Sintering Behavior of Molybdenite Concentrate During Oxidation Roasting Process in Air Atmosphere: Influences of Roasting Temperature and K Content
Abstract
1. Introduction
2. Results
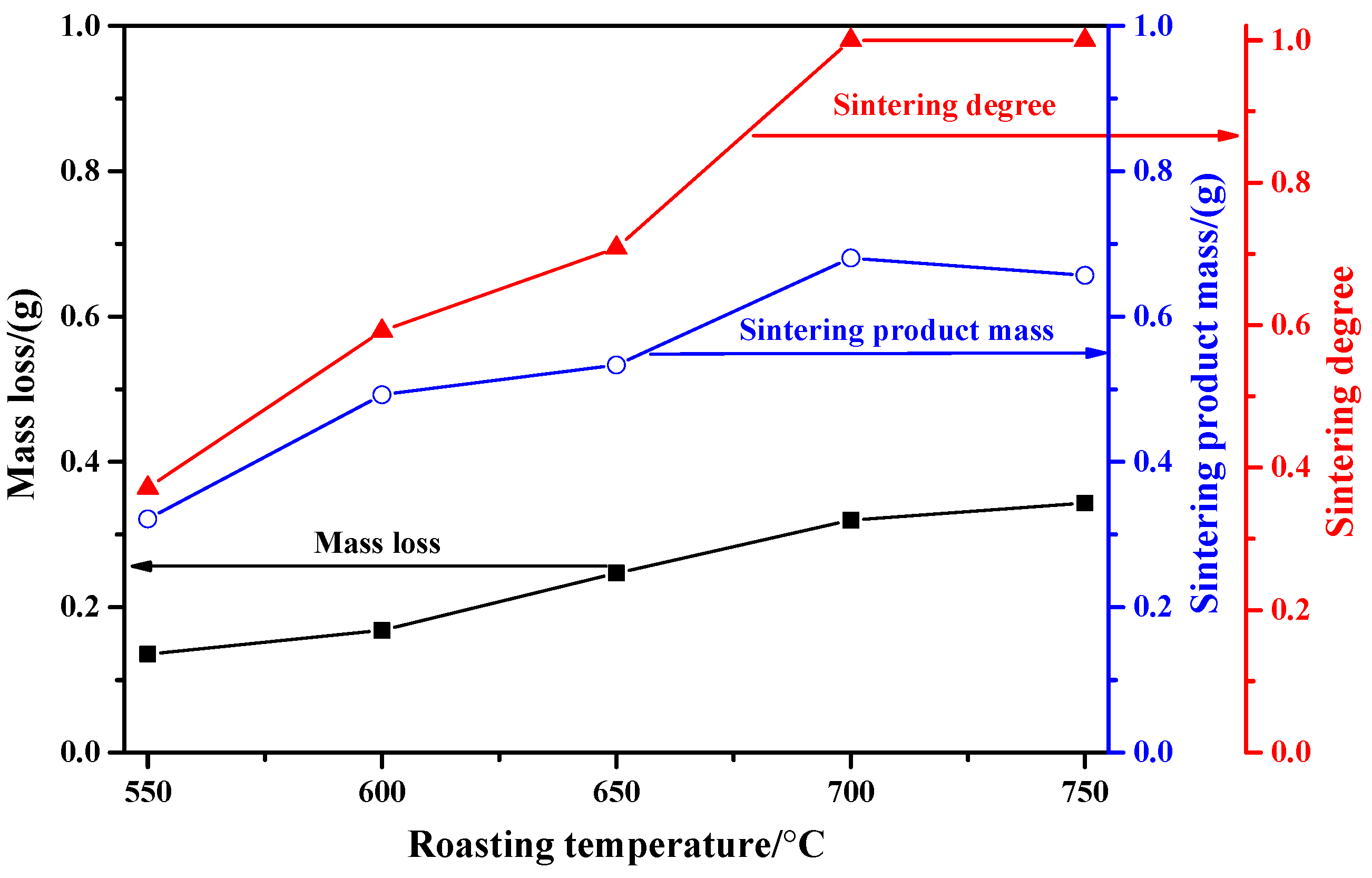
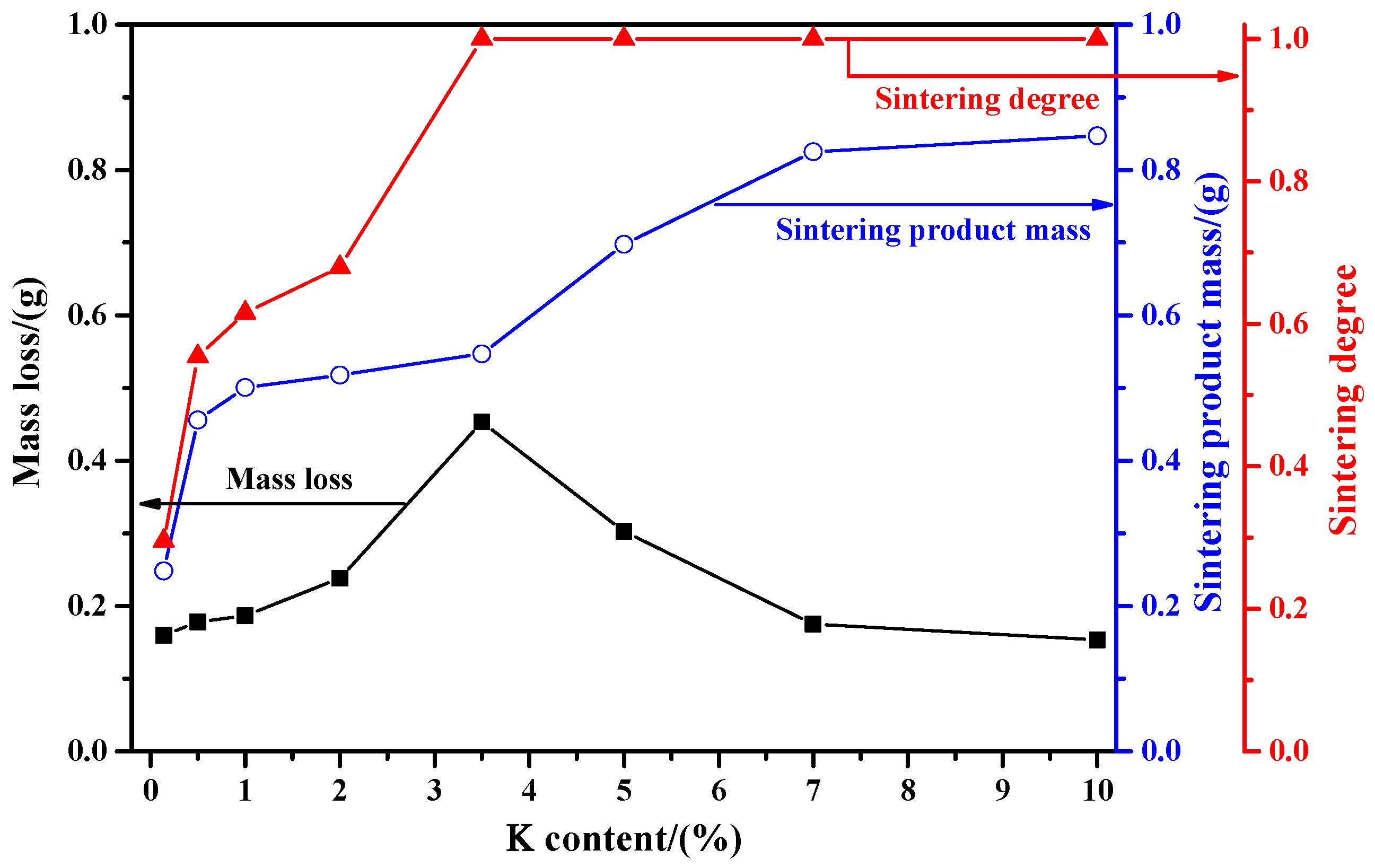
2.1. Mass Loss and Sintering Degree
2.1.1. Influence of Roasting Temperature
2.1.2. Influence of K Content
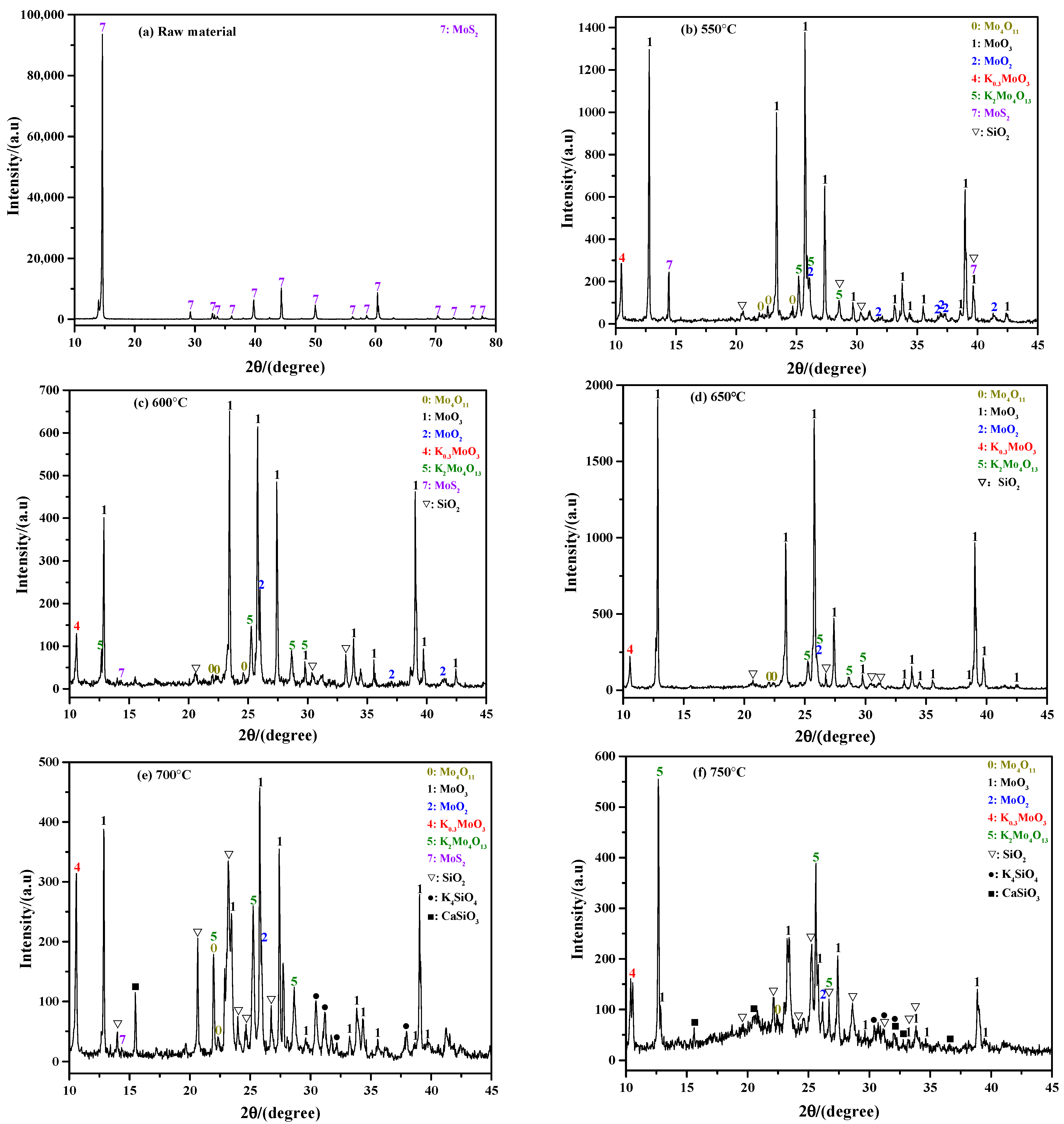
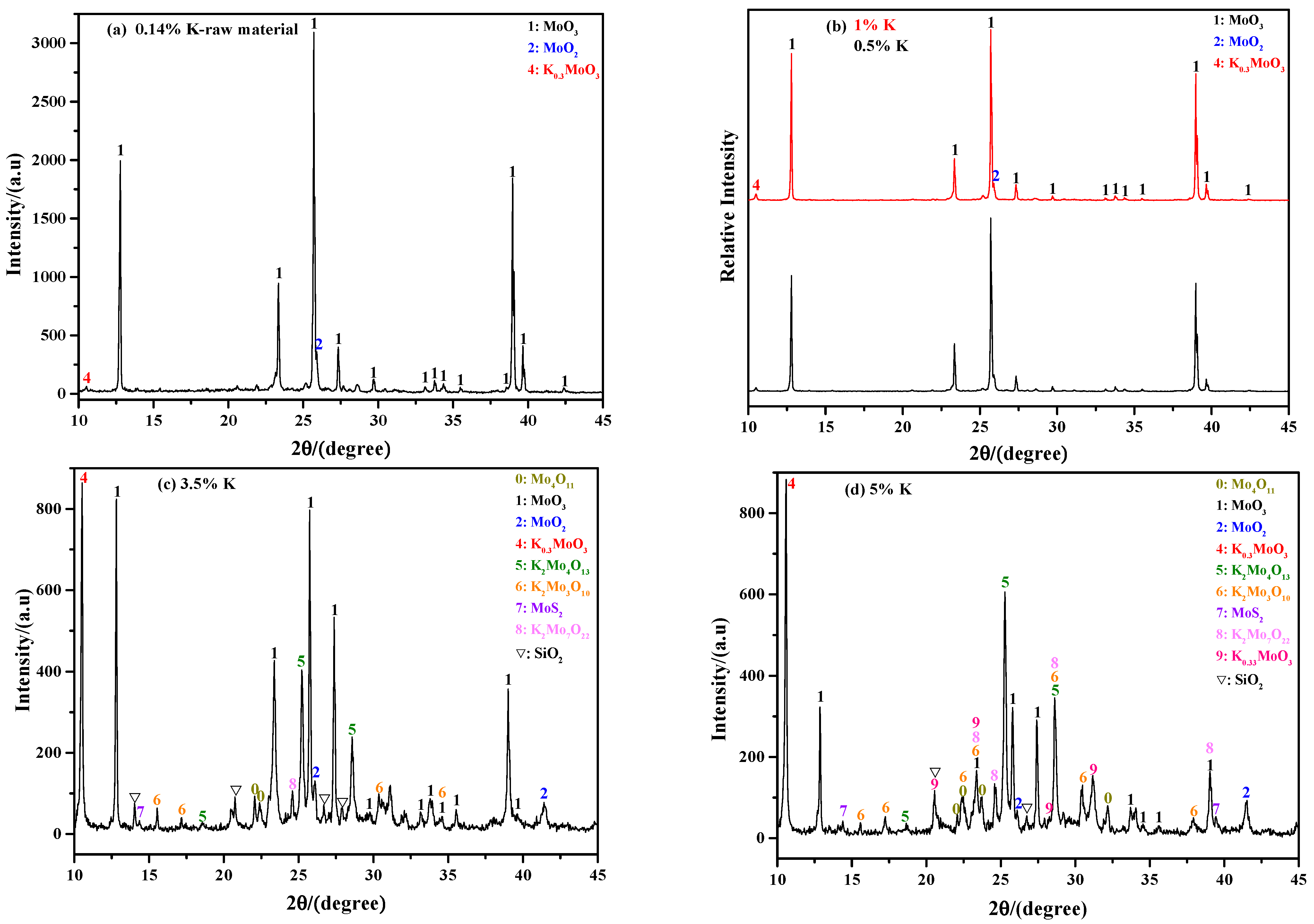
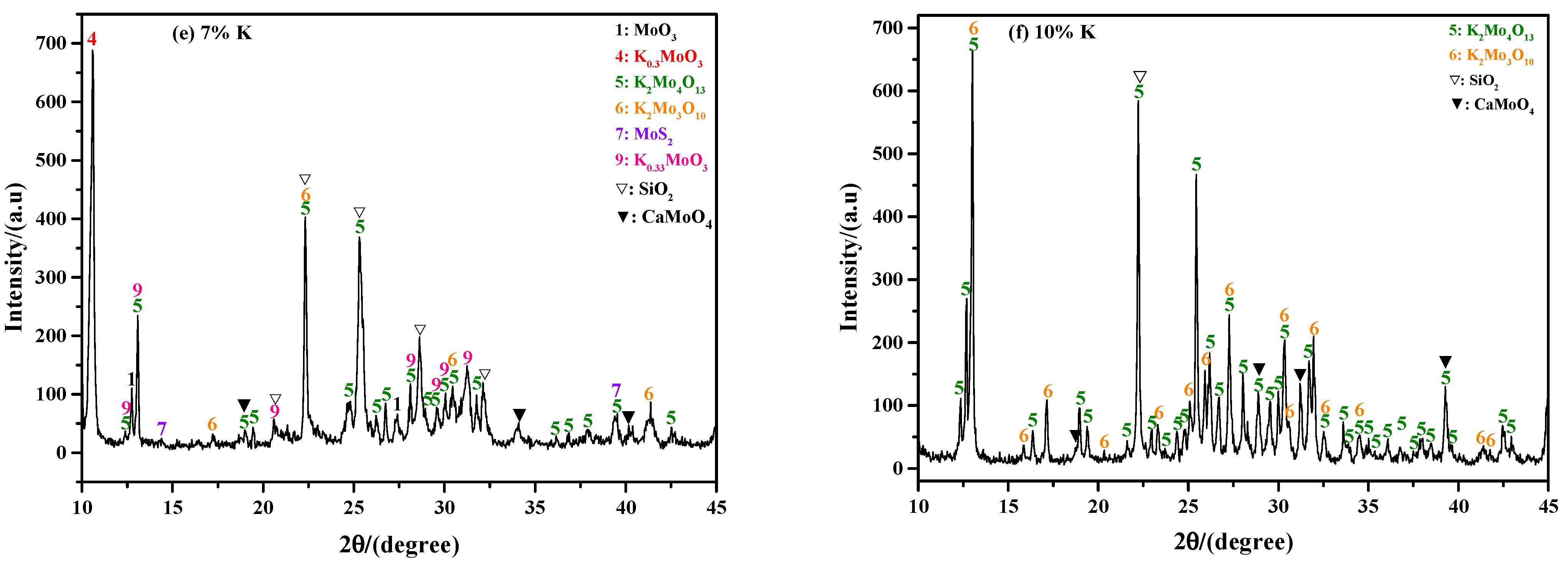
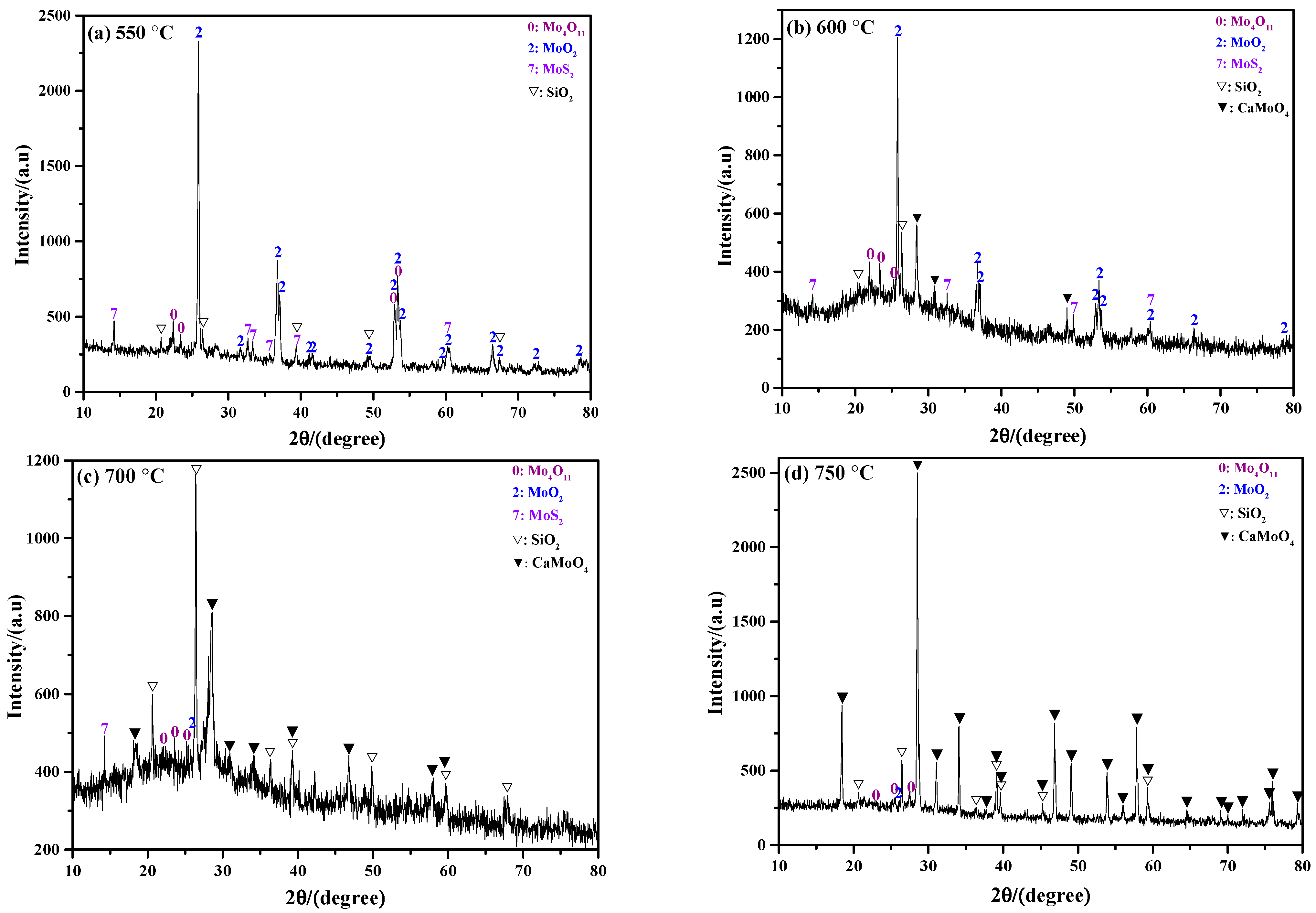
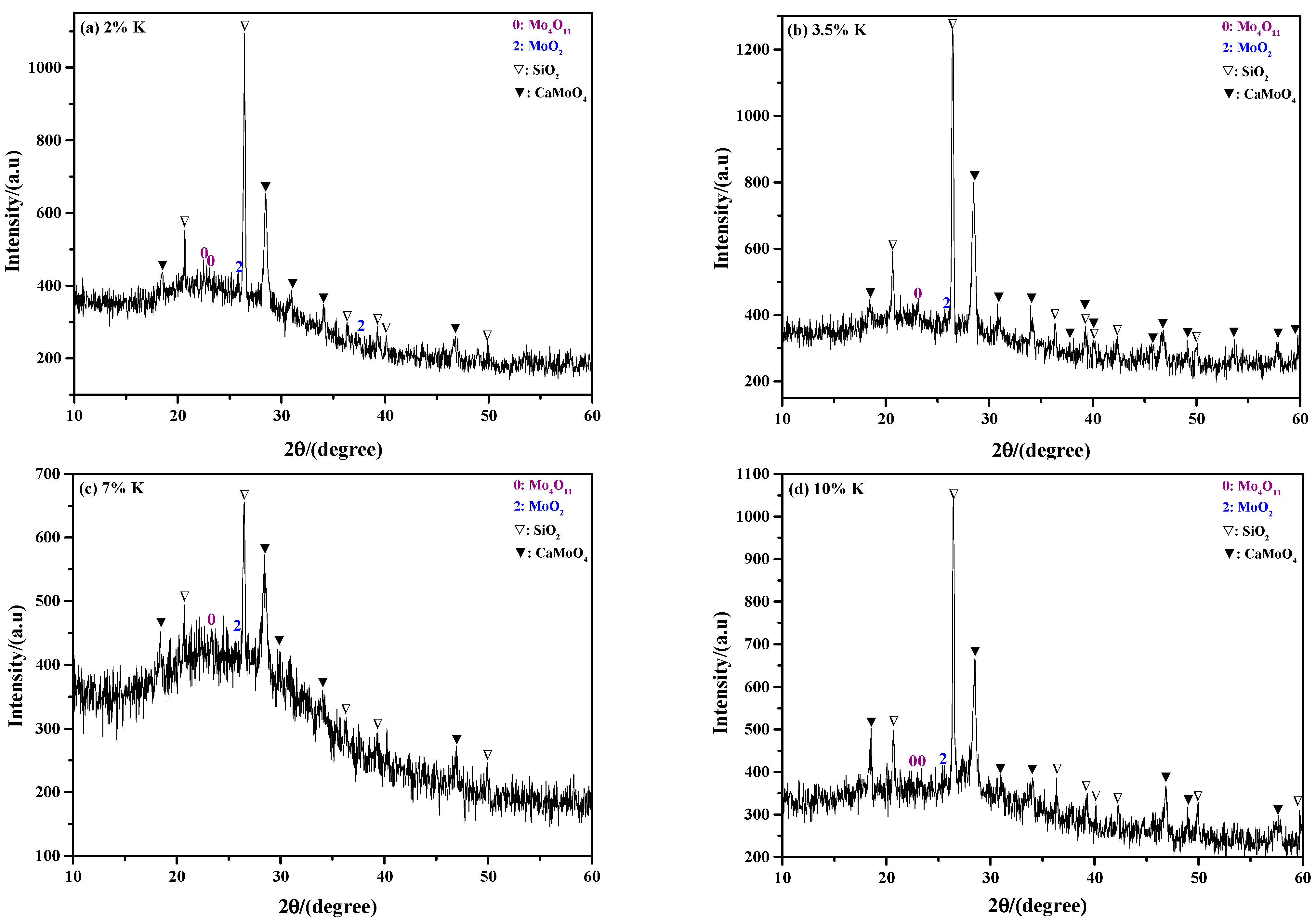
2.2. Phase Composition of Sintering Product
2.2.1. Influence of Roasting Temperature
2.2.2. Influence of K Content
2.3. Morphological Structure of Sintering Product
2.3.1. Influence of Roasting Temperature
2.3.2. Influence of K Content
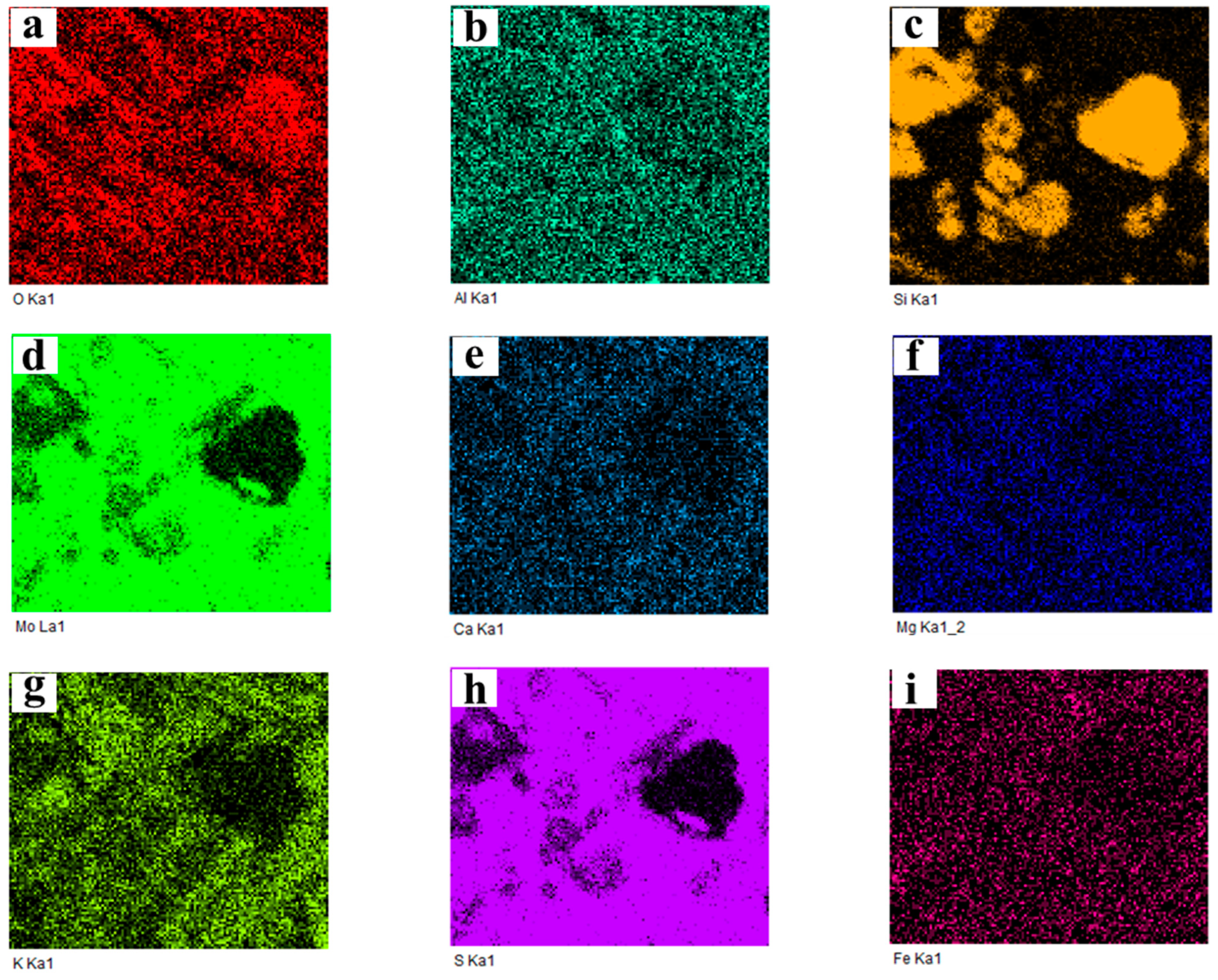
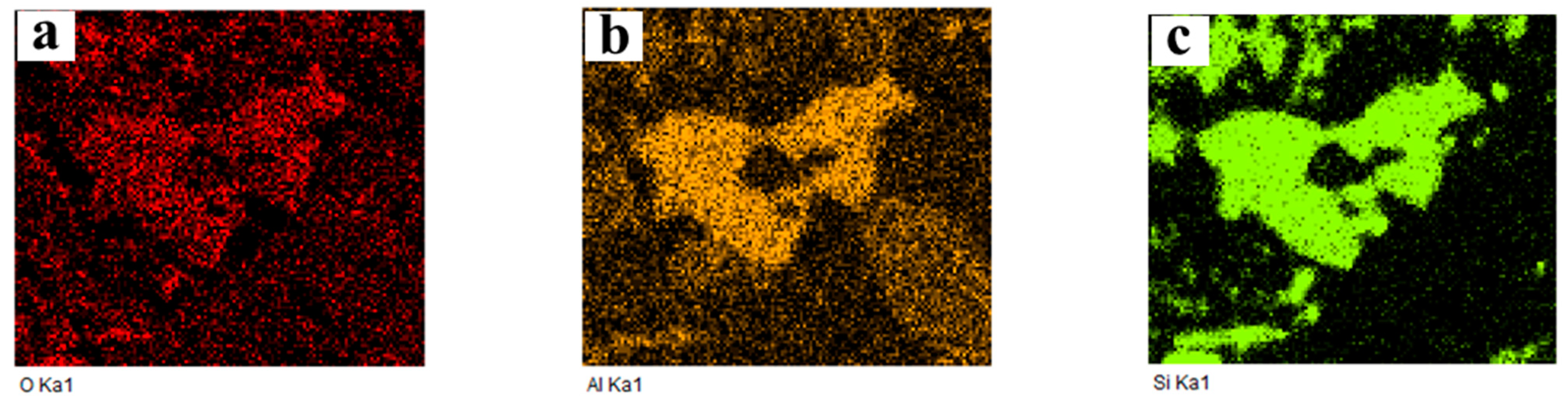
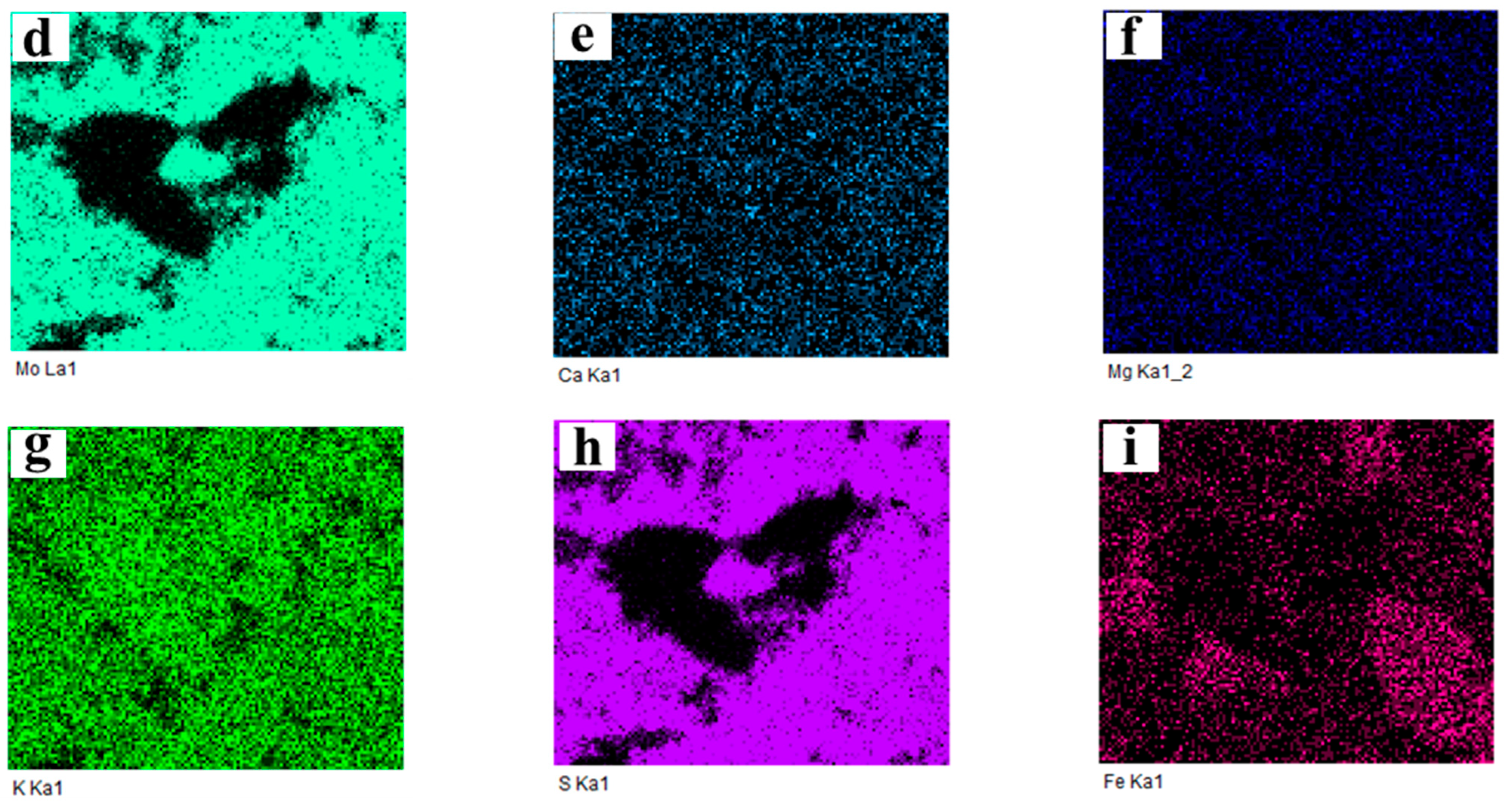
2.3.3. Cross-Section Microstructure
3. Discussion
3.1. Ammonia Leaching of Sintering Product
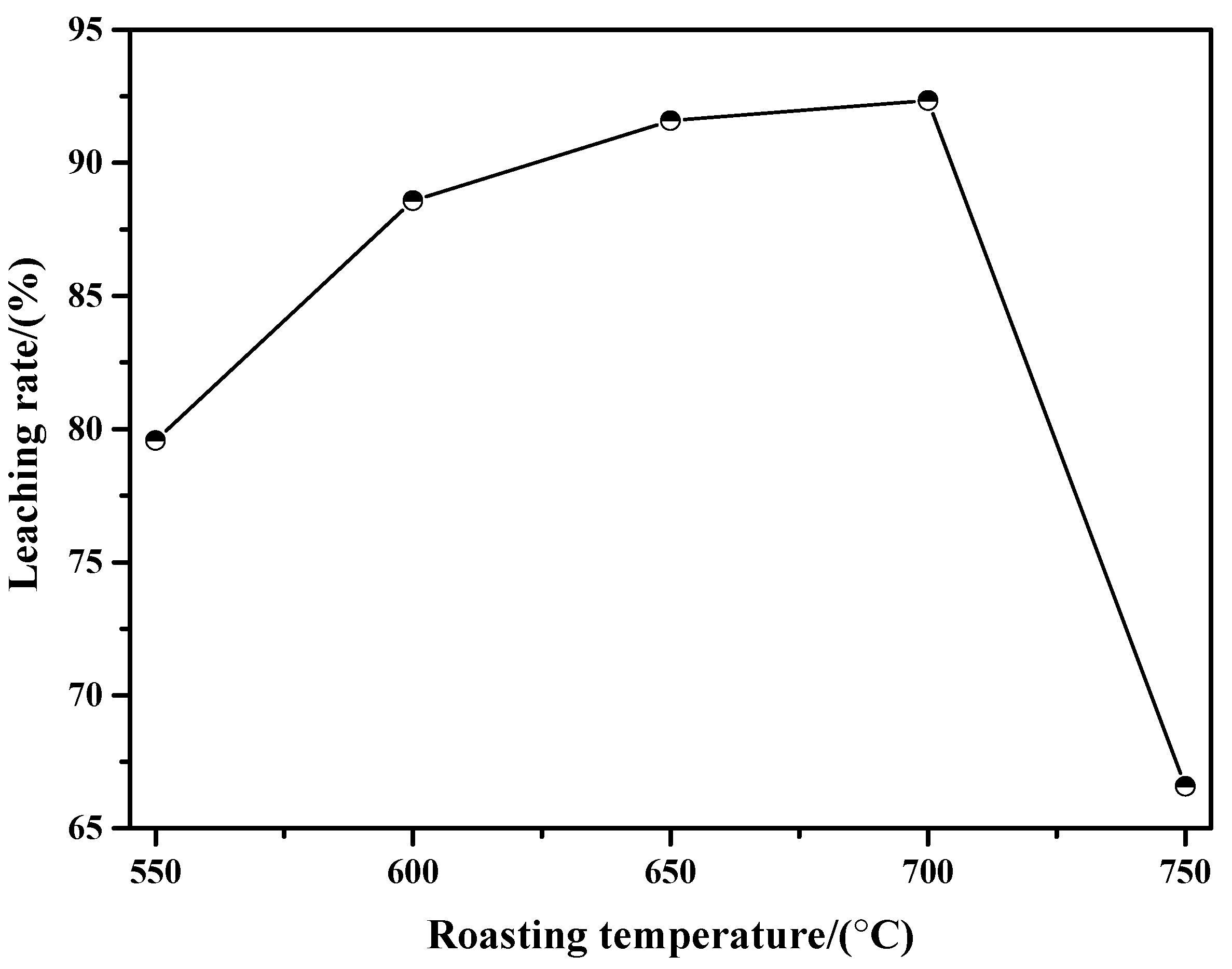
3.1.1. Influence of Roasting Temperature
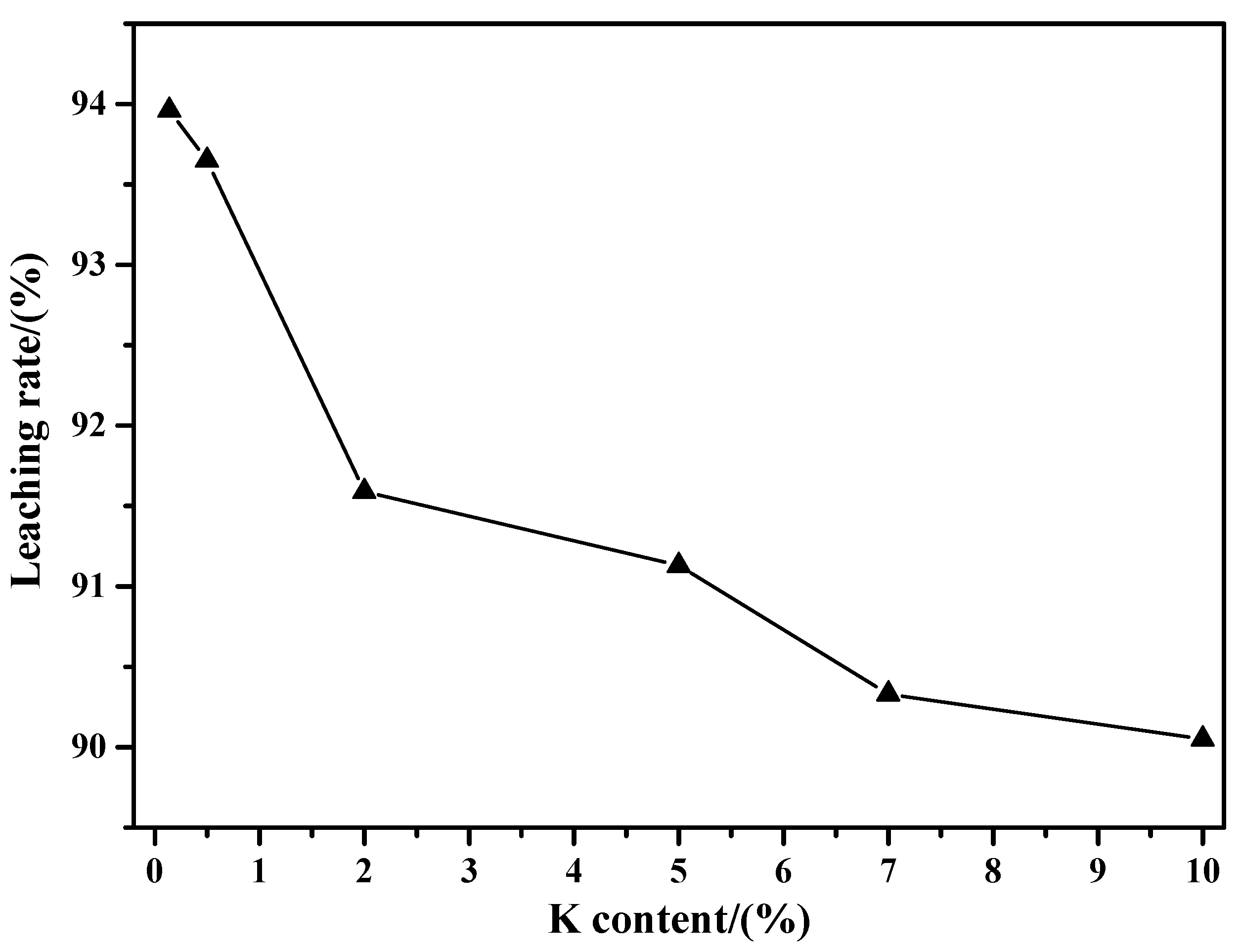
3.1.2. Influence of K Content
3.2. Phase Composition of Ammonia Leaching Residue
3.2.1. Influence of Roasting Temperature
3.2.2. Influence of K Content
3.3. Sintering Mechanism Analysis
4. Materials and Experimental Procedure
4.1. Materials
4.2. Experimental Procedure
4.3. Characterization Methods
5. Conclusions
- (1)
- When the roasting temperature is in the range of 550 °C to 700 °C, the mass loss of the molybdenite concentrate, the mass of the sintering product, and the sintering degree are all increased with the increase in roasting temperature. Meanwhile, when the roasting temperature is above 750 °C, the mass of the sintering product is decreased due to the intense sublimation effect of MoO3.
- (2)
- With the increase in K content, the mass loss of molybdenite concentrate is increased first and then gradually decreased. The maximum mass loss is reached at a K content of 3.5% with a sintering degree close to 1. This work also found that the sintering product mass is continuously increased with the increase in K content.
- (3)
- The phase composition of sintering product has a certain relationship with the roasting temperature and K content. In general, the sintering product not only contains a large amount of MoO3, but also contains numerous unoxidized MoS2, molybdenum sub-oxide, SiO2, and various molybdates; among these, most of the MoO3 and molybdates can be removed after ammonia leaching treatment.
- (4)
- The occurrence of the sintering phenomenon is due to the increase in local reaction temperature and the formation of various low-melting-point eutectics. This work also finds that decreasing the roasting temperature and K content, especially the K content, are effective strategies to reduce the sintering degree of molybdenite concentrate during the oxidation roasting process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, X.K.; Sun, B.; Yang, M.; Yan, J.; Han, X.B.; Zhai, Y.H.; Wang, W.A.; Li, X.M. Thickening mechanism of furnace bed bottom material of multi-hearth furnace oxidation roasting for molybdenum concentrate metallurgy. China Nonferr. Metall. 2023, 52, 73–80. [Google Scholar]
- Liu, H.Z.; Wang, H.F.; Zhang, B.; Wang, W.; Cao, Y.H.; Liu, L.; Wang, H.L. Distribution characteristic of Re during molybdenum concentrate roasting in multiple heart furnace. Nonferr. Met. (Extr. Metall.) 2020, 6, 48–52. [Google Scholar]
- Li, H.X.; Wang, L.; Xue, Z.L. A novel way for preparing hexagonal-shaped Mo2N by NH3 reduction of Fe-doped h-MoO3. Metall. Mater. Trans. B 2024, 55, 2378–2387. [Google Scholar] [CrossRef]
- Yang, H.; Ding, Z.; Li, Y.T.; Li, S.Y.; Wu, P.K.; Hou, Q.H.; Zheng, Y.; Gao, B.; Huo, K.F.; Du, W.J. Recent advances in kinetic and thermodynamic regulation of magnesium hydride for hydrogen storage. Rare Met. 2023, 42, 2906–2927. [Google Scholar] [CrossRef]
- Rakass, S.; Oudghiri Hassani, H.; Abboudi, M.; Kooli, F.; Mohmoud, A.; Aljuhani, A.; Al Wadaani, F. Molybdenum trioxide: Efficient nanosorbent for removal of methylene blue dye from aqueous solutions. Molecules 2018, 23, 2295. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Xue, Z.L. Parameter optimization of ultrafine molybdenum powder during hydrogen reduction of MoO2 based on central composite design method. Int. J. Refract. Met. Hard Mater. 2024, 124, 106845. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Y.; Yang, H.; Lu, Y.; Tan, J.; Li, J.; Li, Q.; Chen, Y.; Shaw, L.L.; Pan, F. Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis. J. Magnes. Alloys 2022, 10, 2946–2967. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, J.F.; Cai, J.J.; Sun, H.C.; Liu, Y. Study on mechanism of molybdenum concentrate roasting. China Molybdenum Ind. 2011, 35, 17–19. [Google Scholar] [CrossRef]
- Utigard, T. Oxidation mechanism of molybdenite concentrate. Metall. Mater. Trans. B 2009, 40, 490–496. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.H.; Zhang, L.B.; Gao, J.Y.; Wang, F.; Dai, Y. Preparation and mechanism of molybdenum trioxide by microwave oxidation roasting of molybdenite. Chin. J. Nonferr. Met. 2023, 33, 2015–2030. [Google Scholar]
- Gan, M.; Fan, X.H.; Zhang, L.; Jiang, T.; Qiu, G.Z.; Wang, Y.; Deng, Q.; Chen, X.L. Reaction behavior of low grade molybdenum concentrates in oxidation roasting process. Chin. J. Nonferr. Met. 2014, 24, 3115–3122. [Google Scholar]
- Sun, H.; Li, G.H.; Bu, Q.Z.; Fu, Z.Q.; Liu, H.B.; Zhang, X.; Luo, J.; Rao, M.J.; Jiang, T. Features and mechanisms of self-sintering of molybdenite during oxidative roasting. Trans. Nonferr. Met. Soc. China 2022, 32, 307–318. [Google Scholar] [CrossRef]
- Li, X.M.; Zhai, Y.H.; Zou, C.; Wang, W.A.; Lin, B. Effect of impurity compounds on sintering behavior of molybdenum concentrate during roasting process. Chin. J. Nonferr. Met. 2024, 34, 549–560. [Google Scholar]
- Ammann, P.R.; Loose, T.A. The oxidation kinetics of molybdenite at 525° to 635 °C. Metall. Trans. 1971, 2, 889–893. [Google Scholar] [CrossRef]
- Marin, T.; Utigard, T.; Hernandez, C. Roasting kinetics of molybdenite concentrates. Can. Metall. Q. 2009, 48, 73–80. [Google Scholar] [CrossRef]
- Fu, Y.F.; Xiao, Q.G.; Gao, Y.Y.; Ning, P.G.; Xu, H.B.; Zhang, Y. Pressure aqueous oxidation of molybdenite concentrate with oxygen. Hydrometallurgy 2017, 174, 131–139. [Google Scholar] [CrossRef]
- Blanco, E.; Sohn, H.Y.; Han, G.; Hakobyan, K.Y. The kinetics of oxidation of molybdenite concentrate by water vapor. Metall. Mater. Trans. B 2007, 38, 689–693. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H.; Dang, J.; Chou, K.C. Oxidation roasting of molybdenite concentrate. Trans. Nonferr. Met. Soc. China 2015, 25, 4167–4174. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H.; Wang, J.S.; Chou, K.C. Influences of different components on agglomeration behavior of MoS2 during oxidation roasting process in Air. Metall. Mater. Trans. B 2016, 47, 2421–2432. [Google Scholar] [CrossRef]
- Bu, C.Y.; Cao, W.C.; Wang, L.; Zhang, G.H.; Sun, G.D.; Wang, D.H.; Chang, H.Q.; Song, C.M.; Zhou, G.Z.; He, K. Study on the sintering phenomenon during the roasting processes of molybdenite by air. China Molybdenum Ind. 2018, 42, 8–11. [Google Scholar]
- Liu, Q.H.; Tian, S.Z.; Yang, S.P.; Wang, L.D.; He, K. Potassium release mechanism of potassium-bearing minerals in ammonium molybdate production process. Chin. J. Process Eng. 2022, 22, 1271–1278. [Google Scholar]
- Liu, Q.H.; Liu, R.L.; Yang, S.P.; He, K.; Wang, L.D. Thermodynamic behavior and analysis of typical impurity elements in the roasting process of molybdenum concentrate. J. Chongqing Univ. 2023, 46, 89–100. [Google Scholar]
- Li, G.H.; Huang, J.H.; Sun, H.; Mang, C.Y.; Hou, Y.R.; Luo, J. Phase and structure optimizations of MoS2 concentrate pellets with Al2O3-SiO2 additives during oxidative and volatilizing roasting process. JOM 2023, 75, 5167–5175. [Google Scholar] [CrossRef]
- Sun, H.; Li, G.; Yu, J.; Luo, J.; Rao, M.; Peng, Z.; Zhang, Y.; Jiang, T. Preparation of high purity MoO3 through volatilization of technical-grade Mo calcine in water vapor atmosphere. Int. J. Refract. Met. Hard Mater. 2018, 77, 1–7. [Google Scholar] [CrossRef]
- Chychko, A.; Teng, L.; Seetharaman, S. MoO3 evaporation studies from binary systems towards choice of Mo precursors in EAF. Steel Res. Int. 2010, 81, 784–791. [Google Scholar] [CrossRef]
- Shibata, K.; Tsuchida, K.; Kato, A. Preparation of ultrafine molybdenum powder by vapour phase reaction of the MoO3-H2 system. J. Less Common Met. 1990, 157, L5–L10. [Google Scholar] [CrossRef]
- Chiang, T.H.; Yeh, H.C. The synthesis of α-MoO3 by ethylene glycol. Materials 2013, 6, 4609–4625. [Google Scholar] [CrossRef]
- Silveira, J.; Moura, J.; Luz-Lima, C.; Freire, P.; Souza Filho, A. Laser-induced thermal effects in hexagonal MoO3 nanorods. Vib. Spectrosc. 2018, 98, 145–151. [Google Scholar] [CrossRef]
- Zhang, S.M.; Jiang, D.S.; Song, J.X.; Che, Y.S.; He, J.L. Investigation of the roasting behavior of mixtures of molybdenum trioxide and molybdenum concentrates. ACS Sustain. Chem. Eng. 2022, 10, 16150–16158. [Google Scholar] [CrossRef]
- Lee, J.R.; Kim, Y.H.; Won, Y.S. Solid-state reaction between MoS2 and MoO3 in a fluidized bed reactor. Korean J. Chem. Eng. 2021, 38, 1791–1796. [Google Scholar] [CrossRef]
- Wilkomirsky, I.; Sáez, E. Kinetics and mechanism of formation of MoO2 by solid state reaction between MoS2 and MoO3. Can. Metall. Q. 2020, 59, 41–50. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, Z.; Wang, Y.F.; Zhang, B.S. Experimental study on oxidation and roasting of molybdenite. China Resour. Compr. Util. 2017, 35, 29–31, 39. [Google Scholar]
- Caillet, P. Anhydrous sodium or potassium polymolybdates and polytungstates. Bull. Soc. Chim. Fr. 1967, 12, 4750–4755. [Google Scholar]



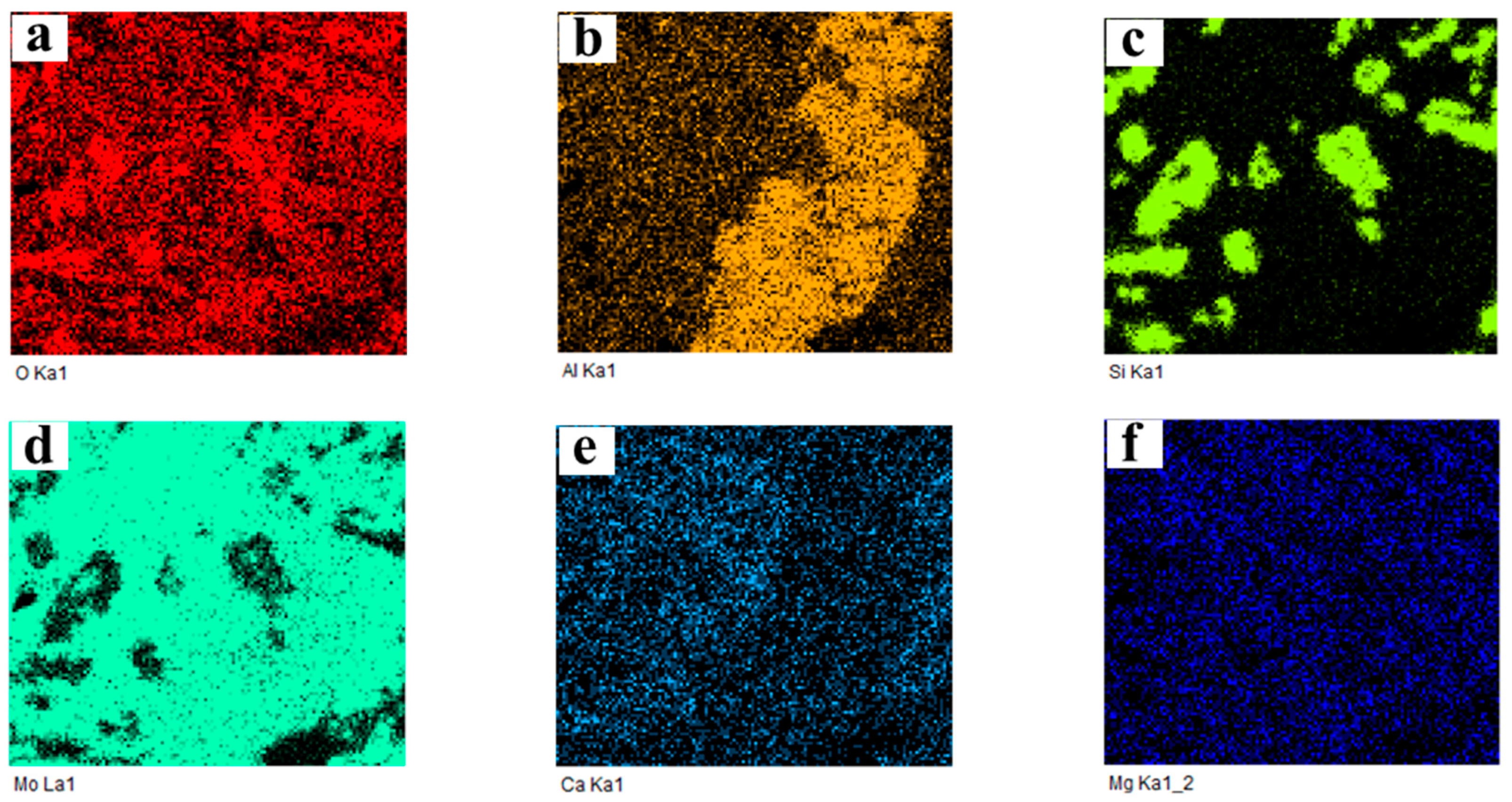
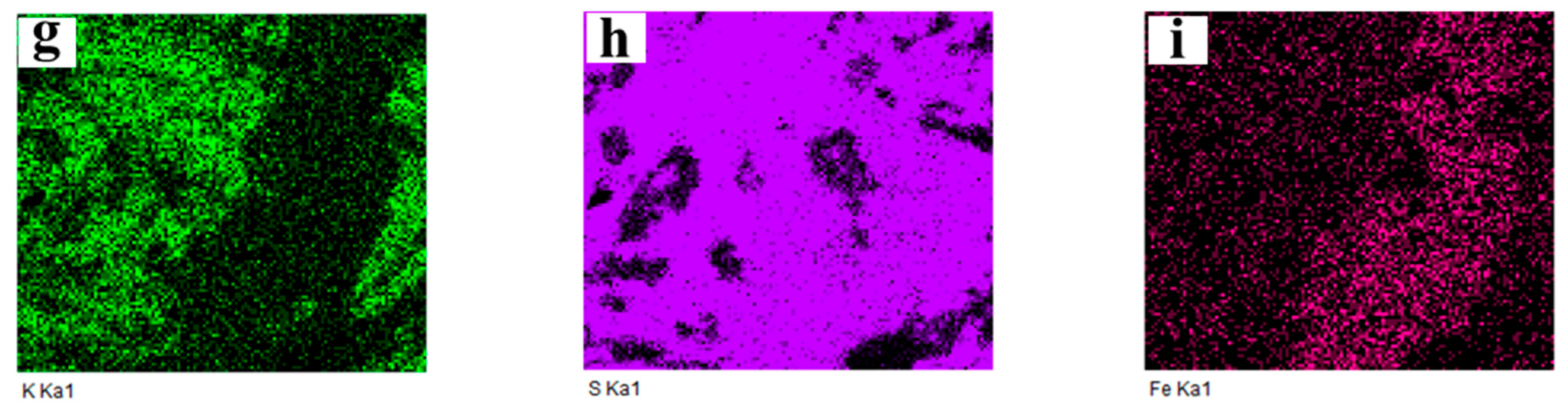
| Total K Content, εK/% | 0.14 | 0.5 | 1 | 2 | 3.5 | 5 | 7 | 10 |
|---|---|---|---|---|---|---|---|---|
| Mass of molybdenite concentrate/g | 1 | 0.9936 | 0.9847 | 0.9670 | 0.9405 | 0.9138 | 0.8783 | 0.8251 |
| Mass of added K2CO3/g | 0 | 0.0064 | 0.0153 | 0.0330 | 0.0595 | 0.0862 | 0.1217 | 0.1749 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, L.; Wu, G. Sintering Behavior of Molybdenite Concentrate During Oxidation Roasting Process in Air Atmosphere: Influences of Roasting Temperature and K Content. Molecules 2024, 29, 5183. https://doi.org/10.3390/molecules29215183
Liu J, Wang L, Wu G. Sintering Behavior of Molybdenite Concentrate During Oxidation Roasting Process in Air Atmosphere: Influences of Roasting Temperature and K Content. Molecules. 2024; 29(21):5183. https://doi.org/10.3390/molecules29215183
Chicago/Turabian StyleLiu, Jiangang, Lu Wang, and Guohuan Wu. 2024. "Sintering Behavior of Molybdenite Concentrate During Oxidation Roasting Process in Air Atmosphere: Influences of Roasting Temperature and K Content" Molecules 29, no. 21: 5183. https://doi.org/10.3390/molecules29215183
APA StyleLiu, J., Wang, L., & Wu, G. (2024). Sintering Behavior of Molybdenite Concentrate During Oxidation Roasting Process in Air Atmosphere: Influences of Roasting Temperature and K Content. Molecules, 29(21), 5183. https://doi.org/10.3390/molecules29215183









