The Lattice Mismatch-Driven Photochemical Self-Assembly of Supported Heterostructures for Stable and Enhanced Electrocatalytic Carbon Dioxide Reduction Reaction
Abstract
1. Introduction
2. Results and Discussion
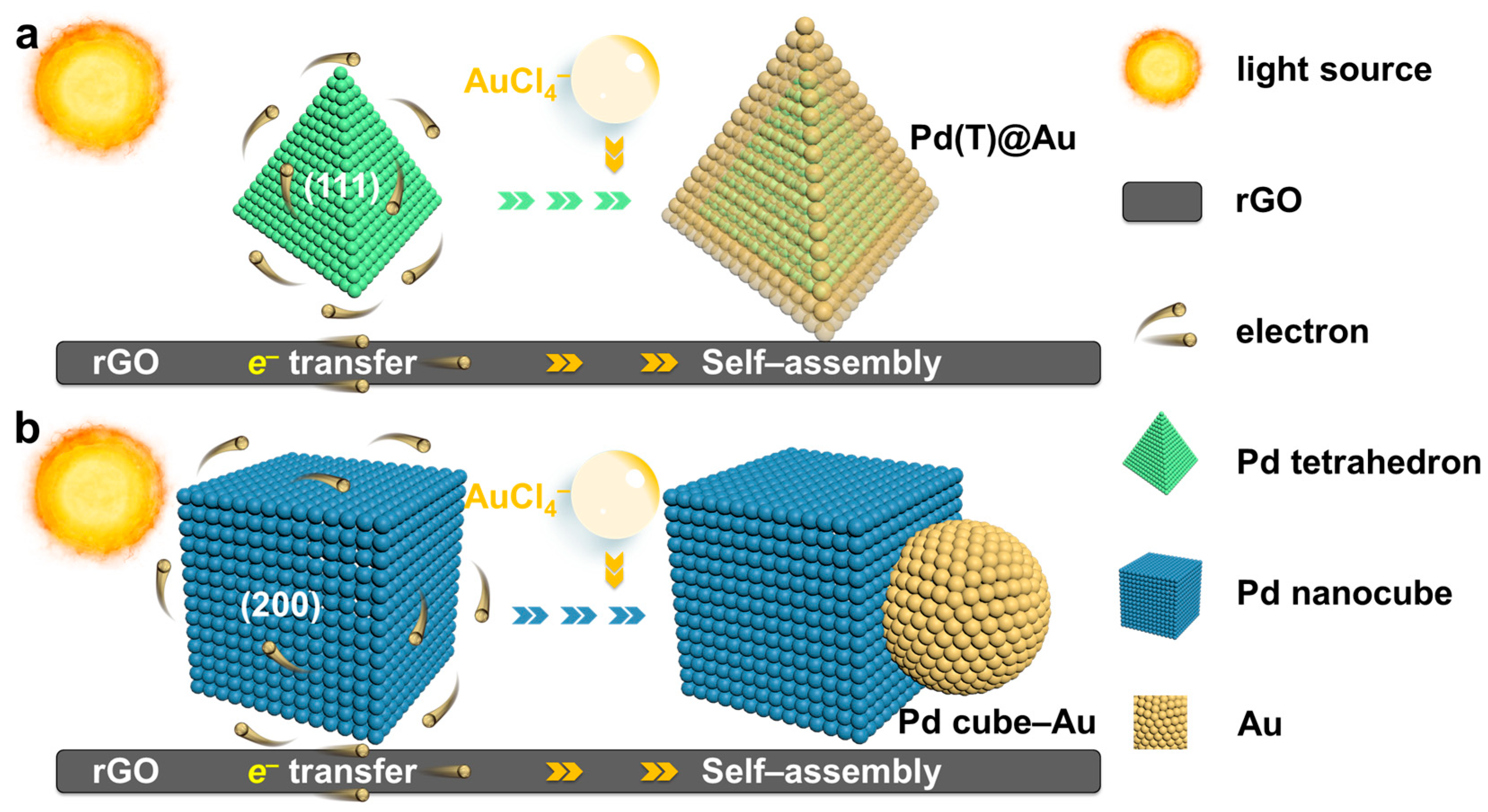
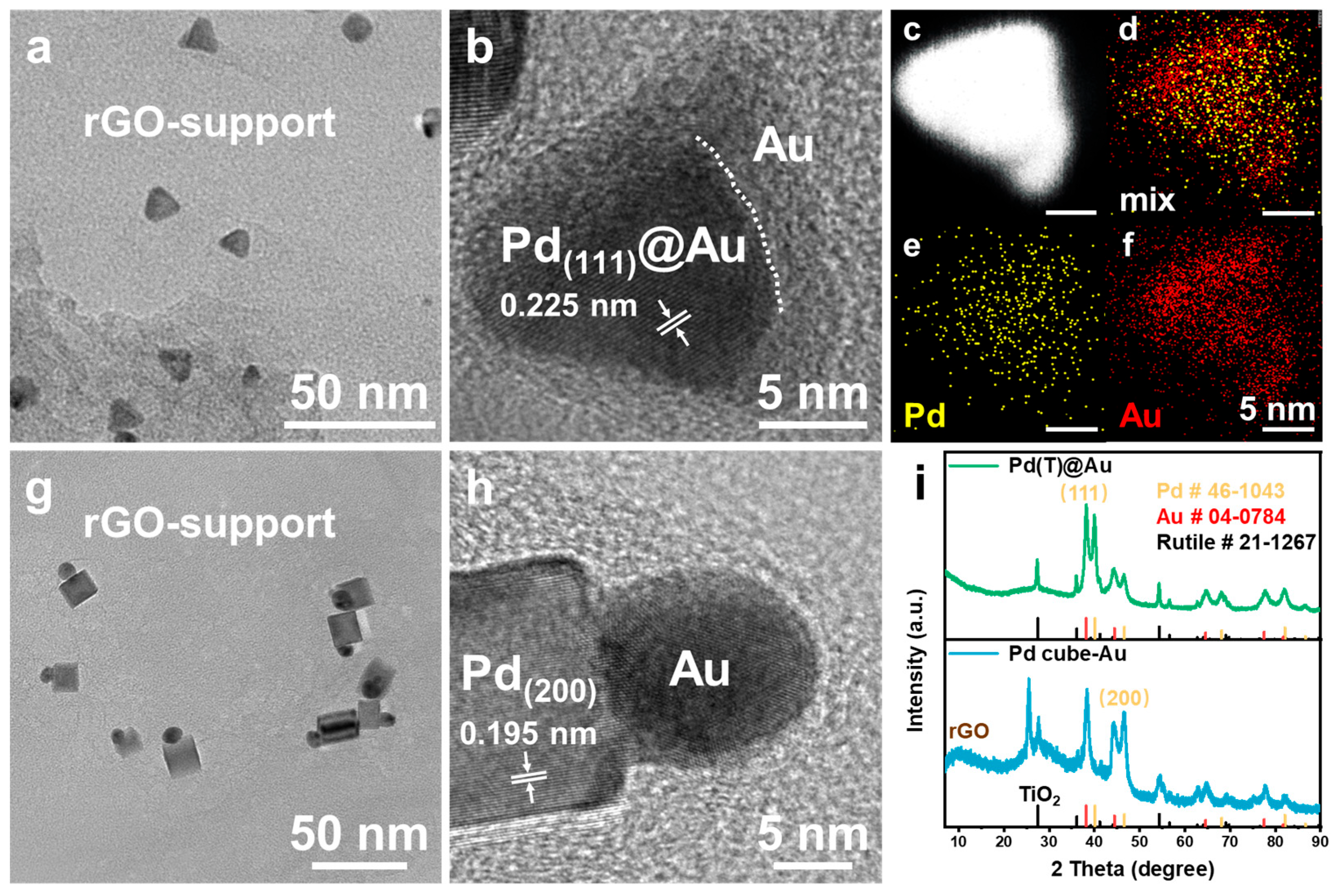
2.1. Synthesis and Characterization of Supported Pd(T)@Au and Pd Cube–Au HNCs
2.2. Self-Assembly Mechanism of Photochemical Deposition
2.3. Electrocatalytic CO2RR Performance
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Rui, K.; Dou, S.X.; Sun, W. Heterostructures for electrochemical hydrogen evolution reaction: A review. Adv. Funct. Mater. 2018, 28, 1803291. [Google Scholar] [CrossRef]
- Zhong, D.-C.; Gong, Y.-N.; Zhang, C.; Lu, T.-B. Dinuclear metal synergistic catalysis for energy conversion. Chem. Soc. Rev. 2023, 52, 3170–3214. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the support in gold-containing nanoparticles as heterogeneous catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef]
- Ren, S.; Lees, E.W.; Hunt, C.; Jewlal, A.; Kim, Y.; Zhang, Z.; Mowbray, B.A.W.; Fink, A.G.; Melo, L.; Grant, E.R.; et al. Catalyst aggregation matters for immobilized molecular CO2RR electrocatalysts. J. Am. Chem. Soc. 2023, 145, 4414–4420. [Google Scholar] [CrossRef]
- Xiao, H.; Bai, M.; Zhao, M.; Fu, Z.; Wang, W.; Zhao, P.; Ma, J.; Zhang, L.; Zhang, J.; He, Y.; et al. Interfacial carbon dots introduced distribution-structure modulation of Pt loading on graphene towards enhanced electrocatalytic hydrogen evolution reaction. J. Colloid Interface Sci. 2024, 656, 214–224. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, D.J.; Xu, Z.J.; Shao-Horn, Y. Best practices for oxygen electrocatalysis. Nat. Sustain. 2024, 7, 371–374. [Google Scholar] [CrossRef]
- Zhu, P.; Xiong, X.; Wang, D.; Li, Y. Advances and regulation strategies of the active moiety in dual-atom site catalysts for efficient electrocatalysis. Adv. Energy Mater. 2023, 13, 2300884. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, X.; Li, H.; Chen, B.; Ye, S.; Zhang, N.; Yu, Z.; Zheng, J.; Chen, B. Enhancing electronic metal support interaction (EMSI) over Pt/TiO2 for efficient catalytic wet air oxidation of phenol in wastewater. J. Hazard. Mater. 2022, 426, 128088. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Huang, Y.; Yu, Z.; Huang, L. Aqueous synthesis of Au10Pt1 nanorods decorated with MnO2 nanosheets for the enhanced electrocatalytic oxidation of methanol. Molecules 2024, 29, 3753. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, Y.; Liu, Y.; Chen, G.; Zhang, M.; Jia, R.; Ling, Y.; Shi, Y.; Wang, X.; Huang, L. Photochemical modulated metal-support-interaction of Pt-rutile TiO2 and the effects on catalytic oxidation of HCHO. J. Catal. 2023, 424, 152–161. [Google Scholar] [CrossRef]
- Van der Hoeven, J.E.S.; Jelic, J.; Olthof, L.A.; Totarella, G.; van Dijk-Moes, R.J.A.; Krafft, J.-M.; Louis, C.; Studt, F.; van Blaaderen, A.; de Jongh, P.E. Unlocking synergy in bimetallic catalysts by core–shell design. Nat. Mater. 2021, 20, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Shi, Q.; Alba, M.; Prieto-Simon, B.; Cheng, W.; Voelcker, N.H. Enzyme-like electrocatalysis from 2D gold nanograss-nanocube assemblies. J. Colloid Interface Sci. 2020, 575, 24–34. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Wang, J.; Pan, H.; Sun, W. Supported sub-nanometer clusters for electrocatalysis applications. Adv. Funct. Mater. 2022, 32, 2202227. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, Z.; Song, S.; Eggert, D.; Liu, Y.; Shi, W.; Yuan, Y.; Kim, K.-S.; Grünwald, M.; Chen, O. Dual atomic coherence in the self-assembly of patchy heterostructural nanocrystals. ACS Nano 2022, 16, 15053–15062. [Google Scholar] [CrossRef]
- Mallapaty, S. How China could be carbon neutral by mid-century. Nature 2020, 586, 482–483. [Google Scholar] [CrossRef]
- Shi, Y.; Lyu, Z.; Zhao, M.; Chen, R.; Nguyen, Q.N.; Xia, Y. Noble-metal nanocrystals with controlled shapes for catalytic and electrocatalytic applications. Chem. Rev. 2021, 121, 649–735. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, X.; Chen, N.; Zhu, J.; Zheng, X.; Chen, Z.; Sheng, T.; Wu, Z.; Xiong, Y. Integration of MnO2 nanosheets with Pd nanoparticles for efficient CO2 electroreduction to methanol in membrane electrode assembly electrolyzers. J. Am. Chem. Soc. 2023, 145, 24852–24861. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, X.; Lin, X.; Liu, G.; Ling, C.; Xi, S.; Chen, B.; Ge, Y.; Tan, C.; Lai, Z.; et al. Phase-dependent growth of Pt on MoS2 for highly efficient H2 evolution. Nature 2023, 621, 300–305. [Google Scholar] [CrossRef]
- Shi, Y.; Lyu, Z.; Cao, Z.; Xie, M.; Xia, Y. How to remove the capping agent from Pd nanocubes without destructing their surface structure for the maximization of catalytic activity? Angew. Chem. Int. Ed. 2020, 59, 19129–19135. [Google Scholar] [CrossRef] [PubMed]
- Wenderich, K.; Mul, G. Methods, mechanism, and applications of photodeposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mei, X.; Han, C.; Gong, X.; Song, P.; Xu, W. Tuning strategies and structure effects of electrocatalysts for carbon dioxide reduction reaction. Chin. J. Catal. 2022, 43, 1618–1633. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Ferrer, B.; García, H. Metal–organic frameworks as photocatalysts for solar-driven overall water splitting. Chem. Rev. 2023, 123, 445–490. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Liu, Y.; Naseri, A.; Li, T.; Ostovan, A.; Asadian, E.; Jia, R.; Shi, L.; Huang, L.; Moshfegh, A.Z. Shape-controlled photochemical synthesis of noble metal nanocrystals based on reduced graphene oxide. ACS Appl. Mater. Interfaces 2022, 14, 16527–16537. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, Y.; Li, Q.; Zhu, Y.; Peng, J.; Jia, R.; Lai, Z.; Shi, L.; Fan, F.; Zheng, G.; et al. A surfactant-free and general strategy for the synthesis of bimetallic core–shell nanocrystals on reduced graphene oxide through targeted photodeposition. ACS Nano 2023, 17, 15085–15096. [Google Scholar] [CrossRef]
- Jia, H.; Yang, Y.; Chow, T.H.; Zhang, H.; Liu, X.; Wang, J.; Zhang, C.-y. Symmetry-broken Au–Cu heterostructures and their tandem catalysis process in electrochemical CO2 reduction. Adv. Funct. Mater. 2021, 31, 2101255. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J. Nanointerface chemistry: Lattice-mismatch-directed synthesis and application of hybrid nanocrystals. Chem. Rev. 2020, 120, 2123–2170. [Google Scholar] [CrossRef]
- Bai, S.; Xiong, Y. Some recent developments in surface and interface design for photocatalytic and electrocatalytic hybrid structures. Chem. Commun. 2015, 51, 10261–10271. [Google Scholar] [CrossRef]
- Liu, Y.; Yodsin, N.; Li, T.; Wu, H.; Jia, R.; Shi, L.; Lai, Z.; Namuangruk, S.; Huang, L. Photochemical engineering unsaturated Pt islands on supported Pd nanocrystals for a robust pH-universal hydrogen evolution reaction. Mater. Horiz. 2024, 11, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Fan, F.; Dittrich, T.; Li, C. Imaging photogenerated charge carriers on surfaces and interfaces of photocatalysts with surface photovoltage microscopy. Chem. Soc. Rev. 2018, 47, 8238–8262. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ren, Z.; Liang, Y.; Zhang, G.; Dittrich, T.; Liu, R.; Liu, Y.; Zhao, Y.; Pang, S.; An, H.; et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. Nature 2022, 610, 296–301. [Google Scholar] [CrossRef]
- Aso, R.; Hojo, H.; Takahashi, Y.; Akashi, T.; Midoh, Y.; Ichihashi, F.; Nakajima, H.; Tamaoka, T.; Yubuta, K.; Nakanishi, H.; et al. Direct identification of the charge state in a single platinum nanoparticle on titanium oxide. Science 2022, 378, 202–206. [Google Scholar] [CrossRef]
- Pizzi, A.; Dhaka, A.; Beccaria, R.; Resnati, G. Anion⋯anion self-assembly under the control of σ- and π-hole bonds. Chem. Soc. Rev. 2024, 53, 6654–6674. [Google Scholar] [CrossRef]
- Jing, H.; Zhu, P.; Zheng, X.; Zhang, Z.; Wang, D.; Li, Y. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. 2022, 1, 100013. [Google Scholar] [CrossRef]
- Jia, B.; Li, L.; Xue, C.; Kang, J.; Liu, L.-m.; Guo, T.; Wang, Z.; Huang, Q.; Guo, S. Restraining interfacial Cu2+ by using amorphous SnO2 as sacrificial protection boosts CO2 electroreduction. Adv. Mater. 2023, 35, 2305587. [Google Scholar] [CrossRef]
- Clarke, T.B.; Krushinski, L.E.; Vannoy, K.J.; Colón-Quintana, G.; Roy, K.; Rana, A.; Renault, C.; Hill, M.L.; Dick, J.E. Single entity electrocatalysis. Chem. Rev. 2024, 124, 9015–9080. [Google Scholar] [CrossRef]
- Yin, A.; Min, X.; Zhang, Y.; Yan, C. Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of monodisperse sub-10 nm Pt–Pd tetrahedrons and cubes. J. Am. Chem. Soc. 2011, 133, 3816–3819. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, J.; Sun, M.; Chen, B.; Zhou, X.; Ye, C.; Guan, Z.; Guo, W.; Wang, G.; Lu, S.; et al. Confined growth of silver–copper Janus nanostructures with {100} facets for highly selective tandem electrocatalytic carbon dioxide reduction. Adv. Mater. 2022, 34, 2110607. [Google Scholar] [CrossRef]
- Hao, J.; Zhuang, Z.; Hao, J.; Cao, K.; Hu, Y.; Wu, W.; Lu, S.; Wang, C.; Zhang, N.; Wang, D.; et al. Strain relaxation in metal alloy catalysts steers the product selectivity of electrocatalytic CO2 reduction. ACS Nano 2022, 16, 3251–3263. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, Z.; Yang, C.; Ji, Y.; Zheng, G. Tuning structures and microenvironments of Cu-based catalysts for sustainable CO2 and CO electroreduction. Acc. Mater. Res. 2023, 4, 264–274. [Google Scholar] [CrossRef]



| MA | MB | Lattice Mismatch (%) |
|---|---|---|
| Pd(T)(111) | Au(111) | 4.7~4.9 |
| Pd cube(200) | Au(111) | 17.4~21.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ren, X.; Ji, Y.; Li, T.; Jia, R.; Shi, L.; Zhou, W.; Qiao, X.; Huang, L. The Lattice Mismatch-Driven Photochemical Self-Assembly of Supported Heterostructures for Stable and Enhanced Electrocatalytic Carbon Dioxide Reduction Reaction. Molecules 2024, 29, 5560. https://doi.org/10.3390/molecules29235560
Liu Y, Ren X, Ji Y, Li T, Jia R, Shi L, Zhou W, Qiao X, Huang L. The Lattice Mismatch-Driven Photochemical Self-Assembly of Supported Heterostructures for Stable and Enhanced Electrocatalytic Carbon Dioxide Reduction Reaction. Molecules. 2024; 29(23):5560. https://doi.org/10.3390/molecules29235560
Chicago/Turabian StyleLiu, Yidan, Xu Ren, Yali Ji, Ting Li, Rongrong Jia, Liyi Shi, Wenlong Zhou, Xiran Qiao, and Lei Huang. 2024. "The Lattice Mismatch-Driven Photochemical Self-Assembly of Supported Heterostructures for Stable and Enhanced Electrocatalytic Carbon Dioxide Reduction Reaction" Molecules 29, no. 23: 5560. https://doi.org/10.3390/molecules29235560
APA StyleLiu, Y., Ren, X., Ji, Y., Li, T., Jia, R., Shi, L., Zhou, W., Qiao, X., & Huang, L. (2024). The Lattice Mismatch-Driven Photochemical Self-Assembly of Supported Heterostructures for Stable and Enhanced Electrocatalytic Carbon Dioxide Reduction Reaction. Molecules, 29(23), 5560. https://doi.org/10.3390/molecules29235560







