Detailed Studies on the Methoxylation and Subsequent Dealkylation of N,N-Diethylbenzenesulfonamide Using a Tailor-Made Electrosynthetic Reactor
Abstract
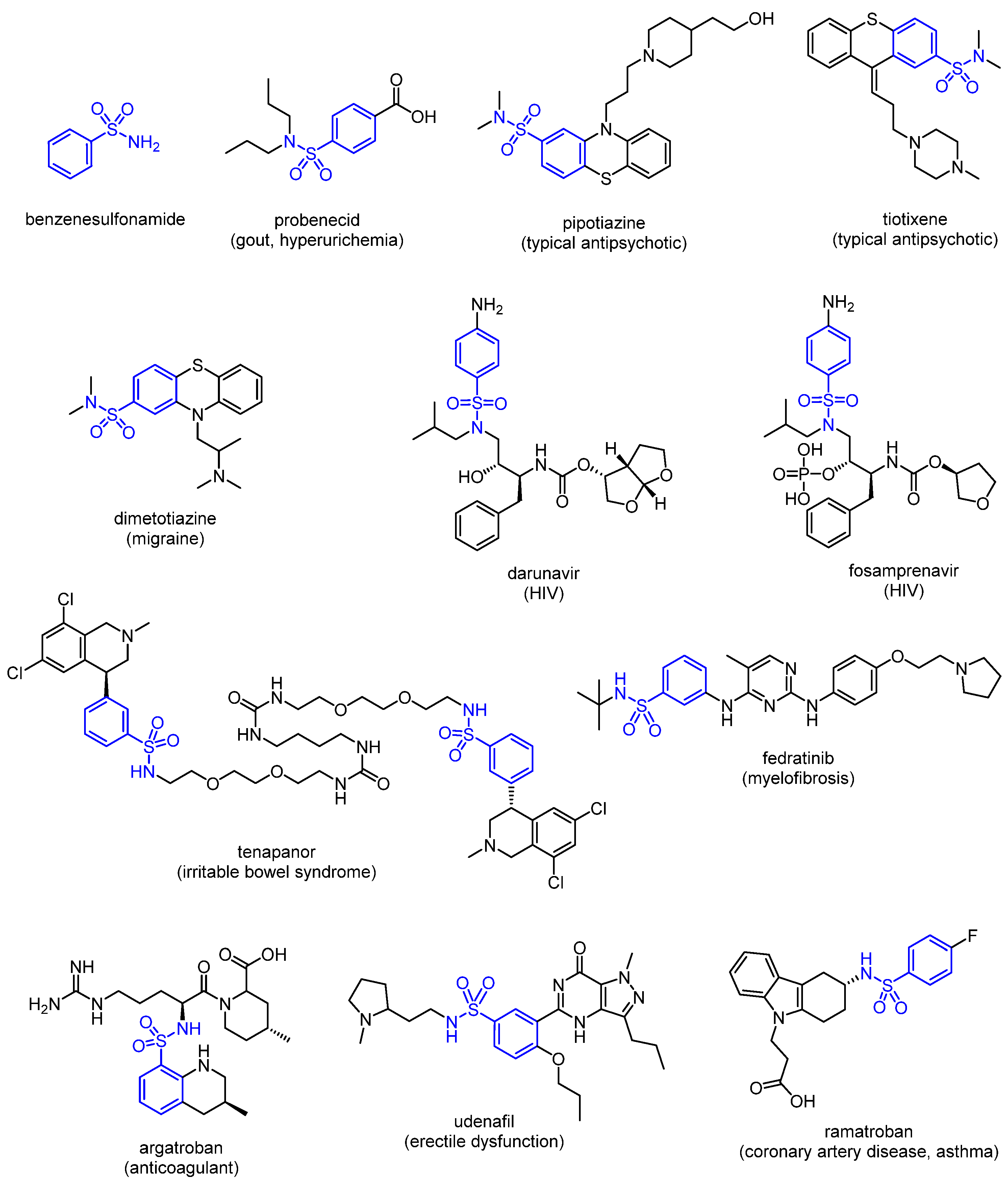
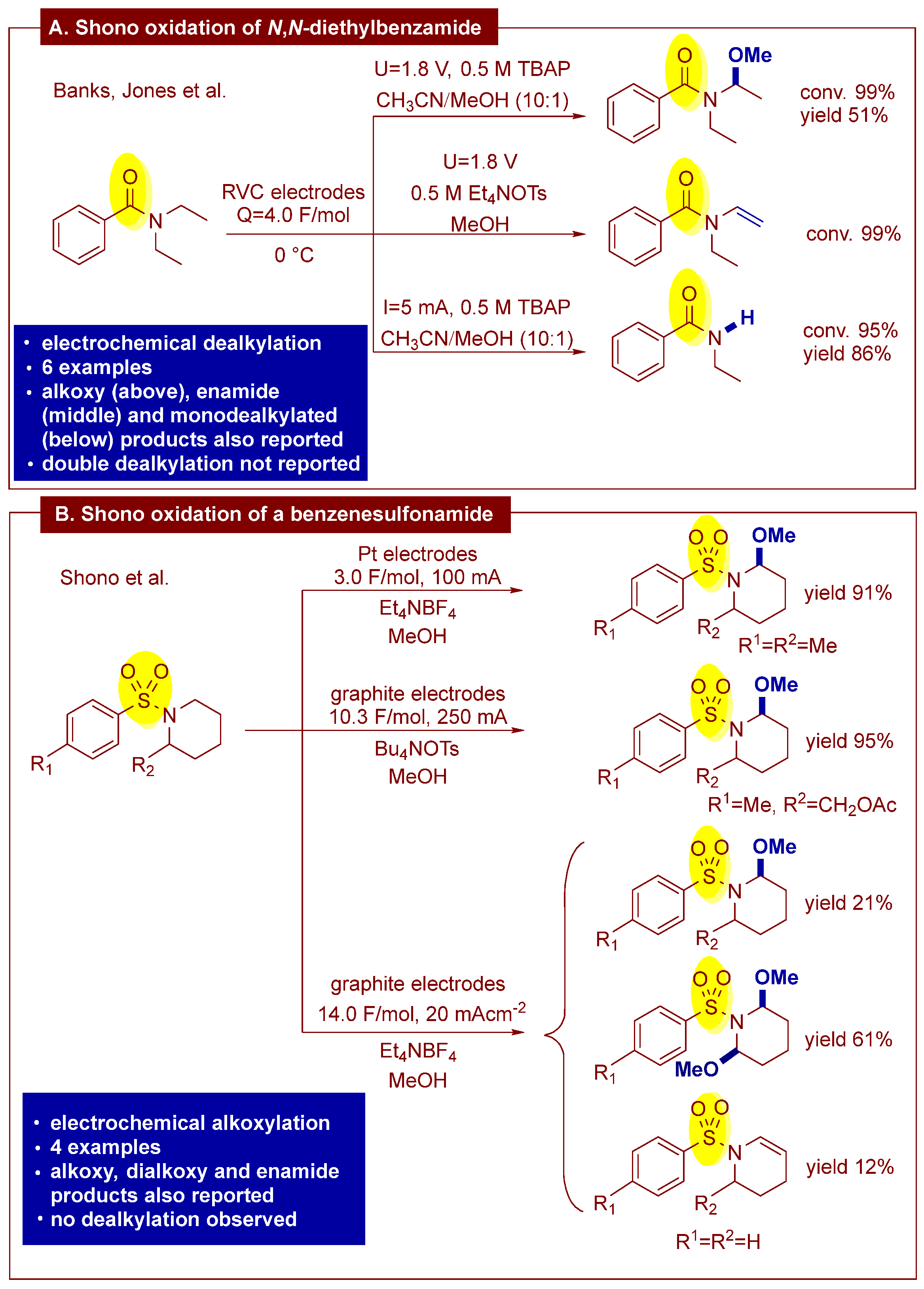
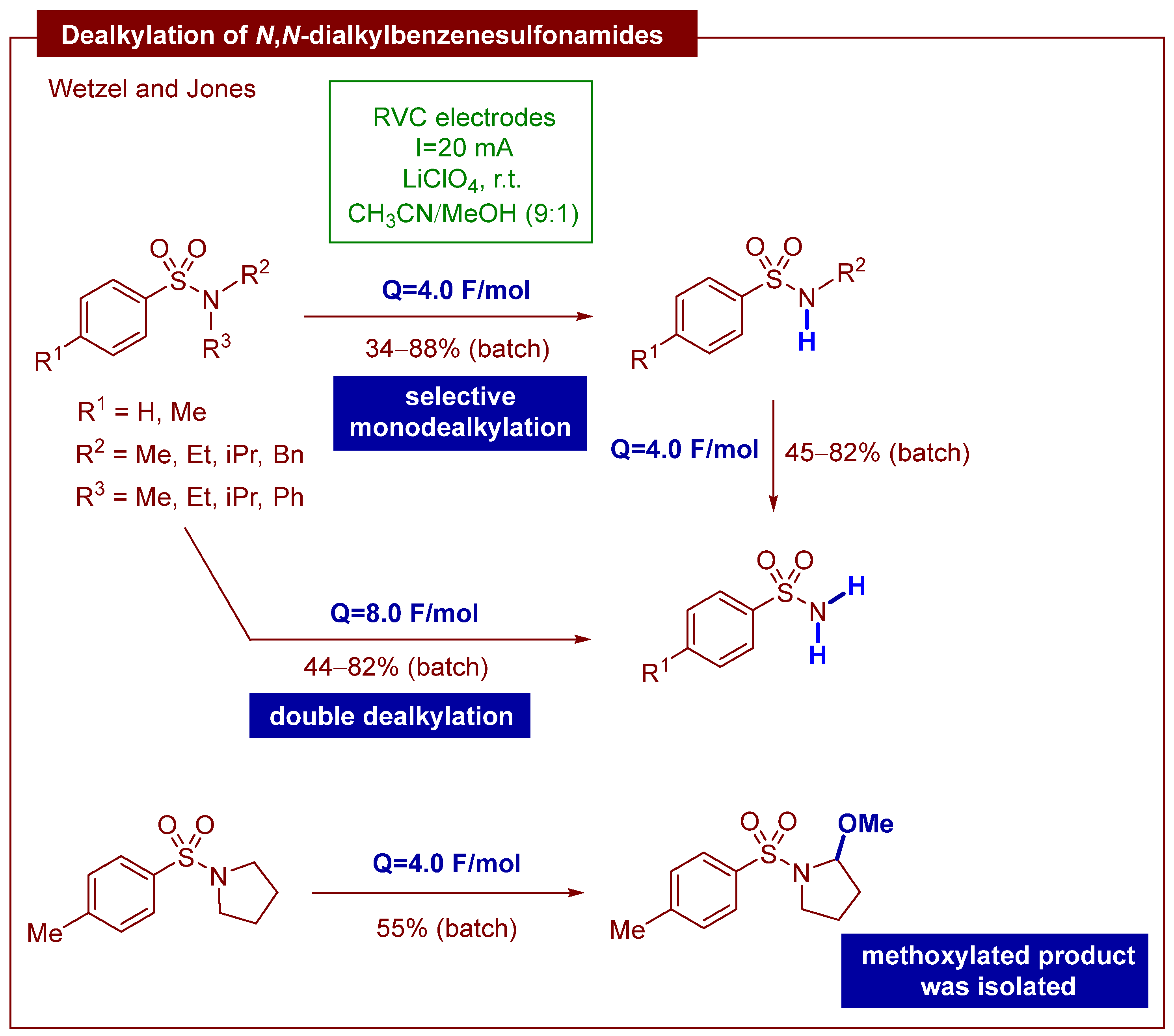
1. Introduction
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, K.A.; Njadarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.M. Ann. Studies on the Effect of Probenecid (‘Benemid’) in Gout. Rheum. Dis. 1954, 13, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.; Locovei, S.; Dahl, G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am. J. Physiol. Cell Physiol. 2008, 295, C761–C777. [Google Scholar] [CrossRef] [PubMed]
- Bechelli, L.P.; Ruffino-Netto, A.; Hetem, G. A double-blind controlled trial of pipotiazine, haloperidol and placebo in recently-hospitalized acute schizophrenic patients. Brazil. J. Med. Biol. Res. 1983, 16, 305–311. [Google Scholar]
- Reynolds, J.E. Anxiolytic sedatives, hypnotics and neuroleptics. In Martindale: The Extra Pharmacopoeia, 30th ed.; Pharmaceutical Press: London, UK, 1993; pp. 364–623. [Google Scholar]
- Vela, J.M.; Buschmann, H.; Holenz, J.; Párraga, A.; Torrens, A. Antidepressants, Antipsychotics, Anxiolytics: From Chemistry and Pharmacology to Clinical Application; Wiley-VCH: Weinheim, Germany, 2007; p. 520. [Google Scholar]
- Shimazawa, M.; Hara, H.; Watano, T.; Sukamoto, T. Effects of Ca2+ channel blockers on cortical hypoperfusion and expression of c-Fos-like immunoreactivity after cortical spreading depression in rats. Br. J. Pharmacol. 1995, 115, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, R. Darunavir: Promising initial results. Lancet 2007, 369, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Perry, C.M.; Keam, S.J. Darunavir: A review of its use in the management of HIV infection in adults. Drugs 2009, 69, 477–503. [Google Scholar] [CrossRef]
- Eron, J.; Yeni, P.; Gathe, J.; Estrada, V.; DeJesus, E.; Staszewski, S.; Lackey, P.; Katlama, C.; Young, B.; Yau, L.; et al. The KLEAN study of fosamprenavir-ritonavir versus lopinavir-ritonavir, each in combination with abacavir-lamivudine, for initial treatment of HIV infection over 48 weeks: A randomised non-inferiority trial. Lancet 2006, 368, 476–482. [Google Scholar] [CrossRef]
- Spencer, A.G.; Labonte, E.D.; Rosenbaum, D.P.; Plato, C.F.; Carreras, C.W.; Leadbetter, M.R.; Kozuka, K.; Kohler, J.; Koo-McCoy, S.; He, L.; et al. Gastrointestinal Inhibition of Sodium-Hydrogen Exchanger 3 Reduces Phosphorus Absorption and Protects against Vascular Calcification in CKD. Sci. Transl. Med. 2014, 6, 227ra36. [Google Scholar]
- Pardanani, A.; Gotlib, J.R.; Jamieson, C.; Cortes, J.E.; Talpaz, M.; Stone, R.M.; Silverman, M.H.; Gilliland, D.G.; Shorr, J.; Tefferi, A.J. Safety and Efficacy of TG101348, a Selective JAK2 Inhibitor, in Myelofibrosis. Clin. Oncol. 2011, 29, 789–796. [Google Scholar] [CrossRef]
- Pardanani, A.; Hood, J.; Lasho, T.; Levine, R.L.; Martin, M.B.; Noronha, G.; Finke, C.; Mak, C.C.; Mesa, R.; Zhu, H.; et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia 2007, 21, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Argatroban: A review of its use in the management of heparin-induced thrombocytopenia. Am. J. Cardiovasc. Drugs 2009, 9, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Kim, J.J. Udenafil: Efficacy and tolerability in the management of erectile dysfunction. Therap. Adv. Urology 2013, 5, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Shichijo, M.; Iino, T.; Manabe, Y.; Watanabe, A.; Shimazaki, M.; Gantner, F.; Bacon, K.B. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J. Pharmacol. Exp. Ther. 2003, 305, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Royer, J.F.; Schratl, P.; Carrillo, J.J.; Jupp, R.; Barker, J.; Weyman-Jones, C.; Beri, R.; Sargent, C.; Schmidt, J.A.; Lang-Loidolt, D.; et al. A novel antagonist of prostaglandin D2 blocks the locomotion of eosinophils and basophils. Eur. J. Clin. Invest. 2008, 38, 663–671. [Google Scholar] [CrossRef]
- Smith, D.A.; Beaumont, K.; Maurer, T.S.; Di, L. Clearance in drug design: Miniperspective. J. Med. Chem. 2019, 62, 2245–2255. [Google Scholar] [CrossRef]
- Testa, B.; Pedretti, A.; Vistoli, G. Reactions and enzymes in the metabolism of drugs and other xenobiotics. J. Drug Discov. Today 2012, 17, 549–560. [Google Scholar] [CrossRef]
- Rose, J.; Castagnoli, N., Jr. The metabolism of tertiary amines. Med. Res. Rev. 1983, 3, 73–88. [Google Scholar] [CrossRef]
- Al-Gailany, K.A.S.; Houston, J.B.; Bridges, J.W. The role of substrate lipophilicity in determining type 1 microsomal P450 binding characteristics. Biochem. Pharmacol. 1978, 27, 783–788. [Google Scholar] [CrossRef]
- Lewis, D.F.V.; Dickins, M. Baseline lipophilicity relationships in human cytochromes P450 associated with drug metabolism. Drug Metab. Rev. 2003, 35, 1–18. [Google Scholar] [CrossRef]
- Cunningham, R.F.; Perel, J.M.; Israili, Z.H.; Dayton, P.G. Probenecid metabolism in vitro with rat, mouse, and human liver preparations. Studies of factors affecting the site of oxidation. Drug Metab. Dispos. 1977, 5, 205–210. [Google Scholar] [PubMed]
- Ku, H.Y.; Ahn, H.J.; Seo, K.A.; Kim, H.; Oh, M.; Bae, S.K.; Shin, J.G.; Shon, J.H.; Liu, K.H. The contributions of cytochromes P450 3A4 and 3A5 to the metabolism of the phosphodiesterase type 5 inhibitors sildenafil, udenafil, and vardenafil. Drug Metab. Dispos. 2008, 36, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, F.; Pei, W.; Yang, M.; Wu, Y.; Ma, D.; Thang, F.; Wang, J. Selective cleavage of the N-propargyl group from sulfonamides and amides under ruthenium catalysis. Tetrahedron Lett. 2018, 59, 1902–1905. [Google Scholar] [CrossRef]
- Inagaki, F.; Hira, S.; Mukai, C. Silver (I)-Catalyzed Deprenylation of Allylsulfonamide Derivatives. Synlett 2017, 28, 2143–2146. [Google Scholar] [CrossRef]
- Moriyama, K.; Nakamure, Y.; Togo, H. Oxidative Debenzylation of N-Benzyl Amides and O-Benzyl Ethers Using Alkali Metal Bromide. Org. Lett. 2014, 16, 3812–3815. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.; Trudell, M.L. Novel N-dealkylation of N-alkyl sulfonamides and N,N-dialkyl sulfonamides with periodic acid catalyzed by chromium (III) acetate hydroxide. Synlett 2004, 11, 1901–1904. [Google Scholar] [CrossRef]
- Vidales, M.J.M.; Robles-Molina, J.; Dominíquez-Romero, J.C.; Canizares, P.; Sáez, C.; Molina-Díaz, A.; Rodrigo, M.A. Removal of sulfamethoxazole from waters and wastewaters by conductive-diamond electrochemical oxidation. J. Chem. Technol. Biotechnol. 2012, 87, 1441–1449. [Google Scholar] [CrossRef]
- Lahcen, A.A.; Amine, A. Mini-review: Recent advances in electrochemical determination of sulfonamides. Anal. Lett. 2018, 51, 424–441. [Google Scholar] [CrossRef]
- Viaud, P.; Coeffard, V.; Thobie-Gautier, C.; Beaudet, I.; Galland, N.; Quintard, J.-P.; Grognec, E.L. Electrochemical cleavage of sulfonamides: An efficient and tunable strategy to prevent β-fragmentation and epimerization. Org. Lett. 2012, 14, 942–945. [Google Scholar] [CrossRef]
- Iwasaki, T.; Matsumoto, K.; Matsuoka, M.; Takahashi, T.; Okumura, K. Detosylation of N-Tosyl Amino Acids and Peptides by Electrolytic Reduction. Bull. Chem. Soc. Jpn. 1973, 46, 852–855. [Google Scholar] [CrossRef]
- Oda, K.; Ohnuma, T.; Ban, Y. A facile removal of the arenesulfonyl group by electrochemical reduction of sulfonamides in a new cooperative system of anthracene and ascorbic acid: The control of crisscross annulation. J. Org. Chem. 1984, 49, 953–959. [Google Scholar] [CrossRef]
- Mairanowsky, V.G. Electro-Deprotection—Electrochemical Removal of Protecting Groups. Angew. Chem. Int. Ed. 1976, 15, 281–292. [Google Scholar] [CrossRef]
- Shono, T.; Matsumura, Y.; Tsubata, K. Electroorganic chemistry. 46. A new carbon-carbon bond forming reaction at the α-position of amines utilizing anodic oxidation as a key step. J. Am. Chem. Soc. 1981, 103, 1172–1176. [Google Scholar] [CrossRef]
- Banks, C.E.; Jones, A.M. The Shono-type electroorganic oxidation of unfunctionalised amides. Carbon–carbon bond formation via electrogenerated N-acyliminium ions. Beilstein J. Org. Chem. 2014, 10, 3056–3072. [Google Scholar]
- Rahman, M.H.; Bal, M.K.; Jones, A.M. Metabolism-inspired electrosynthesis. ChemElectroChem 2019, 6, 4093–4104. [Google Scholar] [CrossRef]
- Alfonso–Súarez, P.; Kolliopoulos, A.V.; Smith, J.P.; Banks, C.E.; Jones, A.M. An experimentalist’s guide to electrosynthesis: The Shono oxidation. Tetrahedron Lett. 2015, 56, 6863–6867. [Google Scholar] [CrossRef]
- Bal, M.K.; Banks, C.E.; Jones, A.M. Metabolism mimicry: An electrosynthetic method for the selective deethylation of tertiary benzamides. ChemElectroChem 2019, 6, 4284–4291. [Google Scholar] [CrossRef]
- Libendi, S.S.; Demizu, Y.; Matsumura, Y.; Onomura, O. High regioselectivity in electrochemical α-methoxylation of N-protected cyclic amines. Tetrahedron 2008, 64, 3935–3942. [Google Scholar] [CrossRef]
- Bodmann, K.; Bug, T.; Steinbeisser, S.; Kreuder, R.; Reiser, O. Electrochemical oxidation of 2-substituted piperidines as a key step towards the synthesis of hydroxylated γ-amino acids. Tetrahedron Lett. 2006, 47, 2061–2064. [Google Scholar] [CrossRef]
- Shono, T.; Matsumura, Y.; Tsubata, K.; Uchida, K.; Kanazawa, T.; Tsuda, K. Electroorganic chemistry. 81. Anodic oxidation of sulfonamides and amidophosphates. J. Org. Chem. 1984, 49, 3711–3716. [Google Scholar] [CrossRef]
- Golub, T.; Becker, J.Y. The effect of N-acyl and N-sulfonyl groups on the anodic methoxylation of piperidine derivatives. Electrochim. Acta 2015, 173, 408–415. [Google Scholar] [CrossRef]
- Wetzel, A.; Jones, A.M. Electrically Driven N(sp2)–C(sp2/3) Bond Cleavage of Sulfonamides. ACS Sustain. Chem. Eng. 2020, 8, 3487–3493. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, D.; Yin, Y.; Wang, Q. Using small molecules to enhance P450 OleT enzyme activity in situ. Chem. Eur. J. 2021, 27, 8940–8945. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Broda, E.; Snieckus, V. Directed ortho-Metalation–Cross-Coupling Strategies. One-Pot Suzuki Reaction to Biaryl and Heterobiaryl Sulfonamides. Org. Lett. 2011, 13, 3588–3591. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A. Giles, M.; Ali., H.I.; Mohamady, S. Metal- and Catalyst-Free Synthesis of 2-Substituted-Phthalimides Using 2-(Arenesulfonyl)Phthalimide as Key Reagents. Eur. J. Org. Chem. 2023, 26, e202300207. [Google Scholar] [CrossRef]
- Zhiuang, Z.; Sun, Y.; Zhong, Y.; He, Q.; Zhang, X.; Yang, C. Visible-Light-Induced Decarboxylative Aminosulfonylation of (Hetero) aryl Carboxylic Oxime Esters. Org. Lett. 2024, 26, 713–718. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, L.; Chen, P.; Cao, W.; Liu, G. Enantioselective Intermolecular Aminoalkynylation of Styrenes via Copper-Catalyzed Radical Relay. Org. Lett. 2021, 23, 129–134. [Google Scholar] [CrossRef]
- Anderson, E.; Biediger, R.J.; Chen, J.; Dupre, B.; Lory, P.; Market, R.V.; Monk, K.A.; Savage, M.M.; Tennyson, R.; Young, B. Modulators of CCR9 receptor and methods of use thereof. US Patent US 8178699, 15 May 2012. [Google Scholar]
- Mizukami, M.; Saito, H.; Higuchi, T.; Imai, M.; Bando, H.; Kawahara, N.; Nagumo, S. Facile synthesis of medium-sized cyclic amines based on Friedel–Crafts reaction via iminium cation by use of acetylene dicobalt complex. Tetrahedron Lett. 2007, 48, 7228–7231. [Google Scholar] [CrossRef]
- Komeyama, K.; Igawa, R.; Morimoto, T.; Takaki, K. Catalytic Cyclization of Alkenyl N,O-Acetals by Fe(OTf)3. Chem. Lett. 2009, 38, 724–725. [Google Scholar] [CrossRef]
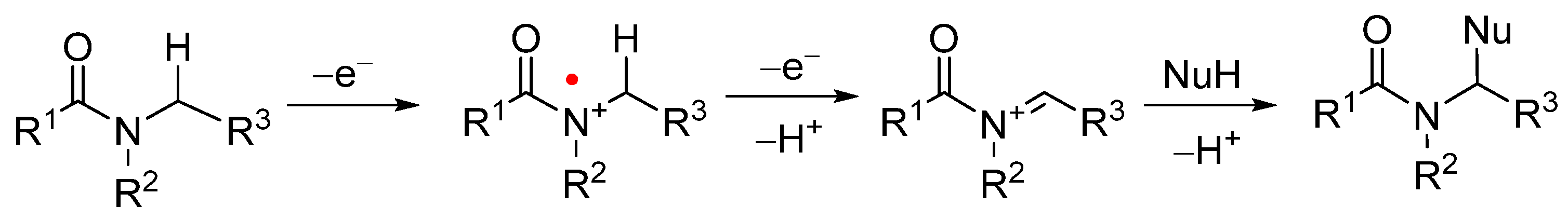
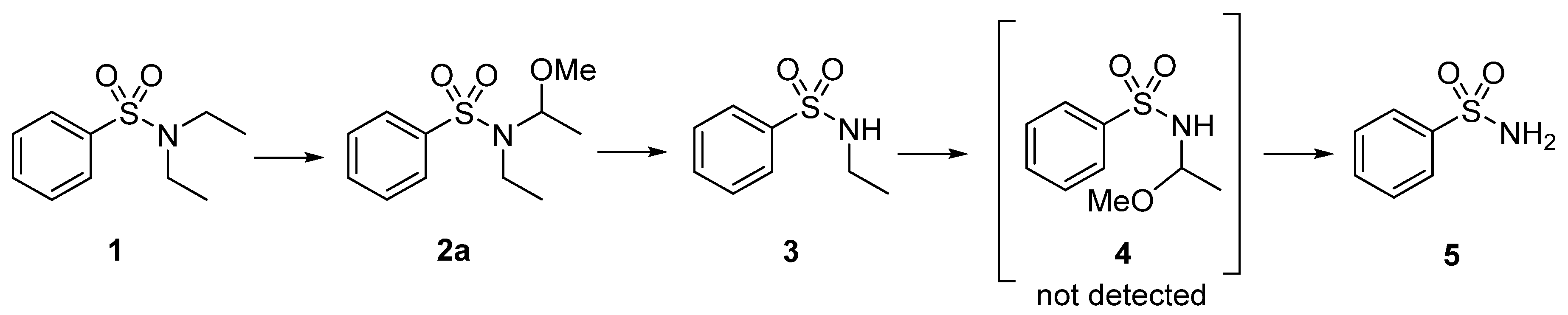
| Electrodes | Q [F/mol] | Solvent | Time [min] | Electrolyte | = 220 nm) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2a | 3 | Other b | |||||
| RVC | 4.5 | CH3CN/MeOH 9:1 | 60 | TBAP | 28 | 43 | 13 | 16 |
| graphite | 4.5 | MeOH | 60 | TBAP | - | 77 | 6 | 17 |
| graphite | 4.5 | CH3CN/MeOH 9:1 | 60 | TBAP | 1 | 46 | 14 | 39 |
| graphite | 4.5 | MeOH | 60 | Bu4NBF4 | 6 | 78 | 2 | 14 |
| RVC | 4.5 | CH3CN/MeOH 9:1 | 60 | Bu4NBF4 | 77 | 16 | 1 | 6 |
| graphite | 4.5 | CH3CN/MeOH 9:1 | 60 | Bu4NBF4 | 1 | 64 | 5 | 30 |
| graphite | 11.25 | MeOH | 150 | Bu4NBF4 | - | 76 | 7 | 17 |
| Electrical Input [mA] | Q [F/mol] | Solvent | Time [min] | Relative Quantity [%] by HPLC/UV = 220 nm) | |||
|---|---|---|---|---|---|---|---|
| 1 | 2a | 3 | Other b | ||||
| 20 | 0.8 | MeOH | 10 | 74 | 24 | 2 | - |
| 20 | 2.3 | MeOH | 30 | 30 | 65 | 5 | - |
| 20 | 3.0 | MeOH | 40 | 20 | 76 | 4 | - |
| 20 | 4.5 | MeOH | 60 | 16 | 78 | 5 | 1 |
| 50 | 1.5 | MeOH | 8 | 58 | 40 | 2 | - |
| 50 | 4.5 | MeOH | 24 | 22 | 73 | 5 | - |
| 100 | 1.5 | MeOH | 4 | 63 | 34 | 3 | - |
| 100 | 4.5 | MeOH | 12 | 40 | 56 | 4 | - |
| 20 | 1.1 | MeOH/H2O 99:1 | 15 | 76 | 19 | 5 | - |
| 20 | 4.5 | MeOH/H2O 99:1 | 60 | 48 | 35 | 17 | - |
| Q [F/mol] | Solvent | Time [h] | Relative Quantity [%] = 220 nm) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2a/2b/2c | 3 | 5 | Other b | |||
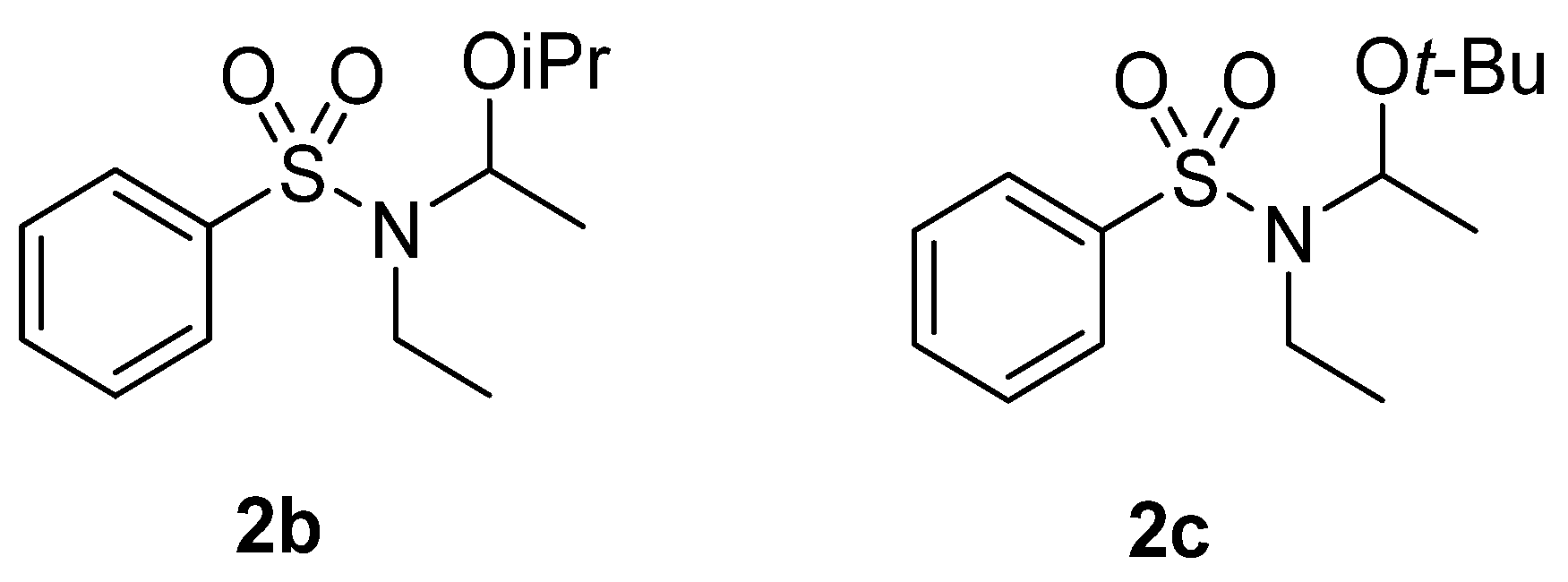
| 4.5 | MeOH/H2O 95:5 | 1 | 17 | 71 (2a) | - | - | 12 |
| 4.5 | CH3CN/MeOH/H2O 85:10:5 | 1 | 10 | 81 (2a) | 9 | - | - |
| 4.5 | CH3CN/IPA/H2O 85:10:5 | 1 | - | - (2b) | 89 | - | 11 |
| 9.0 | CH3CN/t-BuOH/H2O 85:10:5 | 2 | - | - (2c) | 80 | - | 20 |
| 9.0 | CH3CN/IPA/H2O 89:10:1 | 2 | - | - (2b) | 27 | 72 | 1 |
| Q [F/mol] | Time [h] | Conversion of 1 [%] | = 220 nm) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2a | 3 | 5 | Other b | |||
| 1.5 | 1 | 47 | 53 | 47 | - | - | - |
| 3.0 | 2 | 94 | 4 | 92 | 2 | - | 2 |
| 4.5 | 3 | 96 | 6 c | 84 | 3 | - | 7 |
| 6.0 | 4 | 98 | 12 c | 71 | 3 | - | 14 |
| 7.5 | 5 | >99 | 18 c | 61 | 4 | 2 | 15 |
| 9.0 | 6 | >99 | 23 c | 51 | 4 | 3 | 19 |
| 12.0 | 8 | >99 | 31 c | 34 | 5 | 3 | 27 |
| Q [F/mol] | Time [h] | Conversion of 1 [%] | = 220 nm) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2c | 3 | 5 | Other b | |||
| 1.5 | 1 | 29 | 71 | - | 28 | - | 1 |
| 3.0 | 2 | 77 | 23 | - | 70 | 6 | 1 |
| 4.5 | 3 | 94 | 6 | - | 77 | 14 | 3 |
| 6.0 | 4 | 97 | 3 | - | 73 | 19 | 5 |
| 7.5 | 5 | >99 | - | - | 68 | 24 | 8 |
| 9.0 | 6 | >99 | - | - | 64 | 26 | 10 |
| 12.0 | 8 | >99 | - | - | 51 | 27 | 22 |
| Q [F/mol] | Time [h] | Conversion of 1 [%] | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2b | 3 | 5 | Other b | |||
| 1.5 | 1 | 29 | 71 | 14 | 15 | - | - |
| 3.0 | 2 | 70 | 30 | 23 | 32 | 2 | 13 |
| 4.5 | 3 | 91 | 9 | 19 | 52 | 8 | 12 |
| 6.0 | 4 | 98 | 2 | 9 | 58 | 13 | 18 |
| 7.5 | 5 | 98 | 2 | 5 | 51 | 20 | 22 |
| 9.0 | 6 | >99 | 5c | 4 | 45 | 30 | 16 |
| 12.0 | 8 | >99 | 13c | 6 | 21 | 35 | 25 |
| Solvent | Time [h] | Q [F/mol] | Electrolyte | Electrolyte Concentration (M) | Product | Isolated Yield [%] |
|---|---|---|---|---|---|---|
| MeOH | 6.7 | 10.0 | Bu4NBF4 | 0.600 | 2a | 55 |
| CH3CN/t-BuOH/H2O 85:10:5 | 3 | 4.5 | TBAP | 0.167 | 3 | 62 |
| 5 | 25 | |||||
| CH3CN/IPA/H2O 89:10:1 | 9 | 13.5 | TBAP | 0.167 | 5 | 41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Várda, E.F.; Gyűjtő, I.; Ender, F.; Csekő, R.; Balogh, G.T.; Volk, B. Detailed Studies on the Methoxylation and Subsequent Dealkylation of N,N-Diethylbenzenesulfonamide Using a Tailor-Made Electrosynthetic Reactor. Molecules 2024, 29, 5496. https://doi.org/10.3390/molecules29235496
Várda EF, Gyűjtő I, Ender F, Csekő R, Balogh GT, Volk B. Detailed Studies on the Methoxylation and Subsequent Dealkylation of N,N-Diethylbenzenesulfonamide Using a Tailor-Made Electrosynthetic Reactor. Molecules. 2024; 29(23):5496. https://doi.org/10.3390/molecules29235496
Chicago/Turabian StyleVárda, Ernák F., Imre Gyűjtő, Ferenc Ender, Richárd Csekő, György T. Balogh, and Balázs Volk. 2024. "Detailed Studies on the Methoxylation and Subsequent Dealkylation of N,N-Diethylbenzenesulfonamide Using a Tailor-Made Electrosynthetic Reactor" Molecules 29, no. 23: 5496. https://doi.org/10.3390/molecules29235496
APA StyleVárda, E. F., Gyűjtő, I., Ender, F., Csekő, R., Balogh, G. T., & Volk, B. (2024). Detailed Studies on the Methoxylation and Subsequent Dealkylation of N,N-Diethylbenzenesulfonamide Using a Tailor-Made Electrosynthetic Reactor. Molecules, 29(23), 5496. https://doi.org/10.3390/molecules29235496








