Graphene Quantum Dots from Agricultural Wastes: Green Synthesis and Advanced Applications for Energy Storage
Abstract
1. Introduction
2. Results
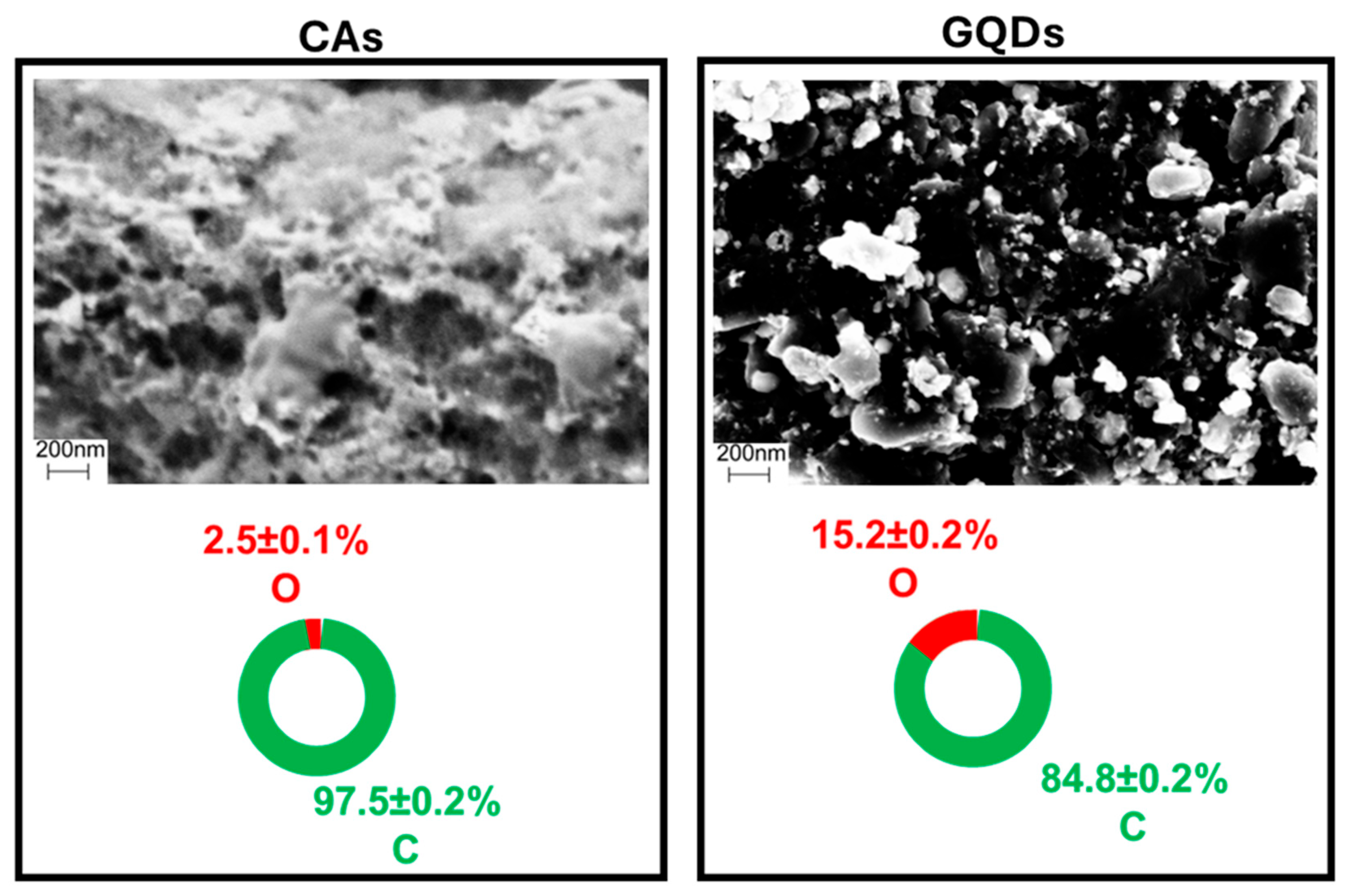
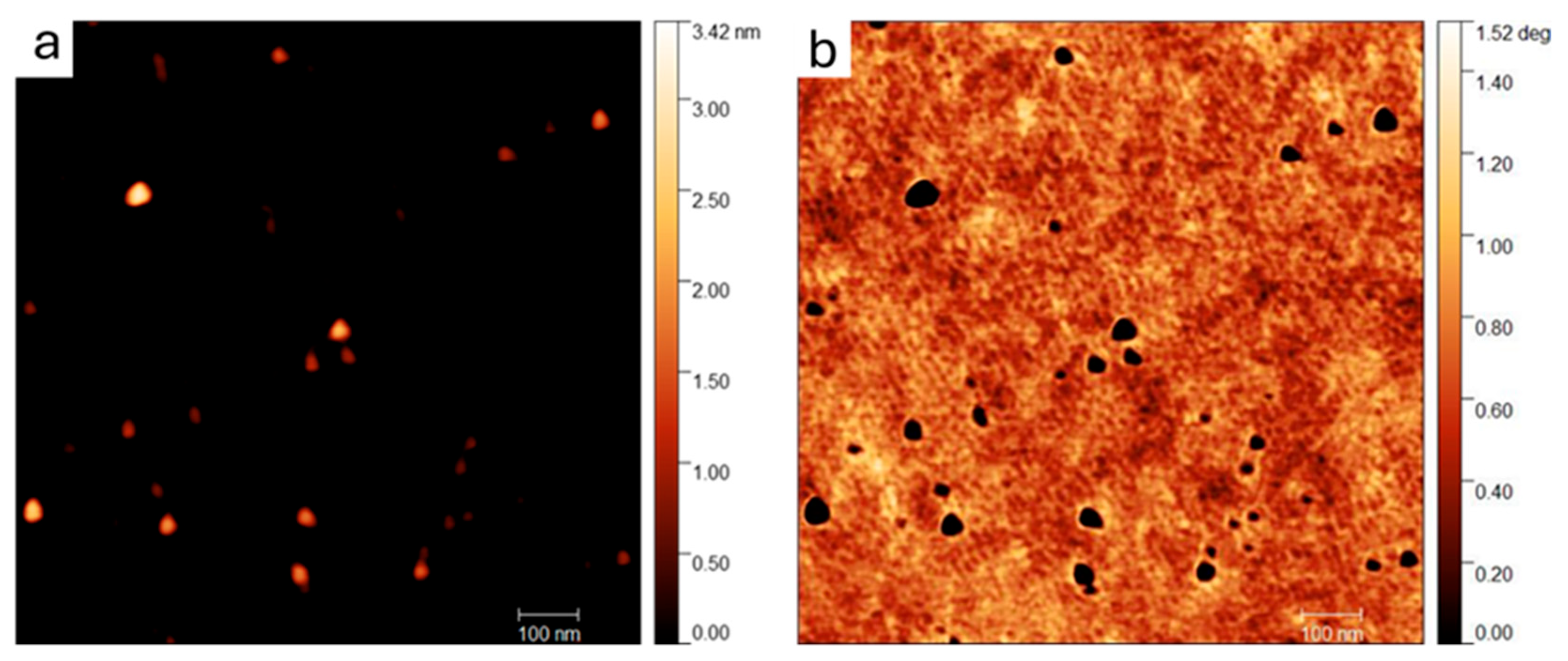
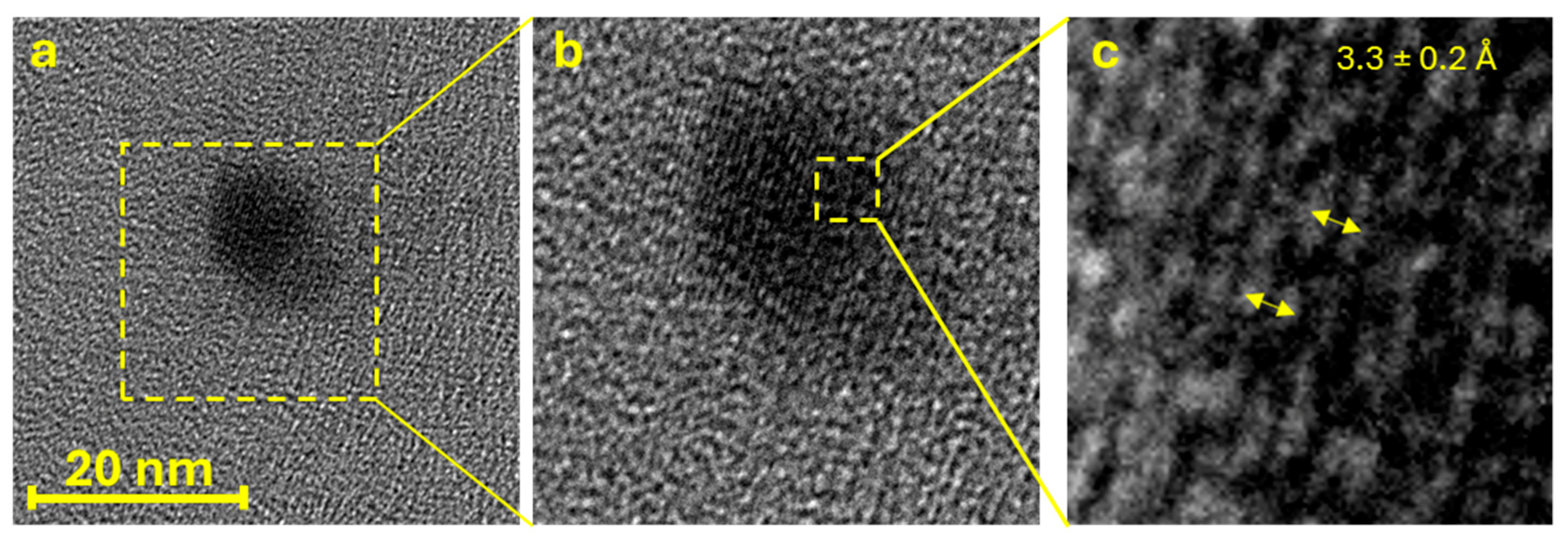
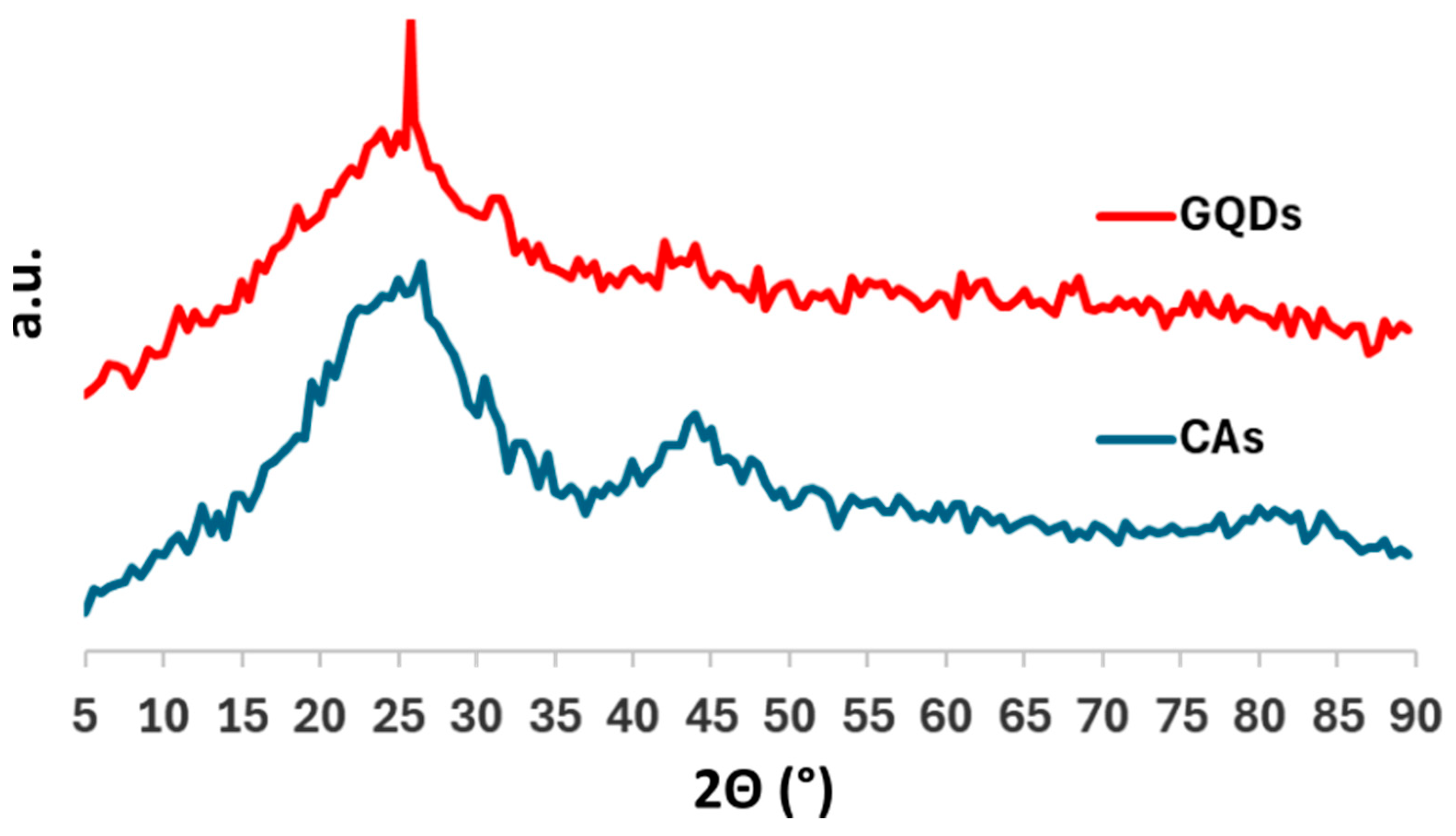
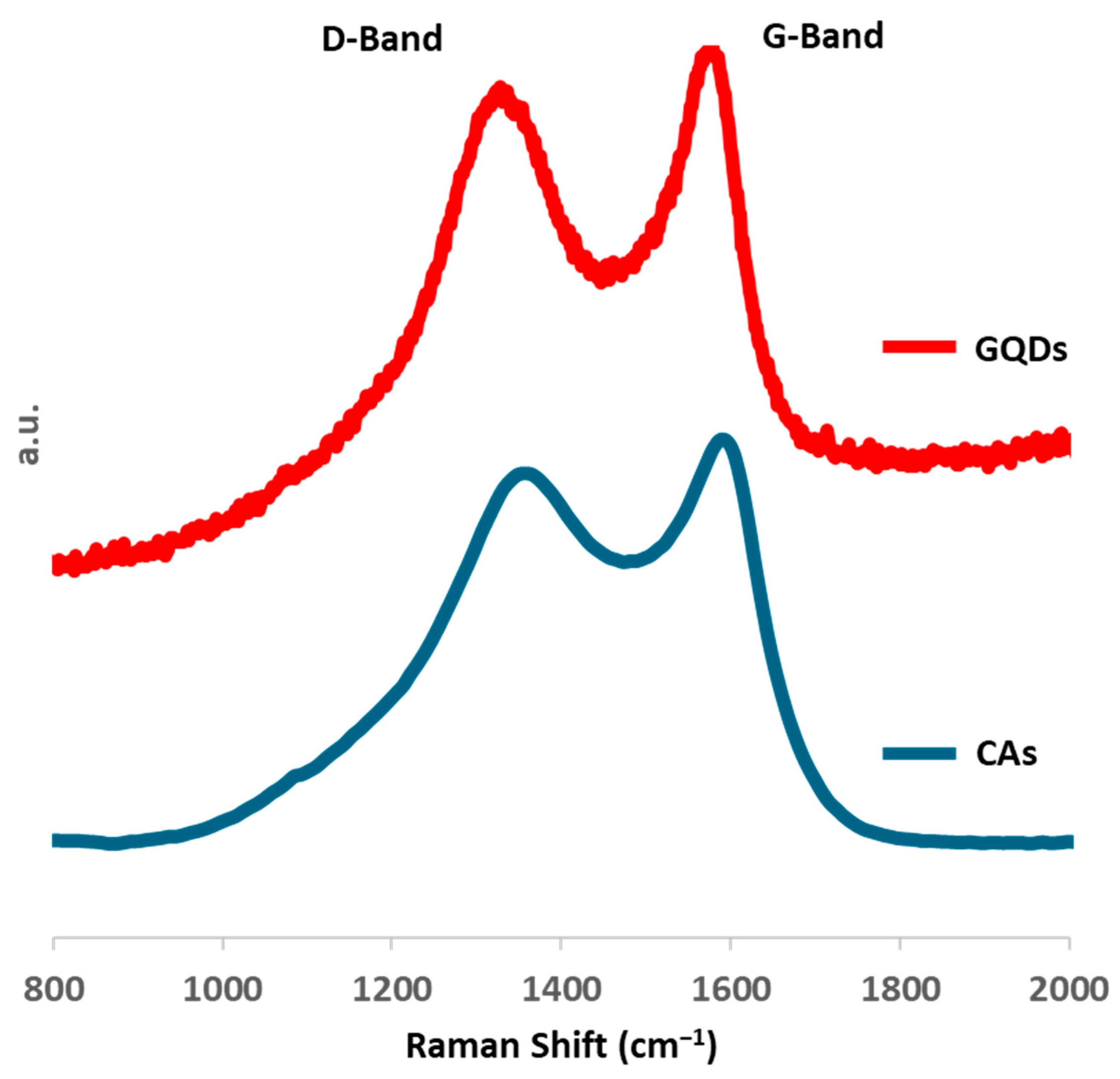
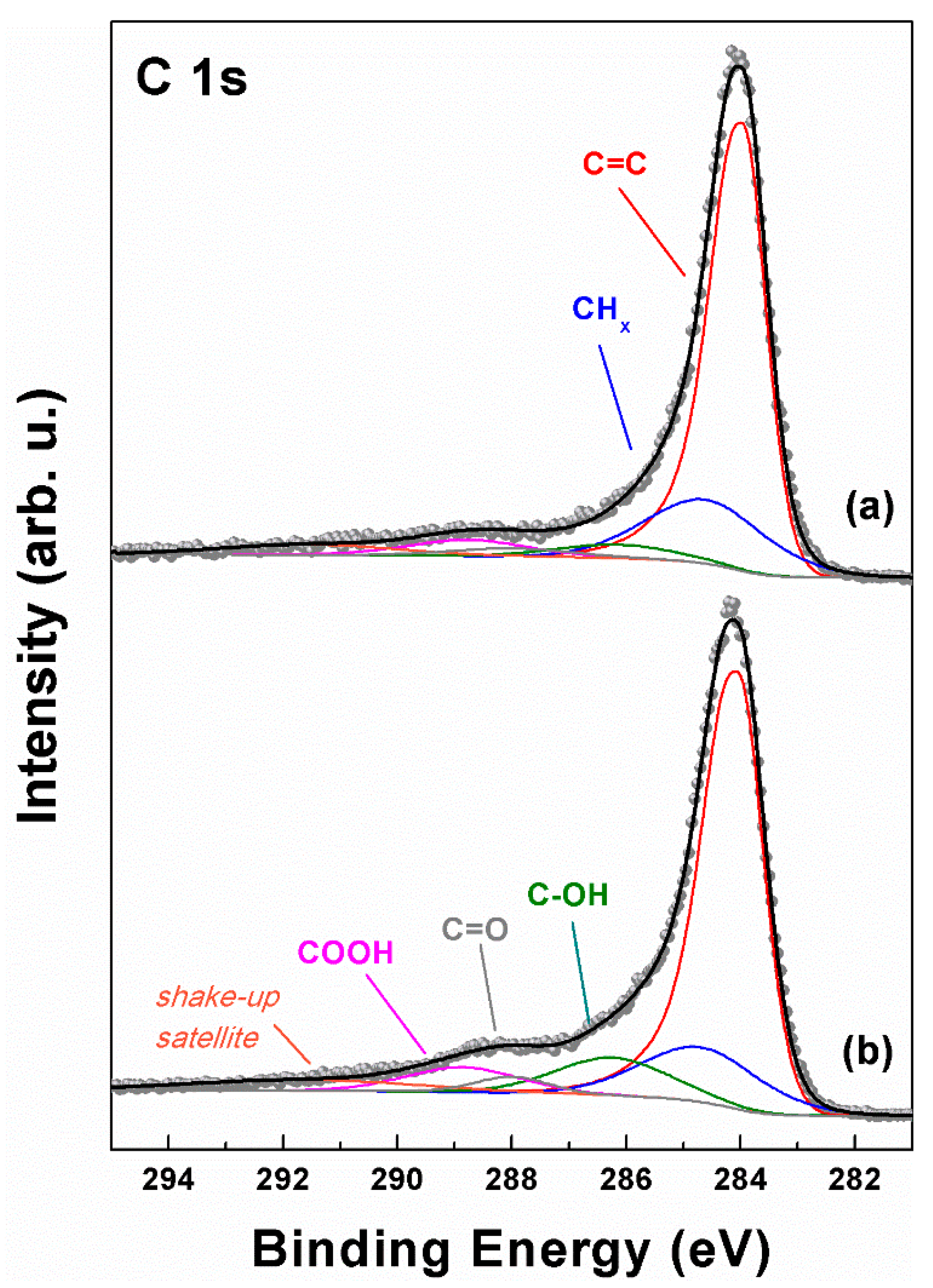
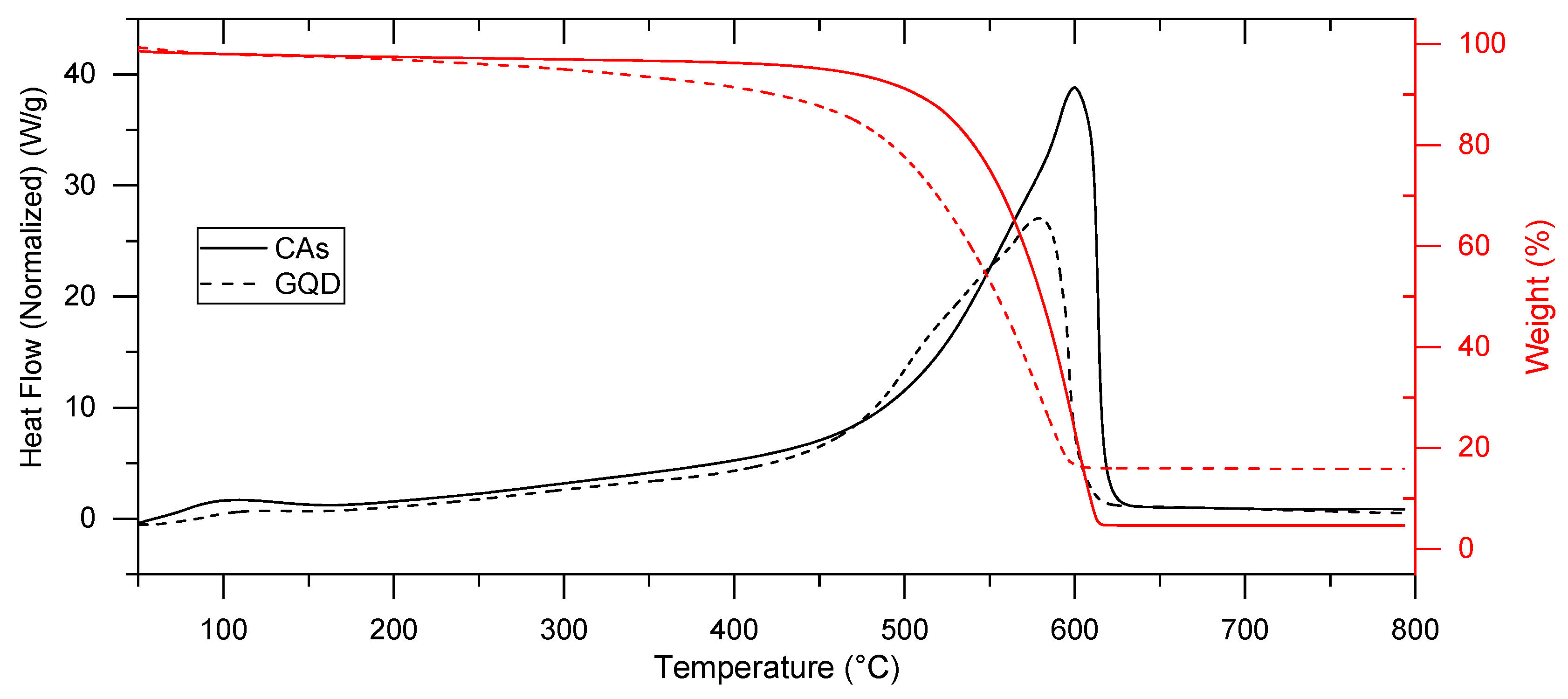
2.1. GQD Characterization
2.2. GQD-Based Electrode Materials for Energy Storage
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Kumar, R.; Kumar, K.; Thakur, N. Sustainable Applications of Biowaste-Derived Carbon. Dots in Eco-Friendly Technological Advancements: A Review. Mater. Sci. Eng. B 2024, 305, 117414. [Google Scholar] [CrossRef]
- Dell’Era, A.; Pasquali, M.; Tarquini, G.; Scaramuzzo, F.A.; De Gasperis, P.; Prosini, P.P.; Mezzi, A.; Tuffi, R.; Cafiero, L. Carbon. Powder Material Obtained from an Innovative High Pressure Water Jet Recycling Process of Tires Used as Anode in Alkali Ion (Li, Na) Batteries. Solid State Ion. 2018, 324, 20–27. [Google Scholar] [CrossRef]
- Kartick, B.; Srivastava, S.K.; Srivastava, I. Green Synthesis of Graphene. J. Nanosci. Nanotechnol. 2013, 13, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Fekry, N.A.; Abdelfattah, A.M. A Novel Nanobiosorbent of Functionalized Graphene Quantum Dots from Rice Husk with Barium Hydroxide for Microwave Enhanced Removal of Lead (II) and Lanthanum (III). Bioresour. Technol. 2020, 298, 122514. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Nordin, N.A.H.M.; Ismail, N.; Zakria, H.S.; Junoh, H.; Aziz, M.H.A. A Review on Sustainable Graphene Production from Rice Husks: Strategies and Key Considerations. Chem. Eng. J. 2024, 497, 154408. [Google Scholar] [CrossRef]
- Childs, N.; Lebeau, B. Rice Outlook: December 2023; USDA, Economic Research Service: Washington, DC, USA, 2023. [Google Scholar]
- Lim, J.S.; Abdul Manan, Z.; Hashim, H.; Wan Alwi, S.R. Towards an Integrated, Resource-Efficient Rice Mill Complex. Resour. Conserv. Recycl. 2013, 75, 41–51. [Google Scholar] [CrossRef]
- Omrani, E.; Menezes, P.L.; Rohatgi, P.K. State of the Art on Tribological Behavior of Polymer Matrix Composites Reinforced with Natural Fibers in the Green Materials World. Eng. Sci. Technol. Int. J. 2016, 19, 717–736. [Google Scholar] [CrossRef]
- Singh, B. Rice Husk Ash. In Waste and Supplementary Cementitious Materials in Concrete: Characterisation, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 417–460. ISBN 9780081021569. [Google Scholar]
- Jessy Mercy, D.; Kiran, V.; Thirumalai, A.; Harini, K.; Girigoswami, K.; Girigoswami, A. Rice Husk Assisted Carbon Quantum Dots Synthesis for Amoxicillin Sensing. Results Chem. 2023, 6, 101219. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.I.; González, L.A. Review on the Physicochemical Treatments of Rice Husk for Production of Advanced Materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Deng, D. Li-Ion Batteries: Basics, Progress, and Challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Dell’Era, A.; Scaramuzzo, F.A.; Stoller, M.; Lupi, C.; Rossi, M.; Passeri, D.; Pasquali, M. Spinning Disk Reactor Technique for the Synthesis of Nanometric Sulfur TiO2 Core-Shell Powder for Lithium Batteries. Appl. Sci. 2019, 9, 1913. [Google Scholar] [CrossRef]
- Roy, K.; Banerjee, A.; Ogale, S. Search for New Anode Materials for High Performance Li-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 20326–20348. [Google Scholar] [CrossRef] [PubMed]
- Dell’Era, A.; Pasquali, M.; Bauer, E.M.; Vecchio Ciprioti, S.; Scaramuzzo, F.A.; Lupi, C. Synthesis, Characterization, and Electrochemical Behavior of LiMnxFe(1−x)PO4 Composites Obtained from Phenylphosphonate-Based Organic-Inorganic Hybrids. Materials 2018, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.; Allam, N.K. Recent Advances in the Design of Cathode Materials for Li-Ion Batteries. RSC Adv. 2020, 10, 21662–21685. [Google Scholar] [CrossRef] [PubMed]
- Tarquini, G.; Dell’Era, A.; Prosini, P.P.; Scaramuzzo, F.A.; Lupi, C.; Pasquali, M. Polysulfide Solution Effects on LiS Batteries Performances. J. Electroanal. Chem. 2020, 870, 114239. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-Ion Batteries toward Na-Ion Chemistries: Challenges and Opportunities. Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, M.; Li, X. Carbon Nanomaterials and Their Composites for Supercapacitors. Carbon Energy 2022, 4, 950–985. [Google Scholar] [CrossRef]
- Dissanayake, K.; Kularatna-Abeywardana, D. A Review of Supercapacitors: Materials, Technology, Challenges, and Renewable Energy Applications. J. Energy Storage 2024, 96, 112563. [Google Scholar] [CrossRef]
- Tyagi, A.; Joshi, M.C.; Shah, A.; Thakur, V.K.; Gupta, R.K. Hydrothermally Tailored Three-Dimensional Ni-V Layered Double Hydroxide Nanosheets as High-Performance Hybrid Supercapacitor Applications. ACS Omega 2019, 4, 3257–3267. [Google Scholar] [CrossRef]
- Yadav, K.K.; Singh, H.; Rana, S.; Sunaina; Sammi, H.; Nishanthi, S.T.; Wadhwa, R.; Khan, N.; Jha, M. Utilization of Waste Coir Fibre Architecture to Synthesize Porous Graphene Oxide and Their Derivatives: An Efficient Energy Storage Material. J. Clean. Prod. 2020, 276, 124240. [Google Scholar] [CrossRef]
- Xiao, J.; Momen, R.; Liu, C. Application of Carbon Quantum Dots in Supercapacitors: A Mini Review. Electrochem. Commun. 2021, 132, 107143. [Google Scholar] [CrossRef]
- Jin, H.; Wu, S.; Li, T.; Bai, Y.; Wang, X.; Zhang, H.; Xu, H.; Kong, C.; Wang, H. Synthesis of Porous Carbon Nano-Onions Derived from Rice Husk for High-Performance Supercapacitors. Appl. Surf. Sci. 2019, 488, 593–599. [Google Scholar] [CrossRef]
- Kalluri, A.; Dharmadhikari, B.; Debnath, D.; Patra, P.; Kumar, C.V. Advances in Structural Modifications and Properties of Graphene Quantum Dots for Biomedical Applications. ACS Omega 2023, 8, 21358–21376. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and Graphene Quantum Dots: A Review on Syntheses, Characterization, Biological and Sensing Applications for Neurotransmitter Determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef]
- Zhang, S.; Sui, L.; Dong, H.; He, W.; Dong, L.; Yu, L. High-Performance Supercapacitor of Graphene Quantum Dots with Uniform Sizes. ACS Appl. Mater. Interfaces 2018, 10, 12983–12991. [Google Scholar] [CrossRef]
- Michenzi, C.; Scaramuzzo, F.; Salvitti, C.; Pepi, F.; Troiani, A.; Chiarotto, I. Photo-Activated Carbon Dots as Catalysts in Knoevenagel Condensation: An Advance in the Synthetic Field. Photochem 2024, 4, 361–376. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and Applications of Graphene Quantum Dots—A Review. Nanotechnol. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Bak, S.; Kim, D.; Lee, H. Graphene Quantum Dots and Their Possible Energy Applications: A Review. Curr. Appl. Phys. 2016, 16, 1192–1201. [Google Scholar] [CrossRef]
- Khaleghi Abbasabadi, M.; Azarifar, D. β-Alanine-Functionalized Magnetic Graphene Oxide Quantum Dots: An Efficient and Recyclable Heterogeneous Basic Catalyst for the Synthesis of 1H-Pyrazolo [1,2-b]Phthalazine-5,10-Dione and 2,3-Dihydroquinazolin-4(1H)-One Derivatives. Appl. Organomet. Chem. 2020, 34, e5872. [Google Scholar] [CrossRef]
- Zahir, N.; Magri, P.; Luo, W.; Gaumet, J.J.; Pierrat, P. Recent Advances on Graphene Quantum Dots for Electrochemical Energy Storage Devices. Energy Environ. Mater. 2022, 5, 201–214. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Jiang, G.; Li, G.; Zhu, J.; Xiao, M.; Zhu, Y.; Gao, R.; Yu, A.; Feng, M.; et al. Graphene Quantum Dots-Based Advanced Electrode Materials: Design, Synthesis and Their Applications in Electrochemical Energy Storage and Electrocatalysis. Adv. Energy Mater. 2020, 10, 2001275. [Google Scholar] [CrossRef]
- Adaikalapandi, S.; Thangadurai, T.D.; Manjubaashini, N.; Nataraj, D.; Babu, T.G.S.; Kumar, S.M. Bamboo Stem Biomass Waste-Derived Excitation-Dependent Carbon Dots for Nanomolar Detection of Fungicide Dodine in Real Samples and Their PH-Sensitive Bacterial Interaction Studies. Diam. Relat. Mater. 2024, 141, 110692. [Google Scholar] [CrossRef]
- Rajkishore, S.K.; Devadharshini, K.P.; Moorthy, P.S.; Kalyan, V.S.R.K.; Sunitha, R.; Prasanthrajan, M.; Maheswari, M.; Subramanian, K.S.; Sakthivel, N.; Sakrabani, R. Novel Synthesis of Carbon Dots from Coconut Wastes and Its Potential as Water Disinfectant. Sustainability 2023, 15, 10924. [Google Scholar] [CrossRef]
- Dhongde, N.R.; Das, N.K.; Banerjee, T.; Rajaraman, P.V. Synthesis of Carbon Quantum Dots from Rice Husk for Anti-Corrosive Coating Applications: Experimental and Theoretical Investigations. Ind. Crops Prod. 2024, 212, 118329. [Google Scholar] [CrossRef]
- Gaayathri, K.H.; Debnath, R.; Roy, M.; Saha, M. Sustainable Production of Graphene Quantum Dots from Rice Husk for Photo-Degradation of Organochlorine Pesticides. Mater. Sci. Eng. Technol. 2024, 55, 487–495. [Google Scholar] [CrossRef]
- Dini, D.; Cognigni, F.; Passeri, D.; Scaramuzzo, F.A.; Pasquali, M.; Rossi, M. Review—Multiscale Characterization of Li-Ion Batteries through the Combined Use of Atomic Force Microscopy and X-ray Microscopy and Considerations for a Correlative Analysis of the Reviewed Data. J. Electrochem. Soc. 2021, 168, 126522. [Google Scholar] [CrossRef]
- Cognigni, F.; Pasquali, M.; Prosini, P.P.; Paoletti, C.; Aurora, A.; Scaramuzzo, F.A.; Rossi, M. X-ray Microscopy: A Non-Destructive Multi-Scale Imaging to Study the Inner Workings of Batteries. ChemElectroChem 2023, 10, e202201081. [Google Scholar] [CrossRef]
- Palser, A.H.R. Interlayer Interactions in Graphite and Carbon Nanotubes. Phys. Chem. Chem. Phys. 1999, 1, 4459–4464. [Google Scholar] [CrossRef]
- Hod, O. Graphite and Hexagonal Boron-Nitride Have the Same Interlayer Distance. Why? J. Chem. Theory Comput. 2012, 8, 1360–1369. [Google Scholar] [CrossRef]
- Lim, D.J.; Marks, N.A.; Rowles, M.R. Universal Scherrer Equation for Graphene Fragments. Carbon N. Y. 2020, 162, 475–480. [Google Scholar] [CrossRef]
- Batool, M.; Hussain, D.; Akrem, A.; Najam-ul-Haq, M.; Saeed, S.; Zaka, S.M.; Nawaz, M.S.; Buck, F.; Saeed, Q. Graphene Quantum Dots as Cysteine Protease Nanocarriers against Stored Grain Insect Pests. Sci. Rep. 2020, 10, 3444. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascòn, J.M.D. Raman Microprobe Studies On Carbon Materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A. Raman Spectroscopic Investigations of Activated Carbon Materials. J. Appl. Phys. 2002, 92, 902–909. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Suneetha, R.B. Spectral, Thermal and Morphological Characterization of Biodegradable Graphene Oxide-Chitosan Nanocomposites. J. Nanosci. Technol. 2018, 4, 342–344. [Google Scholar] [CrossRef]
- Wang, H.; Qi, C.; Yang, A.; Wang, X.; Xu, J. One-Pot Synthesis of Bright Blue Luminescent N-Doped GQDs. Nanomaterials 2021, 11, 2798. [Google Scholar] [CrossRef]
- Sudesh; Kumar, N.; Das, S.; Das, S.; Bernhard, C.; Varma, G.D. Effect of Graphene Oxide Doping on Superconducting Properties of Bulk MgB2. Supercond. Sci. Technol. 2013, 26, 095008. [Google Scholar] [CrossRef]
- Bello, R.H.; Coelho, L.A.F.; Becker, D. Role of Chemical Functionalization of Carbon Nanoparticles in Epoxy Matrices. J. Compos. Mater. 2018, 52, 4. [Google Scholar] [CrossRef]
- Hayyan, M.; Abo-Hamad, A.; AlSaadi, M.A.H.; Hashim, M.A. Functionalization of Graphene Using Deep Eutectic Solvents. Nanoscale Res. Lett. 2015, 10, 324. [Google Scholar] [CrossRef]
- Marrani, A.G.; Coico, A.C.; Giacco, D.; Zanoni, R.; Scaramuzzo, F.A.; Schrebler, R.; Dini, D.; Bonomo, M.; Dalchiele, E.A. Integration of Graphene onto Silicon through Electrochemical Reduction of Graphene Oxide Layers in Non-Aqueous Medium. Appl. Surf. Sci. 2018, 445, 404–414. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate Chemical Analysis of Oxygenated Graphene-Based Materials Using X-ray Photoelectron Spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Larciprete, R.; Lacovig, P.; Gardonio, S.; Baraldi, A.; Lizzit, S. Atomic Oxygen on Graphite Chemical Characterization and Thermal Reduction. J. Phys. Chem. C 2012, 116, 18. [Google Scholar] [CrossRef]
- Lin, C.Y.; Cheng, C.E.; Wang, S.; Shiu, H.W.; Chang, L.Y.; Chen, C.H.; Lin, T.W.; Chang, C.S.; Chien, F.S.S. Synchrotron Radiation Soft X-ray Induced Reduction in Graphene Oxide Characterized by Time-Resolved Photoelectron Spectroscopy. J. Phys. Chem. C 2015, 119, 23. [Google Scholar] [CrossRef]
- Estrade-Szwarckopf, H. XPS Photoemission in Carbonaceous Materials: A “Defect” Peak beside the Graphitic Asymmetric Peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Briggs, J.D.; Seah, M.P. Practical Surface Analysis. In Auger and X-ray Photoelectron Spectroscopy; John Wiley Sons: Chichester, UK, 1990; Volume 1. [Google Scholar]
- Tiberio, F.; Amato, F.; Desiderio, C.; Vincenzoni, F.; Perini, G.; Moretti, I.; Augello, A.; Friggeri, G.; Cui, L.; Giaccari, L.; et al. The Osteoconductive Properties of Graphene-Based Material Surfaces Are Finely Tuned by the Conditioning Layer and Surface Chemistry. Mater. Adv. 2024, 5, 4772–4785. [Google Scholar] [CrossRef]
- Amato, F.; Ferrari, I.; Motta, A.; Zanoni, R.; Dalchiele, E.A.; Marrani, A.G. Assessing the Evolution of Oxygenated Functional Groups on the Graphene Oxide Surface upon Mild Thermal Annealing in Water. RSC Adv. 2023, 13, 29308–29315. [Google Scholar] [CrossRef]
- Morais, A.; Alves, J.P.C.; Lima, F.A.S.; Lira-Cantu, M.; Nogueira, A.F. Enhanced Photovoltaic Performance of Inverted Hybrid Bulk-Heterojunction Solar Cells Using TiO 2 /Reduced Graphene Oxide Films as Electron Transport Layers. J. Photonics Energy 2015, 5, 057408. [Google Scholar] [CrossRef]
- Farivar, F.; Lay Yap, P.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. C 2021, 7, 41. [Google Scholar] [CrossRef]
- Özge Alaş Çolak, M.; Güngör, A.; Akturk, M.B.; Erdem, E.; Genç, R. Unlocking the Full Potential of Citric Acid-Synthesized Carbon Dots as a Supercapacitor Electrode Material via Surface Functionalization. Nanoscale 2023, 16, 719–733. [Google Scholar] [CrossRef]
- Dharmalingam, P.; Ramanan, V.; Karthikeyan, G.G.; Palani, N.S.; Ilangovan, R.; Ramamurthy, P. A Study on the Electrochemical Performance of Nitrogen and Oxygen Co-Doped Carbon Dots Derived from a Green Precursor for Supercapacitor Applications. J. Mater. Sci. Mater. Electron. 2017, 28, 18489–18496. [Google Scholar] [CrossRef]
- Baslak, C.; Demirel, S.; Dogu, S.; Ozturk, G.; Kocyigit, A.; Yıldırım, M. Green Synthesis Capacitor of Carbon Quantum Dots from Stachys Euadenia. Env. Prog. Sustain. Energy 2024, 43, e14340. [Google Scholar] [CrossRef]
- Wei, J.S.; Ding, H.; Zhang, P.; Song, Y.F.; Chen, J.; Wang, Y.G.; Xiong, H.M. Carbon Dots-NiCo2O4 Nanocomposites with Various Morphologies for High Performance Supercapacitors. Small 2016, 12, 5927–5934. [Google Scholar] [CrossRef] [PubMed]
- Song, T.B.; Huang, Z.H.; Niu, X.Q.; Liu, J.; Wei, J.S.; Chen, X.B.; Xiong, H.M. Applications of Carbon Dots in Next-Generation Lithium-Ion Batteries. ChemNanoMat 2020, 6, 1421–1436. [Google Scholar] [CrossRef]
- Atanasio, P.; Zampiva, R.Y.S.; Fornari, A.; Mancini, C.; Rossi, M.; Pasquali, M.; Scaramuzzo, F.A. Innovative and green synthesis of Carbon aerogels for advanced supercapacitors. 2024; submitted. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanasio, P.; Zampiva, R.Y.S.; Buccini, L.; Di Conzo, C.; Proietti, A.; Mura, F.; Aurora, A.; Marrani, A.G.; Passeri, D.; Rossi, M.; et al. Graphene Quantum Dots from Agricultural Wastes: Green Synthesis and Advanced Applications for Energy Storage. Molecules 2024, 29, 5666. https://doi.org/10.3390/molecules29235666
Atanasio P, Zampiva RYS, Buccini L, Di Conzo C, Proietti A, Mura F, Aurora A, Marrani AG, Passeri D, Rossi M, et al. Graphene Quantum Dots from Agricultural Wastes: Green Synthesis and Advanced Applications for Energy Storage. Molecules. 2024; 29(23):5666. https://doi.org/10.3390/molecules29235666
Chicago/Turabian StyleAtanasio, Pierfrancesco, Rubia Y. S. Zampiva, Luca Buccini, Corrado Di Conzo, Anacleto Proietti, Francesco Mura, Annalisa Aurora, Andrea G. Marrani, Daniele Passeri, Marco Rossi, and et al. 2024. "Graphene Quantum Dots from Agricultural Wastes: Green Synthesis and Advanced Applications for Energy Storage" Molecules 29, no. 23: 5666. https://doi.org/10.3390/molecules29235666
APA StyleAtanasio, P., Zampiva, R. Y. S., Buccini, L., Di Conzo, C., Proietti, A., Mura, F., Aurora, A., Marrani, A. G., Passeri, D., Rossi, M., Pasquali, M., & Scaramuzzo, F. A. (2024). Graphene Quantum Dots from Agricultural Wastes: Green Synthesis and Advanced Applications for Energy Storage. Molecules, 29(23), 5666. https://doi.org/10.3390/molecules29235666












