Selective Co(II) and Ni(II) Separation Using the Trihexyl(tetradecyl)phosphonium Decanoate Ionic Liquid
Abstract
1. Introduction
2. Results and Discussion
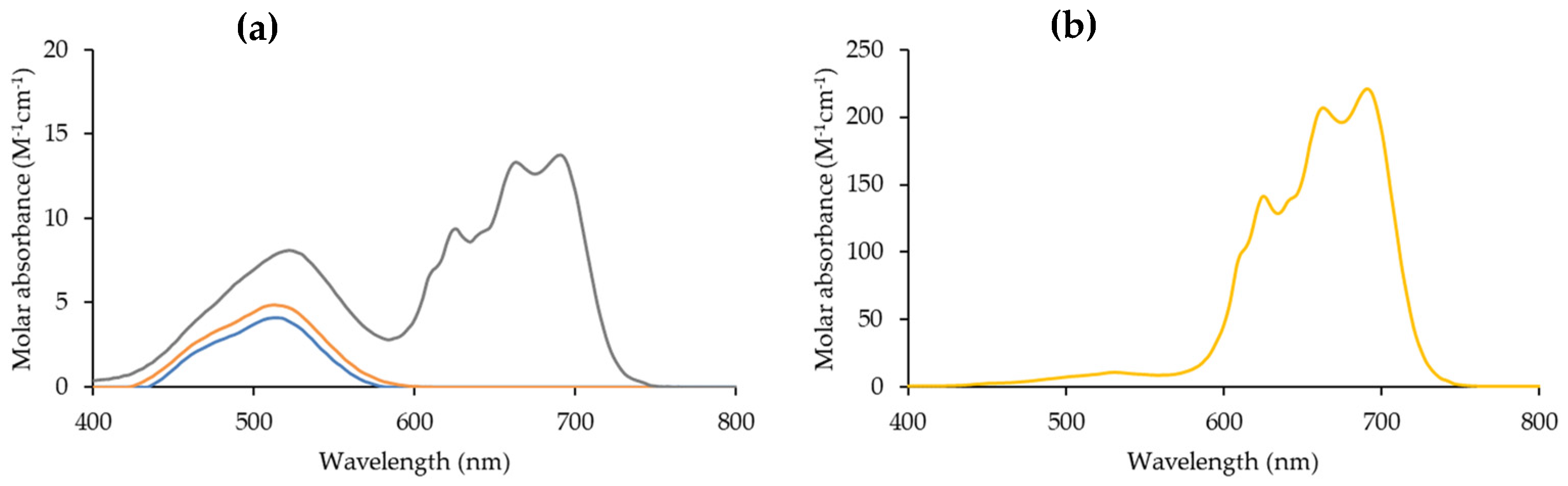
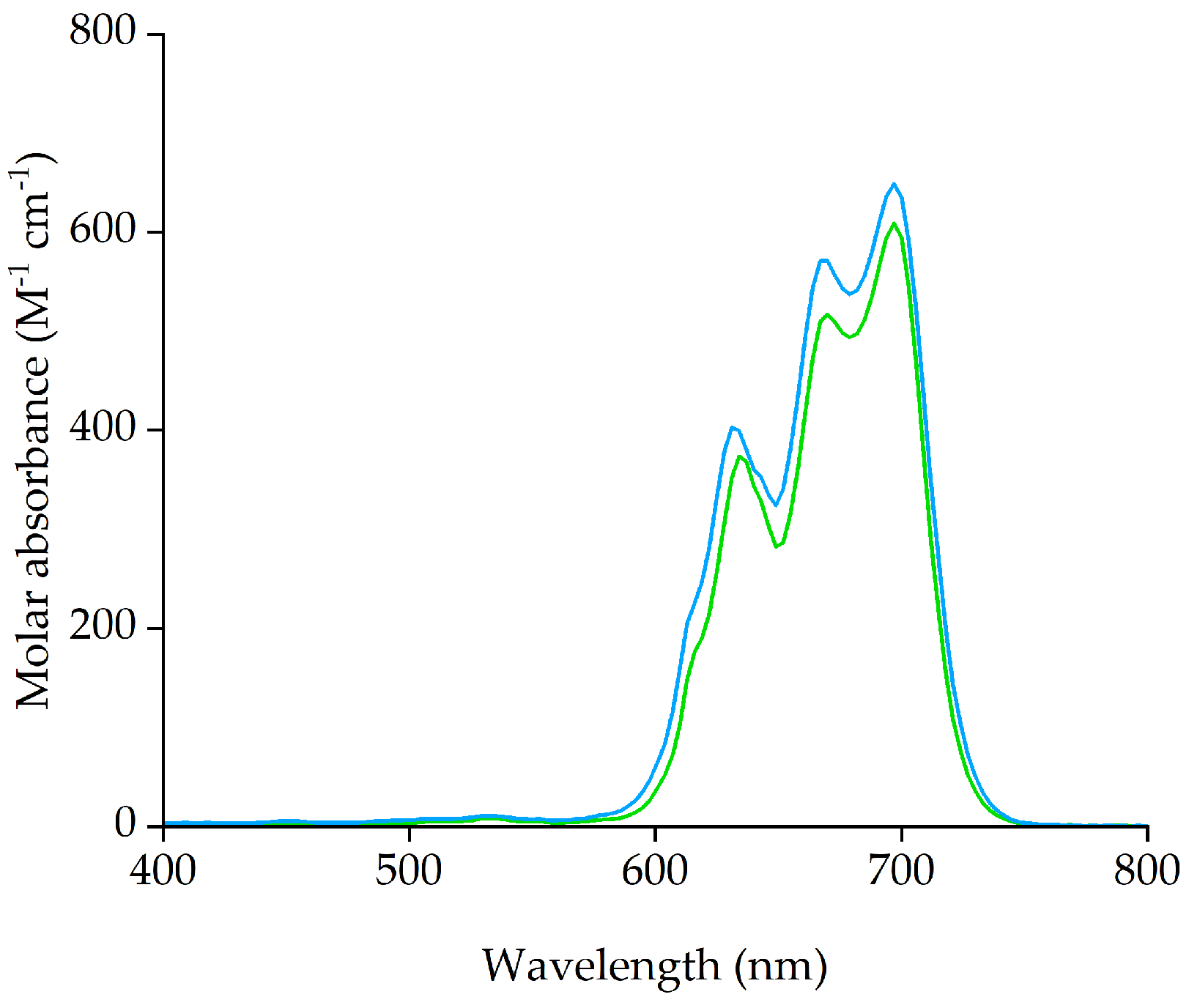
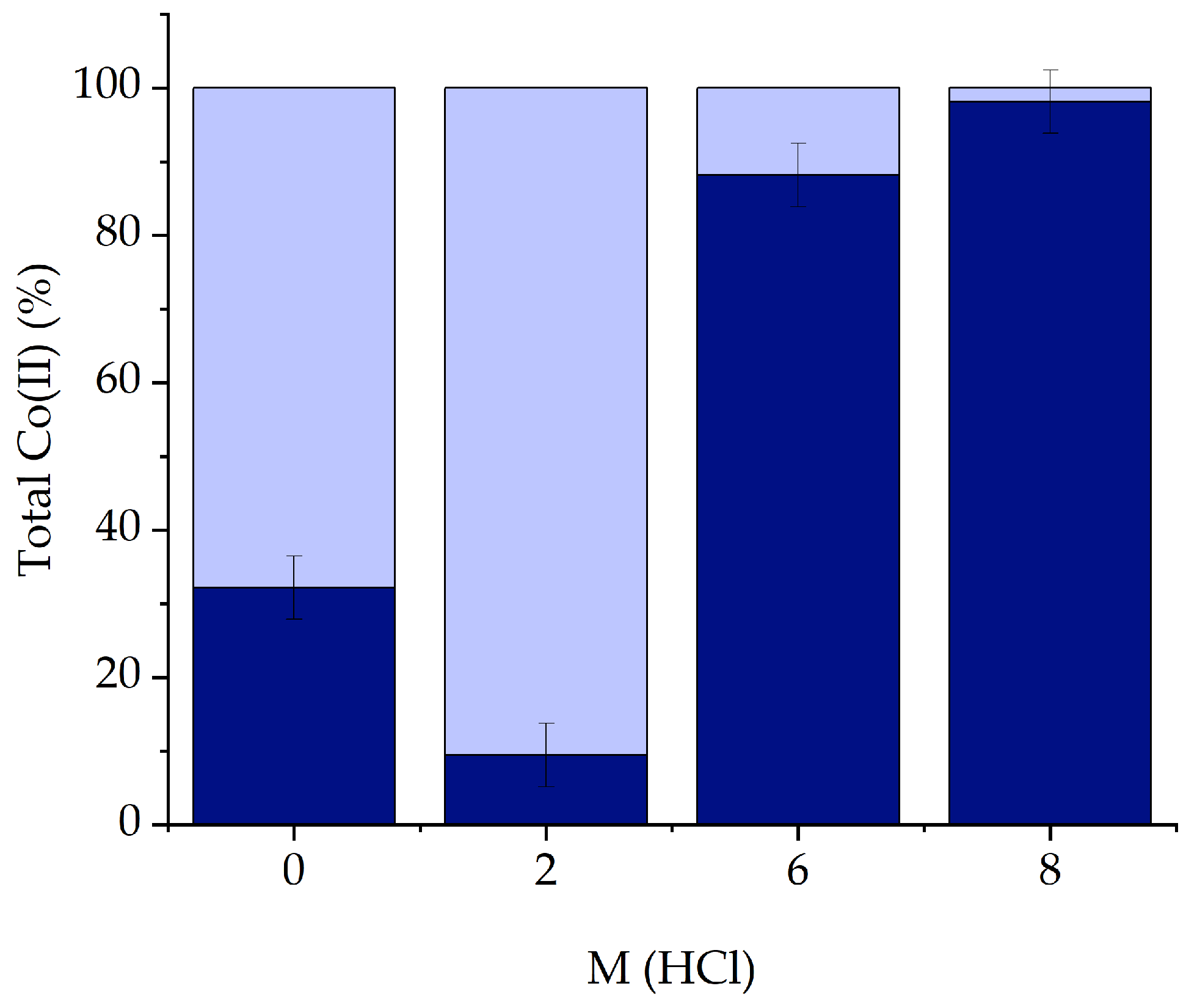
2.1. Liquid-Liquid Extractions
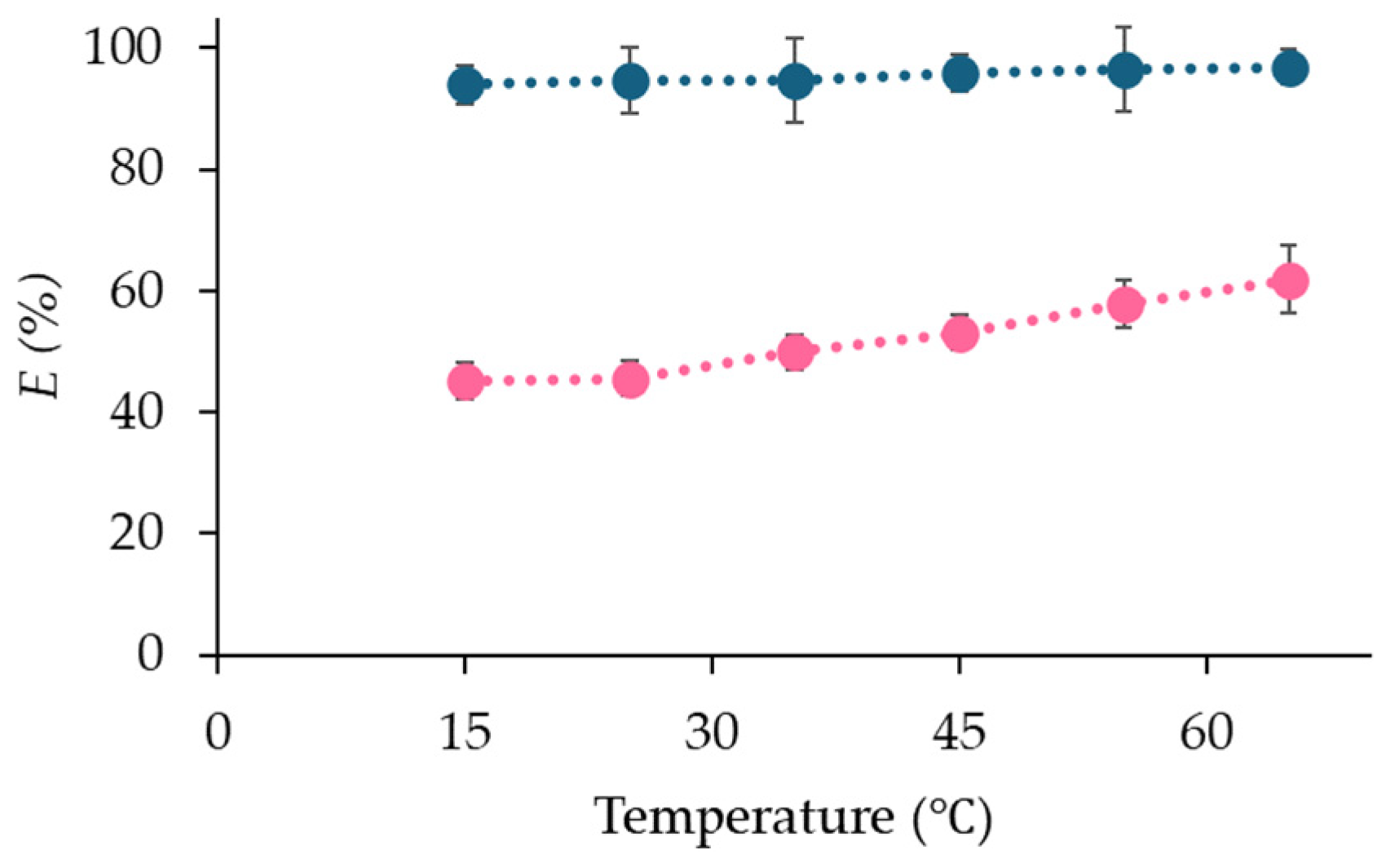
2.2. Effect of Temperature
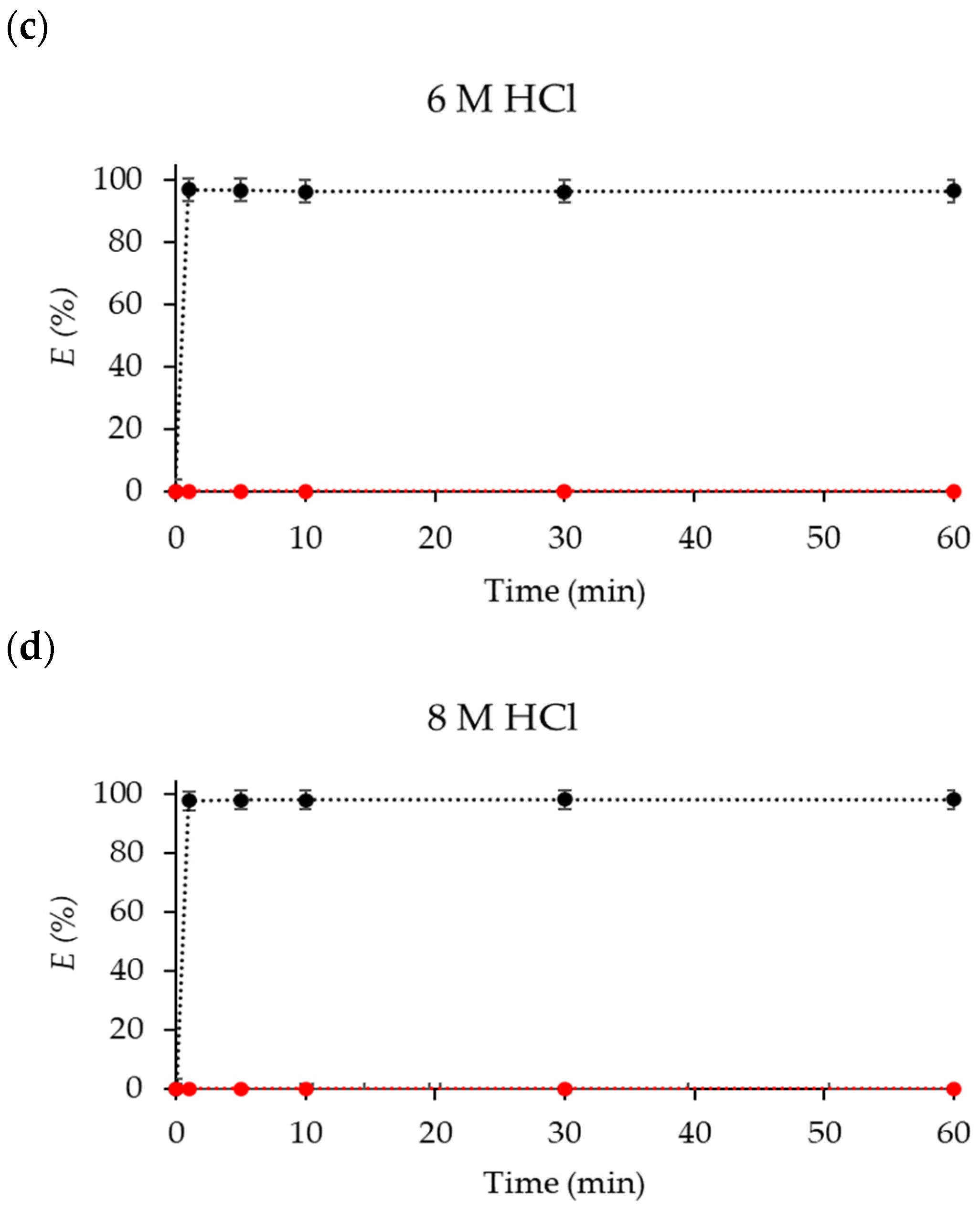
2.3. Stripping of Co(II)
2.4. Membrane Characterization
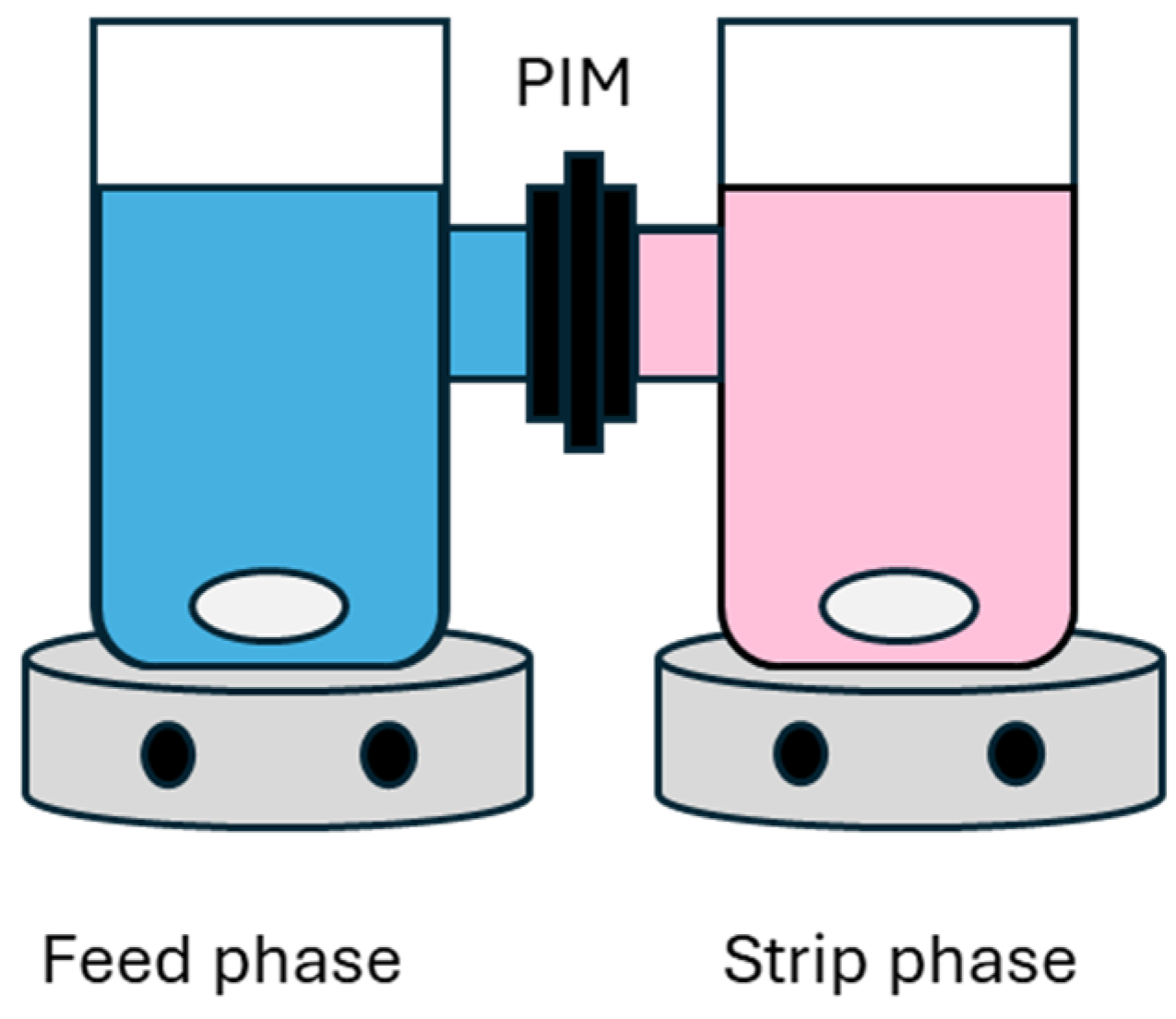
2.5. Separation of Co(II) from Ni(II) with PIMs
3. Materials and Methods
3.1. Chemicals
3.2. Extraction and Stripping Experiments
3.3. Membrane Preparation
3.4. Contact Angle
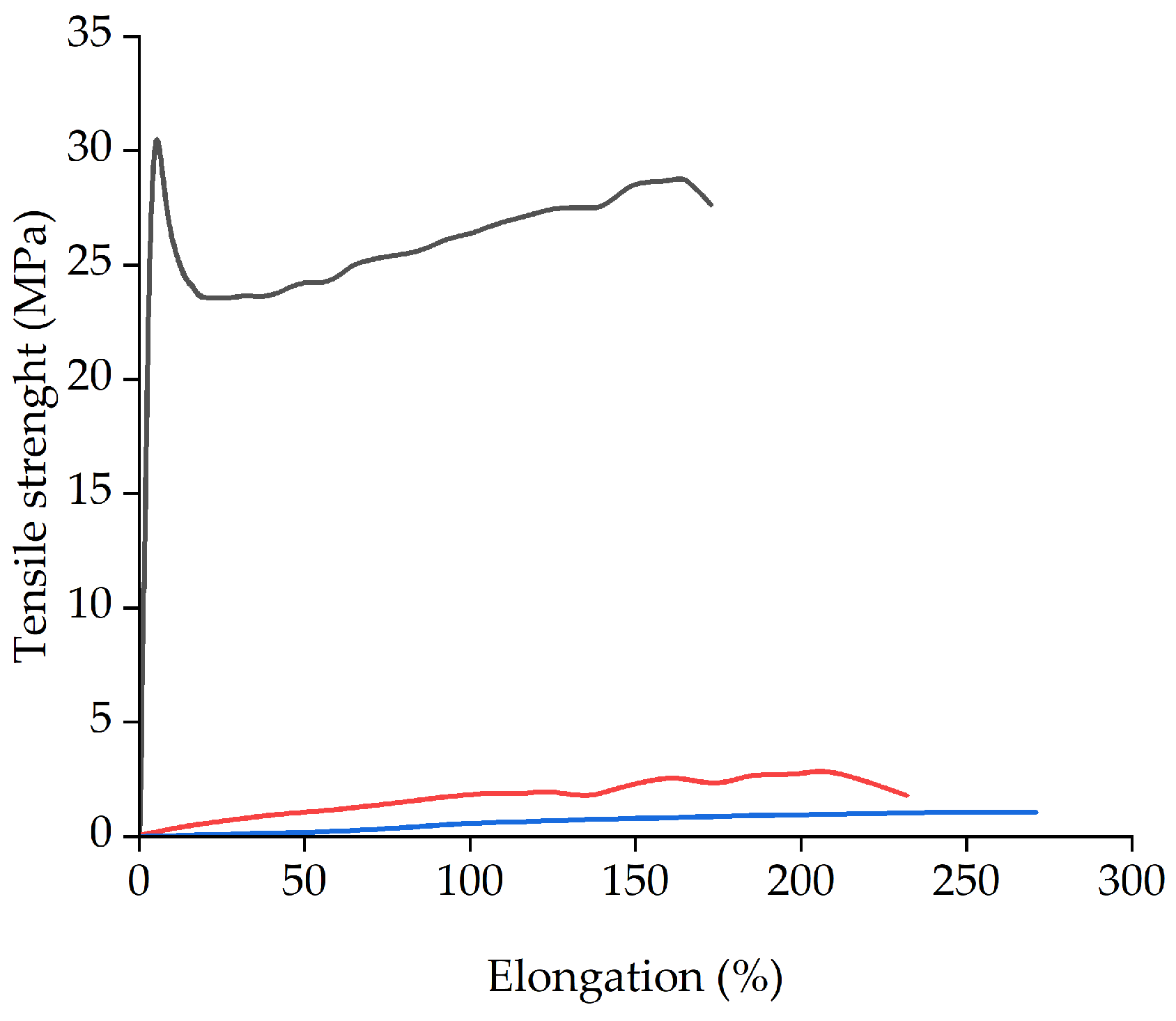
3.5. Elastic Modulus
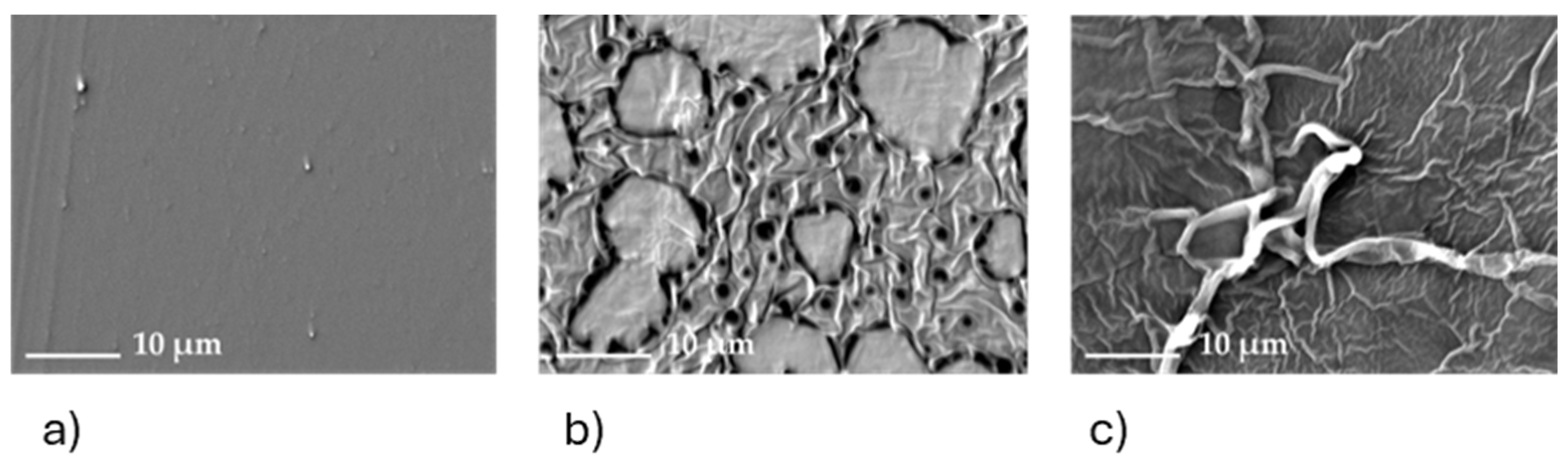
3.6. Microscopy
3.7. FTIR
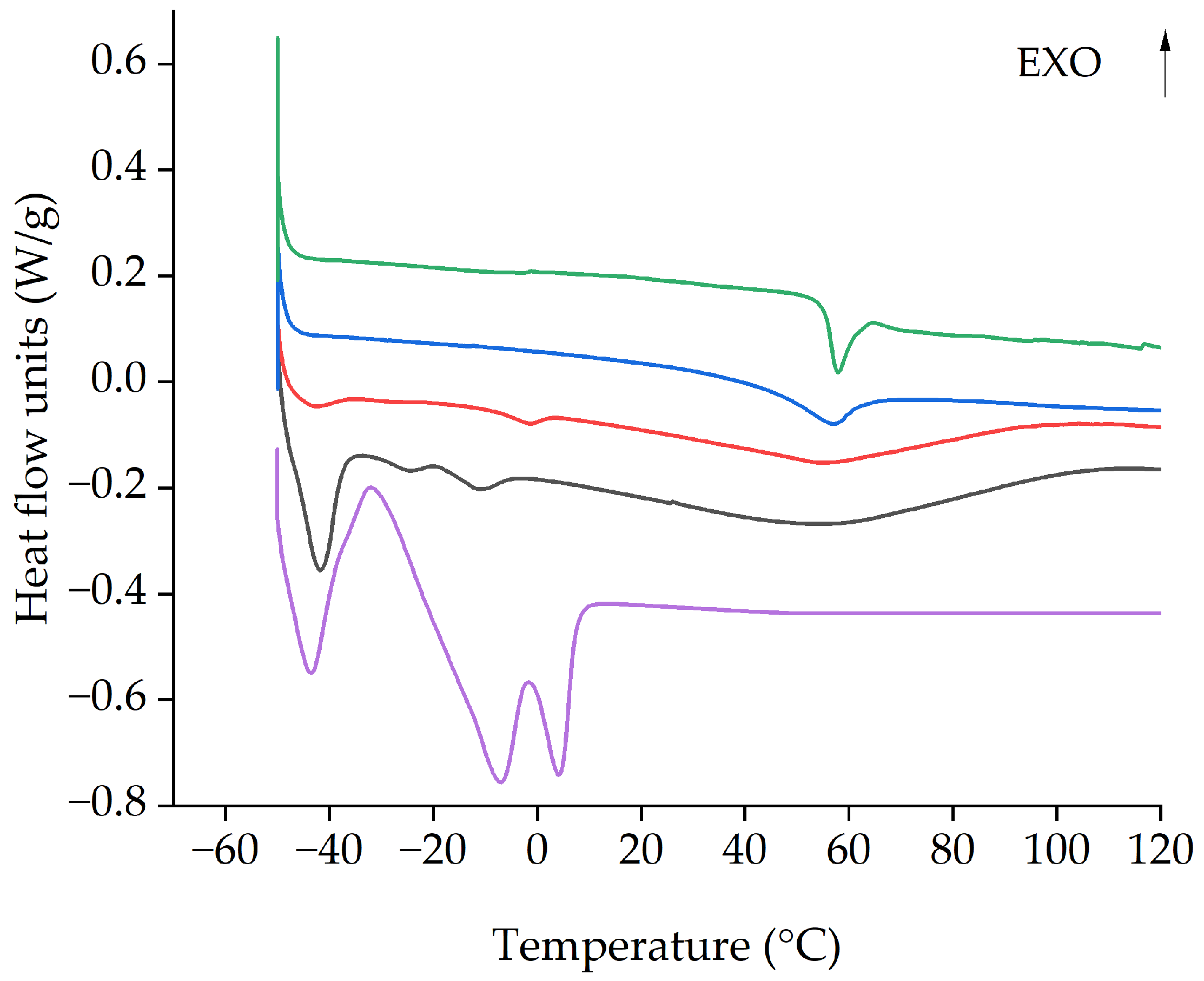
3.8. DSC
3.9. Extraction with PIMs
3.10. Extraction Efficiency in Membrane Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Tisserant, A.; Pauliuk, S. Matching Global Cobalt Demand under Different Scenarios for Co-Production and Mining Attractiveness. J. Econ. Struct. 2016, 5, 4. [Google Scholar] [CrossRef]
- Gianvincenzi, M.; Mosconi, E.M.; Marconi, M.; Tola, F. Battery Waste Management in Europe: Black Mass Hazardousness and Recycling Strategies in the Light of an Evolving Competitive Regulation. Recycling 2024, 9, 13. [Google Scholar] [CrossRef]
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A Perspective on the Sustainability of Cathode Materials Used in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Leal Filho, W.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Cheng, A.L.; Fuchs, E.R.H.; Karplus, V.J.; Michalek, J.J. Electric Vehicle Battery Chemistry Affects Supply Chain Disruption Vulnerabilities. Nat. Commun. 2024, 15, 2143. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and Recovery of Cobalt and Nickel from End of Life Products via Solvent Extraction Technique: A Review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, Y.; Yan, L.; Yang, R. Recovery of Nickel, Copper and Cobalt from Low-Grade Ni–Cu Sulfide Tailings. Hydrometallurgy 2005, 80, 54–58. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Tsakiridis, P.E.; Oustadakis, P.; Karidakis, T.; Katsiapi, A. Hydrometallurgical Process for the Separation and Recovery of Nickel from Sulphate Heap Leach Liquor of Nickeliferrous Laterite Ores. Miner. Eng. 2009, 22, 1181–1192. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Kovačević, A.; Tolazzi, M.; Sanadar, M.; Melchior, A. Hydrometallurgical Recovery of Metals from Spent Lithium-Ion Batteries with Ionic Liquids and Deep Eutectic Solvents. J. Environ. Chem. Eng. 2024, 12, 113248. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical Options for Recycling Spent Lithium-Ion Batteries: A Comprehensive Review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Preston, J.S. Solvent Extraction of Cobalt(II) and Nickel(II) by a Quaternary Ammonium Thiocyanate. Sep. Sci. Technol. 1982, 17, 1697–1718. [Google Scholar] [CrossRef]
- Liu, Y.; Nam, S.-H.; Lee, M.-S. A Study on the Separation of Co(II), Ni(II), and Mg(II) by Solvent Extraction with Cationic Extractants. Bull. Korean Chem. Soc. 2015, 36, 2646–2650. [Google Scholar] [CrossRef]
- Mubarok, M.Z.; Yunita, F.E.; Mubarok, M.Z.; Yunita, F.E. Solvent Extraction of Nickel and Cobalt from Ammonia-Ammonium Carbonate Solution by Using LIX 84-ICNS. Int. J. Nonferr. Metall. 2015, 4, 15–27. [Google Scholar] [CrossRef]
- Devi, N.B.; Nathsarma, K.C.; Chakravortty, V. Separation and Recovery of Cobalt(II) and Nickel(II) from Sulphate Solutions Using Sodium Salts of D2EHPA, PC 88A and Cyanex 272. Hydrometallurgy 1998, 49, 47–61. [Google Scholar] [CrossRef]
- Fleitlikh, I.Y.; Pashkov, G.L.; Grigorieva, N.A.; Nikiforova, L.K.; Pleshkov, M.A.; Shneerson, Y.M. Cobalt and Nickel Recovery from Sulfate Media Containing Calcium, Manganese, and Magnesium with a Mixture of CYANEX 301 and a Trialkylamine. Solvent Extr. Ion Exch. 2011, 29, 782–799. [Google Scholar] [CrossRef]
- He, M.; Jin, X.; Zhang, X.; Duan, X.; Zhang, P.; Teng, L.; Liu, Q.; Liu, W. Combined Pyro-Hydrometallurgical Technology for Recovering Valuable Metal Elements from Spent Lithium-Ion Batteries: A Review of Recent Developments. Green Chem. 2023, 25, 6561–6580. [Google Scholar] [CrossRef]
- Sukhbaatar, T.; Dourdain, S.; Turgis, R.; Rey, J.; Arrachart, G.; Pellet-Rostaing, S. Ionic Liquids as Diluents in Solvent Extraction: First Evidence of Supramolecular Aggregation of a Couple of Extractant Molecules. Chem. Commun. 2015, 51, 15960–15963. [Google Scholar] [CrossRef]
- Wongsawa, T.; Traiwongsa, N.; Pancharoen, U.; Nootong, K. A Review of the Recovery of Precious Metals Using Ionic Liquid Extractants in Hydrometallurgical Processes. Hydrometallurgy 2020, 198, 105488. [Google Scholar] [CrossRef]
- Billard, I.; Ouadi, A.; Gaillard, C. Liquid-Liquid Extraction of Actinides, Lanthanides, and Fission Products by Use of Ionic Liquids: From Discovery to Understanding. Anal. Bioanal. Chem. 2011, 400, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva Rao, P.R.; Venkatesan, K.A.; Rout, A.; Srinivasan, T.G.; Nagarajan, K. Potential Applications of Room Temperature Ionic Liquids for Fission Products and Actinide Separation. Sep. Sci. Technol. 2012, 47, 204–222. [Google Scholar] [CrossRef]
- Kolarik, Z. Ionic Liquids: How Far Do They Extend the Potential of Solvent Extraction of f-Elements? Sep. Sci. Technol. 2012, 31, 24–60. [Google Scholar] [CrossRef]
- Busato, M.; Lapi, A.; D’Angelo, P.; Melchior, A. Coordination of the Co2+ and Ni2+ Ions in Tf2N− Based Ionic Liquids: A Combined X-ray Absorption and Molecular Dynamics Study. J. Phys. Chem. B 2021, 125, 6639–6648. [Google Scholar] [CrossRef]
- Busato, M.; D’Angelo, P.; Melchior, A. Solvation of Zn2+ Ion in 1-Alkyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide Ionic Liquids: A Molecular Dynamics and X-ray Absorption Study. Phys. Chem. Chem. Phys. 2019, 21, 6958–6969. [Google Scholar] [CrossRef]
- Melchior, A.; Gaillard, C.; Gràcia Lanas, S.; Tolazzi, M.; Billard, I.; Georg, S.; Sarrasin, L.; Boltoeva, M. Nickel(II) Complexation with Nitrate in Dry [C4mim][Tf2N] Ionic Liquid: A Spectroscopic, Microcalorimetric, and Molecular Dynamics Study. Inorg. Chem. 2016, 55, 3498–3507. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity-Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Chandrasekhar, K.; Park, J.; Varjani, S.; Sharma, P.; Kumar, D.; Yoon, J.J.; Pandey, A.; Kim, S.H. Synthesis of Fatty Acid-Based Ammonium Ionic Liquids and Their Application for Extraction of Co(II) and Ni(II) Metals Ions from Aqueous Solution. Chemosphere 2022, 307, 135787. [Google Scholar] [CrossRef]
- Vavina, A.V.; Seitkalieva, M.M.; Strukova, E.N.; Ananikov, V.P. Fatty Acid-Derived Ionic Liquids as Soft and Sustainable Antimicrobial Agents. J. Mol. Liq. 2024, 410, 125483. [Google Scholar] [CrossRef]
- Gusain, R.; Khatri, O.P. Fatty Acid Ionic Liquids as Environmentally Friendly Lubricants for Low Friction and Wear. RSC Adv. 2016, 6, 3462–3469. [Google Scholar] [CrossRef]
- Parmentier, D.; Metz, S.J.; Kroon, M.C. Tetraalkylammonium Oleate and Linoleate Based Ionic Liquids: Promising Extractants for Metal Salts. Green Chem. 2013, 15, 205–209. [Google Scholar] [CrossRef]
- Parmentier, D.; Vander Hoogerstraete, T.; Metz, S.J.; Binnemans, K.; Kroon, M.C. Selective Extraction of Metals from Chloride Solutions with the Tetraoctylphosphonium Oleate Ionic Liquid. Ind. Eng. Chem. Res. 2015, 54, 5149–5158. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, D.; Zhang, J.; Zhu, Y.; Zhang, Z.; Qian, C.; Ren, Q.; Xing, H. Long-Chain Fatty Acid-Based Phosphonium Ionic Liquids with Strong Hydrogen-Bond Basicity and Good Lipophilicity: Synthesis, Characterization, and Application in Extraction. ACS Sustain. Chem. Eng. 2015, 3, 309–316. [Google Scholar] [CrossRef]
- Saadeh, S.M.; Yasseen, Z.; Sharif, F.A.; Abu Shawish, H.M. New Room Temperature Ionic Liquids with Interesting Ecotoxicological and Antimicrobial Properties. Ecotoxicol. Environ. Saf. 2009, 72, 1805–1809. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Mahandra, H.; Gupta, B. Optimization of a Solvent Extraction Route for the Recovery of Mo from Petroleum Refinery Spent Catalyst Using Cyphos IL 102. Solvent Extr. Ion Exch. 2018, 36, 401–419. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wisniewski, M. Selective Extraction of Palladium(II) from Hydrochloric Acid Solutions with Phosphonium Extractants. Sep. Purif. Technol. 2011, 80, 385–389. [Google Scholar] [CrossRef]
- Kogelnig, D.; Stojanovic, A.; Jirsa, F.; Körner, W.; Krachler, R.; Keppler, B.K. Transport and Separation of Iron(III) from Nickel(II) with the Ionic Liquid Trihexyl(Tetradecyl)Phosphonium Chloride. Sep. Purif. Technol. 2010, 72, 56–60. [Google Scholar] [CrossRef]
- Vander Hoogerstraete, T.; Binnemans, K. Highly Efficient Separation of Rare Earths from Nickel and Cobalt by Solvent Extraction with the Ionic Liquid Trihexyl(Tetradecyl)Phosphonium Nitrate: A Process Relevant to the Recycling of Rare Earths from Permanent Magnets and Nickel Metal Hydride Batteries. Green Chem. 2014, 16, 1594–1606. [Google Scholar] [CrossRef]
- Vander Hoogerstraete, T.; Wellens, S.; Verachtert, K.; Binnemans, K. Removal of Transition Metals from Rare Earths by Solvent Extraction with an Undiluted Phosphonium Ionic Liquid: Separations Relevant to Rare-Earth Magnet Recycling. Green Chem. 2013, 15, 919–927. [Google Scholar] [CrossRef]
- Ilyas, S.; Srivastava, R.R.; Kim, H. Selective Separation of Cobalt versus Nickel by Split-Phosphinate Complexation Using a Phosphonium-Based Ionic Liquid. Environ. Chem. Lett. 2023, 21, 673–680. [Google Scholar] [CrossRef]
- Satyawirawan, S.A.; Cattrall, R.W.; Kolev, S.D.; Almeida, M.I.G.S. Improving Co(II) Separation from Ni(II) by Solvent Extraction Using Phosphonium-Based Ionic Liquids. J. Mol. Liq. 2023, 380, 121764. [Google Scholar] [CrossRef]
- Mušović, J.; Tekić, D.; Marić, S.; Jocić, A.; Stanković, D.; Dimitrijević, A. Sustainable Recovery of Cobalt and Lithium from Lithium-Ion Battery Cathode Material by Combining Sulfate Leachates and Aqueous Biphasic Systems Based on Tetrabutylphosphonium-Ionic Liquids. Sep. Purif. Technol. 2024, 348, 127707. [Google Scholar] [CrossRef]
- Eyupoglu, V.; Unal, A. The Extraction and the Removal of Cd(II) Using Polymer Inclusion Membrane Containing Symmetric Room Temperature Ionic Liquid as Ion Carrier. J. Environ. Chem. Eng. 2023, 11, 110570. [Google Scholar] [CrossRef]
- Hernández-Fernández, A.; Iniesta-López, E.; Ginestá-Anzola, A.; Garrido, Y.; Pérez de los Ríos, A.; Quesada-Medina, J.; Hernández-Fernández, F.J. Polymeric Inclusion Membranes Based on Ionic Liquids for Selective Separation of Metal Ions. Membranes 2023, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, M.; Safikhani, A.; Vatanpour, V. Polyvinyl Chloride-Based Membranes: A Review on Fabrication Techniques, Applications and Future Perspectives. Sep. Purif. Technol. 2021, 279, 119678. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent Trends in Extraction and Transport of Metal Ions Using Polymer Inclusion Membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; de los Ríos, A.P.; Mateo-Ramírez, F.; Juarez, M.D.; Lozano-Blanco, L.J.; Godínez, C. New Application of Polymer Inclusion Membrane Based on Ionic Liquids as Proton Exchange Membrane in Microbial Fuel Cell. Sep. Purif. Technol. 2016, 160, 51–58. [Google Scholar] [CrossRef]
- Xu, L.; Zeng, X.; He, Q.; Deng, T.; Zhang, C.; Zhang, W. Stable Ionic Liquid-Based Polymer Inclusion Membranes for Lithium and Magnesium Separation. Sep. Purif. Technol. 2022, 288, 120626. [Google Scholar] [CrossRef]
- Baczyńska, M.; Waszak, M.; Nowicki, M.; Prządka, D.; Borysiak, S.; Regel-Rosocka, M. Characterization of Polymer Inclusion Membranes (PIMs) Containing Phosphonium Ionic Liquids as Zn(II) Carriers. Ind. Eng. Chem. Res. 2018, 57, 5070–5082. [Google Scholar] [CrossRef]
- Santos Klienchen Dalari, B.L.; Lisboa Giroletti, C.; Malaret, F.J.; Skoronski, E.; Hallett, J.P.; Matias, W.G.; Puerari, R.C.; Nagel-Hassemer, M.E. Application of a Phosphonium-Based Ionic Liquid for Reactive Textile Dye Removal: Extraction Study and Toxicological Evaluation. J. Environ. Manag. 2022, 304, 114322. [Google Scholar] [CrossRef]
- Skoronski, E.; Fernandes, M.; Malaret, F.J.; Hallett, J.P. Use of Phosphonium Ionic Liquids for Highly Efficient Extraction of Phenolic Compounds from Water. Sep. Purif. Technol. 2020, 248, 117069. [Google Scholar] [CrossRef]
- Marták, J.; Liptaj, T.; Schlosser, Š. Extraction of Butyric Acid by Phosphonium Decanoate Ionic Liquid. J. Chem. Eng. Data 2019, 64, 2973–2984. [Google Scholar] [CrossRef]
- Blaga, A.C.; Dragoi, E.N.; Tucaliuc, A.; Kloetzer, L.; Cascaval, D. Folic Acid Ionic-Liquids-Based Separation: Extraction and Modelling. Molecules 2023, 28, 3339. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Abdollahy, M.; Khodadadi Darban, A.; Mohseni, M. Theoretical and Experimental Comparison of Rare Earths Extraction by [P6,6,6,1,4][Decanoate] Bifunctional Ionic Liquid and D2EHPA Acidic Extractant. Min. Eng. 2022, 180, 107473. [Google Scholar] [CrossRef]
- Xuan, W.; de Souza Braga, A.; Korbel, C.; Chagnes, A. New Insights in the Leaching Kinetics of Cathodic Materials in Acidic Chloride Media for Lithium-Ion Battery Recycling. Hydrometallurgy 2021, 204, 105705. [Google Scholar] [CrossRef]
- Wellens, S.; Thijs, B.; Binnemans, K. An Environmentally Friendlier Approach to Hydrometallurgy: Highly Selective Separation of Cobalt from Nickel by Solvent Extraction with Undiluted Phosphonium Ionic Liquids. Green Chem. 2012, 14, 1657–1665. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Partition Studies on Cobalt and Recycling of Valuable Metals from Waste Li-Ion Batteries via Solvent Extraction and Chemical Precipitation. J. Clean. Prod. 2019, 225, 820–832. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, B.; Zhao, S.; Liao, Z.; Wang, M. Separation of Co(II) and Ni(II) from Hydrochloric Acid Leach Liquor by Solvent Extraction and Crystallization. J. Sustain. Metall. 2022, 8, 91–101. [Google Scholar] [CrossRef]
- Hitchcock, P.B.; Seddon, K.R.; Welton, T. Hydrogen-Bond Acceptor Abilities of Tetrachlorometalate(II) Complexes in Ionic Liquids. J. Chem. Soc. Dalton Trans. 1993, 2639–2643. [Google Scholar] [CrossRef]
- Bowlas, C.J.; Bruce, D.W.; Seddon, K.R. Liquid-Crystalline Ionic Liquids. Chem. Commun. 1996, 1625–1626. [Google Scholar] [CrossRef]
- Skibsted, L.H.; Bjerrum, J. Studies on Cobalt (II) Halide Complex Formation. II. Cobalt (II) Chloride Complexes in 10 M Perchloric Acid Solution. Acta Chem. Scand. A 1978, 32, 429–434. [Google Scholar] [CrossRef]
- Liu, W.; Borg, S.J.; Testemale, D.; Etschmann, B.; Hazemann, J.-L.; Brugger, J. Speciation and Thermodynamic Properties for Cobalt Chloride Complexes in Hydrothermal Fluids at 35–440 °C and 600 Bar: An in-Situ XAS Study. Geochim. Cosmochim. Acta 2011, 75, 1227–1248. [Google Scholar] [CrossRef]
- Bjerrum, J.; Halonin, A.S.; Skibsated, L.H. Studies on Cobalt(II) Halide Complex Formation. I. A Spectrophotometric Study of the Chloro Cobalt(II) Complexes in Strong Aqueous Chloride Solutions. Acta Chem. Scand. A 1975, 29, 326–332. [Google Scholar] [CrossRef]
- Pan, P.; Susak, N.J. Co(II)-Chloride and -Bromide Complexes in Aqueous Solutions up to 5 m NaX and 90 °C: Spectrophotometric Study and Geological Implications. Geochim. Cosmochim. Acta 1989, 53, 327–341. [Google Scholar] [CrossRef]
- Uchikoshi, M. Determination of the Distribution of Cobalt-Chloro Complexes in Hydrochloric Acid Solutions at 298 K. J. Solut. Chem. 2018, 47, 2021–2038. [Google Scholar] [CrossRef]
- Uchikoshi, M.; Shinoda, K. Determination of Structures of Cobalt(II)-Chloro Complexes in Hydrochloric Acid Solutions by X-ray Absorption Spectroscopy at 298 K. Struct. Chem. 2019, 30, 945–954. [Google Scholar] [CrossRef]
- Lee, M.-S.; Nam, S.-H. Chemical Equilibria of Nickel Chloride in HCl Solution at 25 °C. Bull. Korean Chem. Soc. 2009, 30, 2203–2207. [Google Scholar] [CrossRef]
- Rybka, P.; Regel-Rosocka, M. Nickel(II) and Cobalt(II) Extraction from Chloride Solutions with Quaternary Phosphonium Salts. Sep. Sci. Technol. 2012, 47, 1296–1302. [Google Scholar] [CrossRef]
- Chaverra, D.E.; Restrepo-Baena, O.J.; Ruiz, M.C. Cobalt Extraction from Sulfate/Chloride Media with Trioctyl(Alkyl)Phosphonium Chloride Ionic Liquids. ACS Omega 2020, 5, 5643–5650. [Google Scholar] [CrossRef]
- Lommelen, R.; Vander Hoogerstraete, T.; Onghena, B.; Billard, I.; Binnemans, K. Model for Metal Extraction from Chloride Media with Basic Extractants: A Coordination Chemistry Approach. Inorg. Chem. 2019, 58, 12289–12301. [Google Scholar] [CrossRef]
- Lin, B.; McCormick, A.V.; Davis, H.T.; Strey, R. Solubility of Sodium Soaps in Aqueous Salt Solutions. J. Colloid Interface Sci. 2005, 291, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, J.; Wu, M.; Wu, Q.; Liu, J.; Zhang, J. Biobased Plasticizers from Tartaric Acid: Synthesis and Effect of Alkyl Chain Length on the Properties of Poly(Vinyl Chloride. ACS Omega 2021, 6, 13161–13169. [Google Scholar] [CrossRef]
- Yang, J.-X.; Long, Y.-Y.; Pan, L.; Men, Y.-F.; Li, Y.-S. Spontaneously Healable Thermoplastic Elastomers Achieved through One-Pot Living Ring-Opening Metathesis Copolymerization of Well-Designed Bulky Monomers. ACS Appl. Mater. Interfaces 2016, 8, 12445–12455. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Brazel, C.S. Ionic Liquids: New Generation Stable Plasticizers for Poly(Vinyl Chloride). Polym. Degrad. Stab. 2006, 91, 3371–3382. [Google Scholar] [CrossRef]
- Keskin, B.; Yuksekdag, A.; Zeytuncu, B.; Koyuncu, I. Development of Polymer Inclusion Membranes for Palladium Recovery: Effect of Base Polymer, Carriers, and Plasticizers on Structure and Performance. J. Water Process Eng. 2023, 52, 103576. [Google Scholar] [CrossRef]
- Shah, B.L.; Shertukde, V.V. Effect of Plasticizers on Mechanical, Electrical, Permanence, and Thermal Properties of Poly(Vinyl Chloride). J. Appl. Polym. Sci. 2003, 90, 3278–3284. [Google Scholar] [CrossRef]
- Max, J.-J.; Chapados, C. Infrared Spectroscopy of Aqueous Carboxylic Acids: Comparison between Different Acids and Their Salts. J. Phys. Chem. A 2004, 108, 3324–3337. [Google Scholar] [CrossRef]
- Fontàs, C.; Tayeb, R.; Dhahbi, M.; Gaudichet, E.; Thominette, F.; Roy, P.; Steenkeste, K.; Fontaine-Aupart, M.-P.; Tingry, S.; Tronel-Peyroz, E.; et al. Polymer Inclusion Membranes: The Concept of Fixed Sites Membrane Revised. J. Membr. Sci. 2007, 290, 62–72. [Google Scholar] [CrossRef]
- Witt, K.; Radzymińska-Lenarcik, E. Characterization of PVC-Based Polymer Inclusion Membranes with Phosphonium Ionic Liquids. J. Therm. Anal. Calorim. 2019, 138, 4437–4443. [Google Scholar] [CrossRef]
- Zhao, S.; Samadi, A.; Wang, Z.; Pringle, J.M.; Zhang, Y.; Kolev, S.D. Ionic Liquid-Based Polymer Inclusion Membranes for Metal Ions Extraction and Recovery: Fundamentals, Considerations, and Prospects. Chem. Eng. J. 2024, 481, 148792. [Google Scholar] [CrossRef]
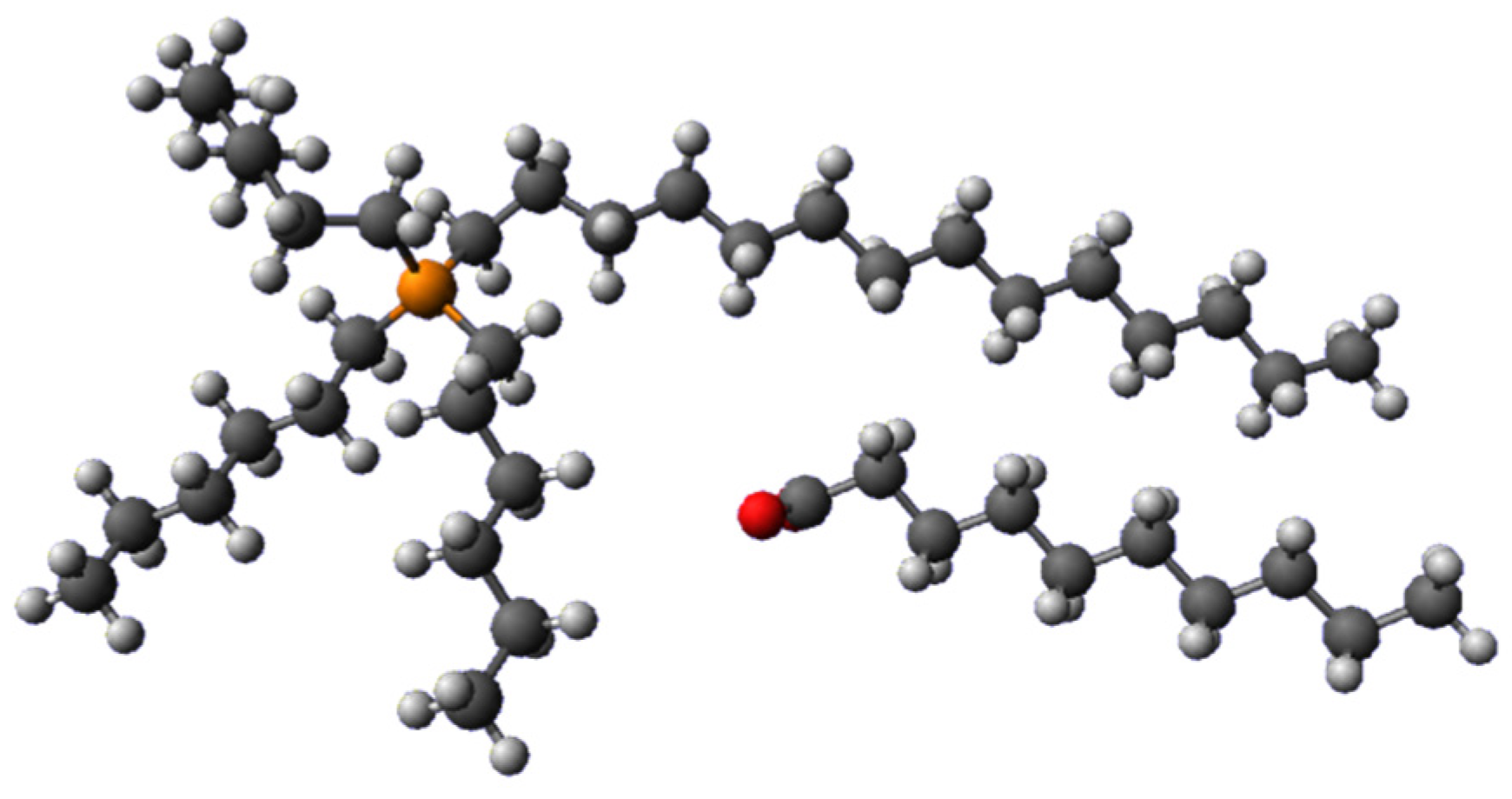

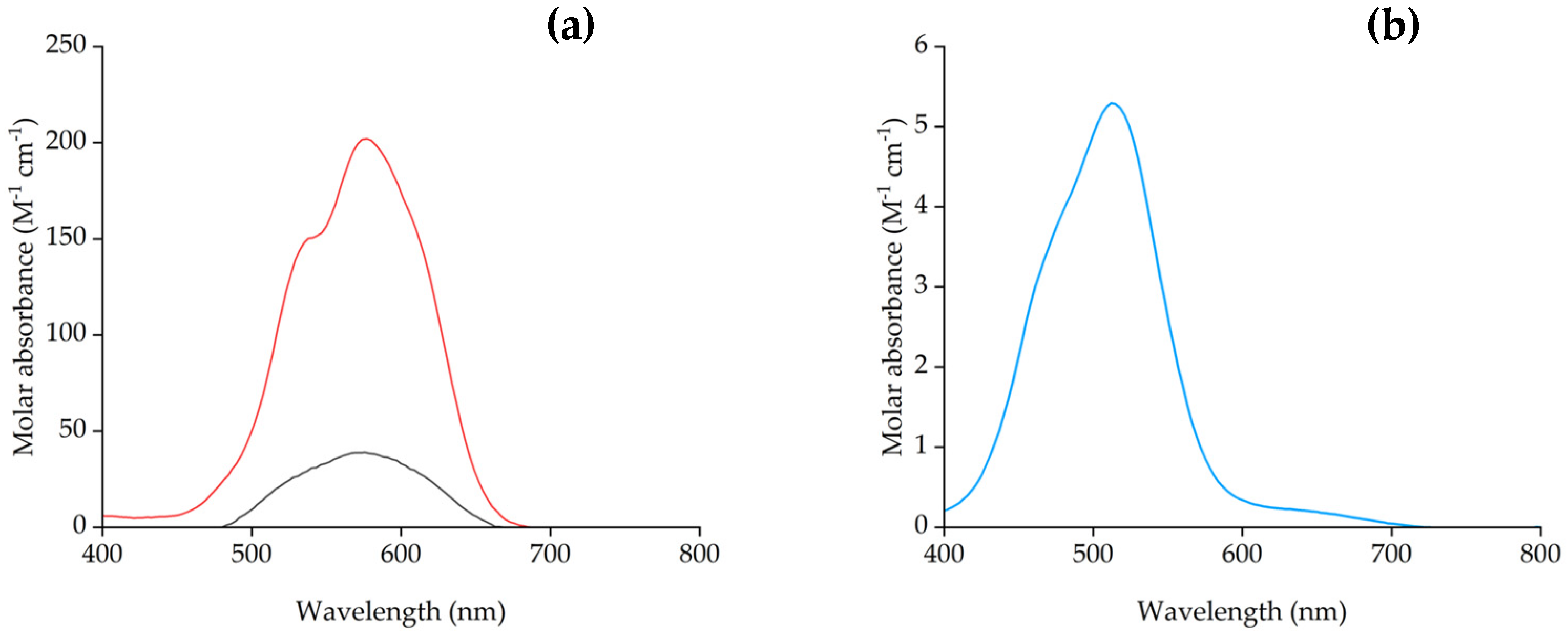

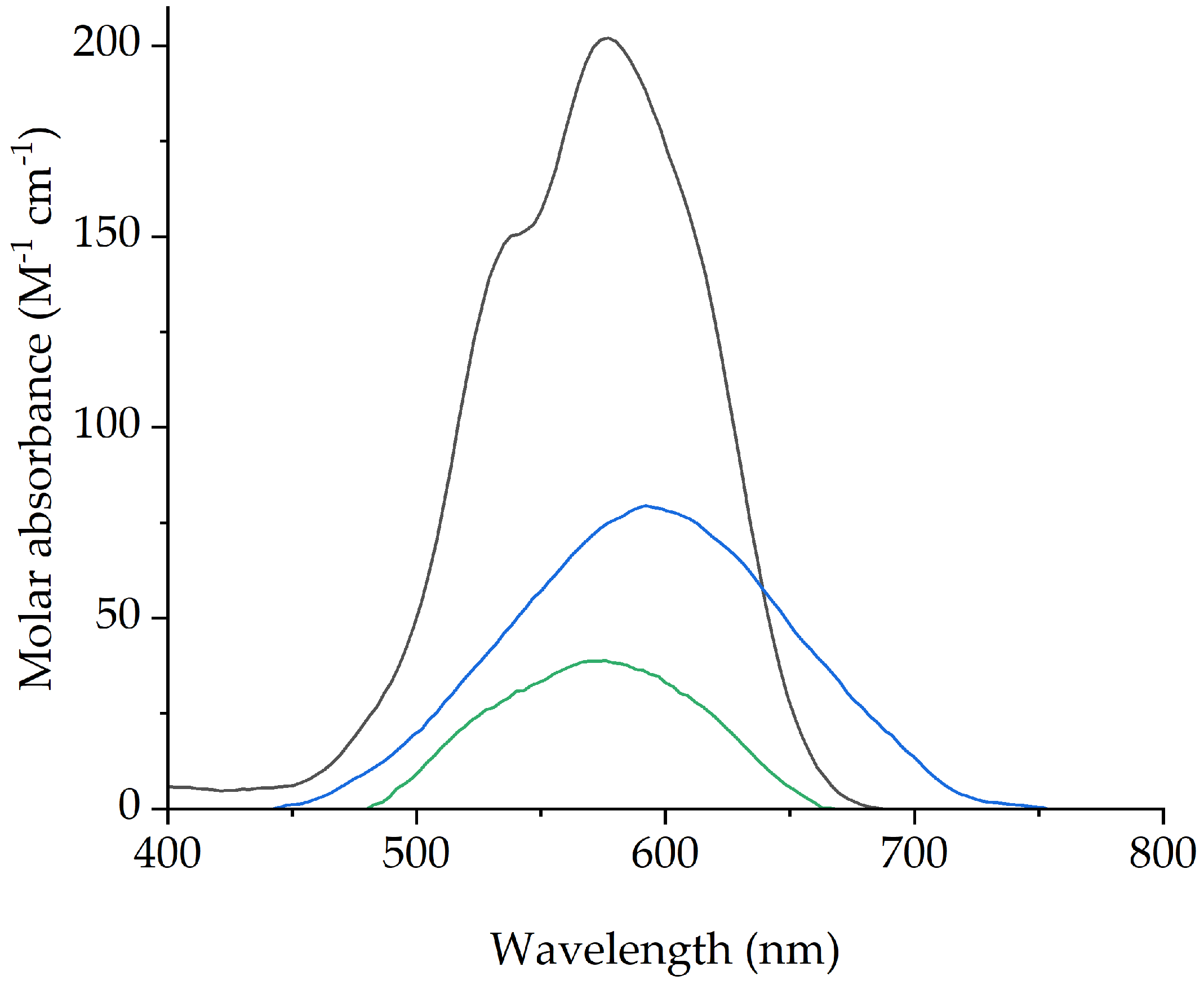



| PVC (%) | [P66614][Dec] (%) | Contact Angle (θ) |
|---|---|---|
| 100 | 0 | 75.7 ± 0.5 |
| 80 | 20 | 74.4 ± 1.6 |
| 50 | 50 | 51.0 ± 2.8 |
| 30 | 70 | 28.1 ± 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, A.; Ricardo García, J.A.; Tolazzi, M.; Melchior, A.; Sanadar, M. Selective Co(II) and Ni(II) Separation Using the Trihexyl(tetradecyl)phosphonium Decanoate Ionic Liquid. Molecules 2024, 29, 4545. https://doi.org/10.3390/molecules29194545
Kovačević A, Ricardo García JA, Tolazzi M, Melchior A, Sanadar M. Selective Co(II) and Ni(II) Separation Using the Trihexyl(tetradecyl)phosphonium Decanoate Ionic Liquid. Molecules. 2024; 29(19):4545. https://doi.org/10.3390/molecules29194545
Chicago/Turabian StyleKovačević, Anđela, José Alejandro Ricardo García, Marilena Tolazzi, Andrea Melchior, and Martina Sanadar. 2024. "Selective Co(II) and Ni(II) Separation Using the Trihexyl(tetradecyl)phosphonium Decanoate Ionic Liquid" Molecules 29, no. 19: 4545. https://doi.org/10.3390/molecules29194545
APA StyleKovačević, A., Ricardo García, J. A., Tolazzi, M., Melchior, A., & Sanadar, M. (2024). Selective Co(II) and Ni(II) Separation Using the Trihexyl(tetradecyl)phosphonium Decanoate Ionic Liquid. Molecules, 29(19), 4545. https://doi.org/10.3390/molecules29194545









