Expedient Synthesis of Substituted Thieno[3,2-b]thiophenes and Selenopheno[3,2-b]selenophenes Through Cascade Cyclization of Alkynyl Diol Derivatives
Abstract
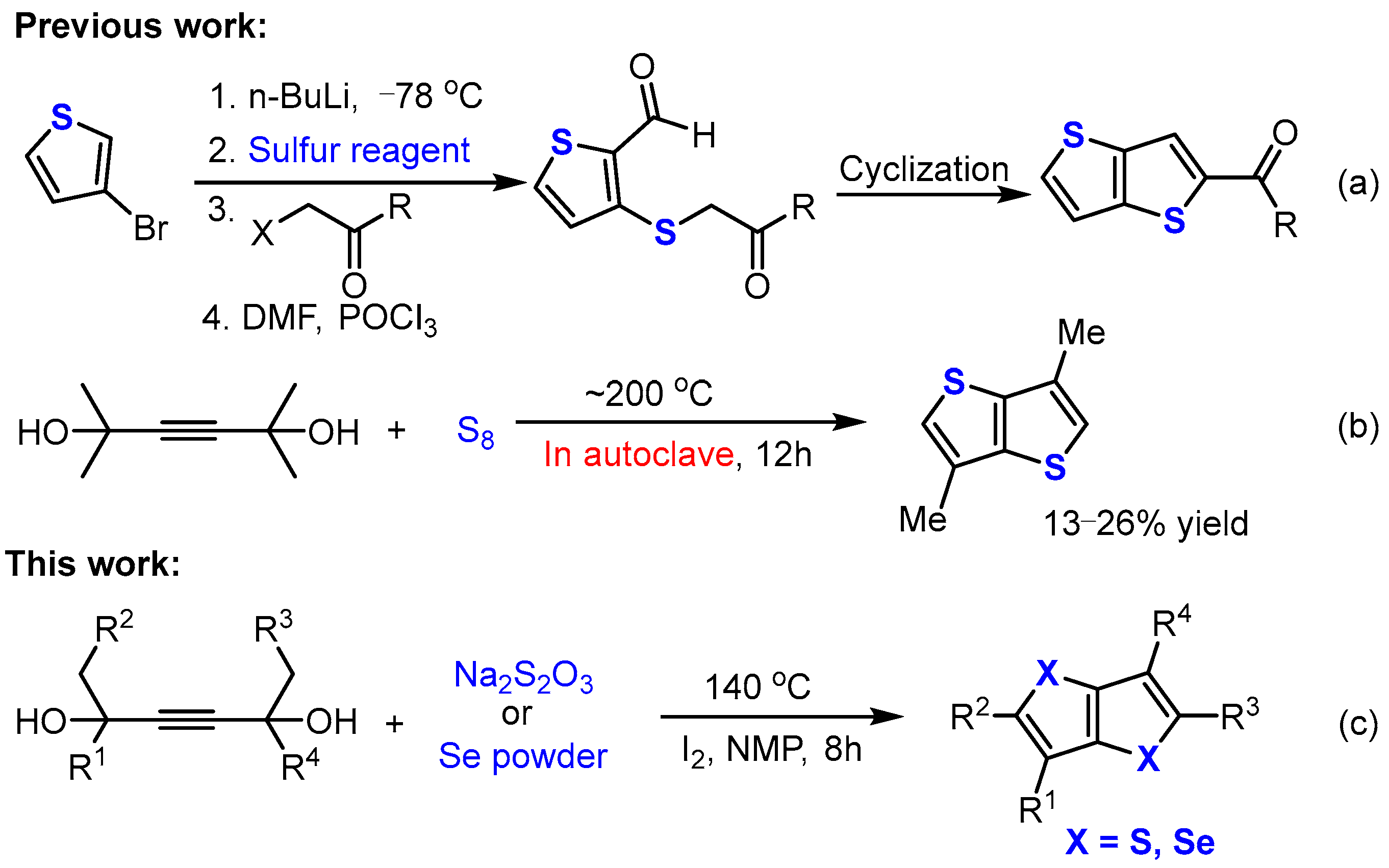
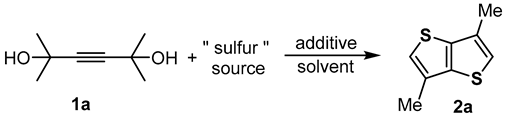
1. Introduction
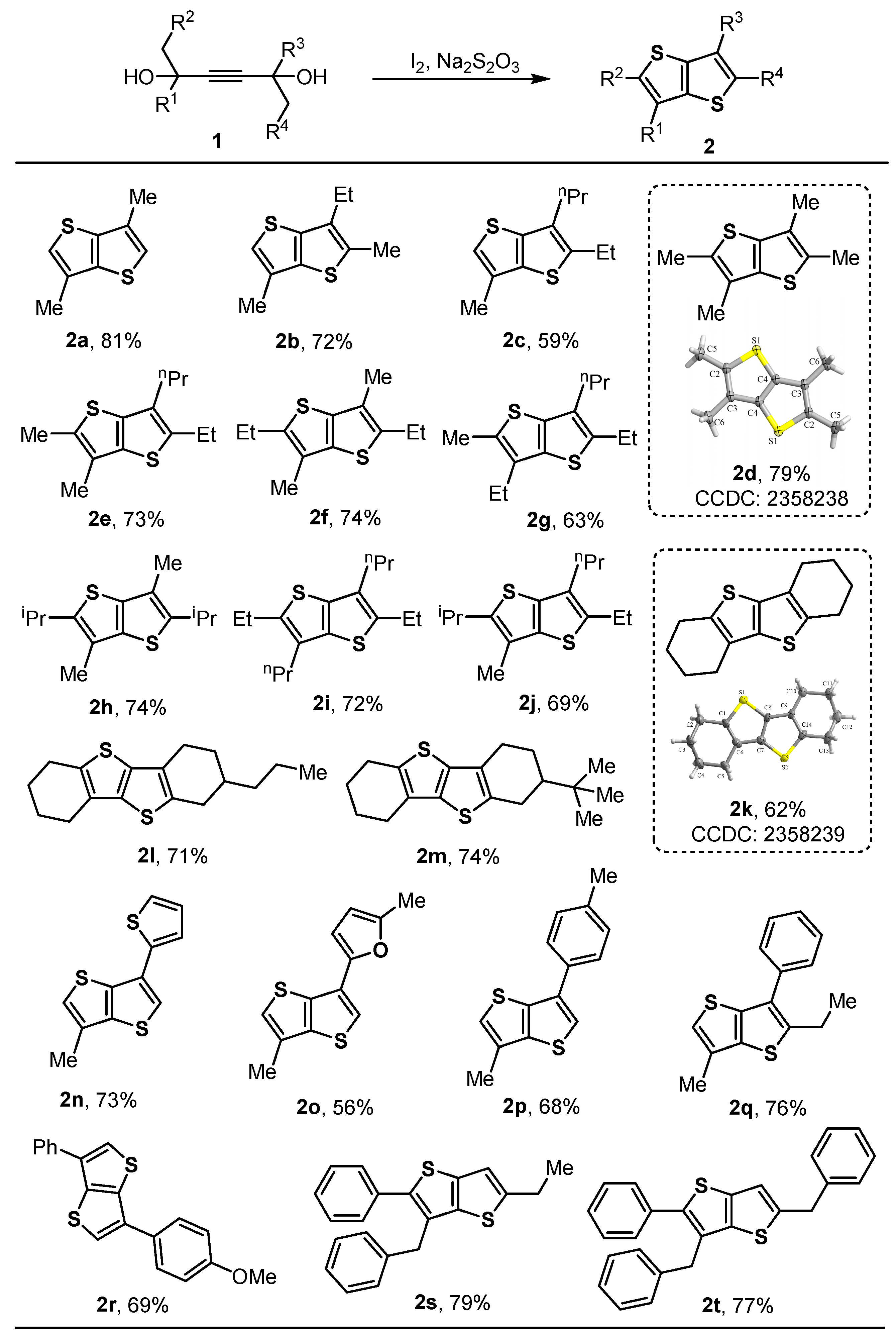
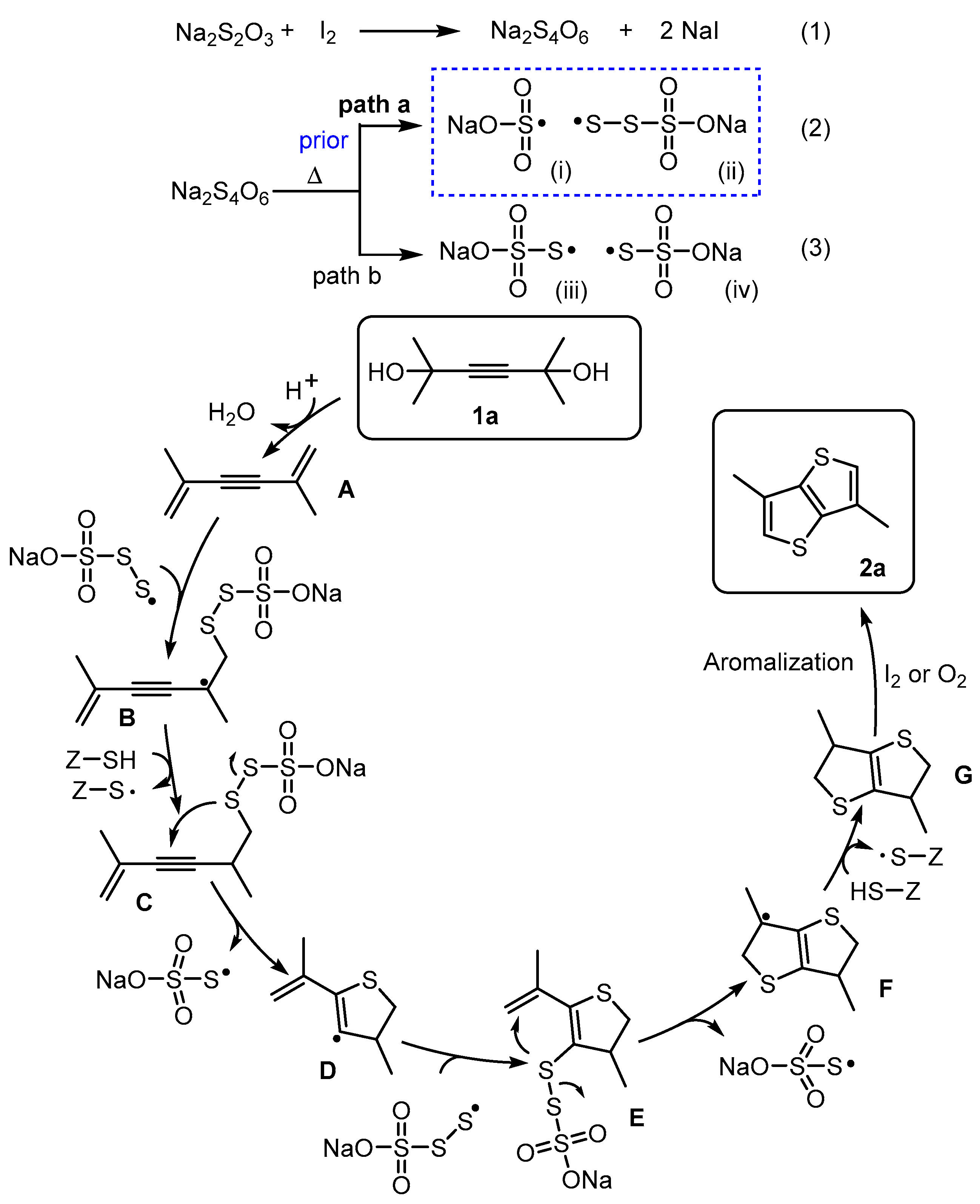
2. Results and Discussion
3. Materials and Methods
3.1. General Methods (Chemistry)
3.2. General Procedures for the Preparation of Compounds 2a–2t
- 3,6-Dimethylthieno[3,2-b]thiophene (2a), Yellow solid (68 mg, 81% yield); Rf = 0.7 (Hexane); 1H NMR (500 MHz, CDCl3) δ 6.96 (s, 2H), 2.36 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 140.0 (2C), 130.3 (2C), 121.8 (2C), 14.6 (2C). ESI-HRMS (m/z) [M + H]+ calcd. for C8H9S2+, 169.0140, found: 169.0138.
- 3-Ethyl-2,6-dimethylthieno[3,2-b]thiophene (2b), Yellow liquid (71 mg, 72% yield); Rf = 0.7 (Hexane); 1H NMR (400 MHz, CDCl3) δ 6.87 (s, 1H), 2.69 (q, J = 7.6 Hz, 2H), 2.48 (s, 3H), 2.34 (s, 3H), 1.27 (t, J = 7.6 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 139.3, 136.1, 133.9, 132.2, 129.9, 119.7, 20.9, 14.7, 14.1, 13.4. ESI-HRMS (m/z): [M]+ calcd. for C10H12S2+, 196.0374, found: 196.0368.
- 2-Ethyl-6-methyl-3-propylthieno[3,2-b]thiophene (2c), Brown liquid (66 mg, 59% yield); Rf = 0.7 (Hexane); 1H NMR (400 MHz, CDCl3) δ 6.87 (s, 1H), 2.86 (q, J = 7.5 Hz, 2H), 2.70–2.62 (m, 2H), 2.34 (s, 3H), 1.82–1.66 (m, 2H), 1.32 (t, J = 7.5 Hz, 3H), 0.98 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 142.5, 130.0, 129.9, 119.1, 29.6, 22.4, 22.3, 16.4, 14.7, 14.0. ESI-HRMS (m/z) [M]+ calcd. for C12H16S2+, 224.0688, found: 224.0679.
- 2,3,5,6-Tetramethylthieno[3,2-b]thiophene (2d), Yellow solid (77 mg, 79% yield); Rf = 0.7 (Hexane); MP: 136–137 °C. 1H NMR (500 MHz, CDCl3) δ 2.45 (s, 6H), 2.21 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 136.1 (2C), 132.0 (2C), 125.3 (2C), 14.0 (2C), 12.5 (2C). ESI-HRMS (m/z) [M + K]+ calcd. for C10H12KS2+, 235.0012, found: 235.0022.
- 2-Ethyl-5,6-dimethyl-3-propylthieno[3,2-b]thiophene (2e), Brown liquid (87 mg, 73% yield); Rf = 0.7 (Hexane); 1H NMR (400 MHz, CDCl3) δ 2.83 (q, J = 7.5 Hz, 2H), 2.62–2.59 (m, 2H), 2.43 (s, 3H), 2.20 (s, 3H), 1.70 (q, J = 7.5 Hz, 2H), 1.30 (t, J = 7.5 Hz, 3H), 0.96 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 140.3, 136.7, 135.1, 132.2, 129.5, 125.38, 29.7, 22.3, 22.2, 16.5, 14.0, 14.0, 12.5. ESI-HRMS (m/z) [M + H]+ calcd. for C13H19S2+, 239.0923, found: 239.0921.
- 2,5-Diethyl-3,6-dimethylthieno[3,2-b]thiophene (2f), Yellow solid (83 mg, 74% yield); Rf = 0.7 (Hexane); MP: 79–81 °C. 1H NMR (500 MHz, CDCl3) δ 2.83 (q, J = 7.5 Hz, 4H), 2.23 (s, 6H), 1.30 (t, J = 7.6 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ 139.9 (2C), 136.2 (2C), 124.5 (2C), 22.3 (2C), 16.0 (2C), 12.4 (2C). ESI-HRMS (m/z) [M + H]+ calcd. for C12H17S2+, 225.0766, found: 225.0766.
- 2,6-Diethyl-5-methyl-3-propylthieno[3,2-b]thiophene (2g), Brown liquid (79 mg, 63% yield); Rf = 0.7 (Hexane); 1H NMR (400 MHz, CDCl3) δ 2.83 (q, J = 7.5 Hz, 2H), 2.68–2.58 (m, 4H), 2.44 (s, 3H), 1.75–1.63 (m, 2H), 1.31–1.23 (m, 6H), 0.96 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 140.3, 135.7, 135.5, 131.8, 131.7, 129.4, 29.7, 22.3, 22.1, 20.9, 16.4, 14.0, 13.8, 13.4. ESI-HRMS (m/z) [M + H]+ calcd. for C14H21S2+, 253.1079, found: 253.1077.
- 2,5-Diisopropyl-3,6-dimethylthieno[3,2-b]thiophene (2h), Yellow solid (93 mg, 74% yield); Rf = 0.7 (Hexane); MP: 141–142 °C. 1H NMR (500 MHz, CDCl3) δ 3.35–3.29 (m, 2H), 2.25 (s, 3H), 1.33 (s, 6H), 1.32 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 146.1 (2C), 135.9 (2C), 123.7 (2C), 28.9 (2C), 24.5 (4C), 12.6 (2C). ESI-HRMS (m/z) [M + H]+ calcd. for C14H21S2+, 253.1079, found: 253.1077.
- 2,5-Diethyl-3,6-dipropylthieno[3,2-b]thiophene (2i), Brown liquid (101 mg, 72% yield); Rf = 0.7 (Hexane); 1H NMR (500 MHz, CDCl3) δ 2.83 (q, J = 7.5 Hz, 4H), 2.64–2.58 (m, 4H), 1.76–1.64 (m, 4H), 1.29 (t, J = 7.5 Hz, 6H), 0.97 (t, J = 7.4 Hz, 6H). 13C NMR (125 MHz, CDCl3) δ 140.4 (2C), 135.7 (2C), 129.5 (2C), 29.7 (2C), 22.3 (2C), 22.2 (2C), 16.5 (2C), 14.1 (2C). ESI-HRMS (m/z) [M + H]+ calcd. for C16H25S2+, 281.1392, found: 281.1391.
- 2-Ethyl-5-isopropyl-6-methyl-3-propylthieno[3,2-b]thiophene (2j), Brown liquid (92 mg, 69% yield); Rf = 0.7 (Hexane); 1H NMR (500 MHz, CDCl3) δ 3.36–3.22 (m, 1H), 2.83 (q, J = 7.5 Hz, 2H), 2.64–2.60 (m, 2H), 2.23 (s, 3H), 1.71 (q, J = 7.5 Hz, 2H), 1.33–1.27 (m, 9H), 0.98 (t, J = 7.3 Hz, 3H). 13C NMR (125 MHz, CDCl3) δ 146.1, 140.4, 136.7, 135.0, 129.8, 123.4, 29.8, 28.9, 24.5 (2C), 22.3, 22.2, 16.6, 14.1, 12.6. ESI-HRMS (m/z) [M + H]+ calcd. for C15H23S2+, 267.1236, found: 267.1233.
- 1,2,3,4,6,7,8,9-Octahydrobenzo[b]benzo[4,5]thieno[2,3-d]thiophene (2k), Yellow solid (77 mg, 62% yield); Rf = 0.7 (Hexane); MP: 126–128 °C. 1H NMR (400 MHz, CDCl3) δ 2.88–2.85 (m, 4H), 2.67–2.63 (m, 4H), 1.97–1.82 (m, 8H). 13C NMR (100 MHz, CDCl3) δ 135.3 (2C), 134.9 (2C), 127.8 (2C), 26.0 (2C), 24.6 (2C), 23.5 (2C), 22.5 (2C). ESI-HRMS (m/z) [M + H]+ calcd. for C14H17S2+, 249.0766, found: 249.0767.
- 2-Propyl-1,2,3,4,6,7,8,9-octahydrobenzo[b]benzo[4,5]thieno[2,3-d]thiophene (2l), Yellow solid (103 mg, 71% yield); Rf = 0.7 (Hexane); MP: 99–101 °C. 1H NMR (400 MHz, CDCl3) δ 2.95–2.90 (m, 1H), 2.86–2.82 (m, 2H), 2.72–2.66 (m, 1H), 2.64–2.28 (m, 3H), 2.52–2.44 (m, 1H), 2.00–1.95 (m, 1H), 1.92–1.83 (m, 5H),1.52–1.37 (m, 5H), 0.94 (t, J = 6.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 135.3, 135.2, 127.9, 127.8, 38.1, 34.6, 32.3, 28.9, 26.0, 24.6, 24.3, 23.5, 22.5, 20.1, 14.2. ESI-HRMS (m/z) [M + H]+ calcd. for C17H23S2+, 291.1235, found: 291.1233.
- 2-(Tert-butyl)-1,2,3,4,6,7,8,9-octahydrobenzo[b]benzo[4,5]thieno[2,3-d]thiophene (2m), Yellow solid (112 mg, 74% yield); Rf = 0.7 (Hexane); MP: 136–138 °C. 1H NMR (400 MHz, CDCl3) δ 2.92–2.84 (m, 3H), 2.78–2.72 (m, 1H), 2.65–2.54 (m, 4H), 2.09–2.05 (m, 1H), 1.92–1.85 (m, 4H), 1.64–1.56 (m, 1H), 1.49–1.38 (m, 1H), 0.98 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 136.2, 135.3, 135.2, 134.5, 127.9, 127.9, 45.5, 32.5, 27.6, 27.3 (3C), 26.0, 25.5, 24.6, 24.1, 23.5, 22.5. ESI-HRMS (m/z) [M + H]+ calcd. for C18H25S2+, 305.1392, found: 305.1391.
- 3-Methyl-6-(thiophen-2-yl)thieno[3,2-b]thiophene (2n), Black liquid (86 mg, 73% yield); Rf = 0.7 (Hexane); 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H), 7.39–7.38 (m, 1H), 7.29–7.28 (m, 1H), 7.13–7.10 (m, 1H), 7.06 (s, 1H), 2.40 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 140.8, 137.5, 136.6, 130.0, 128.7, 127.7, 124.2, 123.8, 122.6, 121.0, 14.6. ESI-HRMS (m/z) [M + H]+ calcd. for C11H9S3+, 236.9861, found: 236.9861.
- 2-Methyl-5-(6-methylthieno[3,2-b]thiophen-3-yl)furan (2o), Yellow solid (66 mg, 56% yield); Rf = 0.7 (Hexane); MP: 114–116 °C. 1H NMR (400 MHz, CDCl3) δ 7.49 (s, 1H), 7.04 (s, 1H), 6.51 (s, 1H), 6.09 (s, 1H), 2.39 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 151.7, 147.8, 140.6, 129.8, 125.6, 122.5, 118.9, 107.4, 106.8, 14.6, 13.6. ESI-HRMS (m/z) [M + H]+ calcd. for C12H11OS2+, 235.0246, found: 235.0247.
- 3-Methyl-6-(p-tolyl)thieno[3,2-b]thiophene (2p), Brown solid (73 mg, 68% yield); Rf = 0.5 (Hexane); MP: 135–137 °C. 1H NMR (400 MHz, CDCl3) δ 7.66 (s, 1H), 7.64 (s, 1H), 7.44 (s, 1H), 7.27 (d, J = 8.0 Hz, 2H), 7.04 (s, 1H), 2.40 (s, 6H). 13C NMR (125 MHz, CDCl3) δ 140.9, 137.4, 137.1, 135.1, 132.0, 130.0, 129.6 (2C), 126.3 (2C), 122.2, 121.1, 21.2, 14.6. ESI-HRMS (m/z) [M + H]+ calcd. for C14H13S2+, 213.0732, found: 213.0733.
- 2-Ethyl-6-methyl-3-phenylthieno[3,2-b]thiophene (2q), Yellow liquid (72 mg, 56% yield); Rf = 0.5 (Hexane); 1H NMR (400 MHz, CDCl3) δ 7.56–7.34 (m, 5H), 6.90 (s, 1H), 2.97 (q, J = 7.5 Hz, 2H), 2.38 (s, 3H), 1.33 (t, J = 7.5 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 144.1, 139.7, 136.3, 135.3, 130.8, 130.0, 128.7 (2C), 128.6 (2C), 127.4, 120.4, 23.0, 16.7, 14.7. ESI-HRMS (m/z) [M + H]+ calcd. for C15H15S2+, 259.0610, found: 259.0609.
- 3-(4-Methoxyphenyl)-6-phenylthieno[3,2-b]thiophene (2r), Yellow solid (111 mg, 69% yield); Rf = 0.5 (Hexane); MP: 143–145 °C. 1H NMR (400 MHz, CDCl3) δ 7.89–7.33 (m, 9H), 7.05–6.99 (m, 2H), 3.88 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.2, 138.2, 138.1, 134.9, 134.6, 134.5, 128.9 (2C), 127.72 (2C), 127.70, 127.4, 126.5 (2C), 122.2, 120.9, 114.4 (2C), 55.3. ESI-HRMS (m/z) [M + H]+ calcd. for C19H15OS2+, 323.0559, found: 323.0556.
- 3-Benzyl-6-ethyl-2-phenylthieno[3,2-b]thiophene (2s), Yellow liquid (132 mg, 79% yield); Rf = 0.5 (Hexane); 1H NMR (400 MHz, CDCl3) δ 7.52–7.13 (m, 10H), 6.89 (s, 1H), 4.12 (s, 2H), 2.84–2.77 (m, 2H), 1.28–1.24 (m, 3H). 13C NMR (125 MHz, CDCl3) δ 149.4, 139.1, 139.1, 138.94, 136.1, 134.7, 129.3 (2C), 128.6 (2C), 128.6 (2C), 128.4 (2C), 128.2, 127.6, 126.3, 115.6, 34.2, 24.3, 15.7. ESI-HRMS (m/z) [M + H]+ calcd. for C21H19S2+, 335.0923, found: 335.0919.
- 3,6-Dibenzyl-2-phenylthieno[3,2-b]thiophene (2t), Yellow liquid (145 mg, 77% yield); Rf = 0.5 (Hexane); 1H NMR (500 MHz, CDCl3) δ 7.51–7.27 (m, 9H), 7.25–7.15 (m, 6H), 6.91 (s, 1H), 4.14 (s, 2H), 4.11 (s, 2H). 13C NMR (125 MHz, CDCl3) δ 145.9, 140.1, 139.7, 139.5, 138.8, 136.1, 133.6, 134.6, 129.3 (2C), 128.6 (2C), 128.6 (2C), 128.5 (2C), 128.4 (2C), 128.2, 127.7 (2C), 126.6, 126.3, 117.5, 37.1, 34.1. ESI-HRMS (m/z) [M]+ calcd. for C26H20S2+, 396.1001, found: 396.0998.
3.3. General Procedures for the Preparation of Compounds 3a–3b
- 3,6-Dimethylselenopheno[3,2-b]selenophene (3a), Yellow solid (87 mg, 69% yield); Rf = 0.7 (Hexane); MP: 96–98 °C. 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 2H), 2.37 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 141.6 (2C), 135.2 (2C), 123.6 (2C), 17.2 (2C). ESI-HRMS (m/z) [M + H]+ calcd. for C8H9Se2+, 252.9148, found: 252.9158.
- 2,3,5,6-Tetramethylselenopheno[3,2-b]selenophene (3b), Brown solid (85 mg, 64% yield); Rf = 0.7 (Hexane); MP: 101–103 °C. 1H NMR (400 MHz, CDCl3) δ 2.51 (s, 6H), 2.16 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 137.6 (2C), 135.8 (2C), 130.2 (2C), 16.2 (2C), 14.5 (2C). ESI-HRMS (m/z) [M + H]+ calcd. for C10H13Se2+, 280.9461, found: 280.9459.
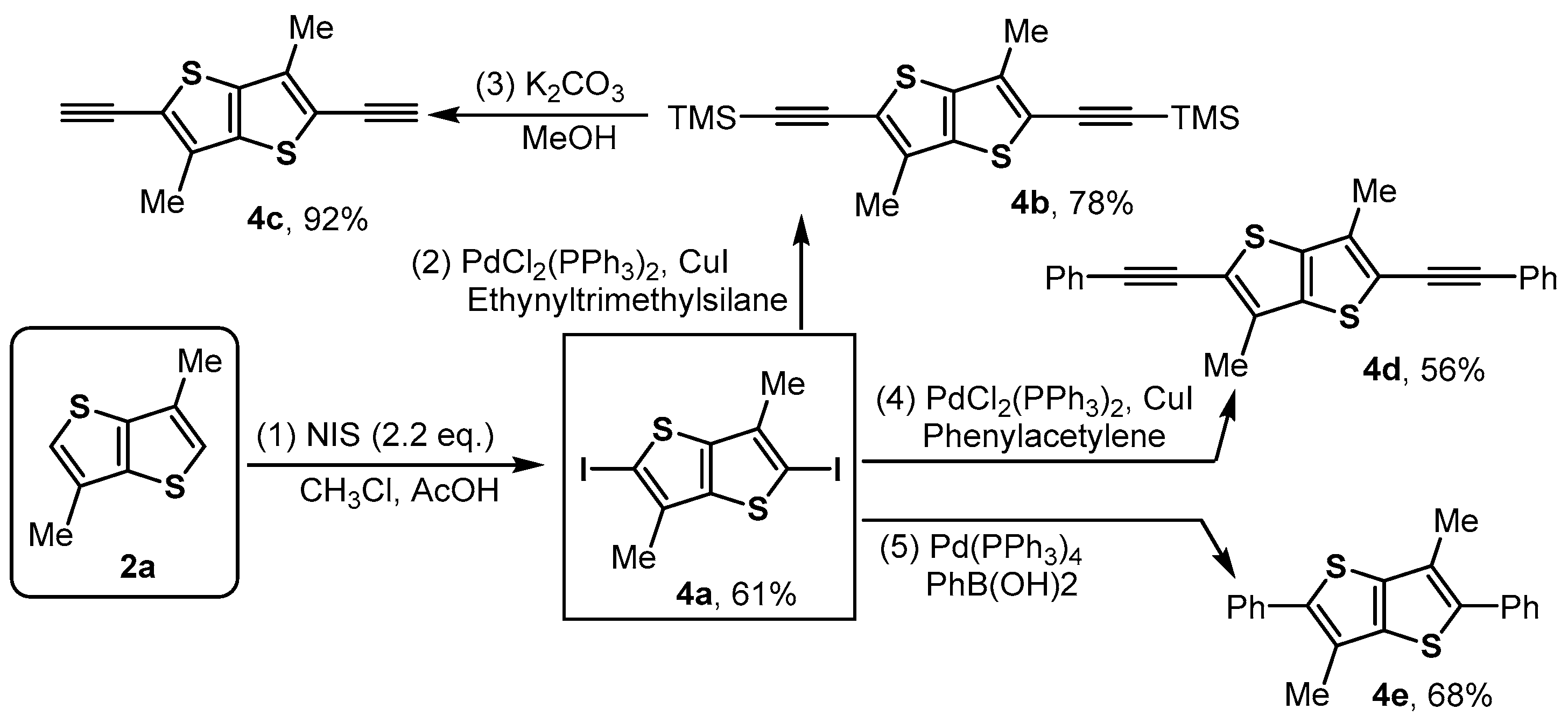
3.4. Synthesis of 2,5-Diiodo-3,6-dimethylthieno[3,2-b]thiophene 4a
- 2,5-Diiodo-3,6-dimethylthieno[3,2-b]thiophene (4a), White solid (512 mg, 61% yield); Rf = 0.7 (Hexane); 1H NMR (400 MHz, CDCl3) δ 2.26 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 140.9 (2C), 134.2 (2C), 76.0 (2C), 16.9 (2C).
3.5. Synthesis of ((3,6-Dimethylthieno[3,2-b]thiophene-2,5-diyl)bis(ethyne-2,1-diyl))bis(trimethylsilane) 4b
- ((3,6-Dimethylthieno[3,2-b]thiophene-2,5-diyl)bis(ethyne-2,1-diyl))bis(trimethylsilane) (4b), Yellow solid (67 mg, 78% yield); MP:157–158 °C. Rf = 0.7 (Hexane); 1H NMR (400 MHz, CDCl3) δ 2.35 (s, 6H), 0.27 (s, 18H). 13C NMR (100 MHz, CDCl3) δ 138.0 (2C), 135.7 (2C), 121.2 (2C), 103.5 (2C), 97.6 (2C), 14.0 (2C), 0.0 (6C). ESI-HRMS (m/z) [M + Na]+ calcd. for C18H24NaS2Si2+, 383.0750, found: 383.0752.
3.6. Synthesis of 2,5-Diethynyl-3,6-dimethylthieno[3,2-b]thiophene 4c
- 2,5-Diethynyl-3,6-dimethylthieno[3,2-b]thiophene (4c), White solid (46 mg, 92% yield); Rf = 0.5 (Hexane); MP:123–124 °C. 1H NMR (400 MHz, CDCl3) δ 3.62 (s, 2H), 2.39 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 138.1 (2C), 136.2 (2C), 120.2 (2C), 85.5 (4C), 13.9 (2C). ESI-HRMS (m/z) [M + Na]+ calcd. for C24H16NaS2+, 238.9960, found: 238.9951.
3.7. Synthesis of 3,6-Dimethyl-2,5-bis(phenylethynyl)thieno[3,2-b]thiophene 4d
- 3,6-Dimethyl-2,5-bis(phenylethynyl)thieno[3,2-b]thiophene (4d), Yellow solid (49 mg, 56% yield); Rf = 0.7 (Hexane); MP:137–139 °C. 1H NMR (400 MHz, CDCl3) δ 7.57–7.52 (m, 4H), 7.40–7.33 (m, 6H), 2.46 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 138.5 (2C), 134.9 (2C), 131.3 (4C), 128.4 (2C), 128.4 (4C), 122.9 (2C), 121.0 (2C), 97.5 (2C), 82.9 (2C), 14.1 (2C). ESI-HRMS (m/z) [M + Na]+ calcd. for C24H16NaS2+, 391.0586, found: 391.0580.
3.8. Synthesis of 3,6-Dimethyl-2,5-diphenylthieno[3,2-b]thiophene 4e
- 3,6-Dimethyl-2,5-diphenylthieno[3,2-b]thiophene (4e), Yellow solid (67 mg, 68% yield); Rf = 0.7 (Hexane); MP:168–170 °C. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 7.3 Hz, 4H), 7.45 (t, J = 7.6 Hz, 4H), 7.36 (d, J = 7.9 Hz, 2H), 2.44 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 139.1 (2C), 138.1 (2C), 135.1 (2C), 129.1 (4C), 128.6 (4C), 127.4 (2C), 125.8 (2C), 14.0 (2C). ESI-HRMS (m/z) [M + H]+ calcd. for C20H17S2+, 321.0766, found: 321.0761.
3.9. Synthesis of 2,5-Diiodo-2,5-dimethylhex-3-yne 5b
- 2,5-Diiodo-2,5-dimethylhex-3-yne (5b), Yellow liquid (141 mg, 78% yield); Rf = 0.6 (Hexane/EtOAc = 30:1); 1H NMR (400 MHz, CDCl3) δ 1.36 (s, 12H). 13C NMR (100 MHz, CDCl3) δ 113.0 (2C), 91.2 (2C), 28.9 (42C). ESI-HRMS (m/z) [M + Na]+ calcd. for C8H12NaI2+, 384.8921, found: 384.8926.
3.10. Synthesis of 2,4-Diphenylthiophene 5d
- 2,4-Diphenylthiophene (5d), Yellow solid (20.6 mg, 35% yield); Rf = 0.5 (Hexane); 1H NMR (400 MHz, CDCl3) δ 7.67–7.61 (m, 4H), 7.60 (s, 1H), 7.44–7.39 (m, 5H), 7.33–7.31 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 145.0, 143.1, 135.9, 134.3, 128.9 (2C), 128.8 (2C), 127.7, 127.3, 126.3 (2C), 125.8 (2C), 122.3, 119.7. ESI-HRMS (m/z) [M + H]+ calcd. for C16H13S+, 237.0732, found: 237.0734.
3.11. Synthesis of 3,6-Dimethylthieno[3,2-b]thiophene-2,5-d2 2a-D
- 3,6-Dimethylthieno[3,2-b]thiophene-2,5-d2 (2a-D), Yellow solid (68 mg, 81% yield); Rf = 0.7 (Hexane); 1H NMR (400 MHz, CDCl3) δ 6.96 (s, 1.68H), 2.36 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 140.0 (2C), 130.3 (2C), 121.8 (2C), 14.6 (2C). 2H NMR (77 MHz, CH2Cl2) δ 6.96 (s, 2D).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, X. Thieno[3,4-b]thiophene-Based Novel Small-Molecule Optoelectronic Materials. Acc. Chem. Res. 2017, 50, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, A.; Aslam, S.; Ahmad, M.; Nazir, M.S.; Farooq, A.; Sultan, S. Recent Synthetic Approaches towards Thienothiophenes: A Potential template for Biologically Active Compounds. Mol. Divers 2024, 28, 1793–1821. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, V.P.; Gol’dfarb, Y.A.L. The Chemistry of Thienothiophenes and Related Systems. Adv. Heterocycl. Chem. 1976, 19, 123–214. [Google Scholar] [CrossRef]
- Podlesný, J.J.; Bureš, F. Thienothiophene Scaffolds as Building Blocks for (Opto)Electronics. Organics 2022, 3, 446–469. [Google Scholar] [CrossRef]
- Isci, R.; Varzeghani, A.R.; Kaya, K.; Sütay, B.; Tekin, E.; Ozturk, T. Triphenylamine/Tetraphenylethylene Substituted 4-Thieno[3,2-b]thiophen-3-ylbenzonitriles: Synthesis, Photophysical-Electronic Properties, and Applications. ACS Sustain. Chem. Eng. 2022, 10, 1605–1615. [Google Scholar] [CrossRef]
- Mishra, A. Material Perceptions and Advances in Molecular Heteroacenes for Organic Oolar cells. Energy Environ. Sci. 2020, 13, 4738–4793. [Google Scholar] [CrossRef]
- Bronstein, H.; Chen, Z.; Ashraf, R.S.; Zhang, W.; Du, J.; Durrant, J.R.; Tuladhar, P.S.; Song, K.; Watkins, S.E.; Geerts, Y.; et al. Thieno[3,2-b]thiophene−Diketopyrrolopyrrole-Containing Polymers for High-Performance Organic Field-Effect Transistors and Organic Photovoltaic Devices. J. Am. Chem. Soc. 2011, 133, 3272–3275. [Google Scholar] [CrossRef]
- McCulloch, I.; Heeney, M.; Chabinyc, M.L.; DeLongchamp, D.; Kline, R.J.; Cölle, M.; Duffy, W.; Fischer, D.; Gundlach, D.; Hamadani, B.; et al. Semiconducting Thienothiophene Copolymers: Design, Synthesis, Morphology, and Performance in Thin-Film Organic Transistors. Adv. Mater. 2009, 21, 1091–1109. [Google Scholar] [CrossRef]
- Fong, H.H.; Pozdin, V.A.; Amassian, A.; Malliaras, G.G.; Smilgies, D.M.; He, M.Q.; Gasper, S.; Zhang, F.; Sorensen, M. Tetrathienoacene Copolymers as High Mobility, Soluble Organic Semiconductors. J. Am. Chem. Soc. 2008, 130, 13202–13203. [Google Scholar] [CrossRef]
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J.A. A Porous Coordination Copolymer with over 5000 m2/g BET Surface Area. J. Am. Chem. Soc. 2009, 131, 4184–4185. [Google Scholar] [CrossRef] [PubMed]
- Henssler, J.T.; Matzger, A.J. Facile and Scalable Synthesis of the Fused-Ring Heterocycles Thieno[3,2-b]thiophene and Thieno[3,2-b]furan. Org. Lett. 2009, 11, 3144–3147. [Google Scholar] [CrossRef] [PubMed]
- Fuller, L.S.; Iddon, B.; Smith, K.A. Thienothiophenes. Part 2.1 Synthesis, metallation and bromine→lithium exchange reactions of thieno[3,2-b]thiophene and its polybromo derivatives. J. Chem. Soc. Perkin Trans. 1 1997, 22, 3465–3470. [Google Scholar] [CrossRef]
- Mazaki, Y.; Kobayashi, K. Synthesis of tetrathieno-acene and pentathieno-acene: UV-spectral trend in a homologous series of thieno-acenes. Tetrahedron Lett. 1989, 30, 3315–3318. [Google Scholar] [CrossRef]
- Litvinov, V.P. The Chemistry of Thienothiophenes. Adv. Heterocycl. Chem. 2006, 90, 125–203. [Google Scholar] [CrossRef]
- Rutherford, D.R.; Sille, J.K.; Elliott, C.M.; Reichert, V.R. Poly(2,5-ethynylenethiophenediylethynylenes), related heteroaromatic analogs, and poly(thieno[3,2-b]thiophenes): Synthesis and thermal and electrical properties. Macromolecules 1992, 25, 2294–2306. [Google Scholar] [CrossRef]
- Teste, J.; Lozac’h, N. Sulfuration of Organic Compounds. IX. Sulfuration of Alcohols and Acetylenic Glycols. Bull. Soc. Chim. Fr. 1955, 442. [Google Scholar]
- Choi, K.S.; Saeada, K.; Dong, H.; Hoshino, M.; Nakayama, J. A One-Pot Synthesis of Substituted Thieno[3,2-b]thiophenens and Selenolo[3,2-b]-selenophenes. Heterocycles 1994, 38, 143–149. [Google Scholar]
- Chen, Z.; Huang, J.; Zhang, W.; Zhou, Y.; Wei, X.; Wei, J.; Zheng, Y.; Wang, L.; Yu, G. Tunable charge-transport polarity in thienothiophene–bisoxoindolinylidene-benzodifurandione copolymers for high-performance field-effect transistors. J. Mater. Chem. C 2022, 10, 2671–2680. [Google Scholar] [CrossRef]
- Marco, A.B.; Gindre, D.; Iliopoulos, K.; Franco, S.; Andreu, R.; Canevet, D.; Sallé, M. (Super)gelators derived from push–pull chromophores: Synthesis, gelling properties and second harmonic generation. Org. Biomol. Chem. 2018, 16, 2470–2478. [Google Scholar] [CrossRef]
- Leriche, P.; Raimundo, J.-M.; Turbiez, M.; Monroche, V.; Allain, M.; Sauvage, F.-C.; Roncali, J.; Frère, P.; Skabarad, P.J. Linearly extended tetrathiafulvalene analogues with fused thiophene units as π-conjugated spacers. J. Mater. Chem. 2003, 13, 1324–1332. [Google Scholar] [CrossRef]
- Zhang, G.; Yi, H.; Chen, H.; Bian, C.; Liu, C.; Lei, A. Trisulfur Radical Anion as the Key Intermediate for the Synthesis of Thiophene via the Interaction between Elemental Sulfur and NaOtBu. Org. Lett. 2014, 16, 6156–6159. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yan, C.; Yan, D.; Zhuang, R. Metal-Free C–S Bond Formation in Elemental Sulfur and Cyclobutanol Derivatives: The Synthesis of Substituted Thiophenes. Org. Lett. 2022, 24, 5309–5313. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Dong, J.; Xie, Z.; Tan, X.; Zhou, L.; Ai, L.; Li, B.; Wang, Y.; Dong, H. A Domino Protocol toward High-performance Unsymmetrical Dibenzo[d,d′]thieno[2,3-b;4,5-b′]dithiophenes Semiconductors. Angew. Chem. Int. Ed. 2024, 63, e202400803. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Qu, Z.; Ji, X.; Deng, G. Three-component bis-heterocycliation for synthesis of 2-aminobenzo[4,5]thieno[3,2-d]thiazoles. Org. Chem. Front. 2019, 6, 1146–1150. [Google Scholar] [CrossRef]
- Li, J.H.; Huang, Q.; Wang, S.Y.; Ji, S.J. Hedychins A and B, 6,7-Dinorlabdane Diterpenoids with a Peroxide Bridge from Hedychium forrestii. Org. Lett. 2018, 20, 704–4708. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Rao, W.; Wang, S.Y.; Ji, S.J. A Trisulfur Radical anion (S3˙−) involved Sulfur insertion Reaction of 1,3-Enynes: Sulfide Sources Control Chemoselective Synthesis of 2,3,5-Trisubstituted Thiophenes and 3-Thienyl disulfides. Chem. Commun. 2019, 55, 7808–7811. [Google Scholar] [CrossRef]
- Chen, L.; Min, H.; Zeng, W.; Zhu, X.; Liang, Y.; Deng, G.; Yang, Y. Transition-Metal-Free Sulfuration/Annulation of Alkenes: Economical Access to Thiophenes Enabled by the Cleavage of Multiple C–H Bonds. Org. Lett. 2018, 20, 7392–7395. [Google Scholar] [CrossRef]
- Song, P.; Rao, W.; Chivers, T.; Wang, S. Applications of trisulfide radical anion S3− in organic synthesis. Org. Chem. Front. 2023, 10, 3378–3400. [Google Scholar] [CrossRef]
- Hou, X.; Liu, H.; Huang, H. Iron-catalyzed fluoroalkylative alkylsulfonylation of alkenes via radical-anion relay. Nat. Commun. 2024, 15, 1480. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, W.; Ma, J.; Li, J.; Meng, Q.; Shen, C.; Zeng, X. Syn-Selective Chlorosulfonylation of Alkynes via a Copper-Powder-Initiated Atom Transfer Radical Addition Reaction and Mechanistic Studies. Org. Lett. 2023, 25, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zeng, X. Metal–Free Radical Thiocyanatosulfonation of Terminal Alkynes in Aqueous Medium. Org. Lett. 2021, 23, 3326–3330. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Jiang, X. Dithionite-Involved Multicomponent Coupling for Alkenyl and Alkyl Tertiary Sulfones. Org. Lett. 2021, 23, 4657–4661. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Zhou, Z.; Zhao, F.; Mao, G.; Deng, G.; Huang, H. Deoxygenative C–S Bond Coupling with Sulfinates via Nickel/Photoredox Dual Catalysis. Org. Lett. 2022, 24, 1865–1870. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Wei, Z.; Liao, W. Multicomponent Cyclization with an Inorganic Sulfur Dioxide Surrogate: Straightforward Construction of Difluorinated Benzosultams. Org. Lett. 2022, 24, 9112–9117. [Google Scholar] [CrossRef]
- Xiao, F.; Xie, H.; Liu, S.; Deng, G. Iodine-Catalyzed Regioselective Sulfenylation of Indoles with Sodium Sulfinates. Adv. Synth. Catal. 2014, 356, 364–368. [Google Scholar] [CrossRef]
- Liao, W.; Lin, S.; Kuo, Y.; Liang, C. Site-Selective Acylation of Phenols Mediated by a Thioacid Surrogate through Sodium Thiosulfate Catalysis. Org. Lett. 2022, 24, 4207–4211. [Google Scholar] [CrossRef]
- Liu, B.; Chu, X.; Liu, H.; Yin, L.; Wang, S.Y.; Ji, S.J. Aqueous Reaction of Alcohols, Organohalides, and Odorless Sodium Thiosulfate under Transition-Metal-Free Conditions: Synthesis of Unsymmetrical Aryl Sulfides via Dual C–S Bond Formation. J. Org. Chem. 2017, 82, 10174–10180. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Yan, R.; Yan, M.; Xu, Q. Promoting Effect of Crystal Water Leading to Catalyst-Free Synthesis of Heteroaryl Thioether from Heteroaryl Chloride, Sodium Thiosulfate Pentahydrate, and Alcohol. J. Org. Chem. 2019, 84, 11294–11300. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Ji, X.; Liu, Q.; Chen, L.; Huang, Y.; Li, Y. Transition Metal-Free Synthesis of Substituted Isothiazoles via Three-Component Annulation of Alkynones, Xanthate and NH4I. Adv. Syn. Catal. 2021, 363, 1059–1068. [Google Scholar] [CrossRef]
- He, R.; Liu, Y.; Feng, Y.; Chen, L.; Huang, Y.; Xie, F.; Li, Y. Access to Thienopyridine and Thienoquinoline Derivatives via Site-Selective C–H Bond Functionalization and Annulation. Org. Lett. 2022, 24, 3167–3172. [Google Scholar] [CrossRef]
- Huang, G.; Li, J.; Li, J.R.; Li, J.M.; Sun, M.; Zhou, P.; Chen, L.; Huang, Y.; Jiang, S.; Li, Y. Access to Substituted Thiophenes through Xanthate-Mediated Vinyl C(sp2)-Br Bond Cleavage and Heterocyclization of Bromoenynes. J. Org. Chem. 2020, 85, 13037–13049. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Chen, Z.; Li, J.M.; Ji, X.; Chen, L.; Huang, Y.; Liu, Q.; Li, Y. Synthesis of Substituted Thiophenes through Dehydration and Heterocyclization of Alkynols. J. Org. Chem. 2022, 87, 3555–3566. [Google Scholar] [CrossRef]
- Li, J.J. Zaitsev’s elimination rule. In Name Reactions; Springer: Cham, Switzerland, 2014; pp. 650–651. ISBN 978-3-319-03979-4. [Google Scholar] [CrossRef]
- Xiong, H.; Lin, Q.; Lu, Y.; Zheng, D.; Li, Y.; Wang, S.; Xie, W.; Li, C.; Zhang, X.; Lin, Y.; et al. General room-temperature Suzuki–Miyaura polymerization for organic electronics. Nat. Mater. 2024, 23, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Vanderspikken, J.; Liu, Z.; Wu, X.; Beckers, O.; Moro, S.; Quill, T.J.; Liu, Q.; Goossens, A.; Marks, A.; Weaver, K.; et al. On the Importance of Chemical Precision in Organic Electronics: Fullerene Intercalation in Perfectly Alternating Conjugated Polymers. Adv. Func. Mater. 2023, 33, 2309403. [Google Scholar] [CrossRef]
- Darabi, H.R.; Aghapoor, K.; Mohsenzadeh, F. Development of a Synthesis of Diphenylthiophenes via a One-Pot Reaction of Phenylacetylene and Sulfur. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 2483–2489. [Google Scholar] [CrossRef]
- Salamanca, V.; Albéniz, A.C. Deuterium Exchange between Arenes and Deuterated Solvents in the Absence of a Transition Metal: Synthesis of D-Labeled Fluoroarenes. Eur. J. Org. Chem. 2020, 22, 3206–3212. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Ji, X.; He, R.; Liu, Y.; Chen, Z.; Huang, Y.; Liu, Q.; Li, Y. Synthesis of Deuterated (E)-Alkene through Xanthate-Mediated Hydrogen–Deuterium Exchange Reactions. Org. Lett. 2021, 23, 7412–7417. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Zhao, W.; Sivaguru, P.; Zanoni, G.; Wang, Y.; Anderson, E.A.; Bi, X. Synthetic exploration of sulfinyl radicals using sulfinyl sulfones. Nat. Commun. 2021, 12, 5244. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Sivaguru, P.; Wang, Z. Exploring the synthetic application of sulfinyl radicals. Org. Chem. Front. 2022, 9, 6063–6076. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, J.; Zhao, G.; Qi, Y.; Wang, H.; Lei, A. Dioxygen-Triggered Oxidative Radical Reaction: Direct Aerobic Difunctionalization of Terminal Alkynes toward β-Keto Sulfones. J. Am. Chem. Soc. 2013, 135, 11481–11484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, Q.; Feng, C.; Wang, Z.; Zhao, W.; Ning, Y.; Wu, Y. The Sulfinylsulfonation of alkynes for β-Sulfinyl alkenylsulfone. Chem. Asian J. 2022, 17, e202200299. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, J.; Wei, F.; Qi, Y.; Wang, H.; Liu, Z.; Lei, A. Aerobic Oxysulfonylation of Alkenes Leading to Secondary and Tertiary β-Hydroxysulfones. Angew. Chem. Int. Ed. 2013, 52, 7156–7159. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Xia, Y. Gas-Phase Reactivity of Peptide Thiyl (RS•), Perthiyl (RSS•), and Sulfinyl (RSO•) Radical Ions Formed from Atmospheric Pressure Ion/Radical Reactions. J. Am. Soc. Mass Spectrom. 2013, 24, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Chen, S.; Mo, X.; Wu, K.; Wu, J.; Lin, W.; Lin, Z.; Lin, J.; Zhang, H.; Wen, T. Trisulfur radical anion-triggered stitching thienannulation: Rapid access to largely π-extended thienoacenes. Chem. Sci. 2020, 11, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Christidis, P.C.; Rentzeperis, P.J. Experimental charge density in polythionate anions: II. X-ray study of the electron density distribution in potassium tetrathionate, K2S4O6. Z. Für Krist. 1989, 188, 31–42. [Google Scholar] [CrossRef]
- Kurihara, M.; Shigehisa, H. Halocyclization of Alkynoic Thioester and Oxidative Aromatization in One-Pot. J. Org. Chem. 2024, 89, 9700–9704. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Nie, J.; Xie, S.; Peng, X.; Hong, H.; Chen, X.; Chen, L.; Li, Y. Aromatization of cyclic hydrocarbons via thioether elimination reaction. Chem. Commun. 2023, 59, 11232–11235. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; He, R.; Liu, J.; Liu, Y.; Chen, L.; Huang, Y.; Li, Y. Selective Synthesis of Substituted Pyridines and Pyrimidines through Cascade Annulation of Isopropene Derivatives. Org. Lett. 2022, 24, 1620–1625. [Google Scholar] [CrossRef]
- Princival, J.L.; Ferreira, J.G. CeCl3-mediated Addition of Acetylenic Bis-lithium Salts to Aldehydes and Ketones: An Efficient route to Bis-substituted Alkyne Diols. Tetrahedron Lett. 2017, 58, 3525–3528. [Google Scholar] [CrossRef]
- Kong, H.; Jung, Y.K.; Cho, N.S.; Kang, I.; Park, J.; Cho, S.; Shim, H. New Semiconducting Polymers Containing 3,6-Dimethyl (thieno[3,2-b]-thiophene or Selenopheno[3,2-b]selenophene) for Organic Thin-Film Transistors. Chem Mater. 2009, 21, 2650–2660. [Google Scholar] [CrossRef]
- Sato, M.; Kubota, Y.; Tanemura, A.; Maruyama, G.; Fujihara, T.; Nakayama, J.; Takayanagi, T.; Takahashi, K.; Unoura, K. Synthesis and Some Properties of Bis(ruthenocenyl)thiophene Derivatives Possible Spin-Coupling in the Two-Electron Oxidized Species of Dinuclear Ruthenocenes Bridged by Thiophene Derivatives. Eur. J. Inorg. Chem. 2006, 22, 4577–4588. [Google Scholar] [CrossRef]


 | ||||
|---|---|---|---|---|
| Entry | S Sources | Solvents | Additives (mmol) | Yields (%) (b) |
| 1 | S8 | NMP | 21 | |
| 2 | EtOCS2K | NMP | trace | |
| 3 | Thiourea | NMP | 16 | |
| 4 | K2S | NMP | trace | |
| 5 | Na2S·9H2O | NMP | trace | |
| 6 | Na2S2O3 | NMP | 31 | |
| 7 (c) | Na2S2O3 | NMP | 33 | |
| 8 | S8 | NMP | I2 (0.5) | 63 |
| 9 | Na2S2O3 | NMP | I2 (0.5) | 79 |
| 10 | Na2S2O3 | NMP | NH4I (0.5) | 13 |
| 11 | Na2S2O3 | NMP | HCl (1 M, 0.5 mL) | 57 |
| 12 | Na2S2O3 | NMP | I2 (0.25) | 61 |
| 13 | Na2S2O3 | NMP | I2 (0.75) | 77 |
| 14 | Na2S2O3 | DMSO | I2 (0.5) | 12 |
| 15 | Na2S2O3 | DMF | I2 (0.5) | 45 |
| 16 | Na2S2O3 | DMAc | I2 (0.5) | 61 |
| 17 | Na2S2O3 | AcOH | I2 (0.5) | trace |
| 18 (d) | Na2S2O3 | NMP | I2 (0.5) | 82 |
| 19 (e) | Na2S2O3 | NMP | I2 (0.5) | 55 |
| 20 (f) | Na2S2O3 | NMP | I2 (0.5) | 81 |
| 21 (g) | Na2S2O3 | NMP | I2 (0.5) | 80 |
| 22 (h) | Na2S2O3 | NMP | I2 (0.5) | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Zhang, X.; He, Z.; Zhao, M.; Chen, L.; Li, Y.; Luo, X. Expedient Synthesis of Substituted Thieno[3,2-b]thiophenes and Selenopheno[3,2-b]selenophenes Through Cascade Cyclization of Alkynyl Diol Derivatives. Molecules 2024, 29, 5507. https://doi.org/10.3390/molecules29235507
Feng Y, Zhang X, He Z, Zhao M, Chen L, Li Y, Luo X. Expedient Synthesis of Substituted Thieno[3,2-b]thiophenes and Selenopheno[3,2-b]selenophenes Through Cascade Cyclization of Alkynyl Diol Derivatives. Molecules. 2024; 29(23):5507. https://doi.org/10.3390/molecules29235507
Chicago/Turabian StyleFeng, Yingqi, Xuelin Zhang, Ziqing He, Miaoshan Zhao, Lu Chen, Yibiao Li, and Xiai Luo. 2024. "Expedient Synthesis of Substituted Thieno[3,2-b]thiophenes and Selenopheno[3,2-b]selenophenes Through Cascade Cyclization of Alkynyl Diol Derivatives" Molecules 29, no. 23: 5507. https://doi.org/10.3390/molecules29235507
APA StyleFeng, Y., Zhang, X., He, Z., Zhao, M., Chen, L., Li, Y., & Luo, X. (2024). Expedient Synthesis of Substituted Thieno[3,2-b]thiophenes and Selenopheno[3,2-b]selenophenes Through Cascade Cyclization of Alkynyl Diol Derivatives. Molecules, 29(23), 5507. https://doi.org/10.3390/molecules29235507







