Recent Advances in the Domino Annulation Reaction of Quinone Imines
Abstract
1. Introduction
2. Domino Reactions of Ortho-Quinone Imines
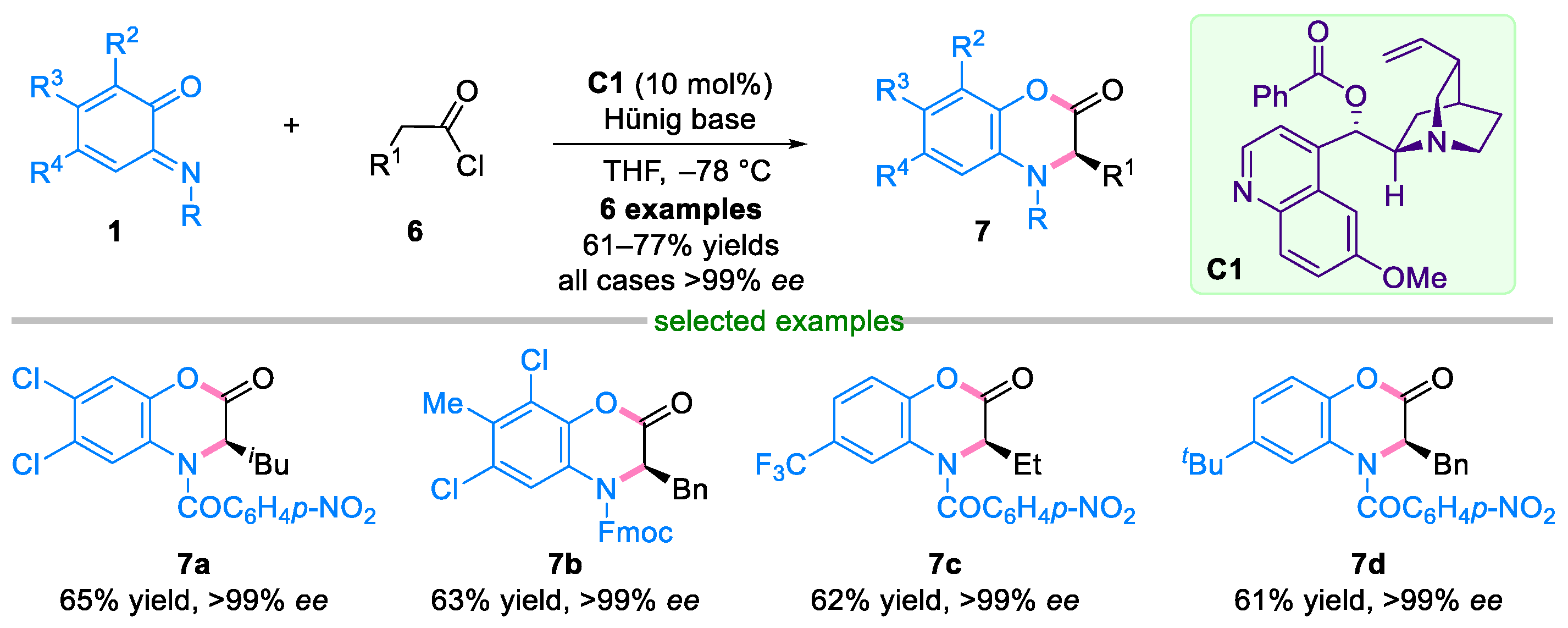
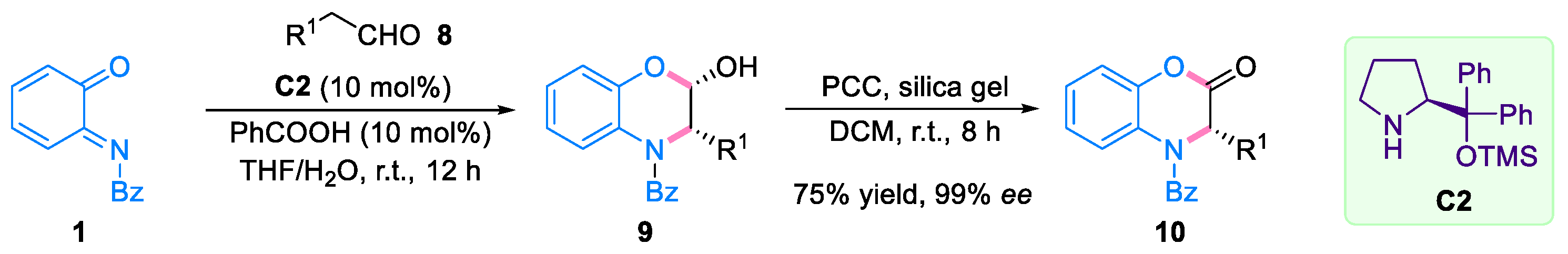
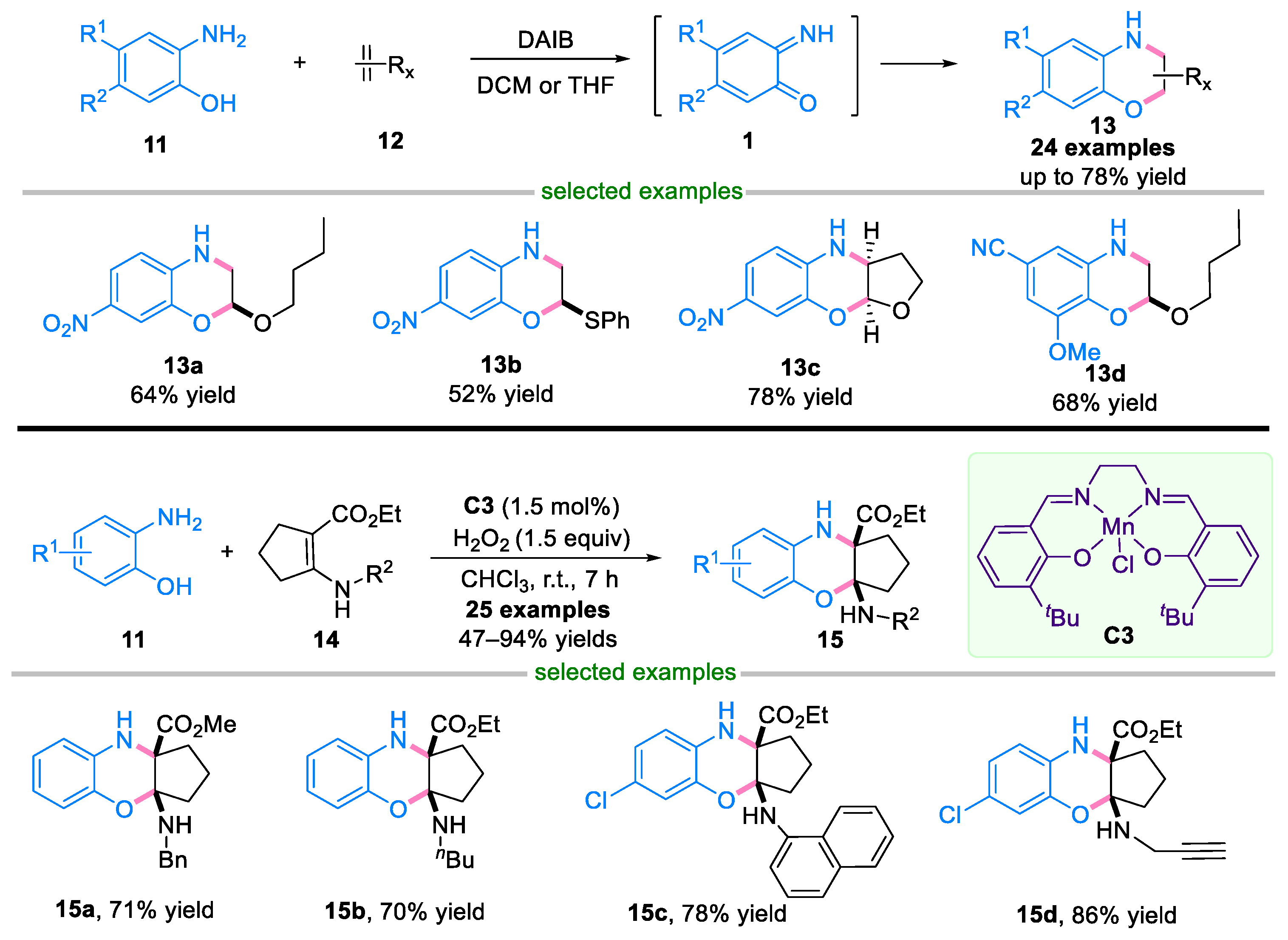
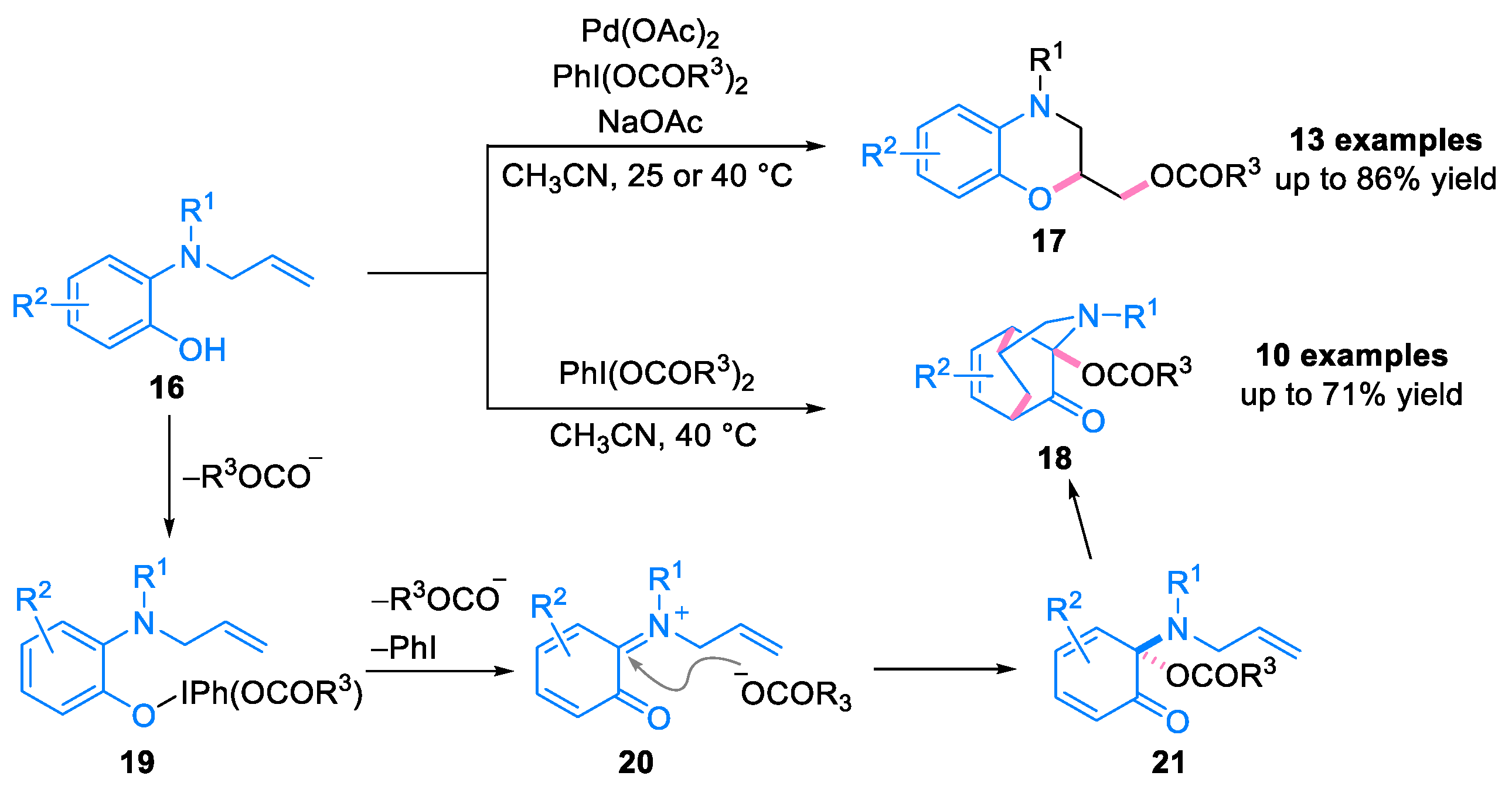
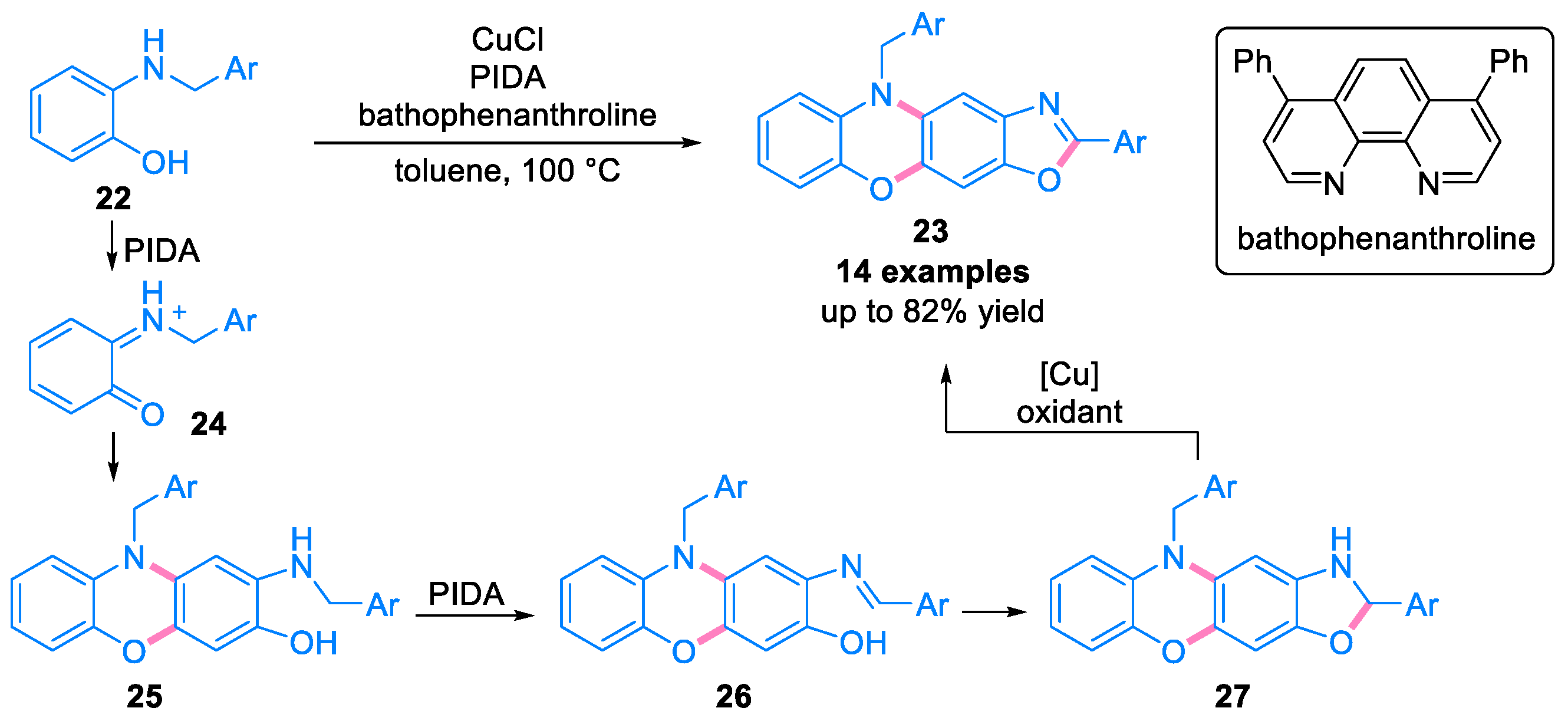
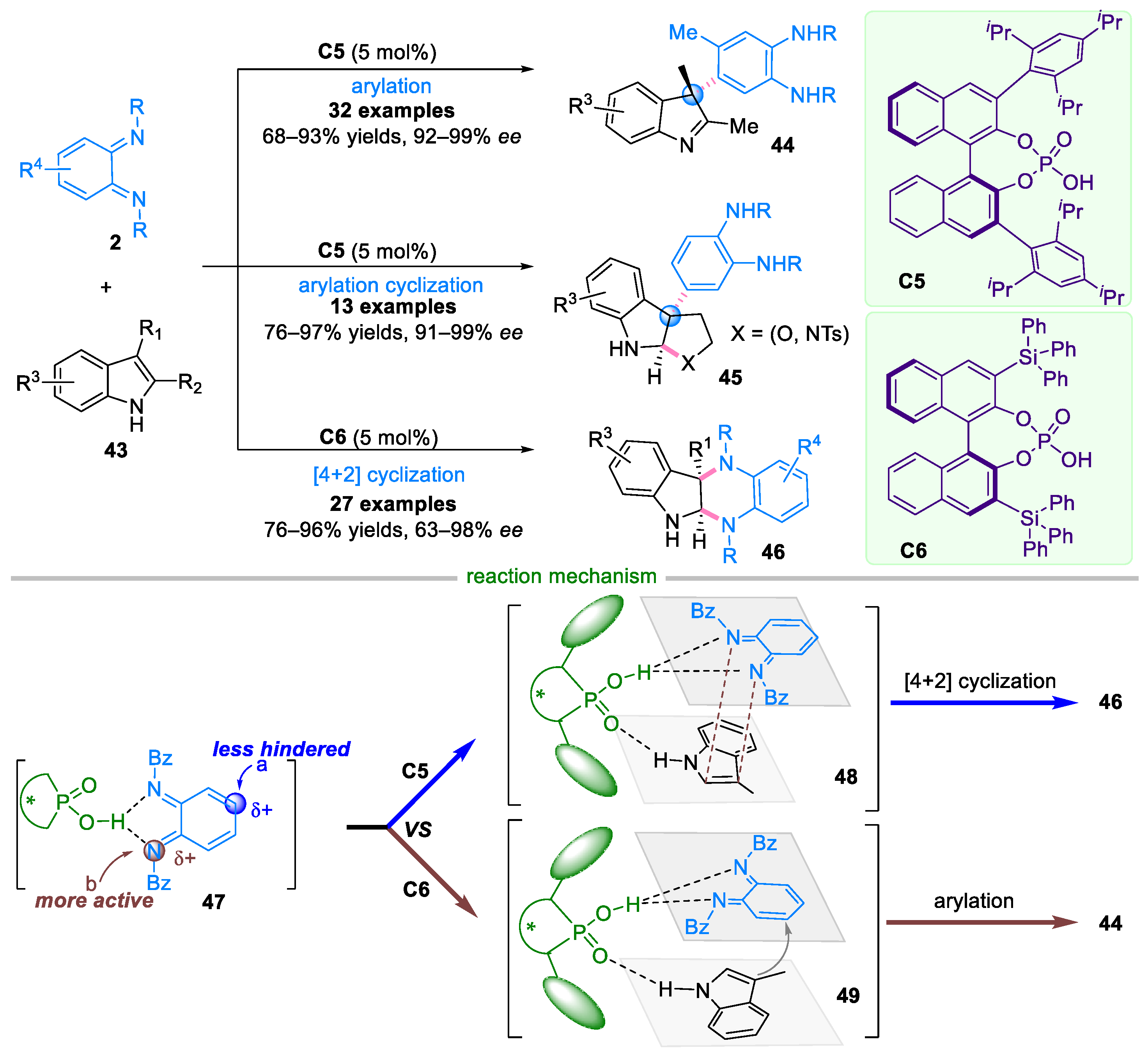
2.1. Domino Reaction of Ortho-Quinone Monoimines
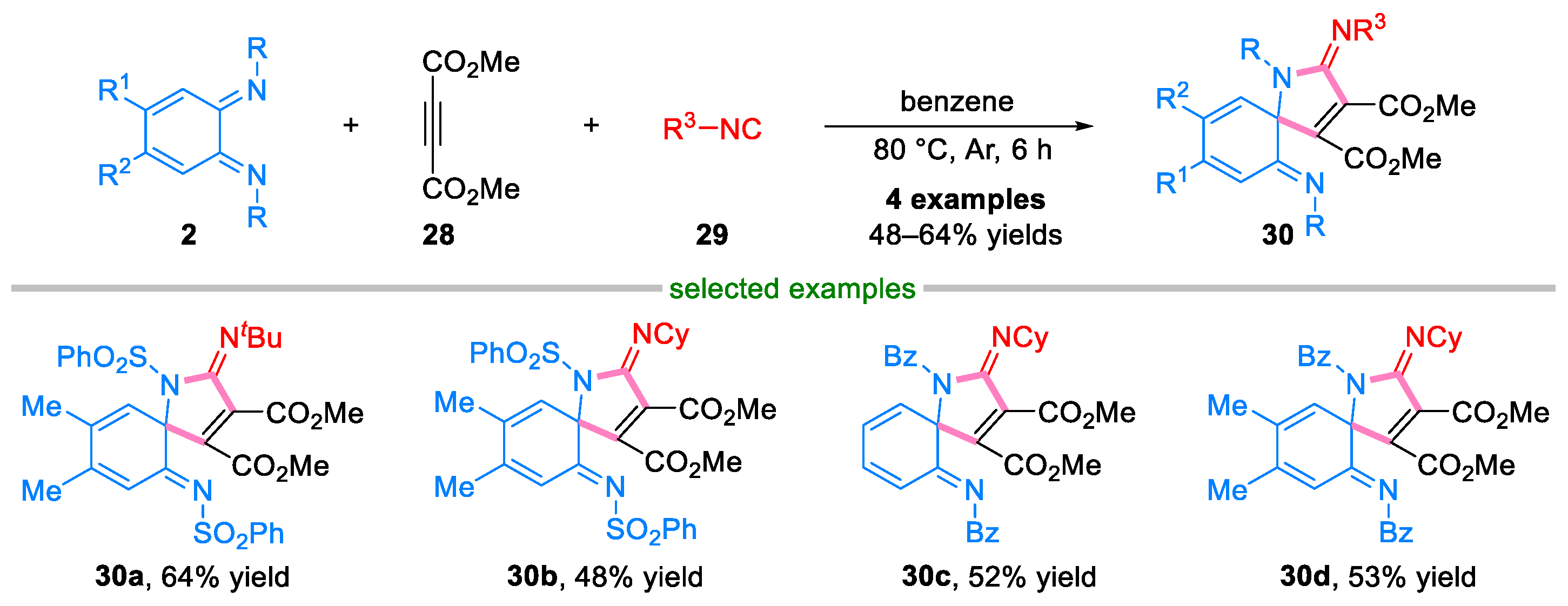
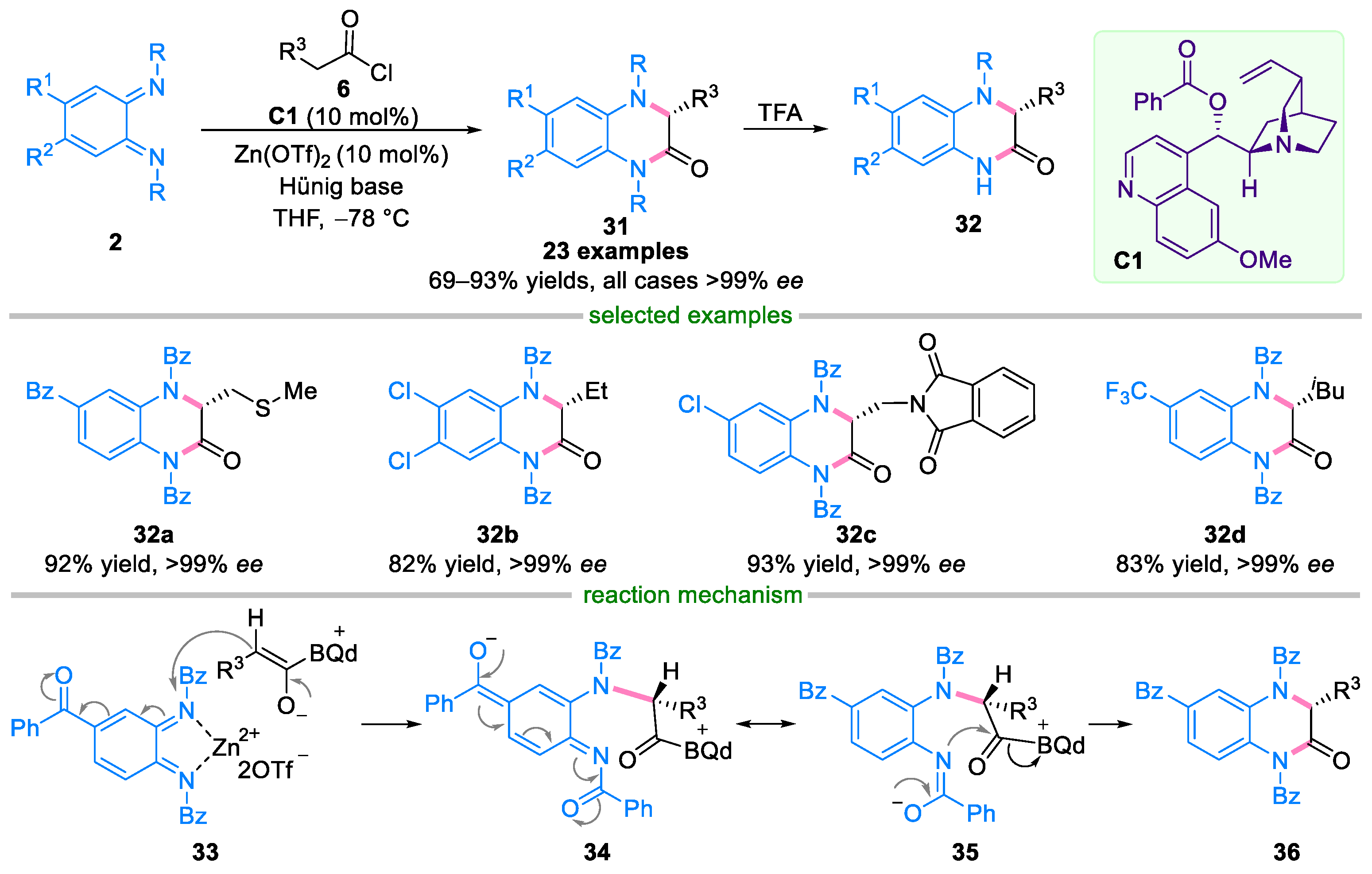
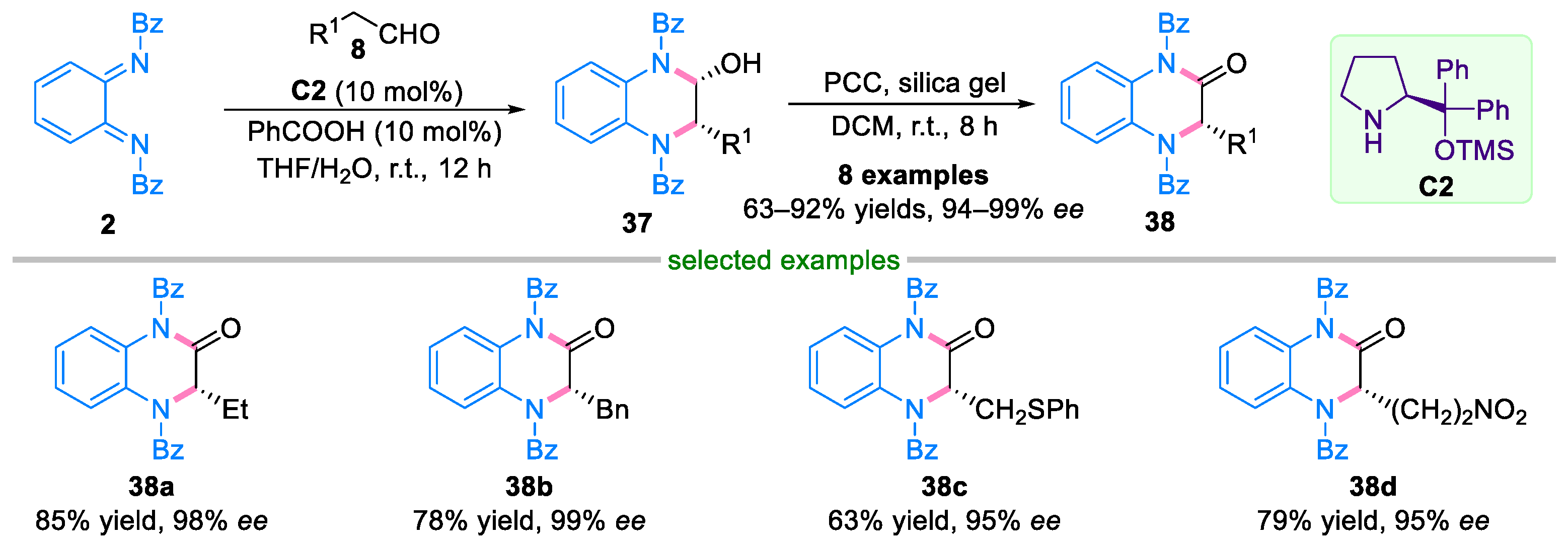
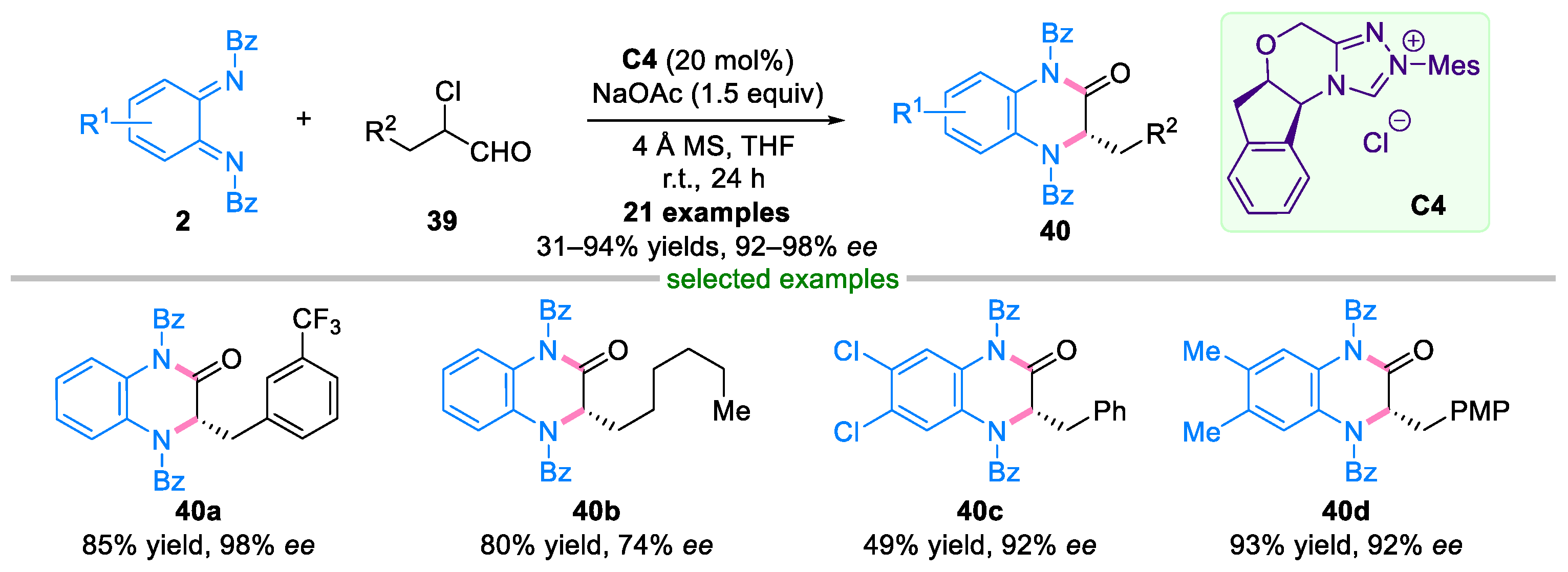
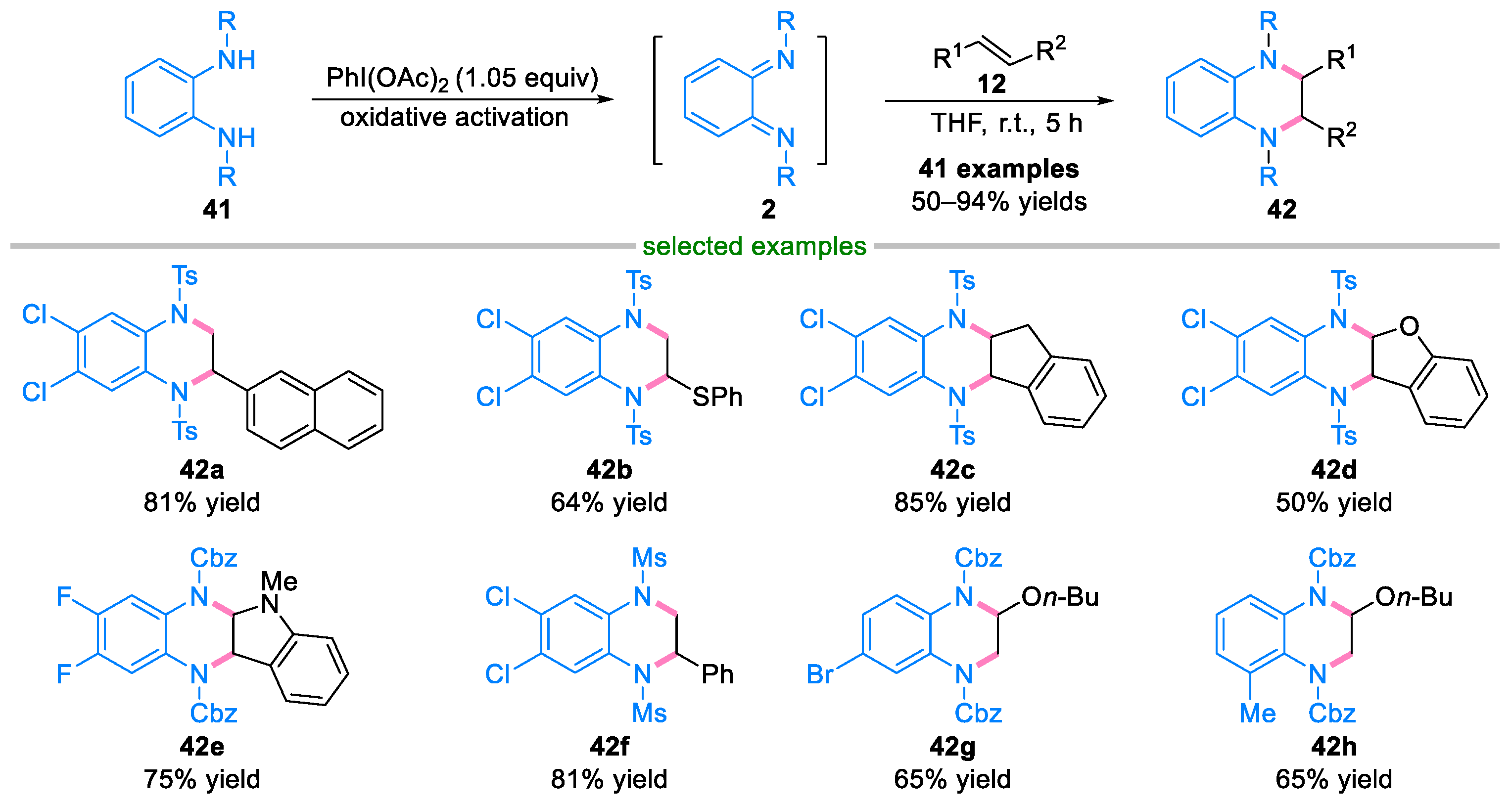
2.2. Domino Reaction of Ortho-Quinone Diimines
3. Domino Reactions of Para-Quinone Imines
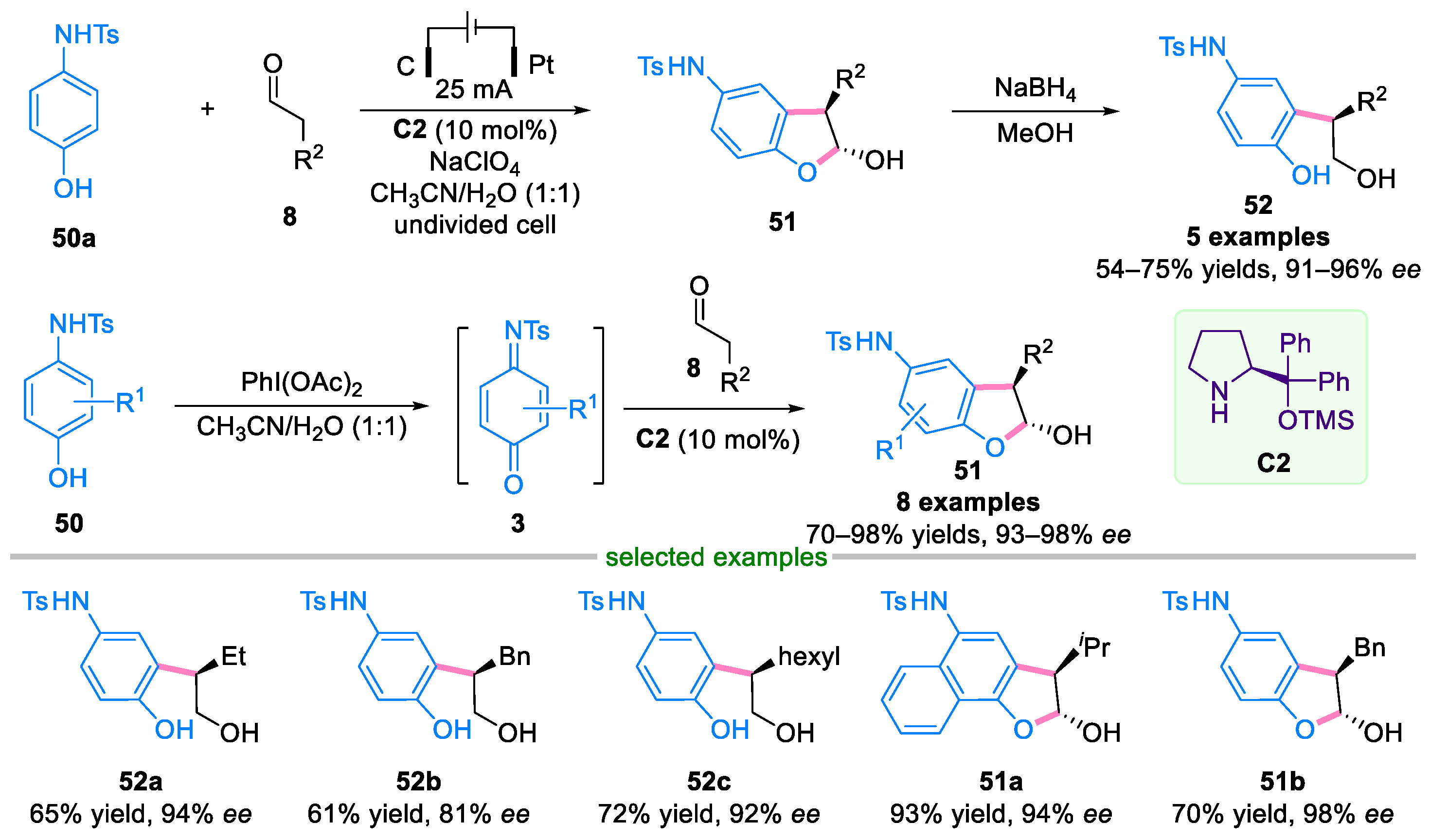
3.1. Domino Reaction of Para-Quinone Monoimines
3.2. Domino Reaction of Para-Quinone Diimines
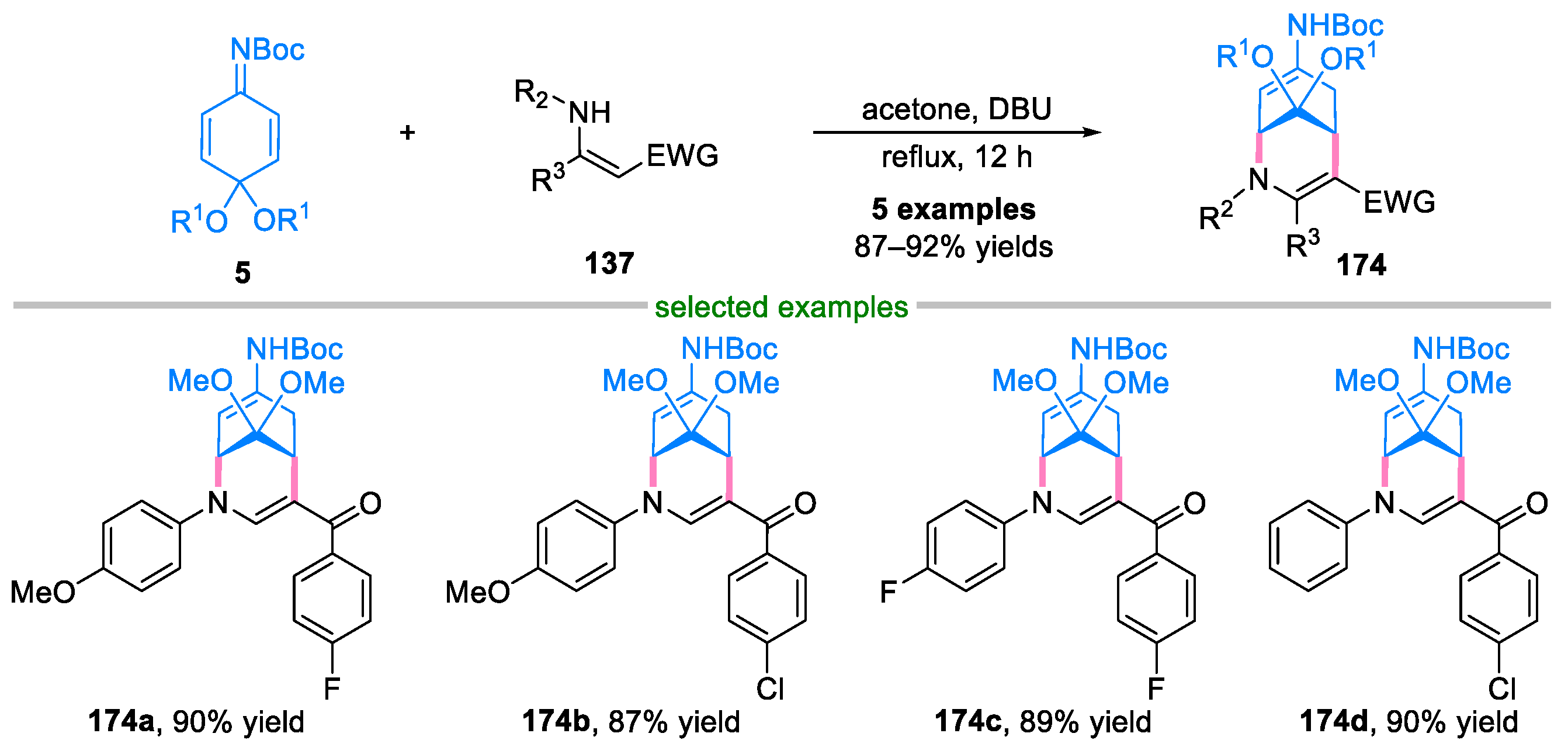
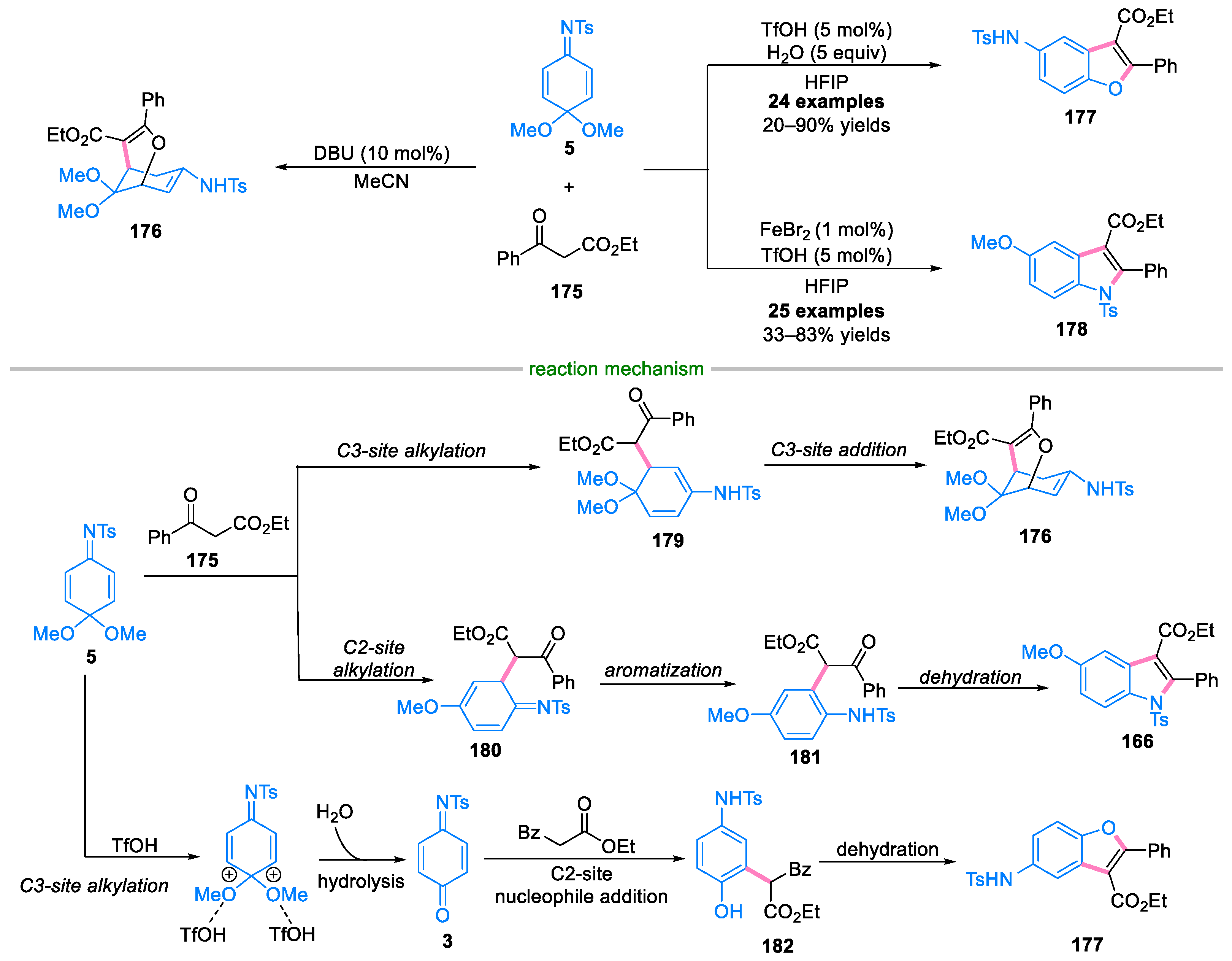
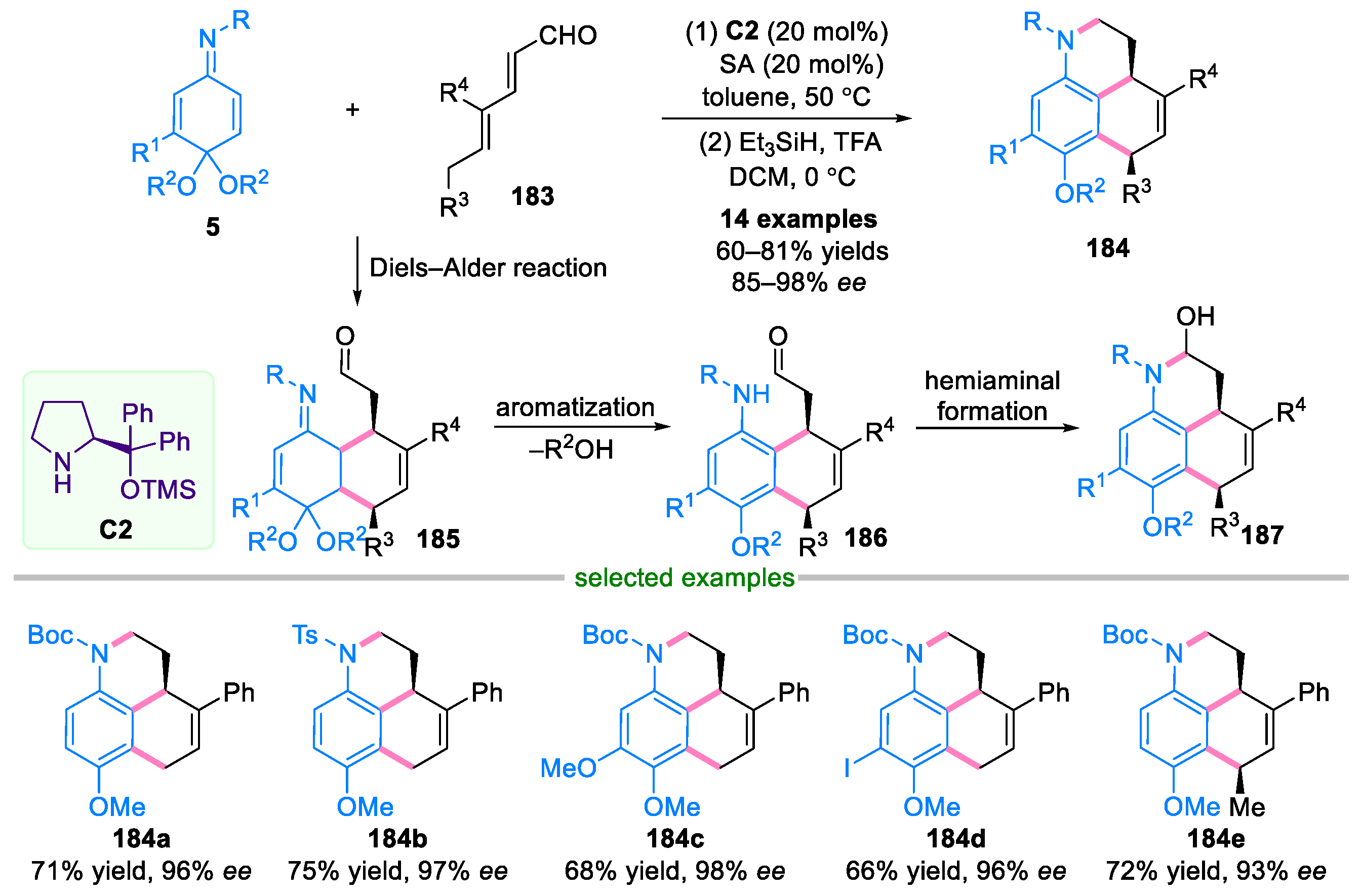
4. Domino Reactions of Quinone Imine Ketals
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Chatterjee, S.; Guidi, M.; Seeberger, P.H.; Gilmore, K. Automated radial synthesis of organic molecules. Nature 2020, 579, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Flores-Bocanegra, L.; Ovalle-Magallanes, B.; Figueroa, M. Natural products from plants targeting key enzymes for the future development of antidiabetic agents. Nat. Prod. Rep. 2023, 40, 1198–1249. [Google Scholar] [CrossRef] [PubMed]
- Cockram, P.E.; Smith, T.K. Active Natural Product Scaffolds against Trypanosomatid Parasites: A Review. J. Nat. Prod. 2018, 81, 2138–2154. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.R.; Huters, A.D.; Towne, T.B.; Reddy, R.E.; Fogle, J.L.; Voight, E.A.; Kym, P.R. Parkinson’s Disease: Advances in Treatment and the Syntheses of Various Classes of Pharmaceutical Drug Substances. Chem. Rev. 2023, 123, 13693–13712. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Improving Drug Design: An Update on Recent Applications of Efficiency Metrics, Strategies for Replacing Problematic Elements, and Compounds in Nontraditional Drug Space. Chem. Res. Toxicol. 2016, 29, 564–616. [Google Scholar] [CrossRef] [PubMed]
- Abdildinova, A.; Gong, Y.-D. Current Parallel Solid-Phase Synthesis of Drug-like Oxadiazole and Thiadiazole Derivatives for Combinatorial Chemistry. ACS Comb. Sci. 2018, 20, 309–329. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.L. Asymmetric Organocatalysis in Drug Development—Highlights of Recent Patent Literature. Org. Process. Res. Dev. 2018, 22, 574–584. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef] [PubMed]
- Kärkäs, M.D.; Porco, J.A., Jr.; Stephenson, C.R.J. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev. 2016, 116, 9683–9747. [Google Scholar] [CrossRef]
- Han, B.; He, X.-H.; Liu, Y.-Q.; He, G.; Peng, C.; Li, J.-L. Asymmetric organocatalysis: An enabling technology for medicinal chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef]
- Ötvös, S.B.; Kappe, C.O. Continuous flow asymmetric synthesis of chiral active pharmaceutical ingredients and their advanced intermediates. Green Chem. 2021, 23, 6117–6138. [Google Scholar] [CrossRef] [PubMed]
- Abbasov, M.E.; Romo, D. The ever-expanding role of asymmetric covalent organocatalysis in scalable, natural product synthesis. Nat. Prod. Rep. 2014, 31, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Stereocontrolled Domino Reactions. Chem. Rev. 2013, 113, 442–524. [Google Scholar] [CrossRef] [PubMed]
- Marson, C.M. Multicomponent and sequential organocatalytic reactions: Diversity with atom-economy and enantiocontrol. Chem. Soc. Rev. 2012, 41, 7712–7722. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Recent Developments in Enantioselective Domino Reactions. Part B: First Row Metal Catalysts. Adv. Synth. Catal. 2023, 365, 768–819. [Google Scholar] [CrossRef]
- Bai, L.; Jiang, X. Catalytic domino reaction: A promising and economic tool in organic synthesis. Chem Catal. 2023, 3, 100752. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino Reactions in Organic Synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Tamanna; Sharma, M.; Kumar, A.; Chauhan, P. Recent development in asymmetric organocatalytic domino re-actions involving 1,6-addition as a key step. Org. Chem. Front. 2022, 9, 572–592. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, Y.; Zhou, W.; Zhou, Y.; Liu, X.-L. Recent advances of chromone-based reactants in the catalytic asymmetric domino annulation reaction. Org. Chem. Front. 2021, 8, 3968–3989. [Google Scholar] [CrossRef]
- Döndaş, H.A.; Retamosa, M.d.G.; Sansano, J.M. Recent Development in Palladium-Catalyzed Domino Reactions: Access to Materials and Biologically Important Carbo- and Heterocycles. Organometallics 2019, 38, 1828–1867. [Google Scholar] [CrossRef]
- Benaglia, M.; Greco, S.J.; Westphal, R.; Filho, E.V.; Medici, F. Stereoselective Domino Reactions in the Synthesis of Spiro Compounds. Synthesis 2022, 54, 2927–2975. [Google Scholar] [CrossRef]
- Pellissier, H. Recent Developments in Enantioselective Metal-Catalyzed Domino Reactions. Adv. Synth. Catal. 2018, 361, 1733–1755. [Google Scholar] [CrossRef]
- Hagiwara, H. Recent Advance of Domino Michael Reaction in Natural Product Synthesis. Nat. Prod. Commun. 2021, 16, 1934578X211049844. [Google Scholar] [CrossRef]
- Manchado, A.; Ramos, V.E.; Díez, D.; Garrido, N.M. Multicomponent Domino Reaction in the Asymmetric Synthesis of Cy-clopentan[c]pyran Core of Iridoid Natural Products. Molecules 2020, 25, 1308. [Google Scholar] [CrossRef] [PubMed]
- Delayre, B.; Wang, Q.; Zhu, J. Natural Product Synthesis Enabled by Domino Processes Incorporating a 1,2-Rearrangement Step. ACS Cent. Sci. 2021, 7, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Volla, C.M.R.; Atodiresei, I.; Rueping, M. Catalytic C–C Bond-Forming Multi-Component Cascade or Domino Reactions: Pushing the Boundaries of Complexity in Asymmetric Organocatalysis. Chem. Rev. 2013, 114, 2390–2431. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.; Paomephan, P.; Boonpratuang, T.; Choeyklin, R.; Boonchird, C.; Surup, F. ent-Clavilactone J and Its Quinone De-rivative, Meroterpenoids from the Fungus Resupinatus sp. J. Nat. Prod. 2023, 86, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-M.; Kuang, B.; Zeng, G.-Z.; Wang, Z.; Wang, J.; Chen, X.-Q.; Tan, N.-H. Nematicidal quinone derivatives from three Rubia plants. Tetrahedron 2018, 74, 2115–2120. [Google Scholar] [CrossRef]
- Zhu, X.; Jing, Y. Natural quinone molecules as effective cathode materials for nonaqueous lithium-ion batteries. J. Power Sources 2023, 531, 231291. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Ni, Y.; Zheng, S.; Yan, Z.; Zhang, K.; Zhao, Q.; Chen, J. Quinone Electrodes for Alkali-Acid Hybrid Batteries. J. Am. Chem. Soc. 2022, 144, 8066–8072. [Google Scholar] [CrossRef]
- Sayil, C.; Deniz, N.G.; Cinarli, A. Synthesis of N-, S-, O-substituted quinone dyes and their dyeability on polyester fibers. Prog. Org. Coat. 2016, 98, 39–42. [Google Scholar] [CrossRef]
- Bao, W.; Chen, Y.-H.; Liu, Y.-W.; Xiang, S.-H.; Tan, B. Atroposelective Synthesis of 2-Arylindoles via Chiral Phosphoric Ac-id-Catalyzed Direct Amination of Indoles. Chin. J. Chem. 2024, 42, 731–735. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Duan, M.; Lin, S.-L.; Liu, Y.-W.; Cheng, J.K.; Xiang, S.-H.; Yu, P.; Houk, K.N.; Tan, B. Organocatalytic aromatization-promoted umpolung reaction of imines. Nat. Chem. 2023, 16, 408–416. [Google Scholar] [CrossRef]
- More, S.G.; Kamble, R.B.; Suryavanshi, G. Oxidative Radical-Mediated Addition of Ethers to Quinone Imine Ketals: An Access to Hemiaminals. J. Org. Chem. 2021, 86, 2107–2116. [Google Scholar] [CrossRef]
- Halder, P.; Humne, V.T.; Mhaske, S.B. Transition-Metal-Free Regioselective One-Pot Synthesis of Aryl Sulfones from Sodium Sulfinates via Quinone Imine Ketal. J. Org. Chem. 2019, 84, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Cheng, F.; Shen, X.; Liu, J.; Lin, J. Highly chemo- and regioselective C–P cross-coupling reaction of quinone imine ketals with Ar2P(O)H to construct ortho-amino triarylphosphine derivatives. Green Chem. 2019, 21, 3536–3541. [Google Scholar] [CrossRef]
- Yadav, N.; Taneja, N.; Musib, D.; Hazra, C.K. Practical Access to meta-Substituted Anilines by Amination of Quinone Imine Ketals Derived from Anisidines: Efficient Synthesis of Anti-Psychotic Drugs. Angew. Chem. Int. Ed. 2023, 62, e202301166. [Google Scholar] [CrossRef]
- Lan, W.; Liu, F.; Hu, J.; Zhu, J.; Hu, S.; Wan, J.-P.; Liao, L. Copper-Catalyzed Regiospecific Amination of Heteroarenes with Quinone imides. J. Org. Chem. 2022, 87, 5592–5602. [Google Scholar] [CrossRef]
- Lan, W.; Zhu, J.; Abulaiti, B.; Chen, G.; Zhang, Z.; Yan, N.; Wan, J.-P.; Zhang, X.; Liao, L. Zinc Trifluoromethanesul-fonate-Catalyzed para-Selective Amination of Free Anilines and Free Phenols with Quinoneimides. J. Org. Chem. 2022, 87, 13895–13906. [Google Scholar] [CrossRef]
- Liu, T. Recent Progress on Quinone Imine Ketals: Synthesis and Applications. Chin. J. Org. Chem. 2020, 40, 2678–2691. [Google Scholar] [CrossRef]
- Wolfer, J.; Bekele, T.; Abraham, C.J.; Dogo-Isonagie, C.; Lectka, T. Catalytic, Asymmetric Synthesis of 1,4-Benzoxazinones: A Remarkably Enantioselective Route to α-Amino Acid Derivatives from o-Benzoquinone Imides. Angew. Chem. Int. Ed. 2006, 45, 7398–7400. [Google Scholar] [CrossRef] [PubMed]
- Paull, D.H.; Alden-Danforth, E.; Wolfer, J.; Dogo-Isonagie, C.; Abraham, C.J.; Lectka, T. An Asymmetric, Bifunctional Catalytic Approach to Non-Natural γ-Amino Acid Derivatives. J. Org. Chem. 2007, 72, 5380–5382. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Han, B.; Jiang, K.; Du, W.; Chen, Y.-C. Organocatalytic enantioselective hetero-Diels–Alder reaction of aldehydes and o-benzoquinone diimide: Synthesis of optically active hydroquinoxalines Bioorg. Med. Chem. Lett. 2009, 19, 3952–3954. [Google Scholar] [CrossRef] [PubMed]
- Bodipati, N.; Peddinti, R.K. Chemical generation of o-quinone monoimines for the rapid construction of 1,4-benzoxazine derivatives. Org. Biomol. Chem. 2012, 10, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.A.; Zuo, H.; Lu, X.; Wu, Y.; Zhong, F. Harnessing structurally unbiased ortho benzoquinone monoimine for biomi-metic oxidative [4+2] cycloaddition with enamines. Chem. Commun. 2020, 56, 5965–5968. [Google Scholar] [CrossRef] [PubMed]
- Giofrè, S.; Keller, M.; Presti, L.L.; Beccalli, E.M.; Molteni, L. Switchable Oxidative Reactions of N-allyl-2-Aminophenols: Palla-dium-Catalyzed Alkoxyacyloxylation vs an Intramolecular Diels−Alder Reaction. Org. Lett. 2021, 23, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Loro, C.; Molteni, L.; Papis, M.; Beccalli, E.M.; Nava, D.; Presti, L.L.; Brenna, S.; Colombo, G.; Foschi, F.; Broggini, G. Direct Synthesis of Fluorescent Oxazolo-phenoxazines by Copper-Catalyzed/Hypervalent Iodine(III)-Mediated Dimeriza-tion/Cyclization of 2-Benzylamino-phenols. J. Org. Chem. 2022, 87, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Torán, R.; Portillo, E.; Sanz-Marco, A.; Vila, C.; Blay, G. Enantioselective construction of quaternary stereocenters via or-ganocatalytic arylation of isoxazolin-5-ones with o-quinone diimides. Org. Chem. Front. 2023, 10, 6081–6086. [Google Scholar] [CrossRef]
- Laviós, A.; Martínez-Pardo, P.; Sanz-Marco, A.; Vila, C.; Pedro, J.R.; Blay, G. Synthesis of α,α-Diaryl-α-amino Acid Precursors by Reaction of Isocyanoacetate Esters with o-Quinone Diimides. Org. Lett. 2023, 25, 5608–5612. [Google Scholar] [CrossRef]
- Nair, V.; Dhanya, R.; Viji, S. The three component reaction involving isocyanides, dimethyl acetylenedicarboxylate and quinoneimides: A facile synthesis of spirofused γ-iminolactams. Tetrahedron 2005, 61, 5843–5848. [Google Scholar] [CrossRef]
- Abraham, C.J.; Paull, D.H.; Scerba, M.T.; Grebinski, J.W.; Lectka, T. Catalytic, Enantioselective Bifunctional Inverse Electron Demand Hetero-Diels−Alder Reactions of Ketene Enolates and o-Benzoquinone Diimides. J. Am. Chem. Soc. 2006, 128, 13370–13371. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Chen, X.; Mou, C.; Luo, G.; Li, Y.; Li, X.; Xue, W.; Jin, Z.; Chi, Y.R. Carbene-Catalyzed α-Carbon Amination of Chloroaldehydes for Enantioselective Access to Dihydroquinoxaline Derivatives. Org. Lett. 2019, 21, 4340–4344. [Google Scholar] [CrossRef]
- Wang, D.; Yu, H.; Sun, S.; Zhong, F. Intermolecular Vicinal Diaminative Assembly of Tetrahydroquinoxalines via Metal-free Oxidative [4+2] Cycloaddition Strategy. Org. Lett. 2020, 22, 2425–2430. [Google Scholar] [CrossRef]
- Gao, H.-J.; Miao, Y.-H.; Sun, W.-N.; Zhao, R.; Xiao, X.; Hua, Y.-Z.; Jia, S.-K.; Wang, M.-C.; Mei, G.-J. Diversity-Oriented Catalytic Asymmetric Dearomatization of Indoles with o-Quinone Diimides. Adv. Sci. 2023, 10, 2305101. [Google Scholar] [CrossRef]
- Jensen, K.L.; Franke, P.T.; Nielsen, L.T.; Daasbjerg, K.; Jørgensen, K.A. Anodic Oxidation and Organocatalysis: Direct Regio- and Stereoselective Access to meta-Substituted Anilines by α-Arylation of Aldehydes. Angew. Chem. Int. Ed. 2010, 49, 129–133. [Google Scholar] [CrossRef]
- Liao, L.; Shu, C.; Zhang, M.; Liao, Y.; Hu, X.; Zhang, Y.; Wu, Z.; Yuan, W.; Zhang, X. Highly Enantioselective [3+2] Coupling of Indoles with Quinone Monoimines Promoted by a Chiral Phosphoric Acid. Angew. Chem. Int. Ed. 2014, 53, 10471–10475. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-S.; Zhu, R.-Y.; Zhang, Y.-C.; Shi, F. Catalytic chemoselective [3+3] cycloadditions of azomethine ylides with quinone monoimides leading to the construction of a dihydrobenzoxazine scaffold. Chem. Commun. 2015, 51, 11798–11801. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qiao, G.; Liu, H.; Zhang, L.; Sun, Z.; Xiao, Y.; Guo, H. Brønsted acid-promoted [3 + 3] cycloaddition of azomethine ylides with quinone monoimine: A practical method towards dihydrobenzoxazine derivatives. RSC Adv. 2015, 5, 84290–84294. [Google Scholar] [CrossRef]
- Sun, X.-X.; Zhang, H.-H.; Li, G.-H.; Meng, L.; Shi, F. Diastereo- and enantioselective construction of an indole-based 2,3-dihydrobenzofuran scaffold via catalytic asymmetric [3+2] cyclizations of quinone monoimides with 3-vinylindoles. Chem. Commun. 2016, 52, 2968–2971. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, S.; Hu, F.; Liao, Y.; Liao, L.; Xu, X.; Yuan, W.; Zhang, X. Highly enantioselective [3+2] coupling of cyclic enamides with quinone monoimines promoted by a chiral phosphoric acid. Chem. Commun. 2016, 52, 8757–8760. [Google Scholar] [CrossRef]
- Bin Kim, U.; Jung, D.J.; Jeon, H.J.; Rathwell, K.; Lee, S.-G. Synergistic Dual Transition Metal Catalysis. Chem. Rev. 2020, 120, 13382–13433. [Google Scholar] [CrossRef]
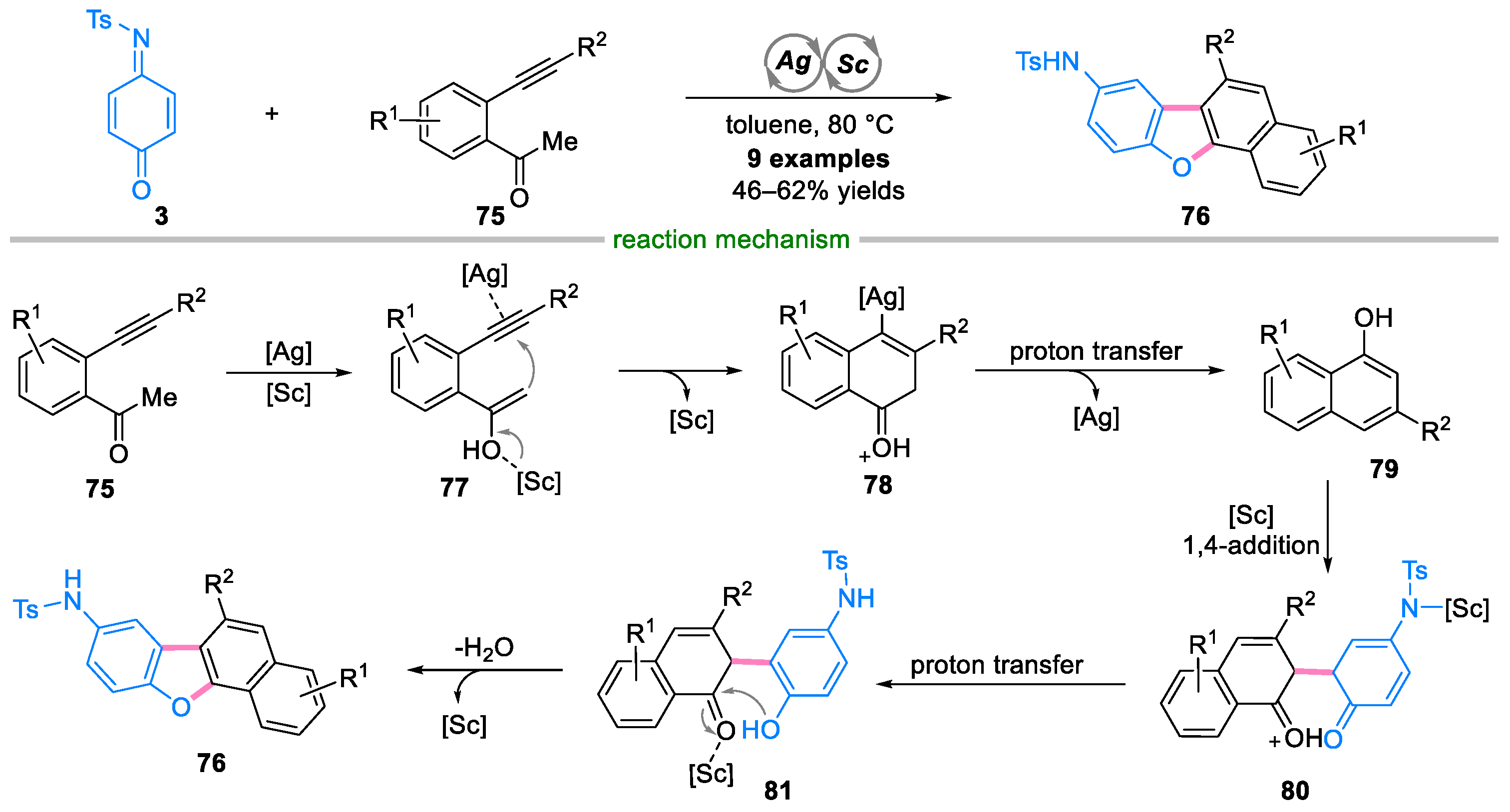
- Chen, K.; Liu, S.; Wang, D.; Hao, W.-J.; Zhou, P.; Tu, S.-J.; Jiang, B. Silver/Scandium-Cocatalyzed Bicyclization of β Alkynyl Ketones Leading to Benzo[c]xanthenes and Naphtho[1,2 b]benzofurans. J. Org. Chem. 2017, 82, 11524–11530. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Qi, J.; Zhou, L.; Xu, Z.; Tung, C.-H. Synthesis of benzannulated spiroketals with gold-catalyzed cycloisomeriza-tion/spiroketalization cascade. Org. Chem. Front. 2018, 5, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, Y.J.; Goh, M.S.; Kim, Y.; Li, Z.; Park, J.U.; Ahn, Y.; Seon, J.H.; Yoo, H.M.; Ko, K.C.; et al. Seven new secondary metabolites isolated from roots of Lespedeza bicolor. Fitoterapia 2023, 170, 105671. [Google Scholar] [CrossRef]
- Kowalski, K.; Szczupak, Ł.; Oehninger, L.; Ott, I.; Hikisz, P.; Koceva-Chyła, A.; Therrien, B. Ferrocenyl derivatives of ptero-carpene and coumestan: Synthesis, structure and anticancer activity studies. J. Organomet. Chem. 2014, 772–773, 49–59. [Google Scholar] [CrossRef]
- Njamen, D.; Talla, E.; Mbafor, J.T.; Fomum, Z.T.; Kamanyi, A.; Mbanya, J.-C.; Cerdá-Nicolás, M.; Giner, R.M.; Recio, M.; Rıos, J.L. Anti-inflammatory activity of erycristagallin, a pterocarpene from Erythrina mildbraedii. Eur. J. Pharmacol. 2003, 468, 67–74. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, S.; Cheng, S.; Dai, X.; Xu, X.; Yuan, W.; Zhang, X. Synthesis of Novel Pterocarpen Analogues via [3 + 2] Coupling-Elimination Cascade of α,α-Dicyanoolefins with Quinone Monoimines. J. Heterocycl. Chem. 2019, 56, 1672–1683. [Google Scholar] [CrossRef]
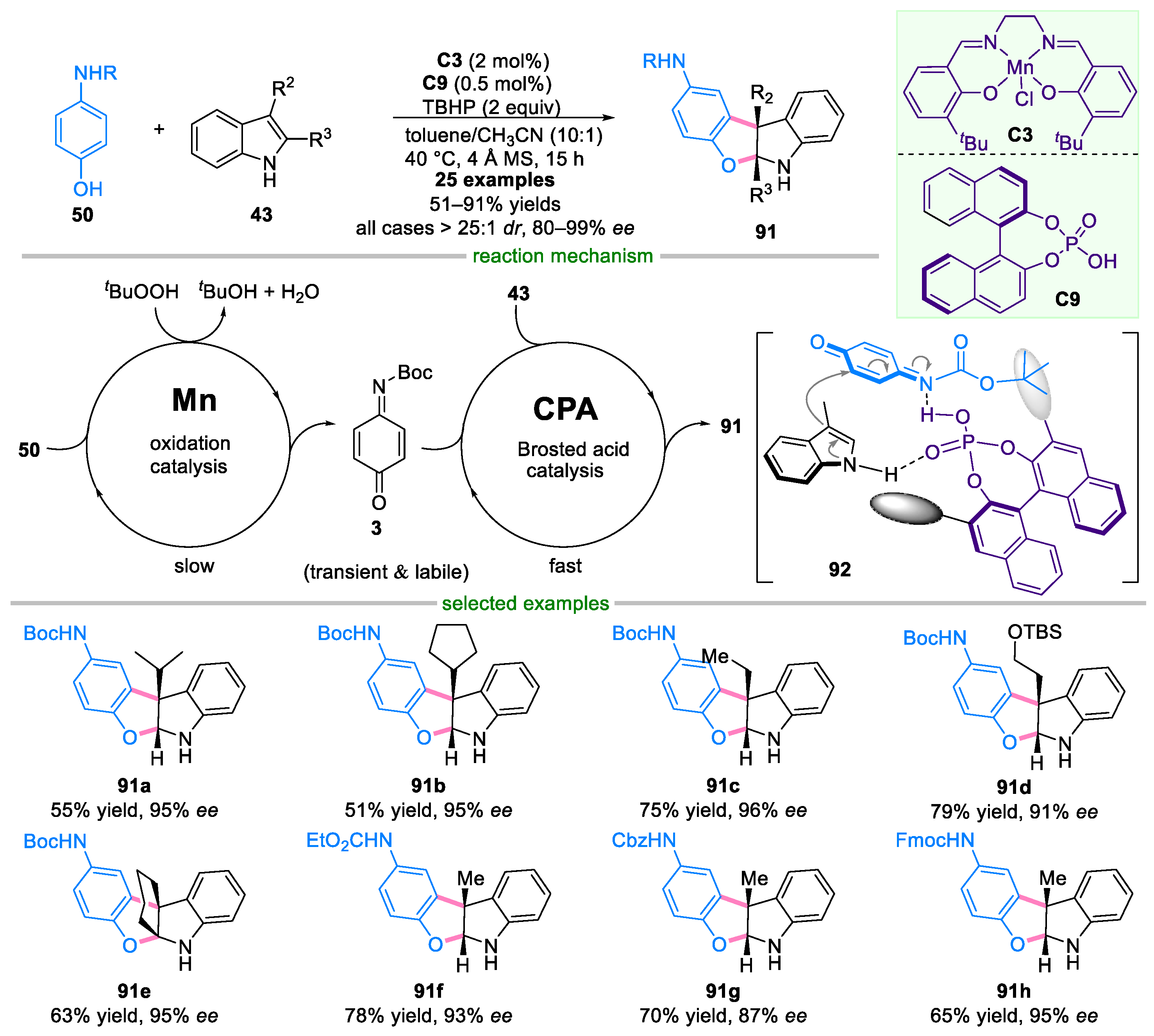
- Yu, Q.; Fu, Y.; Huang, J.; Qin, J.; Zuo, H.; Wu, Y.; Zhong, F. Enantioselective Oxidative Phenol-Indole [3 + 2] Coupling Enabled by Biomimetic Mn(III)/Brønsted Acid Relay Catalysis. ACS Catal. 2019, 9, 7285–7291. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.-L. Advances in Catalytic Asymmetric Dearomatization. ACS Cent. Sci. 2021, 7, 432–444. [Google Scholar] [CrossRef]
- Xia, Z.-L.; Xu-Xu, Q.-F.; Zheng, C.; You, S.-L. Chiral phosphoric acid-catalyzed asymmetric dearomatization reactions. Chem. Soc. Rev. 2020, 49, 286–300. [Google Scholar] [CrossRef]
- Sheng, F.-T.; Wang, J.-Y.; Tan, W.; Zhang, Y.-C.; Shi, F. Progresses in organocatalytic asymmetric dearomatization reactions of indole derivatives. Org. Chem. Front. 2020, 7, 3967–3998. [Google Scholar] [CrossRef]
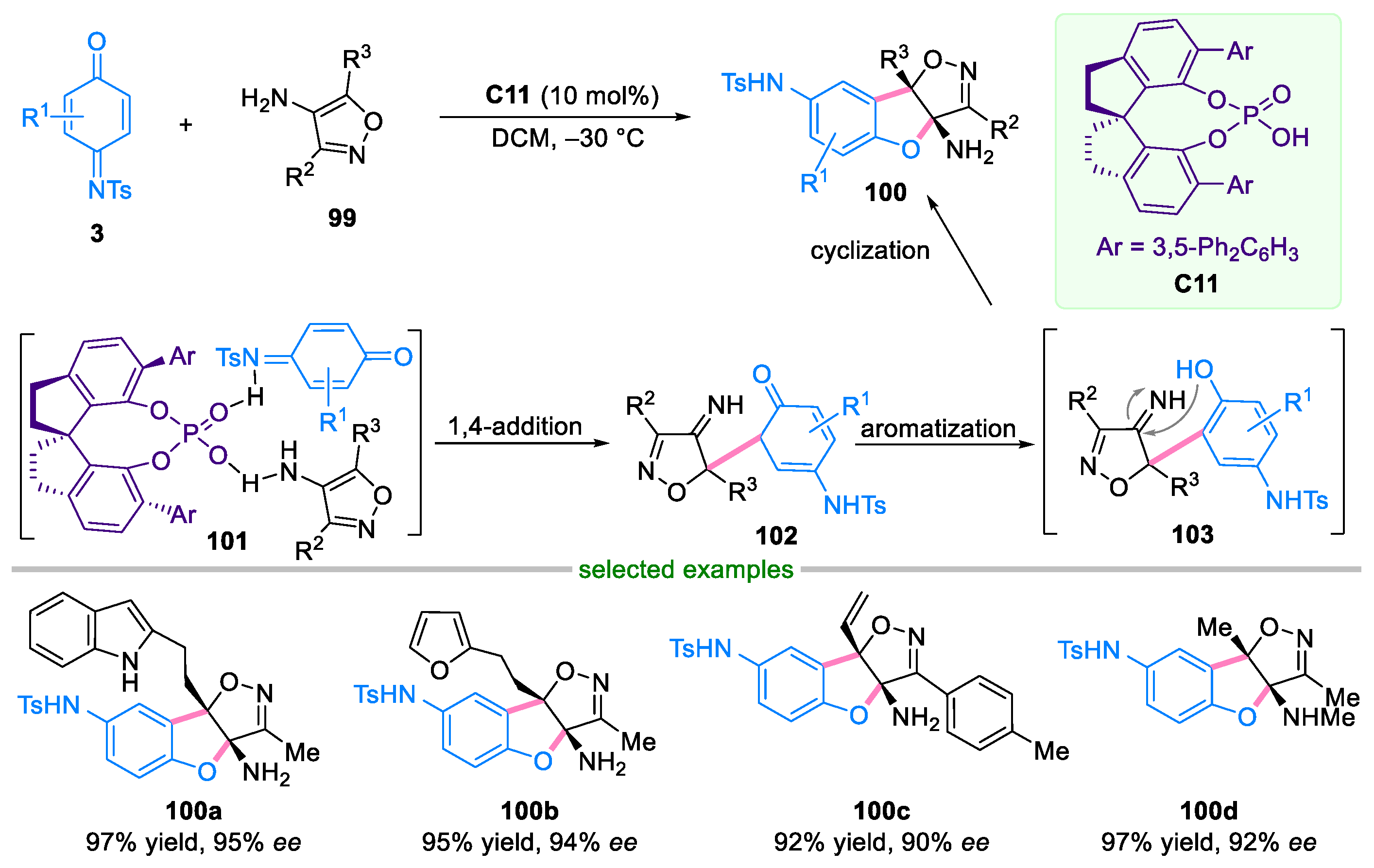
- Liu, H.; Yan, Y.; Zhang, J.; Liu, M.; Cheng, S.; Wang, Z.; Zhang, X. Enantioselective dearomative [3+2] annulation of 5-amino-isoxazoles with quinone monoimines. Chem. Commun. 2020, 56, 13591–13594. [Google Scholar] [CrossRef] [PubMed]
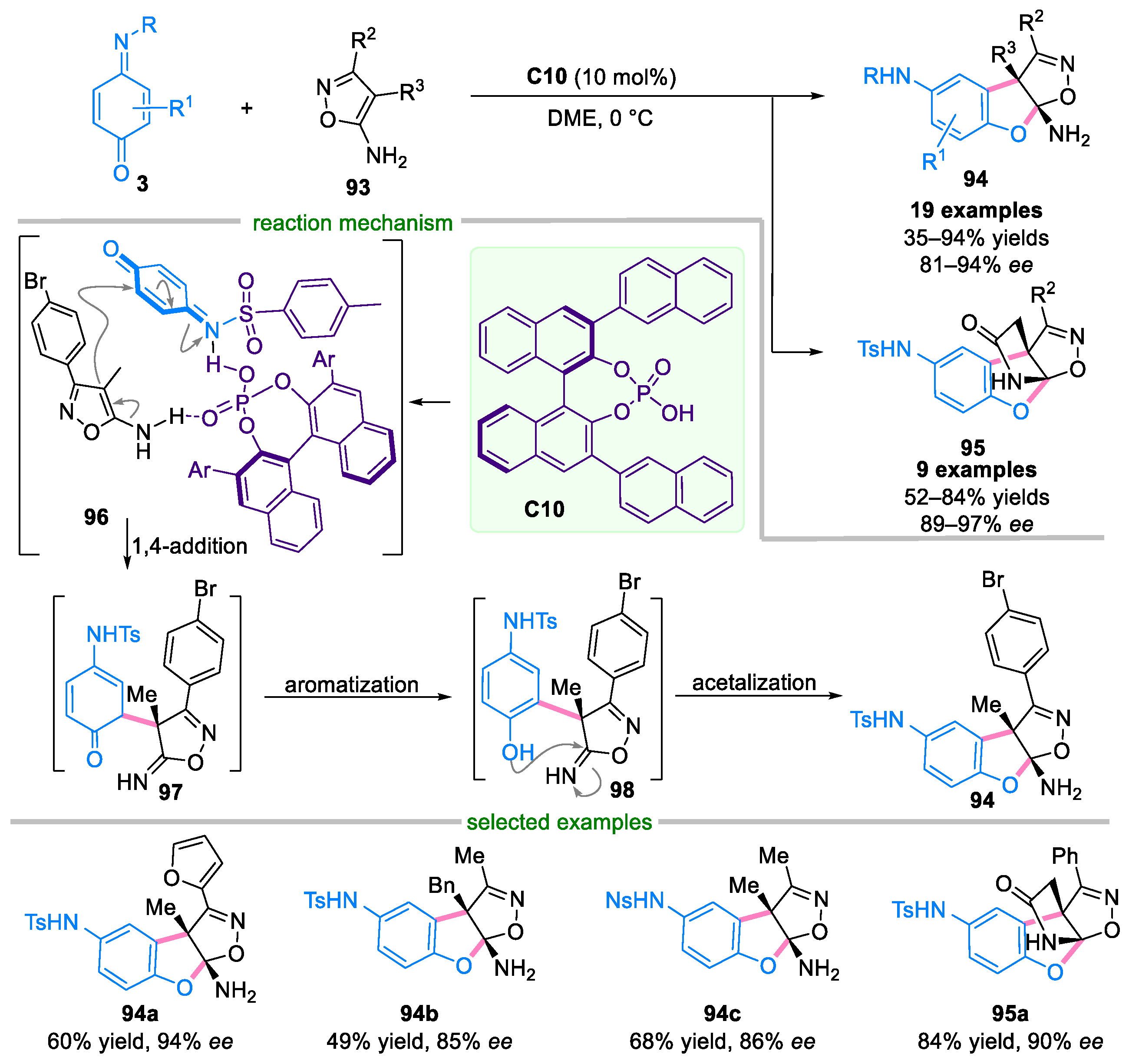
- Yan, Y.-K.; Bao, A.-L.; Li, M.; Xie, X.-S.; Li, W.-Z.; Zhang, X.-M. Highly enantioselective [3+2] annulation of 4-amino-isoxazoles with quinone monoimines to access structurally diverse isoxazoline fused dihydrobenzofurans and antifungal evaluation. J. Mol. Struct. 2023, 1294, 136277. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Huang, M.; Liu, H.; Yan, Y.; Zhang, X. Enantioselective [3 + 2] annulation of 3-hydroxymaleimides with quinone monoimines. Org. Chem. Front. 2021, 8, 2268–2273. [Google Scholar] [CrossRef]
- Qin, L.-Z.; Cheng, Y.-L.; Wen, X.; Xu, Q.-L.; Zhen, L. Synthesis of indole-fused scaffolds via [3+3] cyclization reaction of 2-indolylmethanols with quinone imines. Tetrahedron 2021, 77, 131742. [Google Scholar] [CrossRef]
- Jing, Y.; Chen, H.; Zhao, S.; Cheng, S.; Xu, X.; Yuan, W.; Zhang, X. Unprecedented Tandem Conjugate Addition/C-O Ester Migration of α-Cyano Arylacetates with a Quinone Monoimine. ChemistrySelect 2019, 4, 4156–4158. [Google Scholar] [CrossRef]
- Cheng, S.-B.; Jing, Y.; Cao, L.-Y.; Li, W.-Z.; Zhang, X.-M. Tandem Reaction of Phenyl α-Cyano-α-arylacetates with Quinone Monoimines. ChemistrySelect 2021, 6, 8923–8927. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, P.; Li, S.; Ma, Y.; Zhang, P.; Xu, W. Efficient synthesis of spiro diheterocycles via multi-component dicyclization reaction. Org. Biomol. Chem. 2022, 20, 8461–8464. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.N.; Reddy, V.R.; Dinda, S.; Nanubolu, J.B.; Chandra, R. Asymmetric Reaction of p-Quinone Diimide: Organocatalyzed Michael Addition of α-Cyanoacetates. Org. Lett. 2018, 20, 2572–2575. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-Y.; Gelis, C.; Bouchet, D.; Retailleau, P.; Moreau, X.; Neuville, L.; Masson, G. Chiral Phosphoric Acid-Catalyzed En-antioselective Constructionof 2,3-DisubstitutedIndolines. Org. Lett. 2021, 23, 442–448. [Google Scholar] [CrossRef]
- Zhong, Z.; Liao, L.; Liu, Y.; Zhang, M.; Wan, J.-P. Annulation of enaminones with quinonediimides/quinoneimides for selective synthesis of indoles and 2-aminobenzofurans. Chem. Commun. 2023, 59, 6885–6888. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Chou, C.T.; Swenton, J.S. Synthetically and biologically interesting N-acyl quinone imine ketals and N-acyl quinol imine ethers from anodic oxidation of anilides. J. Am. Chem. Soc. 1987, 109, 946–948. [Google Scholar] [CrossRef]
- Chou, C.T.; Swenton, J.S. A convergent strategy for synthesis of Erythrina alkaloids. J. Am. Chem. Soc. 1987, 109, 6898–6899. [Google Scholar] [CrossRef]
- Chuang, K.V.; Navarro, R.; Reisman, S.E. Benzoquinone-derived sulfinylimines as versatile intermediates for alkaloid syn-thesis: Total synthesis of (–)-3-demethoxyerythratidinone. Chem. Sci. 2011, 2, 1086–1089. [Google Scholar] [CrossRef]
- Liu, T.; He, C.; Wang, F.; Shen, X.; Li, Y.; Lang, M.; Li, G.; Huang, C.; Cheng, F. Organocatalyzed [2+2] Cycloaddition Reactions between Quinone Imine Ketals and Allenoates. Synthesis 2020, 52, A. [Google Scholar] [CrossRef]
- Song, R.; Han, Z.; He, Q.; Fan, R. Amine-Mediated Transimination and Aromatization-Triggered Domino Reaction in the Synthesis of Polyfunctionalized 4-Aminoquinolines. Org. Lett. 2016, 18, 5328–5331. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nakatsu, H.; Maruoka, K. Catalytic Asymmetric Diels–Alder Reaction of Quinone Imine Ketals: A Site-Divergent Approach. Angew. Chem. Int. Ed. 2015, 54, 4617–4621. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Liao, L.; Liao, Y.; Hu, X.; Zhang, Y.; Yuan, W.; Zhang, X. Lewis Acid Catalyzed [3+2] Coupling of Indoles with Quinone Monoacetals or Quinone Imine Ketal. Eur. J. Org. Chem. 2014, 2014, 4467–4471. [Google Scholar] [CrossRef]
- Liao, L.-H.; Zhang, M.-M.; Liao, Y.-J.; Yuan, W.-C.; Zhang, X.-M. Lewis Acid Catalyzed [3+2] Coupling of Quinone Monoacetals or Quinone Imine Ketals with Vinylcarbamates. Synlett 2015, 26, 1720–1724. [Google Scholar] [CrossRef]
- Yan, Y.; Li, M.; Liu, M.; Huang, M.; Cao, L.; Li, W.; Zhang, X. Sc(OTf)3-Catalyzed Dearomative [3+2] Annulation of 5-Aminoisox-azoles with Quinone Imine Ketals or Quinone Monoacetals. Eur. J. Org. Chem. 2022, 2022, e202200067. [Google Scholar] [CrossRef]
- Hu, X.-M.; Zhou, B.; Yang, C.-L.; Lin, J.; Yan, S.-J. Site-Selective Reaction of Enaminones and Enamine Esters for the Synthesis of Novel Diverse Morphan Derivatives. ACS Omega 2018, 3, 5994–6005. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, S.; Deng, P.; Chang, X.; Zhao, Y.; Ma, Y.; Zhang, D.; Xia, F.; Yang, L.; Wang, J.; et al. Lewis Acid Regulated Divergent Catalytic Reaction between Quinone Imine Ketals (QIKs) and 1,3-Dicarbonyl Compounds: Switchable Access to Multiple Products Including 2-Aryl-1,3-Dicarbonyl Compounds, Indoles, and Benzofurans. Adv. Synth. Catal. 2022, 364, 94–102. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, B.-X.; Chen, Y.-R.; Du, W.; Chen, Y.-C. Asymmetric Diels–Alder and Cascade Reaction of Quinone Imine Ketals and 2,4-Dienals: Construction of Chiral Benzo[de]quinolone Derivatives. Adv. Synth. Catal. 2016, 358, 296–302. [Google Scholar] [CrossRef]

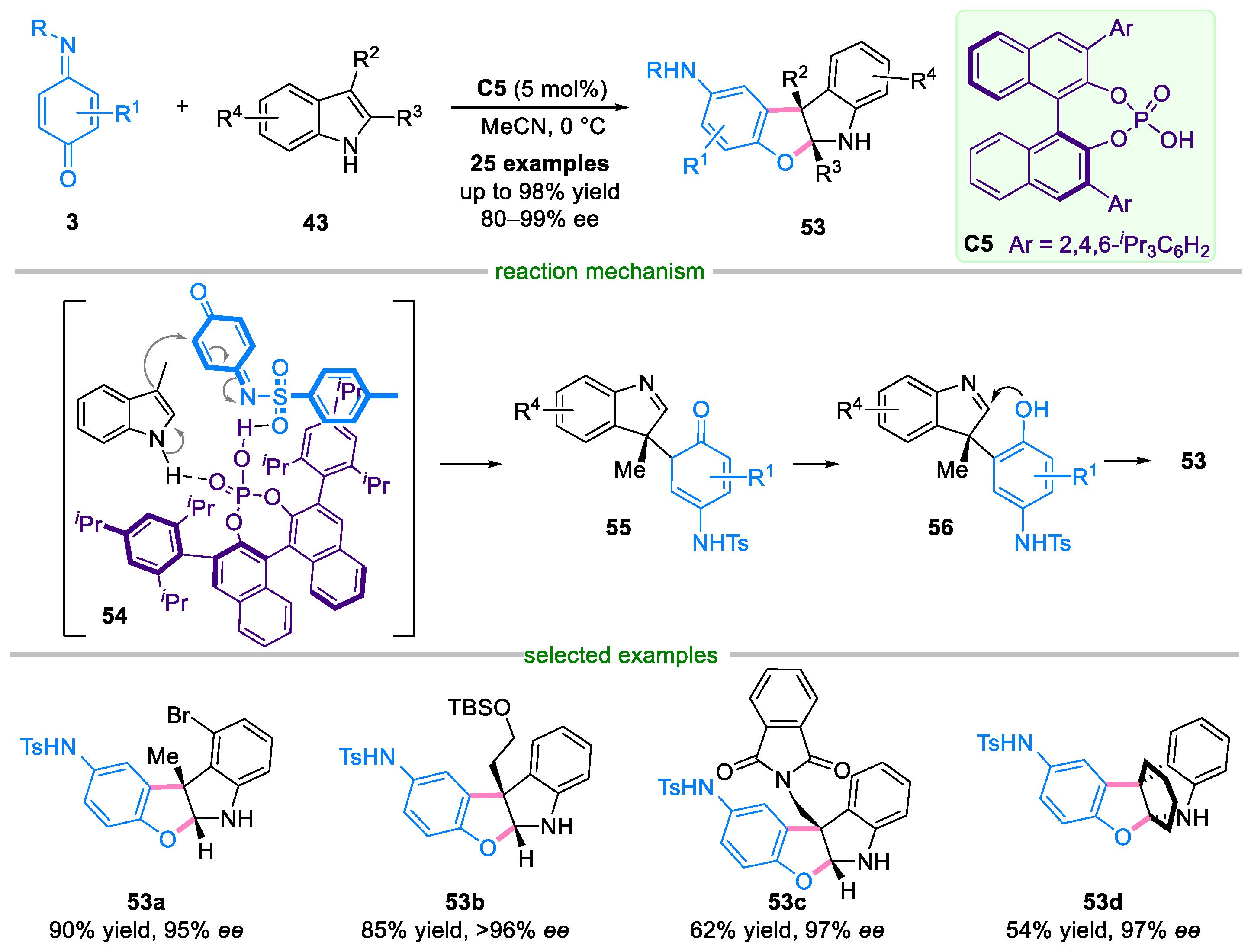
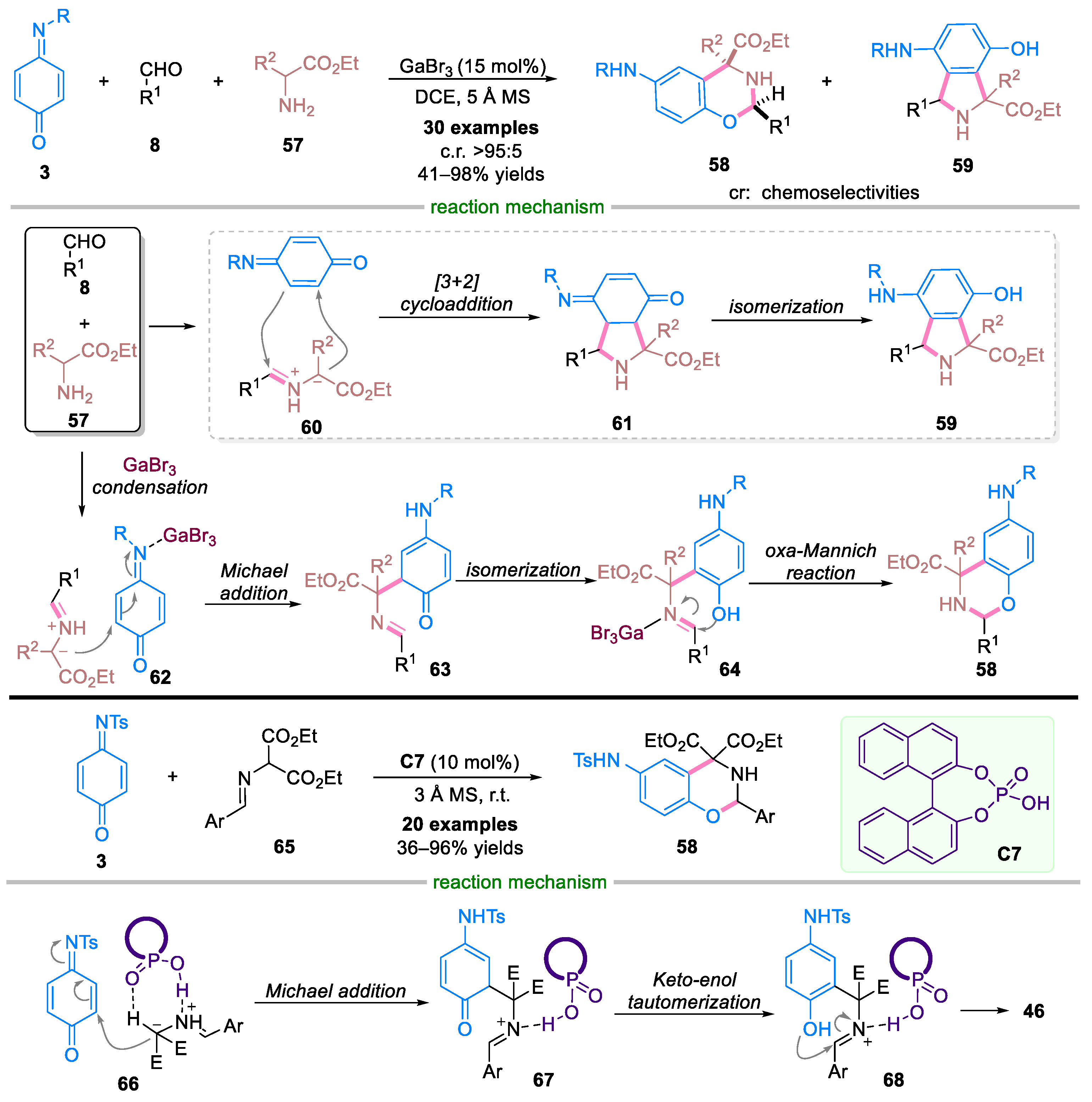
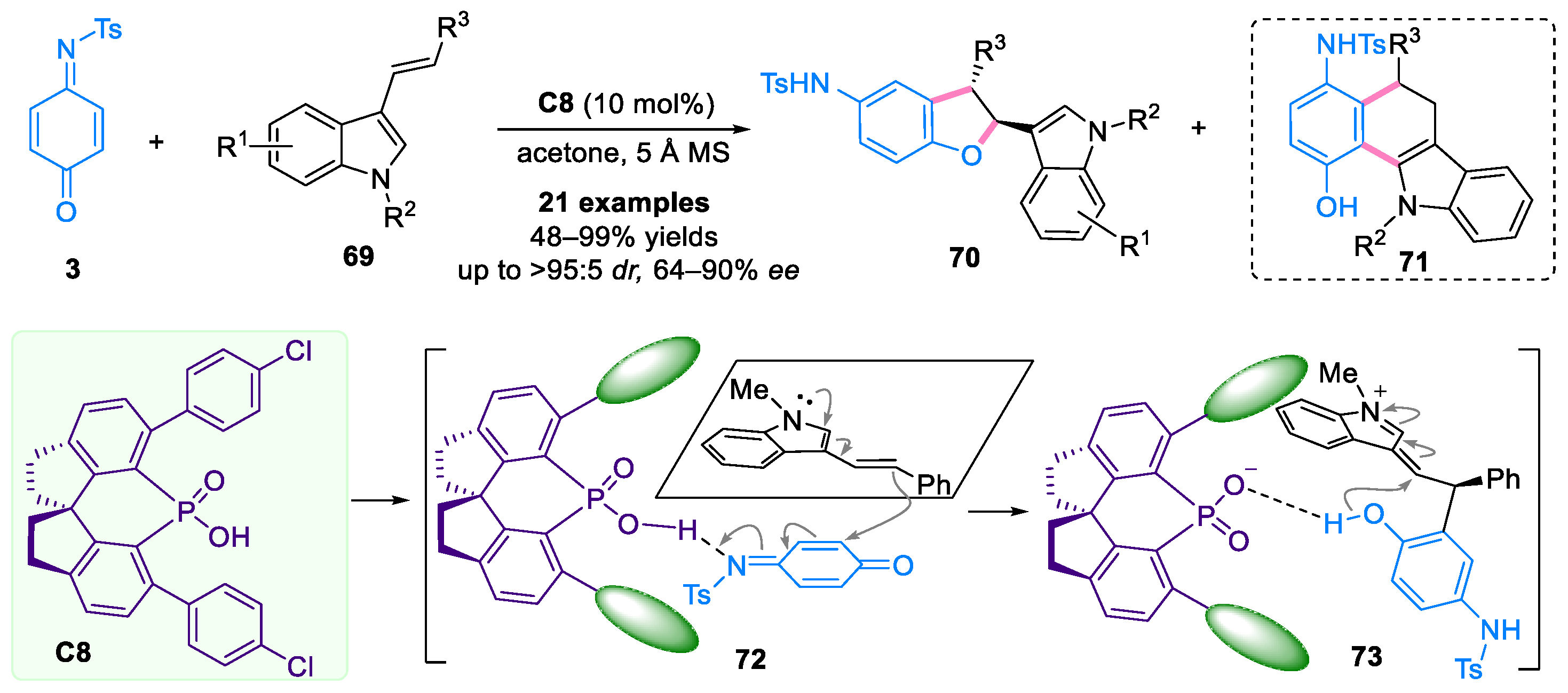
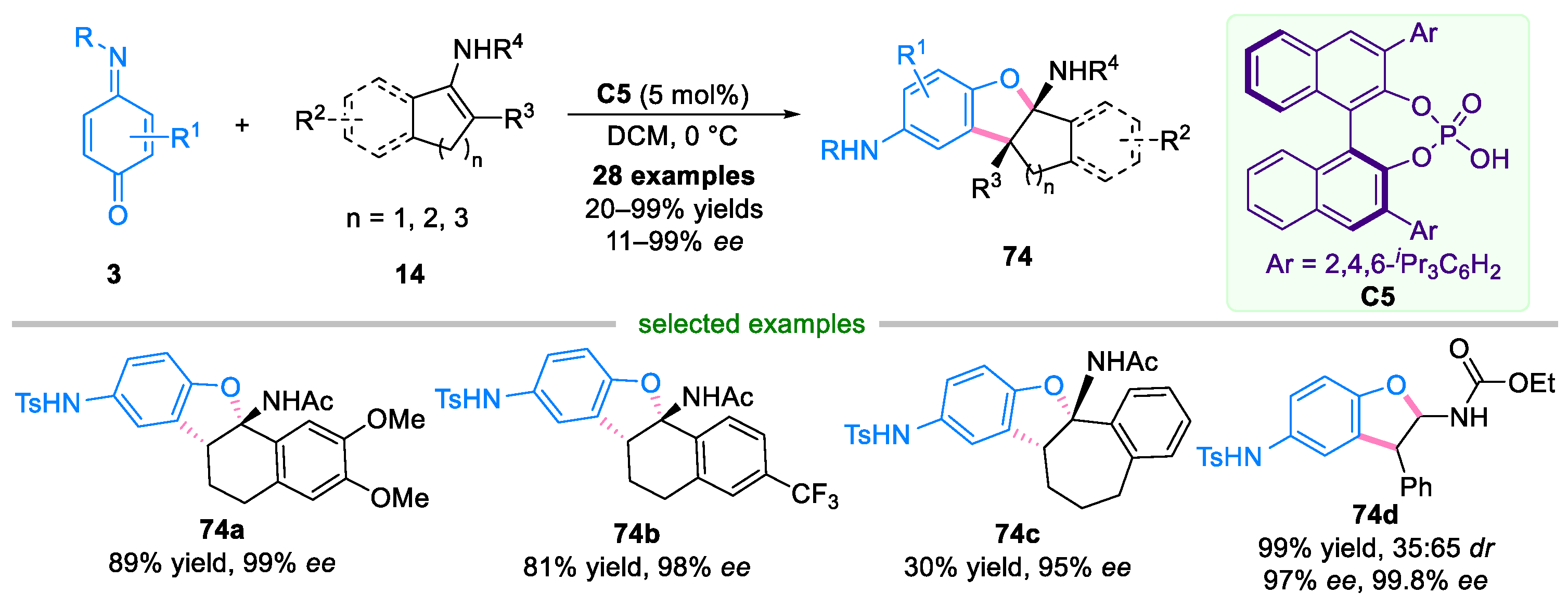


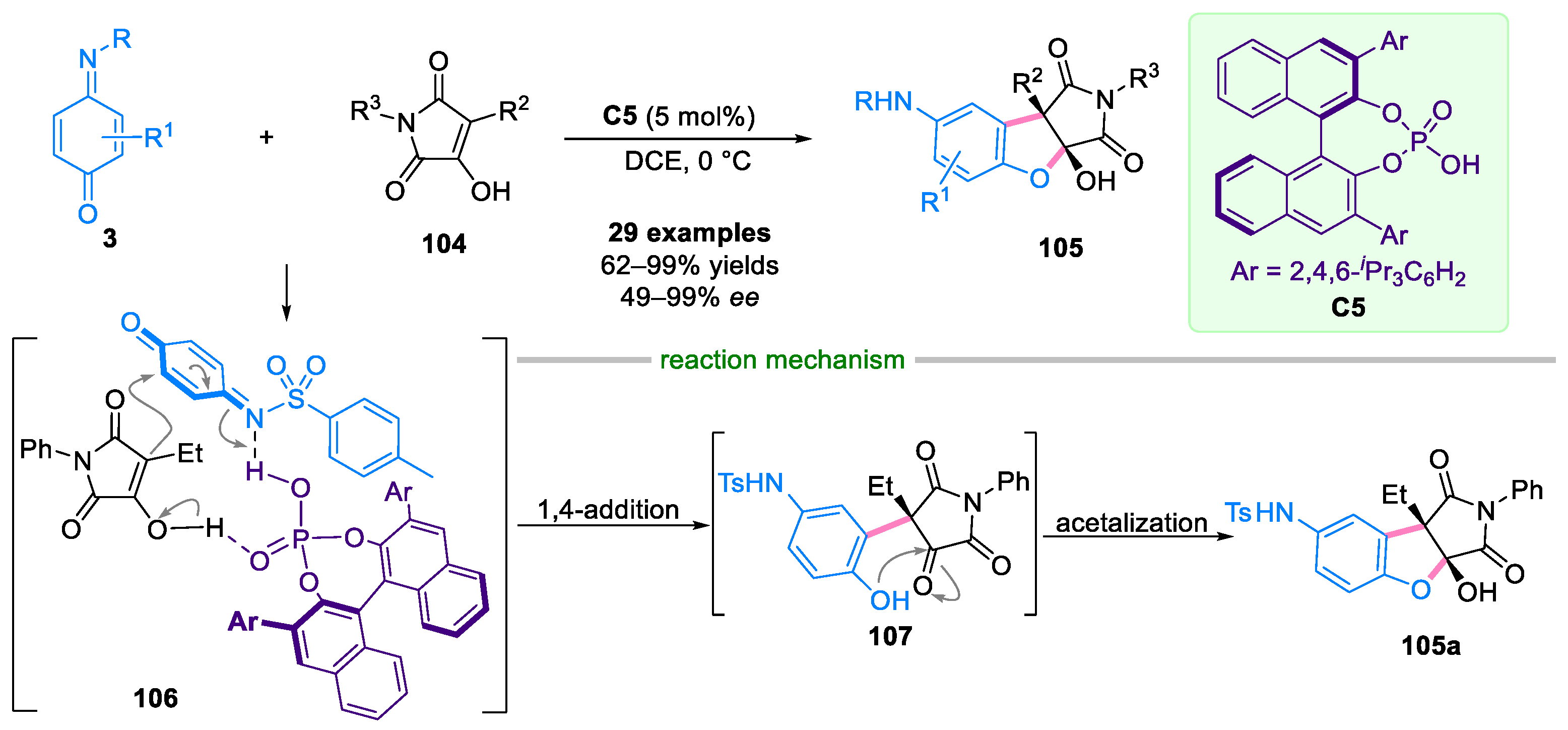
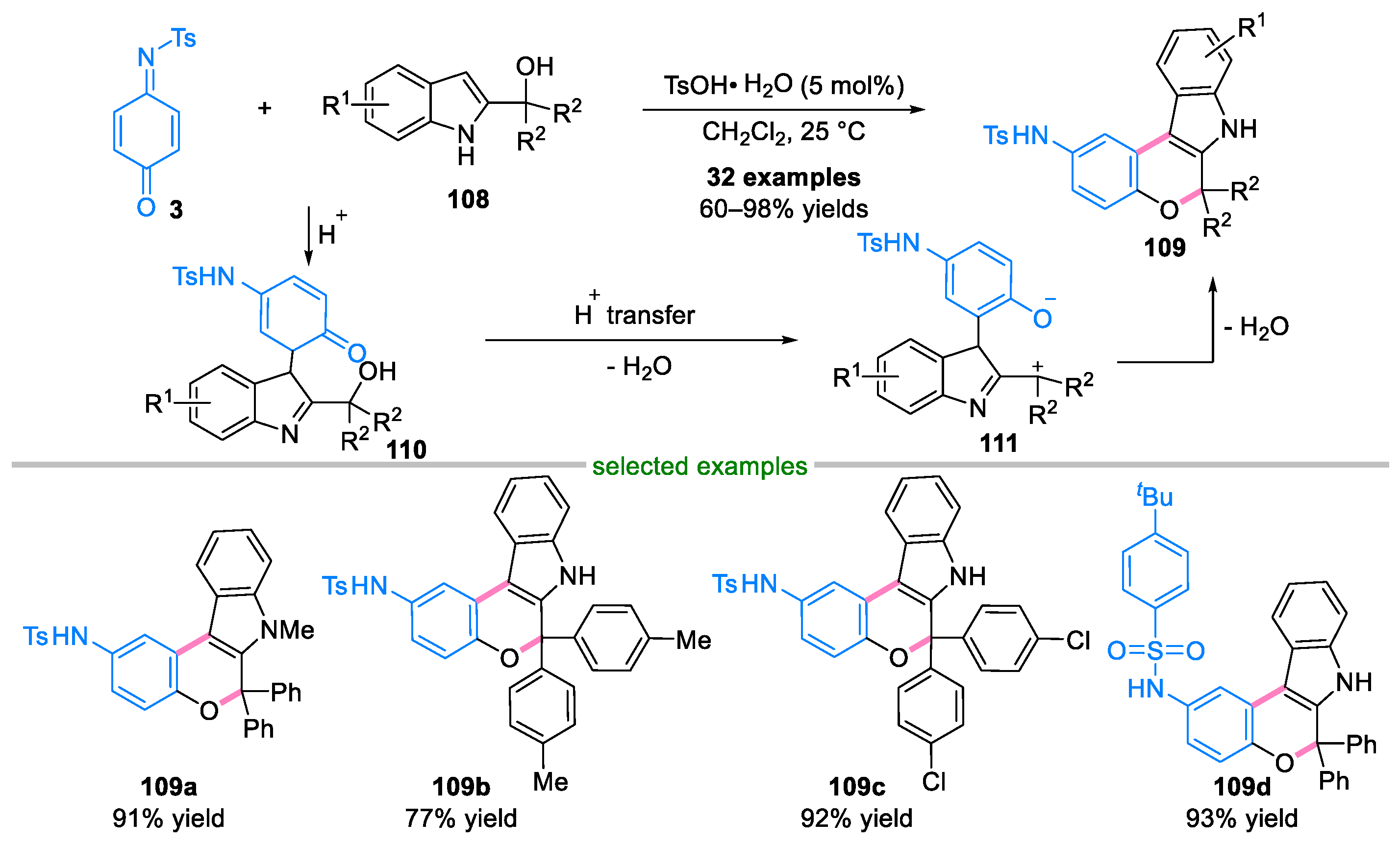
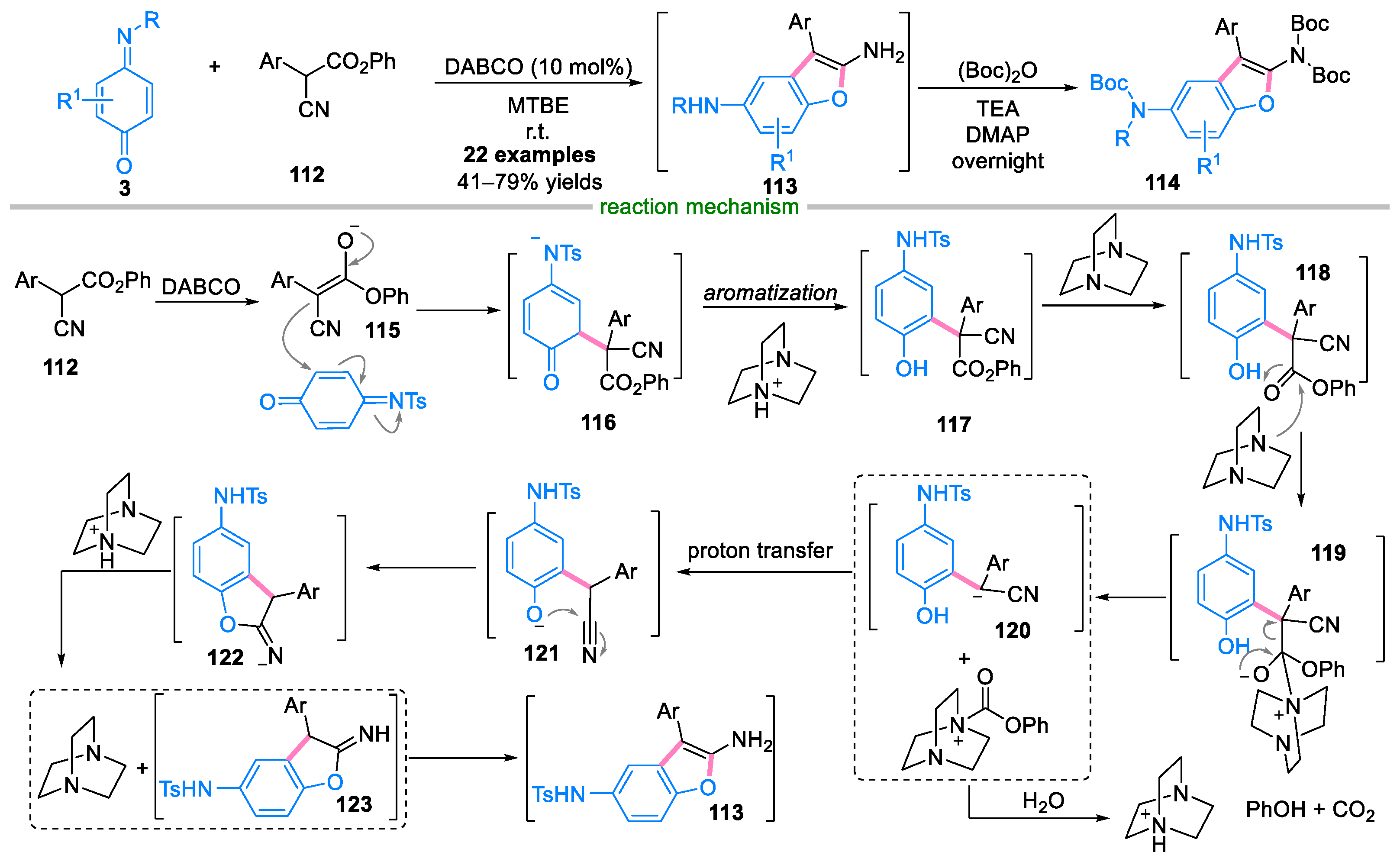


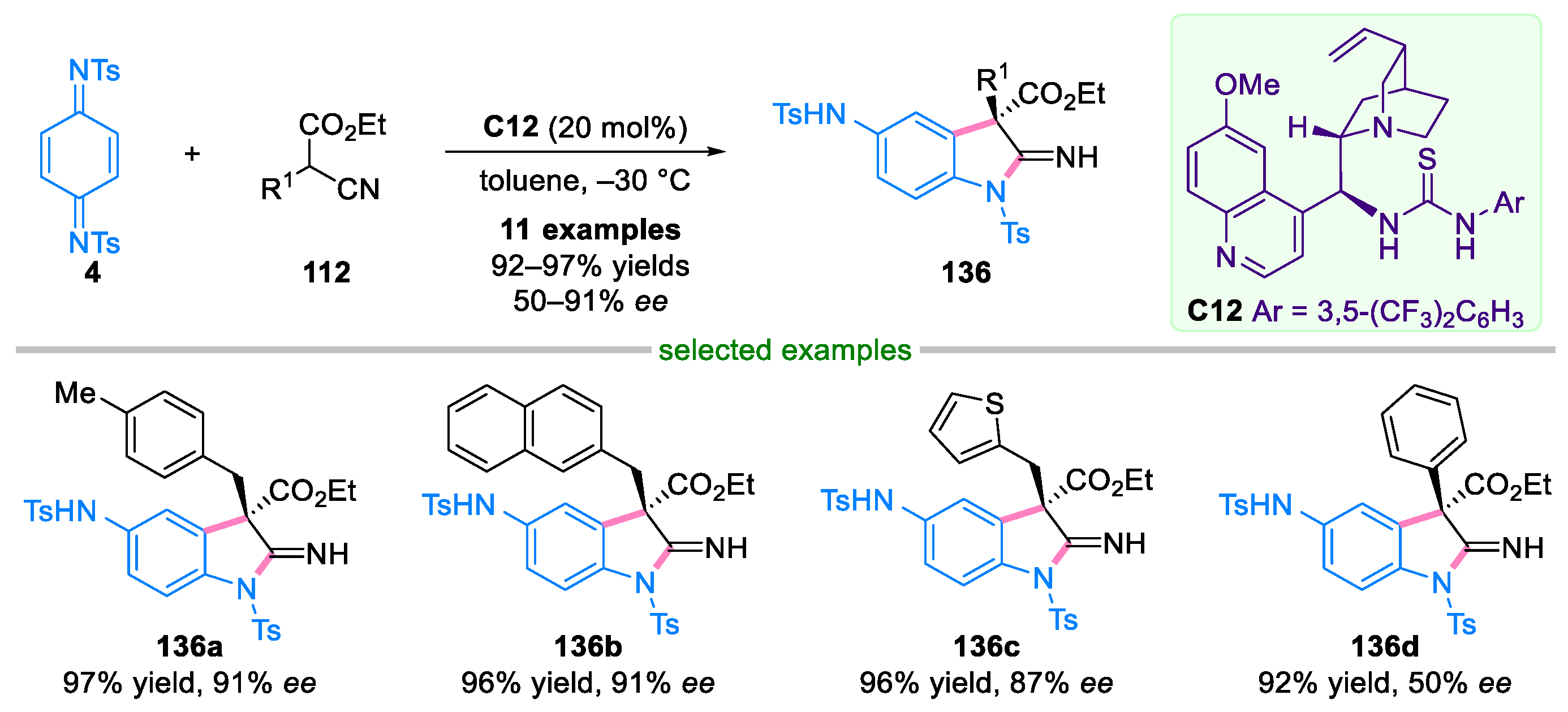



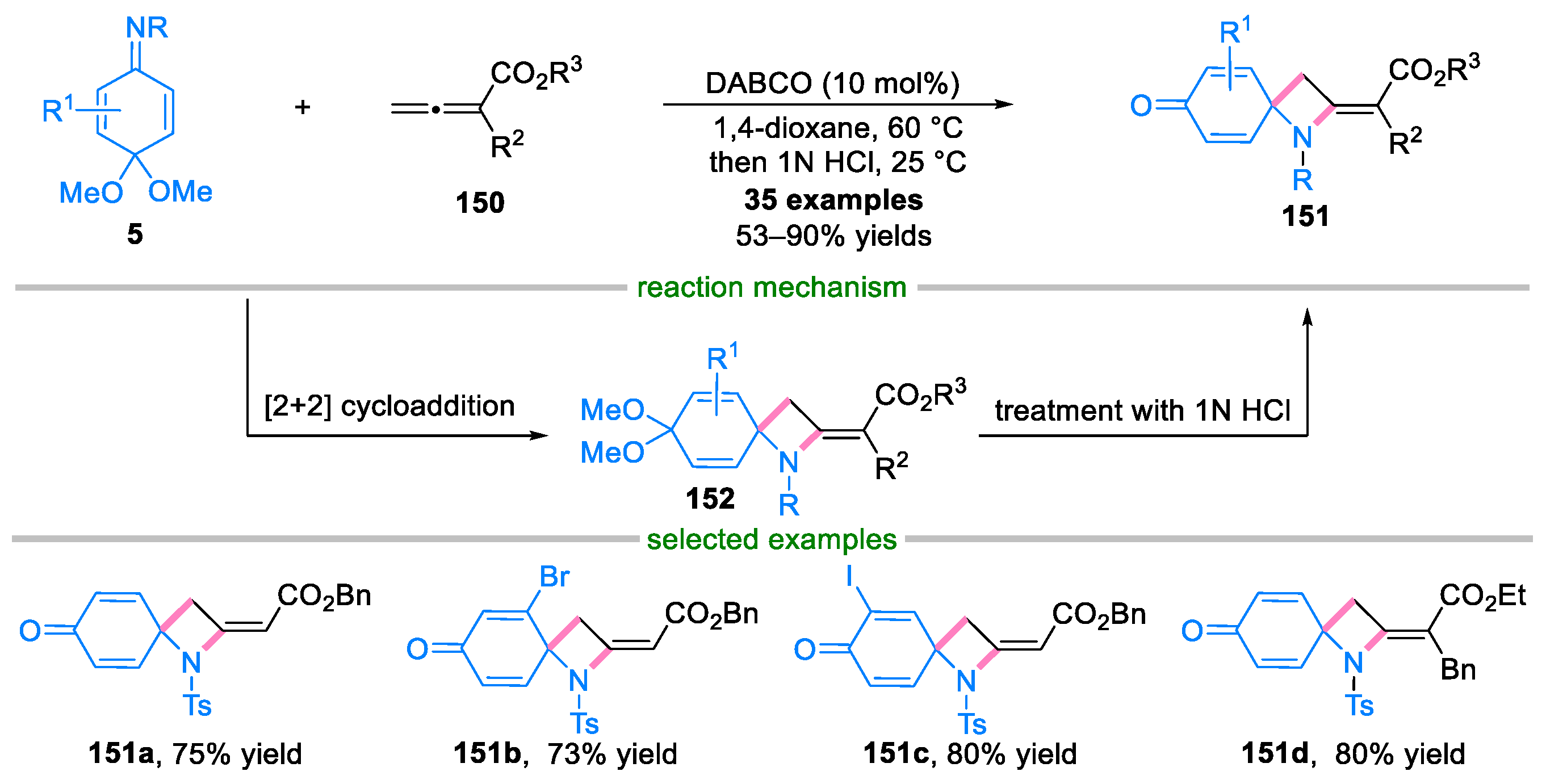
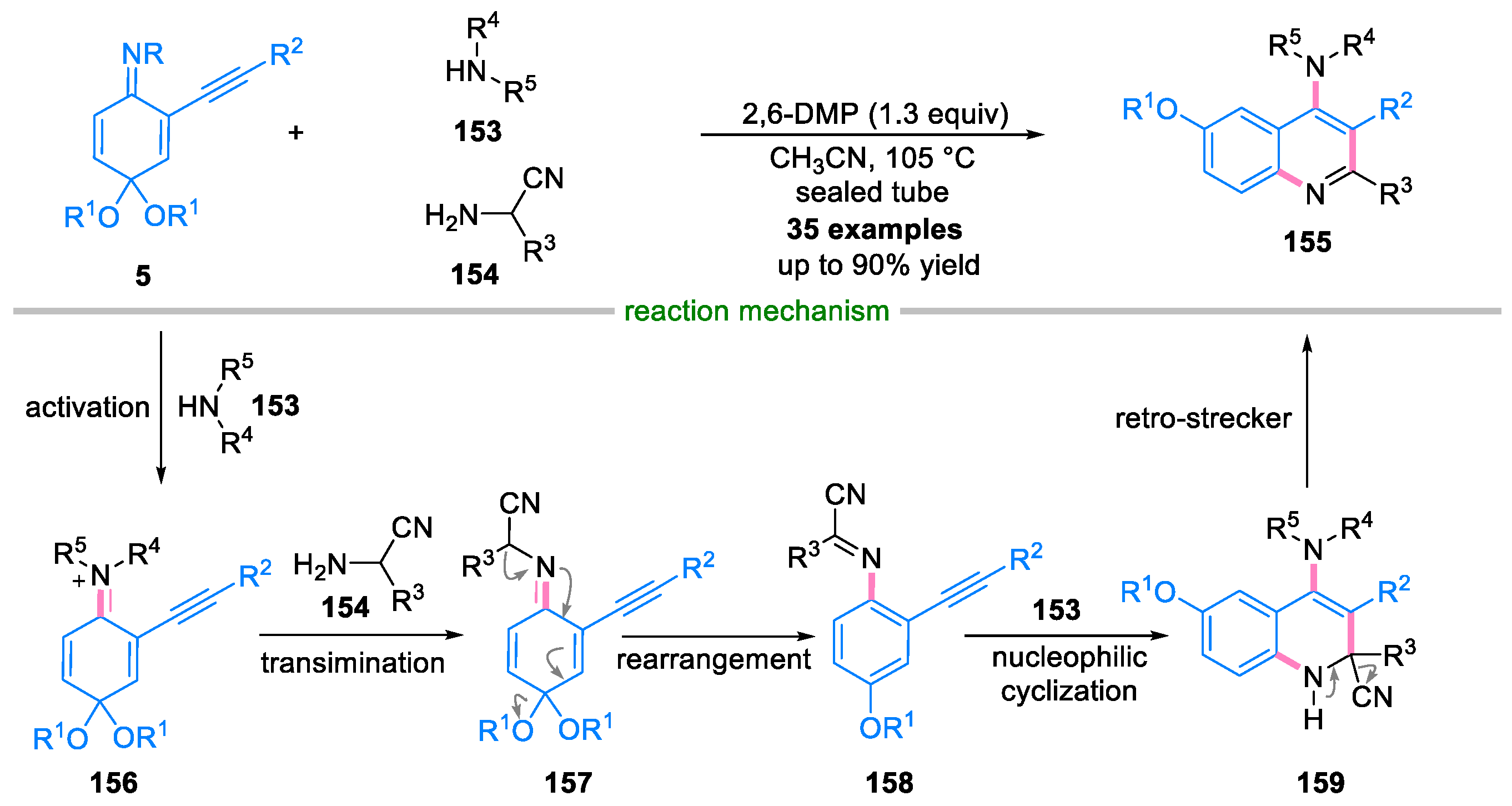

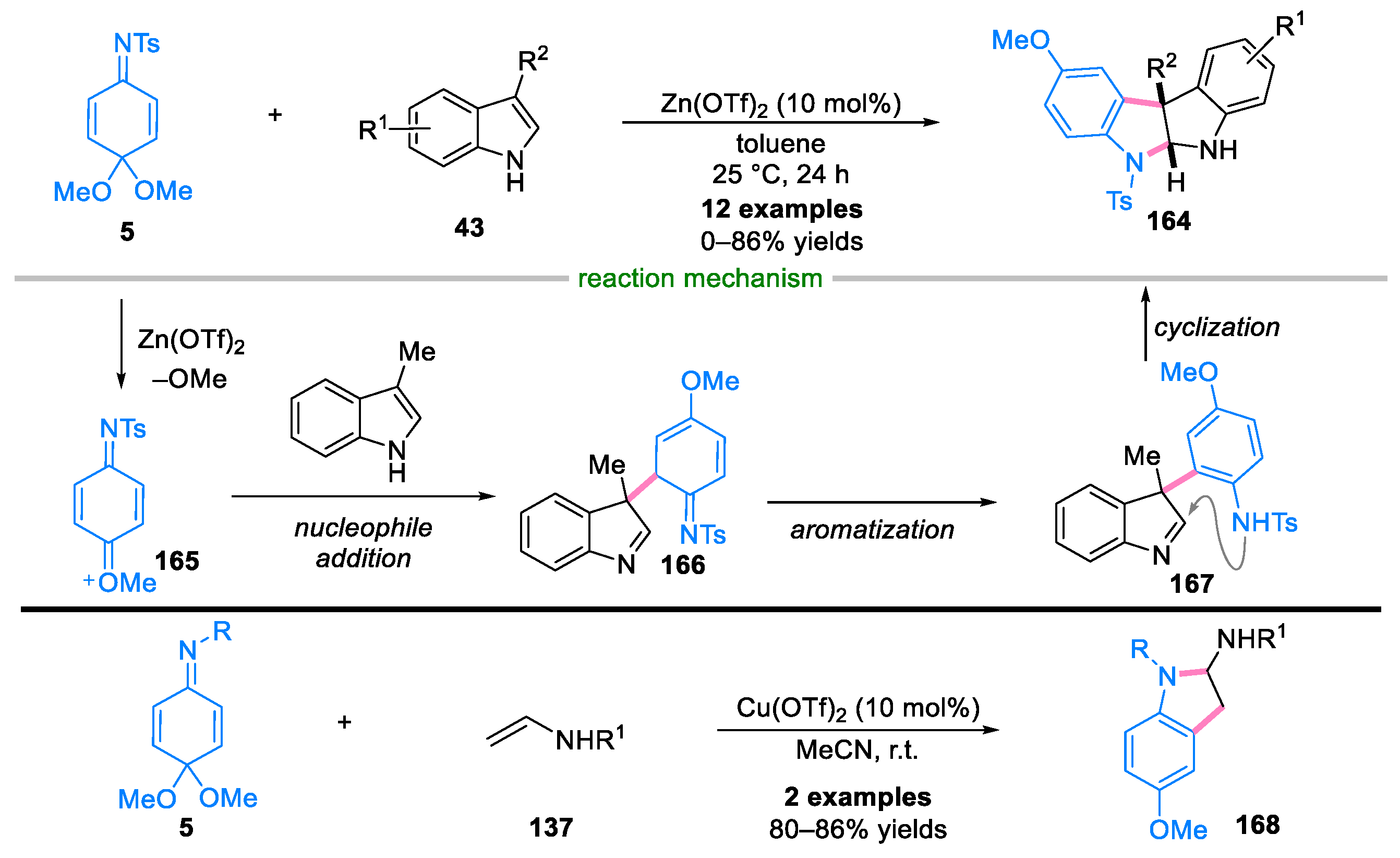
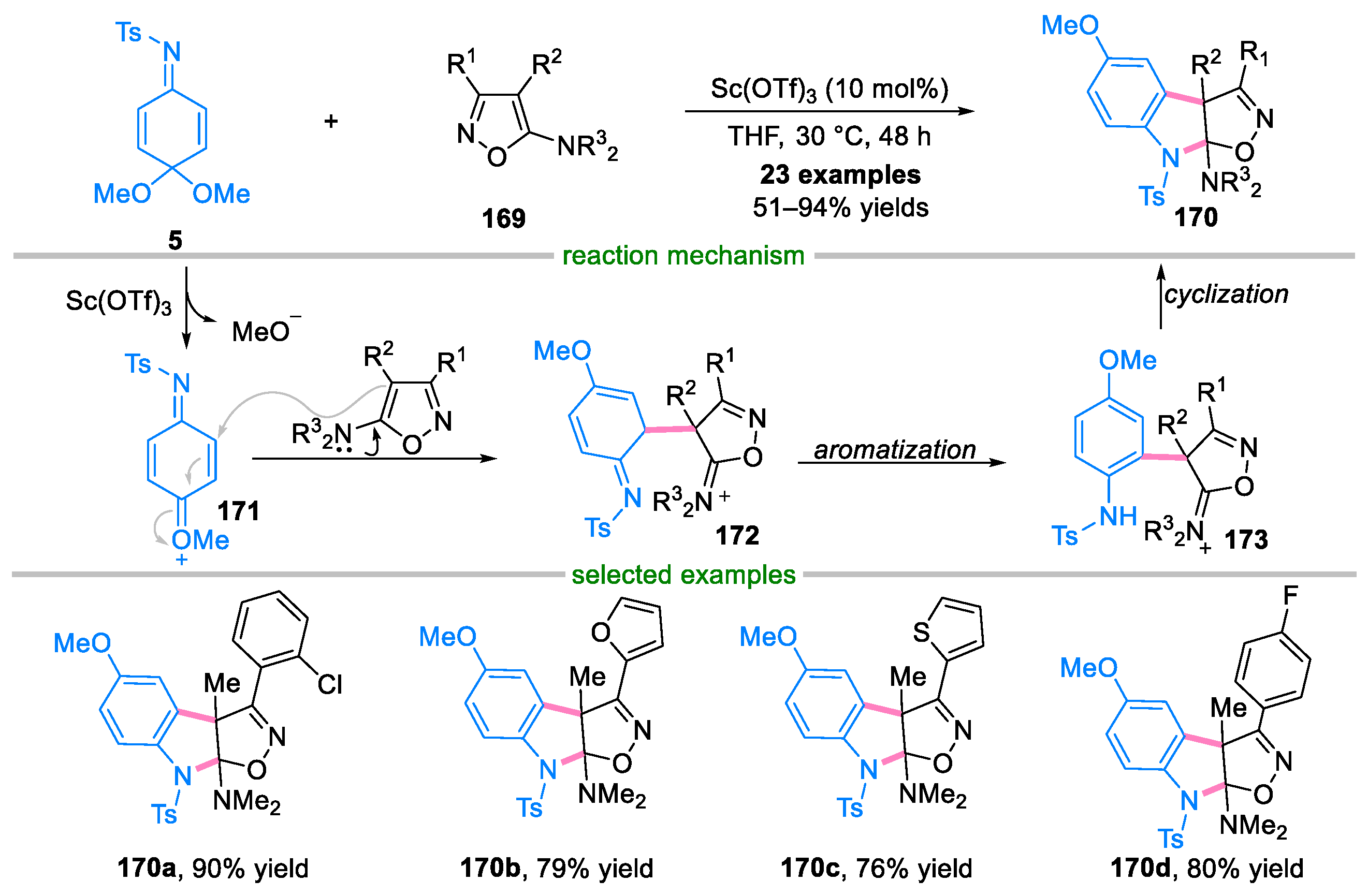
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-H.; Fu, X.-H.; Li, Q.; You, Y.; Yang, L.; Zhao, J.-Q.; Zhang, Y.-P.; Yuan, W.-C. Recent Advances in the Domino Annulation Reaction of Quinone Imines. Molecules 2024, 29, 2481. https://doi.org/10.3390/molecules29112481
Wang Z-H, Fu X-H, Li Q, You Y, Yang L, Zhao J-Q, Zhang Y-P, Yuan W-C. Recent Advances in the Domino Annulation Reaction of Quinone Imines. Molecules. 2024; 29(11):2481. https://doi.org/10.3390/molecules29112481
Chicago/Turabian StyleWang, Zhen-Hua, Xiao-Hui Fu, Qun Li, Yong You, Lei Yang, Jian-Qiang Zhao, Yan-Ping Zhang, and Wei-Cheng Yuan. 2024. "Recent Advances in the Domino Annulation Reaction of Quinone Imines" Molecules 29, no. 11: 2481. https://doi.org/10.3390/molecules29112481
APA StyleWang, Z.-H., Fu, X.-H., Li, Q., You, Y., Yang, L., Zhao, J.-Q., Zhang, Y.-P., & Yuan, W.-C. (2024). Recent Advances in the Domino Annulation Reaction of Quinone Imines. Molecules, 29(11), 2481. https://doi.org/10.3390/molecules29112481








