The Far-Infrared Absorption Spectrum of HD16O: Experimental Line Positions, Accurate Empirical Energy Levels, and a Recommended Line List
Abstract
1. Introduction
2. Experiment and Line List
2.1. FTS Recordings
2.2. Global Experimental Line List
3. Rovibrational Assignments
3.1. H2XO (X = 16, 17, and 18)
3.2. D2XO (X = 16, 17, and 18)
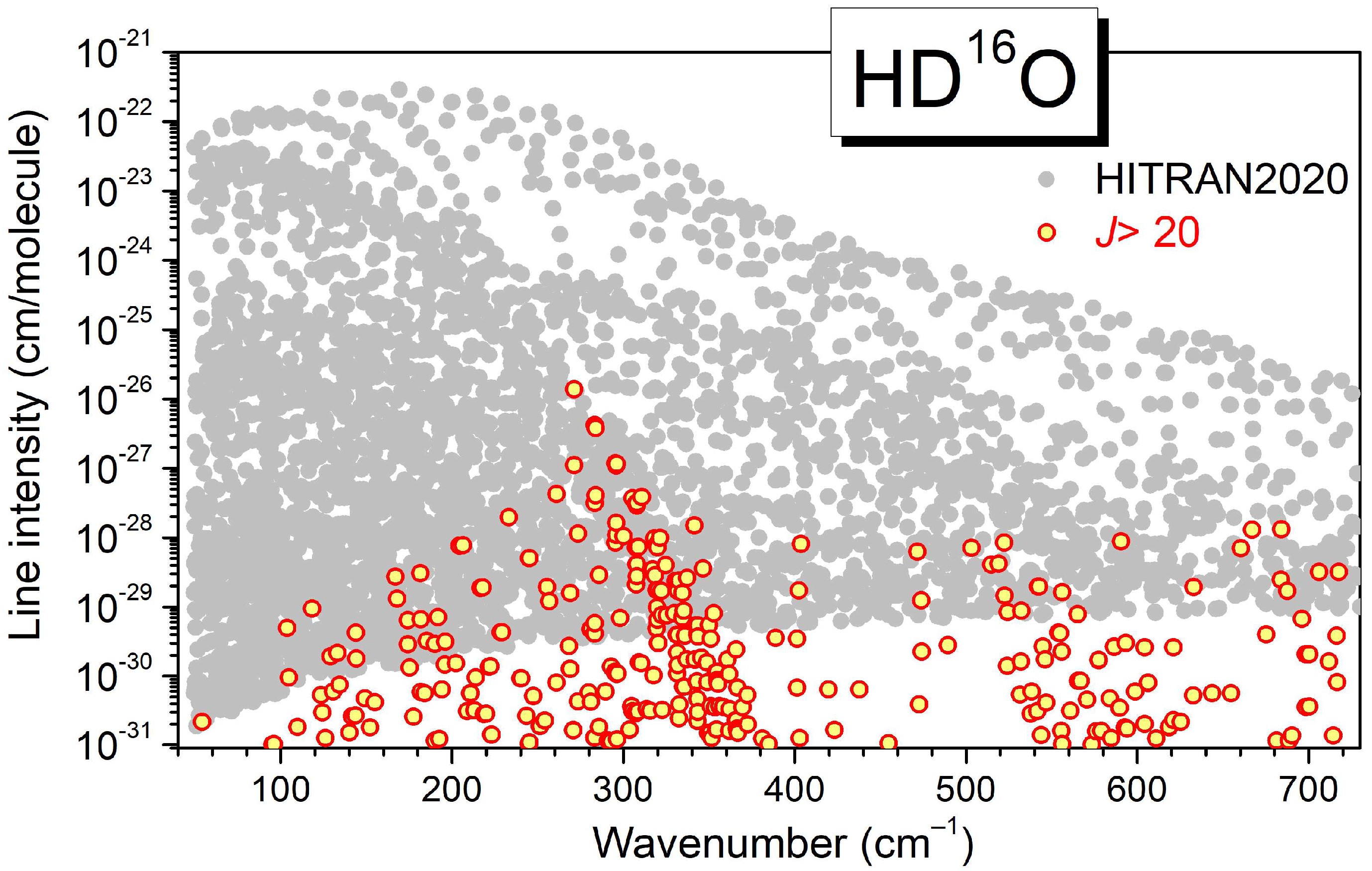
3.3. HD16O
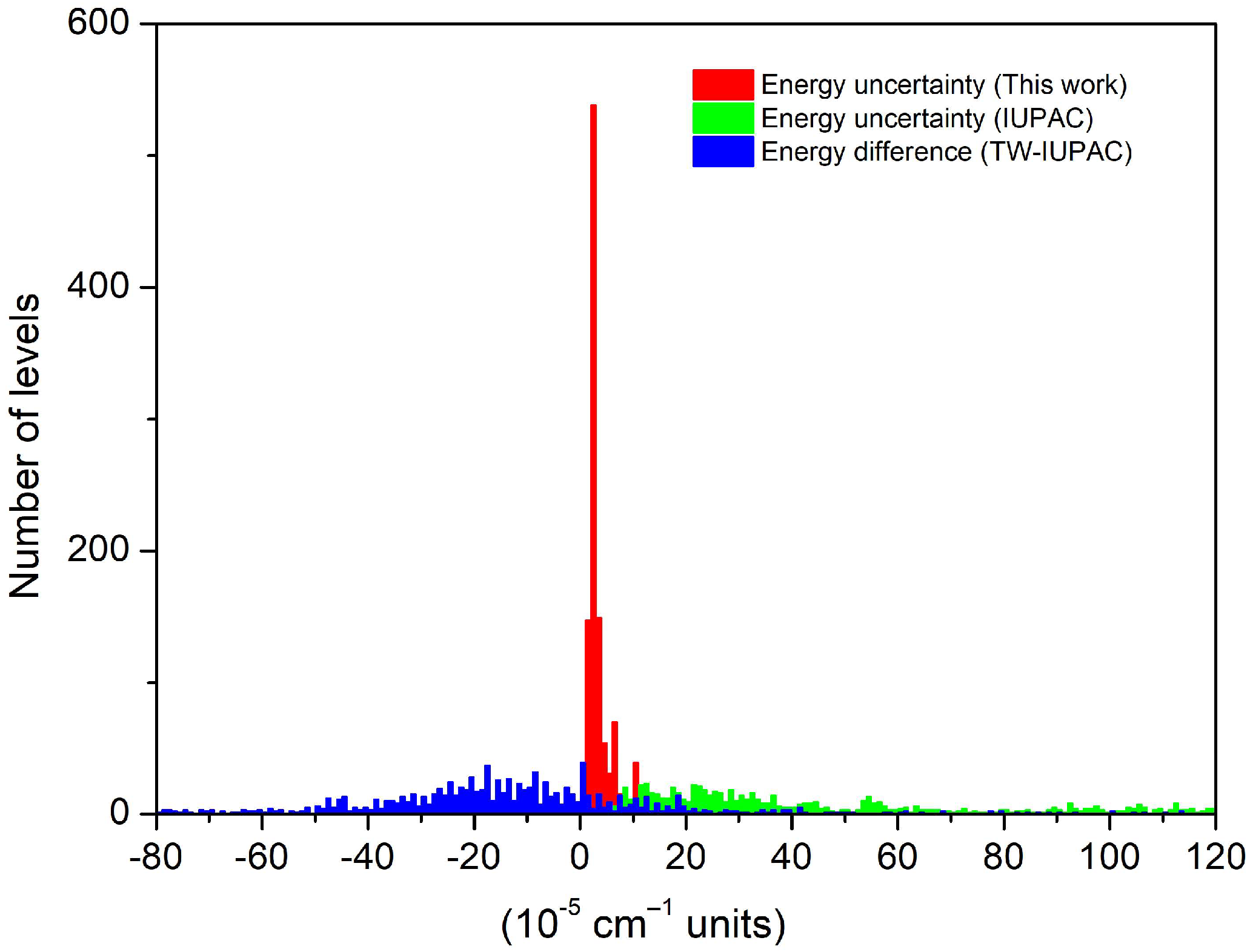
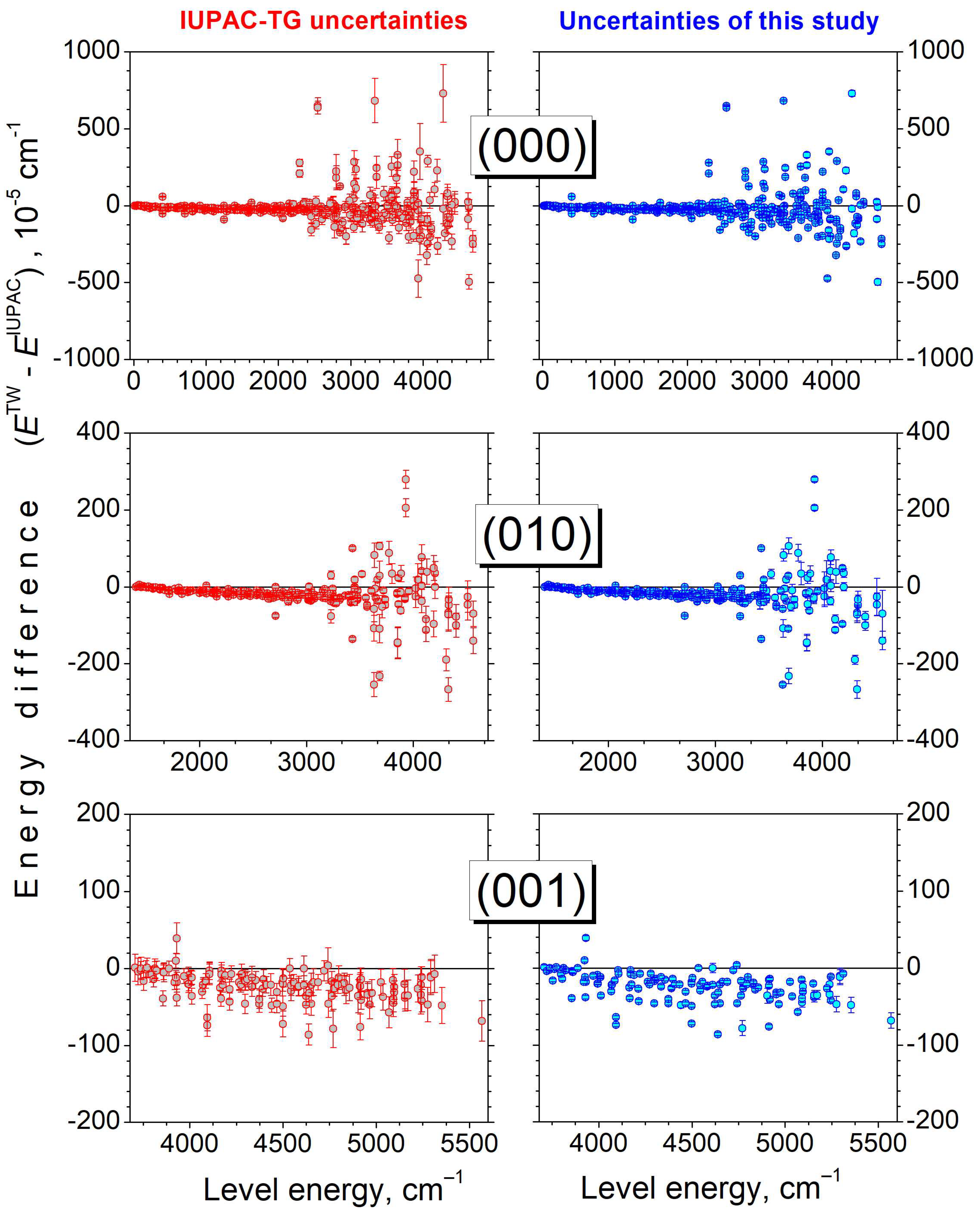
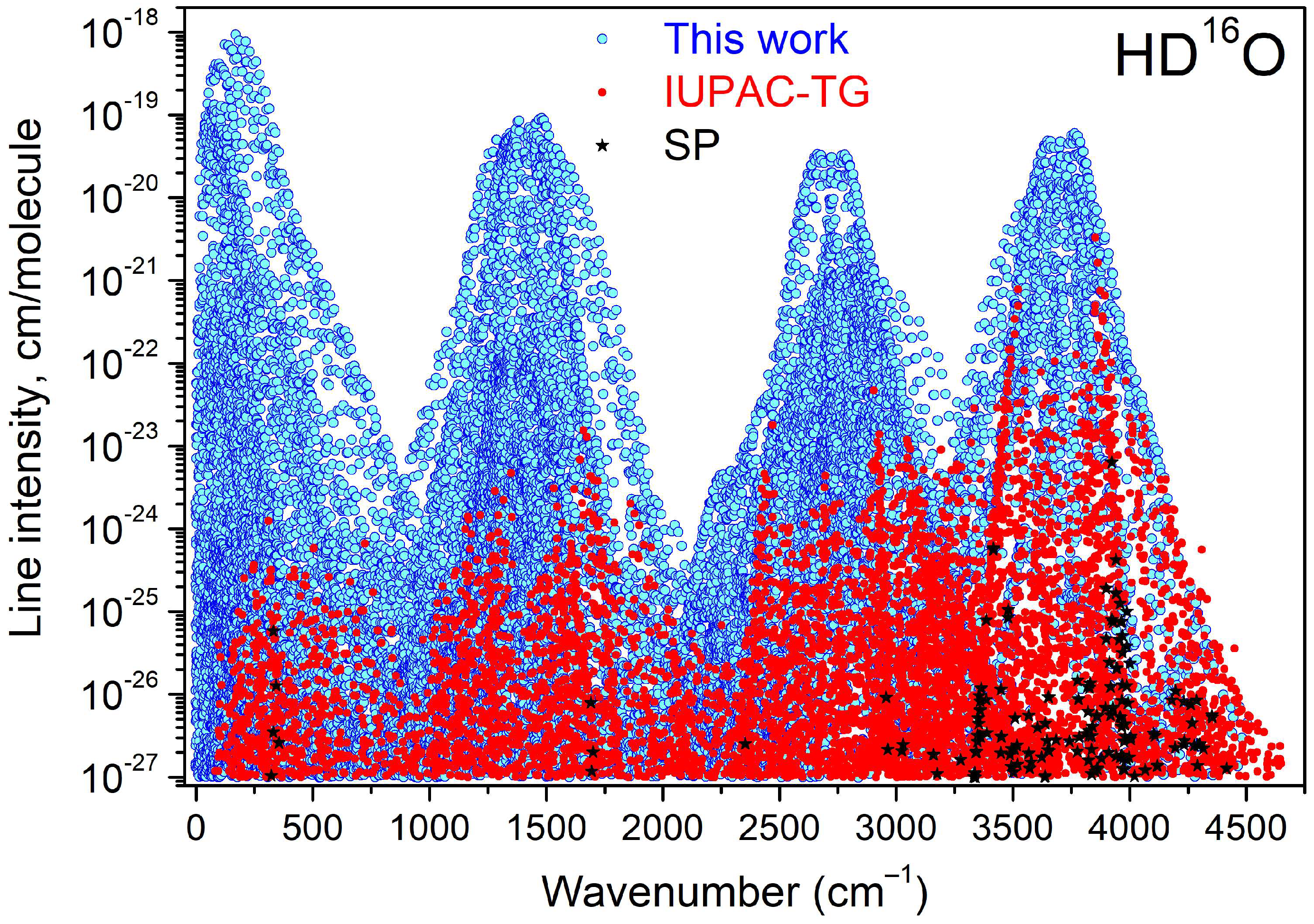
4. Empirical Energy Levels and a Recommended Line List for HD16O
- (i)
- The (010) 20 0 20 and (010) 20 1 20 energies (dE = −0.096 cm−1) were obtained from the 1643.8755 cm−1 line position assigned by Toth to the 20 0 20–19 1 19 and 20 1 20–19 0 19 transitions of the ν2 band [53],
- (ii)
- The (100) 13 7 7 and (100) 15 2 14 levels with the dE values of −0.097 and −0.061 cm−1, respectively, were determined on the basis of the results of Papineau et al. [46]. The (100) 13 7 7 energy relies on the 3078.740 cm−1 wavenumber assigned to the 13 7 7–126 6 transition of the ν1 band. The (100) 15 2 14 energy was obtained from the 2904.200 and 2904.655 cm−1 wavenumbers assigned to the 15 2 14–14 2 13and 15 2 14–141 13 transitions of the same ν1 band,
- (iii)
- (The 9 9 1, 9 9 0, 10 8 3, 10 9 2, 10 9 1, and 15 2 14 levels of the (001) vibrational states have dE values of −0.097, −0.097, 0.081, −0.140, −0.140 and 0.266 cm−1, respectively. Our energy value relies on line positions of ν3 transitions given by Toth and Brault [48]: 9 9 1–8 8 0 and 9 9 0–8 8 1 at 4007.7146 cm−1, 10 8 3–9 7 2 at 4012.5627 cm−1, 10 9 2–9 8 1 and 10 9 1–9 8 2 at 4021.6755 cm−1, 15 2 14–14 2 13 at 3895.4251 cm−1.
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toureille, M.; Koroleva, A.O.; Mikhailenko, S.N.; Pirali, O.; Campargue, A. Water vapor absorption spectroscopy in the far-infrared (50–720 cm−1). Part 1: Natural water. J. Quant. Spectrosc. Radiat. Transf. 2022, 291, 108326. [Google Scholar] [CrossRef]
- Karlovets, E.V.; Mikhailenko, S.N.; Koroleva, A.O.; Campargue, A. Water vapor absorption spectroscopy and validation tests of databases in the far-infrared (50–720 cm−1). Part 2: H217O and HD17O. J. Quant. Spectrosc. Radiat. Transf. 2024, 314, 108829. [Google Scholar] [CrossRef]
- Mikhailenko, S.N.; Karlovets, E.V.; Koroleva, A.O.; Campargue, A. The far infrared absorption spectrum of D216O, D217O, and D218O: Experimental line positions, empirical energy levels and recommended line lists. J. Phys. Chem. Ref. Data 2024, 53, 023102. [Google Scholar] [CrossRef]
- Mikhailenko, S.N.; Béguier, S.; Odintsova, T.A.; Tretyakov, M.Y.; Pirali, O.; Campargue, A. The far-infrared spectrum of 18O enriched water vapour (40–700 cm−1). J. Quant. Spectrosc. Radiat. Transf. 2020, 253, 107105. [Google Scholar] [CrossRef]
- Gordon, I.E.; Rothman, L.S.; Hargreaves, R.J.; Hashemi, R.; Karlovets, E.V.; Skinner, F.M.; Yurchenko, S.N. The HITRAN2020 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107949. [Google Scholar] [CrossRef]
- Delahaye, T.; Armante, R.; Scott, N.A.; Jacquinet-Husson, N.; Chédin, A.; Crépeau, L.; Yurchenko, S. The 2020 edition of the GEISA spectroscopic database. J. Mol. Spectrosc. 2021, 380, 111510. [Google Scholar] [CrossRef]
- Furtenbacher, T.; Tóbiás, R.; Tennyson, J.; Polyansky, O.L.; Kyuberis, A.A.; Ovsyannikov, R.I.; Császár, A.G. W2020: A database of validated rovibrational experimental transitions and empirical energy levels of water isotopologues. II. H217O and H218O with an update to H216O. J. Phys. Chem. Ref. Data 2020, 49, 43103. [Google Scholar] [CrossRef]
- Partridge, H.; Schwenke, D.W. The determination of an accurate isotope dependent potential energy surface for water from extensive ab initio calculations and experimental data. J. Chem. Phys. 1997, 106, 4618–4639. [Google Scholar] [CrossRef]
- Schwenke, D.W.; Partridge, H. Convergence testing of the analytic representation of an ab initio dipole moment function for water: Improved fitting yield improved intensity. J. Chem. Phys. 2000, 113, 6592–6597. [Google Scholar] [CrossRef]
- Odintsova, T.A.; Tretyakov, M.Y.; Simonova, A.; Ptashnik, I.; Pirali, O.; Campargue, A. Measurement and temperature dependence of the water vapor self-continuum in the 70–700 cm−1 range. J. Mol. Struct. 2020, 1210, 128046. [Google Scholar] [CrossRef]
- Odintsova, T.A.; Koroleva, A.O.; Simonova, A.A.; Campargue, A.; Tretyakov, M.Y. The atmospheric continuum in the “terahertz gap”: Review of experiments at SOLEIL synchrotron and modeling. J. Mol. Spectrosc. 2022, 386, 111603. [Google Scholar] [CrossRef]
- Koroleva, A.O.; Odintsova, T.A.; Tretyakov, M.Y.; Pirali, O.; Campargue, A. The foreign-continuum absorption of water vapour in the far-infrared (50–500 cm−1). J. Quant. Spectrosc. Radiat. Transf. 2021, 261, 107486. [Google Scholar] [CrossRef]
- Toth, R.A.; Brown, L.R.; Plymate, C. Self-broadened widths and frequency shifts of water vapor lines between 590 and 2400 cm−1. J. Quant. Spectrosc. Radiat. Transf. 1998, 59, 529–562. [Google Scholar] [CrossRef]
- Podobedov, V.B.; Plusquellic, D.F.; Fraser, G.T. THz laser study of self-pressure and temperature broadening and shifts of water vapor lines for pressures up to 1.4 kPa. J. Quant. Spectrosc. Radiat. Transf. 2004, 87, 377–385. [Google Scholar] [CrossRef]
- Tóbiás, R.; Diouf, M.L.; Cozijn, F.M.J.; Ubachs, W.; Császár, A.G. A paths lead to hubs in the spectroscopic networks of water isotopologues H216O and H218O. Commun. Chem. 2024, 7, 34. [Google Scholar] [CrossRef]
- Mellau, G.; Mikhailenko, S.N.; Starikova, E.N.; Tashkun, S.A.; Over, H.; Tyuterev, V.G. Rotational levels of the (000) and (010) states of D216O from hot emission spectra in the 320–860 cm−1 region. J. Mol. Spectrosc. 2004, 224, 32–60. [Google Scholar] [CrossRef]
- Zobov, N.F.; Ovsannikov, R.I.; Shirin, S.V.; Polyansky, O.L.; Tennyson, J.; Janka, A.; Bernath, P.F. Infrared emission spectrum of hot D2O. J. Mol. Spectrosc. 2006, 240, 112–119. [Google Scholar] [CrossRef]
- Tennyson, J.; Bernath, P.F.; Brown, L.R.; Campargue, A.; Császár, A.G.; Daumont, L.; Vasilenko, I.A. IUPAC critical evaluation of the rotational-vibrational spectra of water vapor. Part IV: Energy levels and transition wavenumbers for D216O, D217O, and D218O. J. Quant. Spectrosc. Radiat. Transf. 2014, 142, 93–108. [Google Scholar] [CrossRef]
- Kaupinen, J.; Karkkainen, T.; Kyro, E. High-resolution spectrum of water vapor between 30 and 720 cm−1. J. Mol. Spectrosc. 1978, 71, 15–45. [Google Scholar] [CrossRef]
- Johns, J.W.C. High-resolution far-infrared (20–350 cm−1) spectra of several species of H2O. J. Opt. Soc. Am. B 1985, 2, 1340–1354. [Google Scholar] [CrossRef]
- Paso, R.; Horneman, V.M. High-resolution rotational absorption spectra of H216O, HD16O, and D216O between 110 and 500 cm−1. J. Opt. Soc. Am. B 1995, 12, 1813–1837. [Google Scholar] [CrossRef]
- Toth, R.A. HDO and D2O low pressure, long path spectra in the 600–3100 cm−1 region. I. HDO line positions and strengths. J. Mol. Spectrosc. 1999, 195, 73–97. [Google Scholar] [CrossRef] [PubMed]
- Janca, A.; Tereszchuk, K.; Bernath, P.F.; Zobov, N.F.; Shirin, S.V.; Polyansky, O.L.; Tennyson, J. Emission spectrum of hot HDO below 4000 cm−1. J. Mol. Spectrosc. 2003, 219, 132–135. [Google Scholar] [CrossRef]
- Kyuberis, A.A.; Zobov, N.F.; Naumenko, O.V.; Voronin, B.A.; Polyansky, O.L.; Lodi, L.; Tennyson, J. Room temperature line lists for deuterated water. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 175–185. [Google Scholar] [CrossRef]
- Tennyson, J.; Bernath, P.F.; Brown, L.R.; Campargue, A.; Császár, A.G.; Daumont, L.; Voronin, B.A. IUPAC critical evaluation of the rotational-vibrational spectra of water vapor. Part II. Energy levels and transition wavenumbers for HD16O, HD17O, and HD18O. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2160–2184. [Google Scholar] [CrossRef]
- Townes, C.H.; Merrit, F.R. Water spectrum near one-centimeter wave-length. Phys. Rev. 1946, 70, 558–559. [Google Scholar] [CrossRef]
- Strandberg, M.W.P.; Wentink, T., Jr.; Hillger, R.E.; Wannier, G.H.; Deutsch, M.L. Stark spectrum of HDO. Phys. Rev. 1948, 73, 188. [Google Scholar] [CrossRef]
- McAfee, K.B., Jr. A Study of the Absorption of Some Gases at Microwave Frequencies. Ph.D. Thesis, Harvard University, Harvard, MA, USA, 1949. [Google Scholar]
- Strandberd, M.W.P. Rotational absorption spectrum of HDO. J. Chem. Phys. 1949, 17, 901–904. [Google Scholar] [CrossRef]
- Beers, Y.; Weisbaum, S. An ultra-high-frequency rotational line of HDO. Phys. Rev. 1953, 91, 1014. [Google Scholar] [CrossRef]
- Jen, C.K.; Bianco, D.R.; Massey, J.T. Some heavy water rotational absorption lines. J. Chem. Phys. 1953, 21, 520–525. [Google Scholar] [CrossRef]
- Crawford, H.D. Two new lines in the microwave spectrum of heavy water. J. Chem. Phys. 1953, 21, 2099. [Google Scholar] [CrossRef]
- Posener, D.W.; Strandberg, M.W.P. Centrifugal distortion in asymmetric top molecules. III. H2O, D2O, and HDO. Phys. Rev. 1954, 95, 374–384. [Google Scholar] [CrossRef]
- Weisbaum, S.; Beers, Y.; Herrmann, G. Low-frequency rotational spectrum of HDO. J. Chem. Phys. 1955, 23, 1601–1605. [Google Scholar] [CrossRef]
- Erlandson, G.; Cox, J. Millimeter-wave lines of heavy water. J. Chem. Phys. 1956, 25, 778–779. [Google Scholar] [CrossRef]
- Posener, D.W. Hyperfine structure in the microwave spectrum of water. Austr. J. Phys. 1957, 10, 276–285. [Google Scholar] [CrossRef]
- Treacy, E.B.; Beers, Y. Hyperfine structure of the rotational spectrum of HDO. J. Chem. Phys. 1962, 36, 1473–1480. [Google Scholar] [CrossRef]
- Verhoeven, J.; Bluyssen, H.; Dymanus, A. Hyperfine structure of HDO and D2O by beam maser spectroscopy. Phys. Lett. 1968, A26, 424–425. [Google Scholar] [CrossRef]
- Steenbeckeliers, G.; Bellet, J. Spectre de rotation de l’eau lourde. Comp. Rend. Acad. Sci. 1970, B270, 1039–1041. [Google Scholar]
- Bellet, J.; Steenbeckeliers, G. Calcul des constantes rotationnelles des molecules H2O, HDO et D2O dans leurs etats fondamentaux de vibration. Comp. Rend. Acad. Sci. 1970, B271, 1208–1211. [Google Scholar]
- De Lucia, F.C.; Cook, R.L.; Helminger, P.; Gordy, W. Millimeter and submillimeter wave rotational spectrum and centrifugal distortion effects of HDO. J. Chem. Phys. 1971, 55, 5334–5339. [Google Scholar] [CrossRef]
- Lafferty, W.; Bellet, J.; Steenbeckeliers, G. Spectre microonde des transitions de faible intensite de la molecule HDO. Etude de la molecule dans l’etat vibrationnel excite2. Comp. Rend. Acad. Sci. 1971, B273, 388. [Google Scholar]
- Messer, J.K.; De Lucia, F.C.; Helminger, P. Submillimeter spectroscopy of the major isotopes of water. J. Mol. Spectrosc. 1984, 105, 139–155. [Google Scholar] [CrossRef]
- Baskakov, O.I.; Alekseev, V.A.; Alekseev, E.A.; Polevoi, B.I. New submillimeter rotational lines of water and its isotopes. Opt. Spectrosc. 1987, 63, 1016–1018. [Google Scholar]
- Goyette, T.M.; Ferguson, D.W.; De Lucia, F.C.; Dutta, J.M.; Jones, C.R. The pressure broadening of HDO by O2, N2, H2, and He between 100 and 600 K. J. Mol. Spectrosc. 1993, 162, 366–374. [Google Scholar] [CrossRef]
- Papineau, N.; Camy-Peyret, C.; Flaud, J.M.; Guelachvili, G. The 2ν2 and ν1 bands of HD16O. J. Mol. Spectrosc. 1982, 92, 451–468. [Google Scholar] [CrossRef]
- Toth, R.A.; Gupta, V.D.; Brault, J.W. Line positions and strengths of HDO in the 2400–3300 cm−1 region. Appl. Opt. 1982, 21, 3337–3345. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.A.; Brault, J.W. Line positions and strengths in the (001), (110), and (030) bands of HDO. Appl. Opt. 1983, 22, 908–926. [Google Scholar] [CrossRef]
- Guelachvili, G. Experimental Doppler-limited spectra of the ν2 bands of H216O, H217O, H218O, and HDO by Fourier-transform spectroscopy: Secondary wave-number standards between 1066 and 2296 cm−1. J. Opt. Soc. Am. 1983, 73, 137–150. [Google Scholar] [CrossRef]
- Flaud, J.-M.; Camy-Peyret, C.; Mahmoudi, A. The ν2 band of HD16O. Int. J. IR MMW 1986, 7, 1063–1090. [Google Scholar] [CrossRef]
- Rinsland, C.P.; Smith, M.A.H.; Malathy Devi, V.; Benner, D.C. Measurements of Lorentz-broadening coefficients and pressure-induced line shift coefficients in the ν2 band of HD16O. J. Mol. Spectrosc. 1991, 150, 640–646. [Google Scholar] [CrossRef]
- Rinsland, C.P.; Smith, M.A.H.; Malathy Devi, V.; Benner, D.C. Measurements of Lorentz-broadening coefficients and pressure-induced line-shift coefficients in the ν1 band of HD16O and the ν3 band of D216O. J. Mol. Spectrosc. 1992, 156, 507–511. [Google Scholar] [CrossRef]
- Toth, R.A. HD16O, HD18O, and HD17O transition frequencies and strengths in the ν2 bands. J. Mol. Spectrosc. 1993, 162, 20–40. [Google Scholar] [CrossRef]
- Siemsen, K.J.; Bernard, J.E.; Madej, A.A.; Marmet, L. Absolute frequency measurement of an HDO absorption line near 1480 cm−1. J. Mol. Spectrosc. 2000, 199, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.A. Line Lists of Water Vapor Parameters from 500- to 8000 cm−1. Available online: https://mark4sun.jpl.nasa.gov/h2o.html (accessed on 11 November 2024).
- Malathy Devi, V.; Benner, D.C.; Sung, K.; Crawford, T.J.; Gamache, R.R.; Renaud, C.L.; Villanueva, G.L. Line parameters for CO2– and self–broadening in the ν1 band of HD16O. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 133–157. [Google Scholar] [CrossRef]
- Malathy Devi, V.; Benner, D.C.; Sung, K.; Crawford, T.J.; Gamache, R.R.; Renaud, C.L.; Villanueva, G.L. Line parameters for CO2– and self–broadening in the ν3 band of HD16O. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 158–174. [Google Scholar] [CrossRef]
- Tashkun, S.A.; Perevalov, V.I.; Teffo, J.-L. Global fittings of the vibrational-rotational line positions of the 12C16O17O and 12C16O18O isotopic species of carbon dioxide. J. Mol. Spectrosc. 2001, 210, 137–145. [Google Scholar] [CrossRef]
- Tashkun, S.A.; Velichko, T.I.; Mikhailenko, S.N. Critical evaluation of measured pure-rotation and rotation-vibration line positions and an experimental dataset of energy levels of 12C16O in X1Σ+ state. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1106–1116. [Google Scholar] [CrossRef]
- Tashkun, S.; Barbe, A.; Mikhailenko, S.; Starikova, E.; Tyuterev, V. Complete RITZ set of rovibrational energy levels of 16O3 deduced from experimental spectra: Critical analysis of transition frequencies in spectroscopic databases. J. Phys. Chem. Ref. 2024, accepted. [Google Scholar]
- Mellau, G.C.; Mikhailenko, S.N.; Tyuterev, V.G. Hot water emission spectra: Rotational energy levels of the (000) and (010) states of HD17O. J. Mol. Spectrosc. 2015, 308–309, 6–19. [Google Scholar] [CrossRef]
- Schwenke, D.W. Variational calculations of rovibrational energy levels and transition intensities for tetratomic molecules. J. Phys. Chem. 1996, 100, 2867–2884, Erratum in J. Phys. Chem. 1996, 100, 18884. [Google Scholar] [CrossRef]
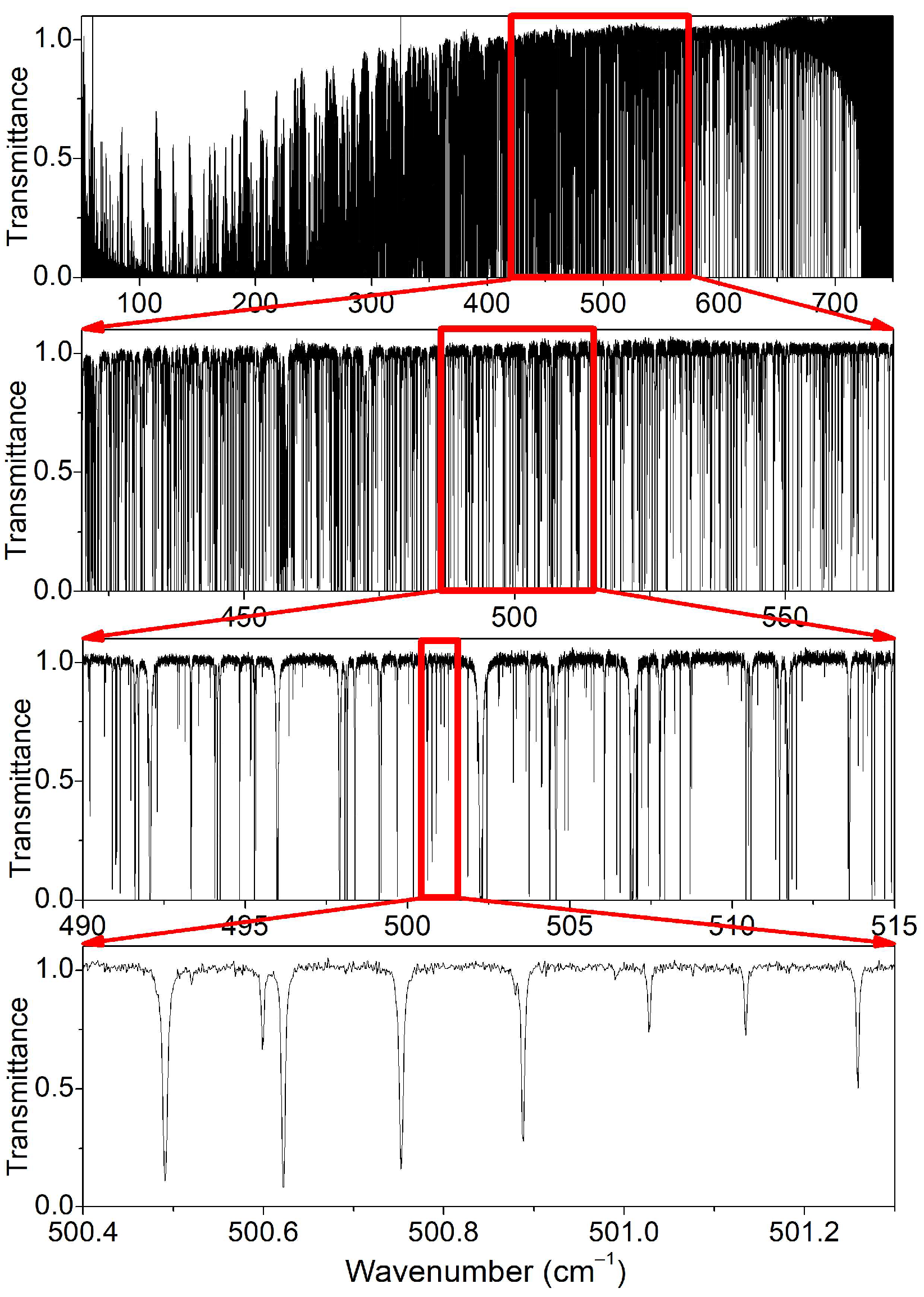

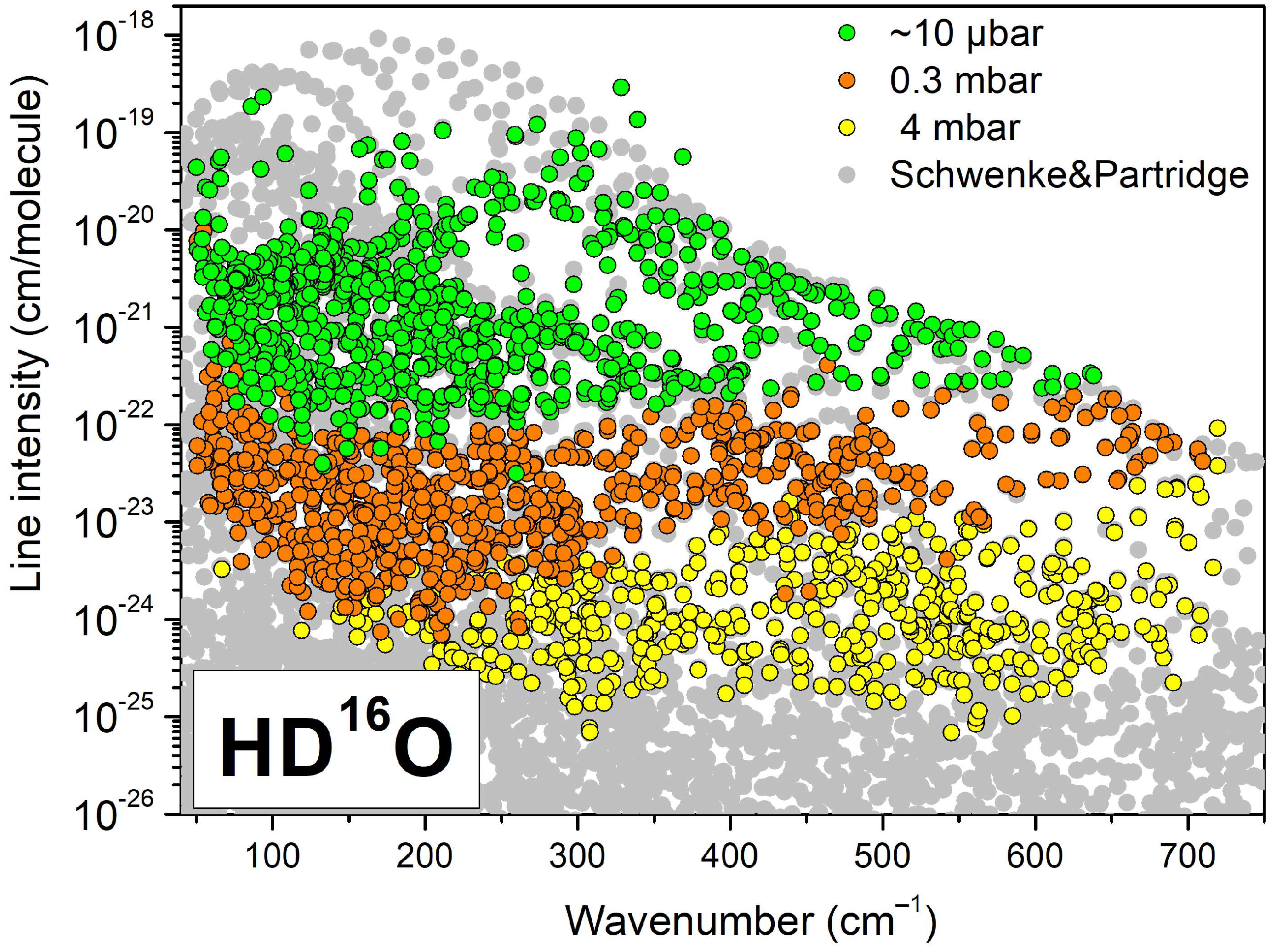
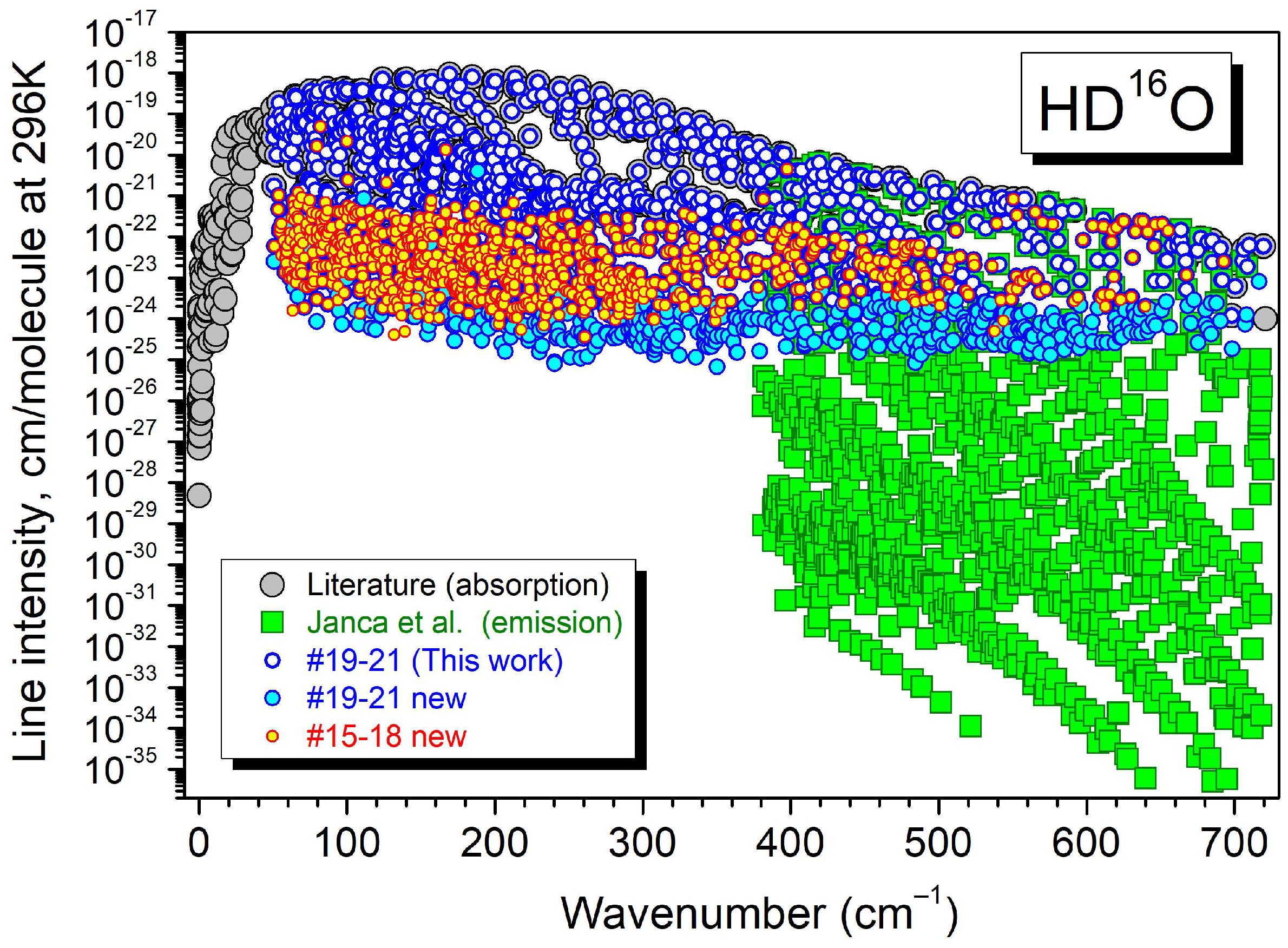
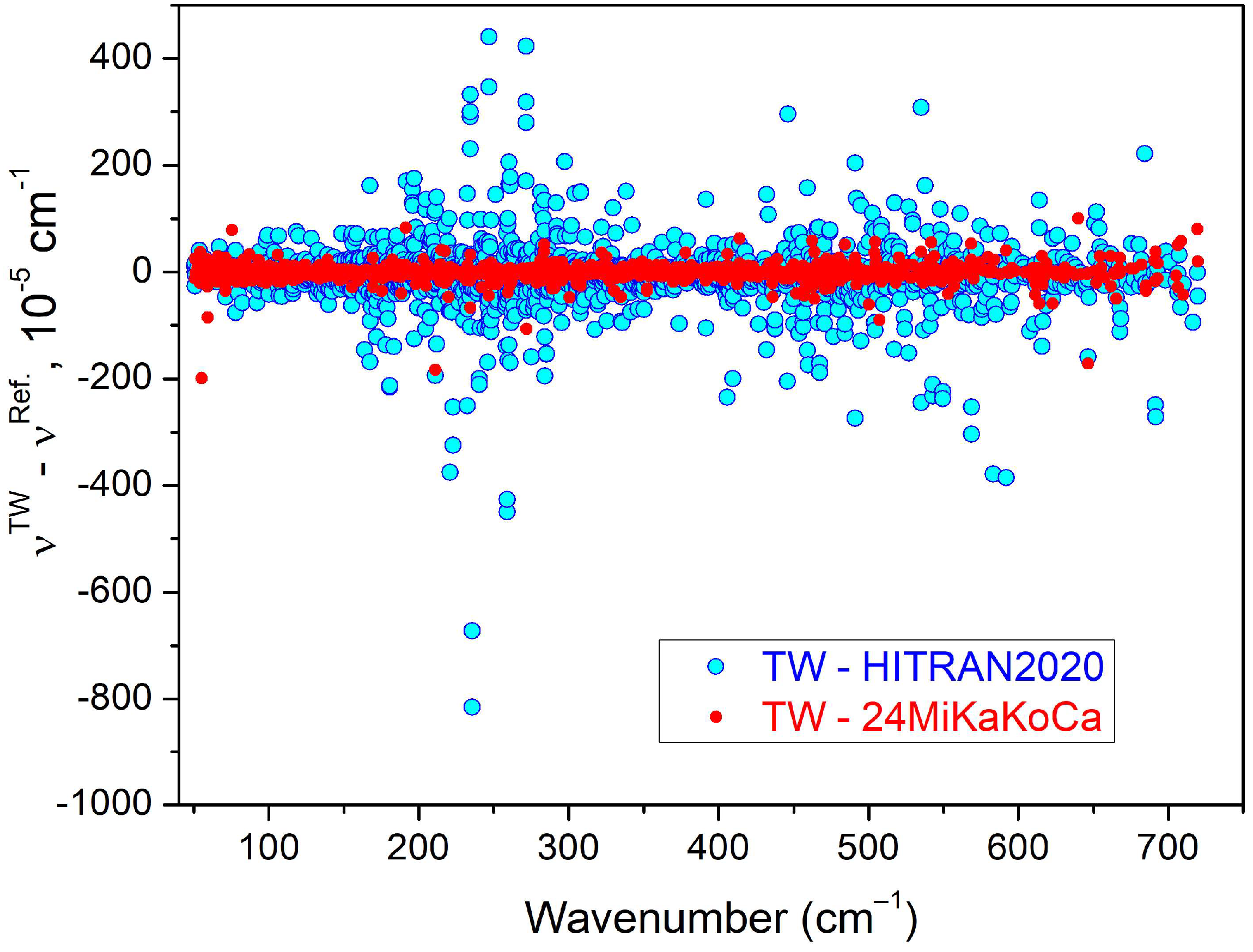

| Label | Sample a | Max. Pressure (mbar) | Nb of P b | Ref. |
|---|---|---|---|---|
| #1–5 | Natural | 6.7 | 5 | [1] |
| #6–9 | 41% H217O, 27% H218O, 22% H216O | 3.85 | 4 | [2] |
| #10–14 | 50% D2O 20.5% H217O 13.5% H218O 11% H216O | 8.0 | 5 | |
| #15–18 | D2O | 8.0 | 4 | [3] |
| #19–21 | 50% H216O 50% D2O | 4.0 | 3 | This work |
| Label | Recording | Pressure | Resolution, cm−1 | Number of Scans |
|---|---|---|---|---|
| #19 | Sample | ≈4 mbar | 0.002 | 160 |
| Baseline | Pumping on the cell | 0.05 | 200 | |
| #20 | Sample | ≈0.3 mbar | 0.001 | 100 |
| Baseline | Pumping on the cell | 0.05 | 200 | |
| #21 | Sample | ≈10 µbar a | 0.001 | 160 |
| Baseline | Pumping on the cell | 0.05 | 200 |
| Molecule | Abundance, Min–Max, in % | Factor a (%) | Number of Transitions b | Jmax | Kamaxc | Region, cm−1 |
|---|---|---|---|---|---|---|
| H216O | 24.650–24.910 | 25.0.0 | 1035 | 21 | 13 | 51.434–718.657 |
| H217O | 0.025–0.140 | 0.15 | 356 | 16 | 11 | 53.509–667.815 |
| H218O | 0.070–0.210 | 0.2 | 406 | 17 | 11 | 53.569–702.589 |
| HD16O | 49.250–49.785 | 50 | 2443 | 25 | 14 | 50.277–719.550 |
| HD17O | 0.045–0.320 | 0.3 | 701 | 19 | 11 | 50.134–631.440 |
| HD18O | 0.170–0.430 | 0.45 | 851 | 20 | 12 | 52.187–654.961 |
| D216O | 24.330–25.865 | 25 | 2152 | 29 | 17 | 50.210–690.430 |
| D217O | 0.025–0.200 | 0.52 | 538 | 21 | 14 | 50.425–490.178 |
| D218O | 0.070–0.470 | 0.2 | 704 | 23 | 14 | 51.538–516.600 |
| Position | dF | Int_SP | Iso | V′ | J′ | Ka′ | Kc′ | V″ | J″ | Ka″ | Kc″ | Pos_calc | dν |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50.21024 | 21 | 2.129 × 10−23 | D216O | 010 | 3 | 3 | 0 | 010 | 3 | 2 | 1 | 50.21043 | −19 |
| 124.23846 | 3 | 1.338 × 10−23 | 010 | 7 | 7 | 1 | 010 | 7 | 6 | 2 | 124.23852 | −6 | |
| 124.25938 | 13 | 7.880 × 10−25 | 000 | 20 | 6 | 15 | 000 | 20 | 5 | 16 | 124.25936 | 2 | |
| 124.31025 | 12 | 2.093 × 10−24 | 010 | 14 | 3 | 12 | 010 | 14 | 2 | 13 | 124.31029 | −4 | |
| 140.98902 | 32 | 2.371 × 10−23 | 000 | 15 | 9 | 6 | 000 | 15 | 8 | 7 | 140.98887 | 15 | |
| 167.54655 | 22 | 2.078 × 10−25 | 010 | 12 | 10 | 3 | 010 | 12 | 9 | 4 | 167.54651 | 4 | |
| 212.70450 | 30 | 3.205 × 10−25 | 020 | 9 | 5 | 4 | 020 | 8 | 4 | 5 | 212.70449 | 1 | |
| 231.59471 | 3 | 5.643 × 10−25 | 020 | 9 | 6 | 4 | 020 | 8 | 5 | 3 | 231.59484 | −13 | |
| 252.97401 | 8 | 2.135 × 10−25 | 020 | 8 | 8 | 1 | 020 | 7 | 7 | 0 | 252.97418 | −17 | |
| 261.54655 | 6 | 2.118 × 10−25 | 000 | 27 | 0 | 27 | 000 | 26 | 1 | 26 | 261.54785 | −130 | |
| 261.78073 | 6 | 2.006 × 10−25 | 000 | 26 | 2 | 25 | 000 | 25 | 1 | 24 | 261.78130 | −57 | |
| 262.52074 | 50 | 8.240 × 10−26 | 000 | 13 | 8 | 6 | 000 | 14 | 3 | 11 | 262.52090 | −16 | |
| 265.59588 | 10 | 1.397 × 10−25 | 020 | 9 | 8 | 1 | 020 | 8 | 7 | 2 | 265.59627 | −39 | |
| 328.10894 | 10 | 1.818 × 10−24 | 010 | 12 | 11 | 2 | 010 | 11 | 10 | 1 | 328.10904 | −10 | |
| 365.73560 | 12 | 1.967 × 10−25 | 010 | 15 | 11 | 4 | 010 | 14 | 10 | 5 | 365.73541 | 19 | |
| 460.35106 | 81 | 5.608 × 10−26 | 000 | 23 | 7 | 16 | 000 | 22 | 6 | 17 | |||
| 462.29782 | 79 | 5.180 × 10−26 | 000 | 17 | 11 | 7 | 000 | 17 | 8 | 10 | 462.29779 | 2 | |
| 544.67069 | 22 | 1.538 × 10−24 | 000 | 10 | 10 | 1 | 000 | 9 | 7 | 2 | 544.67053 | 16 | |
| 656.61455 | 38 | 1.844 × 10−25 | 000 | 13 | 12 | 1 | 000 | 12 | 9 | 4 | 656.61476 | −21 | |
| 683.17223 | 47 | 1.504 × 10−25 | 000 | 15 | 12 | 3 | 000 | 14 | 9 | 6 | 683.17338 | −115 | |
| 62.28061 | 35 | 5.340 × 10−23 | D217O | 000 | 4 | 4 | 1 | 000 | 4 | 3 | 2 | 62.28059 | 2 |
| 78.61542 | 9 | 6.384 × 10−23 | 000 | 9 | 5 | 5 | 000 | 9 | 4 | 6 | 78.61536 | 6 | |
| 99.78119 | 15 | 9.088 × 10−25 | 000 | 14 | 5 | 9 | 000 | 13 | 6 | 8 | 99.78101 | 18 | |
| 96.21984 | 14 | 1.755 × 10−22 | D218O | 000 | 9 | 2 | 7 | 000 | 8 | 3 | 6 | 96.22005 | −21 |
| 103.97704 | 17 | 2.675 × 10−25 | 010 | 6 | 6 | 1 | 010 | 6 | 5 | 2 | 103.97646 | 58 | |
| 177.97477 | 6 | 9.695 × 10−24 | 010 | 6 | 6 | 0 | 010 | 5 | 5 | 1 | 177.97499 | −22 | |
| 285.06436 | 46 | 2.464 × 10−25 | 010 | 11 | 9 | 2 | 010 | 10 | 8 | 3 | 285.06453 | −17 | |
| 312.95597 | 21 | 2.174 × 10−25 | 010 | 11 | 5 | 7 | 010 | 10 | 2 | 8 | 312.95560 | 37 |
| Band | NTa | Jmax | Ka max | Region, cm−1 |
|---|---|---|---|---|
| (000)–(000) | 1778 | 25 | 14 | 50.27–719.55 |
| (010)–(010) | 617 | 18 | 11 | 50.73–698.49 |
| (020)–(020) | 18 | 9 | 6 | 145.53–310.23 |
| (100)–(100) | 30 | 9 | 8 | 96.60–350.15 |
| Total | 2443 | 25 | 14 | 50.27–719.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailenko, S.N.; Karlovets, E.V.; Koroleva, A.O.; Campargue, A. The Far-Infrared Absorption Spectrum of HD16O: Experimental Line Positions, Accurate Empirical Energy Levels, and a Recommended Line List. Molecules 2024, 29, 5508. https://doi.org/10.3390/molecules29235508
Mikhailenko SN, Karlovets EV, Koroleva AO, Campargue A. The Far-Infrared Absorption Spectrum of HD16O: Experimental Line Positions, Accurate Empirical Energy Levels, and a Recommended Line List. Molecules. 2024; 29(23):5508. https://doi.org/10.3390/molecules29235508
Chicago/Turabian StyleMikhailenko, Semen N., Ekaterina V. Karlovets, Aleksandra O. Koroleva, and Alain Campargue. 2024. "The Far-Infrared Absorption Spectrum of HD16O: Experimental Line Positions, Accurate Empirical Energy Levels, and a Recommended Line List" Molecules 29, no. 23: 5508. https://doi.org/10.3390/molecules29235508
APA StyleMikhailenko, S. N., Karlovets, E. V., Koroleva, A. O., & Campargue, A. (2024). The Far-Infrared Absorption Spectrum of HD16O: Experimental Line Positions, Accurate Empirical Energy Levels, and a Recommended Line List. Molecules, 29(23), 5508. https://doi.org/10.3390/molecules29235508







