE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A. Biochemistry of Tellurium. Biol. Trace Elem. Res. 1996, 55, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Harry, G.J.; Goodrum, J.F.; Bouldin, T.W.; Wagner-Recio, M.; Toews, A.D.; Morell, P. Tellurium-Induced Neuropathy: Metabolic Alterations Associated with Demyelination and Remyelination in Rat Sciatic Nerve. J. Neurochem. 1989, 52, 938–945. [Google Scholar] [CrossRef]
- Irfan, M.; Rehman, R.; Razali, M.R.; Shafiq-Ur-Rehman; Ateeq-Ur-Rehman; Iqbal, M.A. Organotellurium Compounds: An Overview of Synthetic Methodologies. Rev. Inorg. Chem. 2020, 40, 193–232. [Google Scholar] [CrossRef]
- Sredni, B.; Caspi, R.R.; Klein, A.; Kalechmans, Y.; Danziger, Y.; BenYa’akov, M.; Tamari, T.; Shalit, F.; Albeck, M. A New Immunomodulating Compound (AS-101) with Potential Therapeutic Application. Nature 1987, 330, 173–176. [Google Scholar] [CrossRef]
- Ba, L.A.; Döring, M.; Jamier, V.; Jacob, C. Tellurium: An Element with Great Biological Potency and Potential. Org. Biomol. Chem. 2010, 8, 4203–4216. [Google Scholar] [CrossRef]
- Giles, G.I.; Giles, N.M.; Collins, C.A.; Holt, K.; Fry, F.H.; Lowden, P.A.S.; Gutowski, N.J.; Jacob, C. Electrochemical, in Vitro and Cell Culture Analysis of Integrated Redox Catalysts: Implications for Cancer Therapy. Chem. Commun. 2003, 2030–2031. [Google Scholar] [CrossRef]
- Turner, R.J.; Weiner, J.H.; Taylor, D.E. Tellurite-Mediated Thiol Oxidation in Escherichia Coli. Microbiology 1999, 145, 2549–2557. [Google Scholar] [CrossRef]
- Lin, Z.; Lee, C.; Chang, H.; Chang, H. Antibacterial Activities of Tellurium Nanomaterials. Chem. Asian J. 2012, 7, 930–934. [Google Scholar] [CrossRef]
- Morena, A.G.; Bassegoda, A.; Hoyo, J.; Tzanov, T. Hybrid Tellurium–Lignin Nanoparticles with Enhanced Antibacterial Properties. ACS Appl. Mater. Interfaces 2021, 13, 14885–14893. [Google Scholar] [CrossRef]
- Andersson, C.; Brattsand, R.; Hallberg, A.; Engman, L.; Persson, J.; Moldéus, P.; Cotgreave, I. Diaryl Tellurides as Inhibitors of Lipid Peroxidation in Biological and Chemical Systems. Free Rad. Res. 1994, 20, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Gay, B.M.; Luchese, C.; Nogueira, C.W.; Wendler, P.; Macedo, A.; Dos Santos, A.A. Antioxidant Effect of Functionalized Alkyl-Organotellurides: A Study in Vitro. J. Enzyme Inhib. Med. Chem. 2010, 25, 467–475. [Google Scholar] [CrossRef]
- Kim, C.; Lim, Y.J.; Kim, Y.E.; Cho, H.; Lee, S.H. Studies on the Selective Syntheses of Sodium Ditelluride and Dialkyl Ditellurides. Molecules 2022, 27, 8991. [Google Scholar] [CrossRef]
- Lim, Y.J.; Shin, N.H.; Kim, C.; Kim, Y.E.; Cho, H.; Park, M.; Lee, S.H. An Efficient and Practical Method for the Selective Synthesis of Sodium Diselenide and Diorganyl Diselenides through Selenium Reduction. Tetrahedron 2020, 76, 131720. [Google Scholar] [CrossRef]
- Shin, N.H.; Lim, Y.J.; Kim, C.; Kim, Y.E.; Jeong, Y.R.; Cho, H.; Park, M.; Lee, S.H. An Efficient Method for Selective Syntheses of Sodium Selenide and Dialkyl Selenides. Molecules 2022, 27, 5224. [Google Scholar] [CrossRef]
- Rossi, R.A.; Penenory, A.B. Direct (One Pot) Synthesis of Organoselenium and Organotellurium Compounds from the Metals. J. Org. Chem. 1981, 46, 4580–4582. [Google Scholar] [CrossRef]
- Higa, K.T.; Harris, D.C.; Sliwka, M.J.; Lincoln, D.E.; Nissim, S.H. Diallyl Telluride and Synthesis of Diorgano Tellurides. US Patent 5,035,874, 30 July 1991. [Google Scholar]
- Karaghiosoff, K.; Klapötke, T.M.; Krumm, B.; Ruscitti, O.P. Synthesis and Characterization of Dicyclohexyl Telluride; Raman Spectra and Multinuclear NMR Studies of Dialkyl Tellurides. J. Organomet. Chem. 1999, 577, 69–75. [Google Scholar] [CrossRef]
- Suzuki, H.; Nakamura, T.; Sakaguchi, T.; Ohta, K. A Convenient Synthesis of Functionalized Dibenzotellurophenes and Related Compounds via the Intramolecular Telluro Coupling Reaction. the Positive Effect of Heavy Chalcogen Atoms on the Molecular Hyperpolarizability of a Captodative Conjugation System. J. Org. Chem. 1995, 60, 5274–5278. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Huang, F. A Simple Copper Salt-Catalyzed Synthesis of Unsymmetrical Diaryl Selenides and Tellurides from Arylboronic Acids with Diphenyl Diselenide and Ditelluride. Synlett 2005, 13, 2007–2010. [Google Scholar] [CrossRef]
- Saba, S.; Rafique, J.; Braga, A.L. Synthesis of Unsymmetrical Diorganyl Chalcogenides under Greener Conditions: Use of an Iodine/DMSO System, Solvent- and Metal-Free Approach. Adv. Synth. Catal. 2015, 357, 1446–1452. [Google Scholar] [CrossRef]
- Saba, S.; Botteselle, G.V.; Godoi, M.; Frizon, T.E.A.; Galetto, F.Z.; Rafique, J.; Braga, A.L. Copper-Catalyzed Synthesis of Unsymmetrical Diorganyl Chalcogenides (Te/Se/S) from Boronic Acids under Solvent-Free Conditions. Molecules 2017, 22, 1367. [Google Scholar] [CrossRef] [PubMed]
- Goldani, B.; do Sacramento, M.; Lenardão, E.J.; Schumacher, R.F.; Barcellos, T.; Alves, D. Synthesis of Symmetrical and Unsymmetrical Tellurides via Silver Catalysis. New J. Chem. 2018, 42, 15603–15609. [Google Scholar] [CrossRef]
- Liu, J.; Tian, M.; Li, Y.; Shan, X.; Li, A.; Lu, K.; Fagnoni, M.; Protti, S.; Zhao, X. Metal-Free Synthesis of Unsymmetrical Aryl Selenides and Tellurides via Visible Light-Driven Activation of Arylazo Sulfones. Eur. J. Org. Chem. 2020, 47, 7358–7367. [Google Scholar] [CrossRef]
- Sun, N.; Zheng, K.; Zhang, M.; Zheng, G.; Jin, L.; Hu, B.; Shen, Z.; Hu, X. Cu-Catalyzed Chan–Lam Synthesis of Unsymmetrical Aryl Chalcogenides under Aqueous Micellar Conditions. Green Chem. 2023, 25, 2782–2789. [Google Scholar] [CrossRef]
- Detty, M.R. Mild Reductions of Oxides of the Group 6a Elements Sulfur, Selenium, and Tellurium with (Phenylseleno)trimethylsilane. J. Org. Chem. 1979, 44, 4528–4531. [Google Scholar] [CrossRef]
- Balfe, M.P.; Chaplin, C.A.; Phillips, H. The Oxidation of Certain Alkyl Tellurides. J. Chem. Soc. 1938, 341–347. [Google Scholar] [CrossRef]
- Balfe, M.P.; Nandi, K.N. Further Experiments on the Oxidation of Alkyl Tellurides. J. Chem. Soc. 1941, 70–72. [Google Scholar] [CrossRef]
- You, Y.; Ahsan, K.; Detty, M.R. Mechanistic Studies of the Tellurium(II)/Tellurium(IV) Redox Cycle in Thiol Peroxidase-Like Reactions of Diorganotellurides in Methanol. J. Am. Chem. Soc. 2003, 125, 4918–4927. [Google Scholar] [CrossRef]
- Suzuki, H.; Nakamura, T. Sodium Telluride in N-Methyl-2-Pyrrolidone; an Efficient Telluration System for the Synthesis of Aromatic Tellurides and Ditellurides. Synthesis 1992, 6, 549–551. [Google Scholar] [CrossRef]
- Kirsch, G.; Goodman, M.M.; Knapp, F.F. Organotellurium Compounds of Biological Interest-Unique Properties of the N-Chlorosuccinimide Oxidation Product of 9-Telluraheptadecanoic Acid. Organometallics 1983, 2, 357–363. [Google Scholar] [CrossRef]
- Neto, P.B.R.; Santana, S.O.; Levitre, G.; Galdino, D.; Oliveira, J.L.; Ribeiro, R.T.; Barros, M.E.S.B.; Bieber, L.W.; Menezes, P.H.; Navarro, M. Electrochemical Synthesis of Organochalcogenides in Aqueous Medium. Green Chem. 2016, 18, 657–661. [Google Scholar] [CrossRef]
- Suzuki, H.; Inouye, M. A Convenient Synthesis of Symmetrical Diaryl Tellurides using Tellurium/Rongalite as Telluration System. Chem. Lett. 1985, 14, 389–390. [Google Scholar] [CrossRef]
- Engman, L.; Perssorv, N.J.; Andersson, C.M.; Berglundb, M. Application of the Hammett Equation to the Electrochemical Oxidation of Diary1 Chalcogenides and Aryl Methyl Chalcogenides. J. Chem. Soc. Perkin Trans. 2 1992, 8, 1309–1313. [Google Scholar] [CrossRef]
- Engman, L. New General Synthesis of Diaryl Tellurides from Aromatic Amines. J. Org. Chem. 1983, 48, 2920–2922. [Google Scholar] [CrossRef]
- Degrand, C. Electrochemical Synthesis by the SRN1 Mechanism of 4-Phenylselenobenzonitrile and 4-Phenyltellurobenzonitrile. J. Chem. Soc. Chem. Commun. 1986, 315, 1113–1115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).