The Composition, Antioxidant and Antibacterial Activity of Essential Oils from Five Species of the Magnoliaceae Family
Abstract
1. Introduction
2. Results
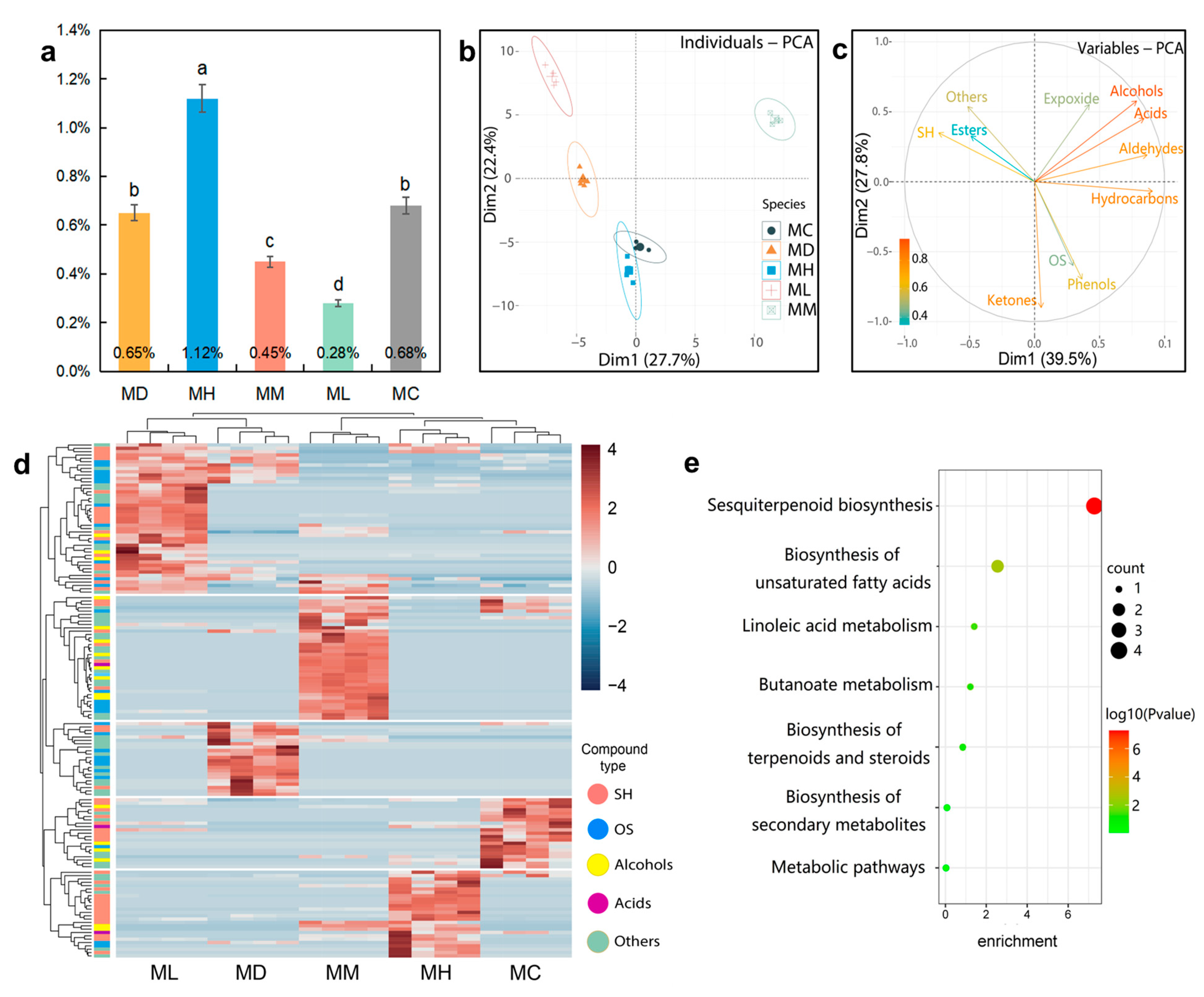
2.1. GC–MS Analysis
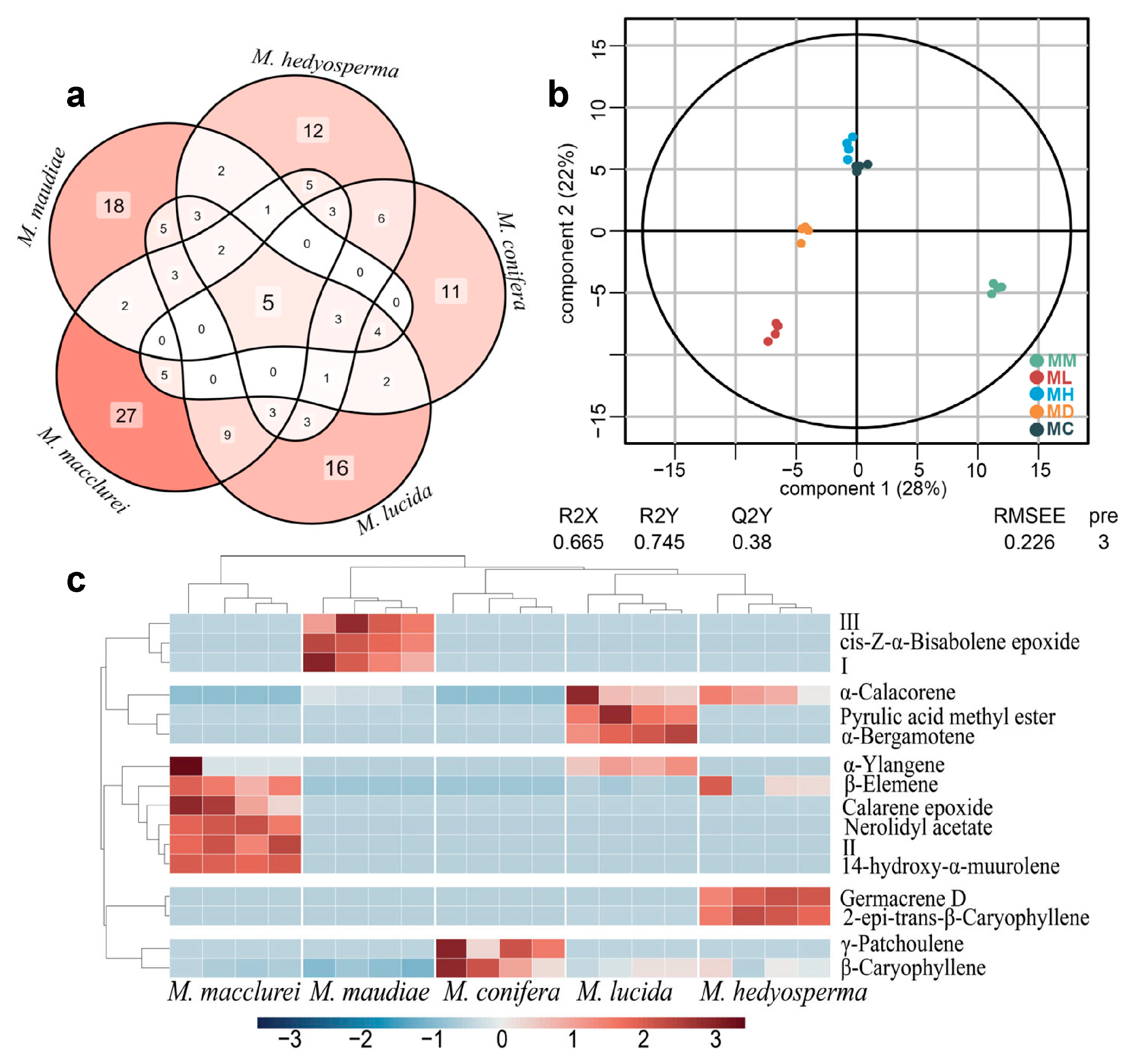
2.2. Components Identification
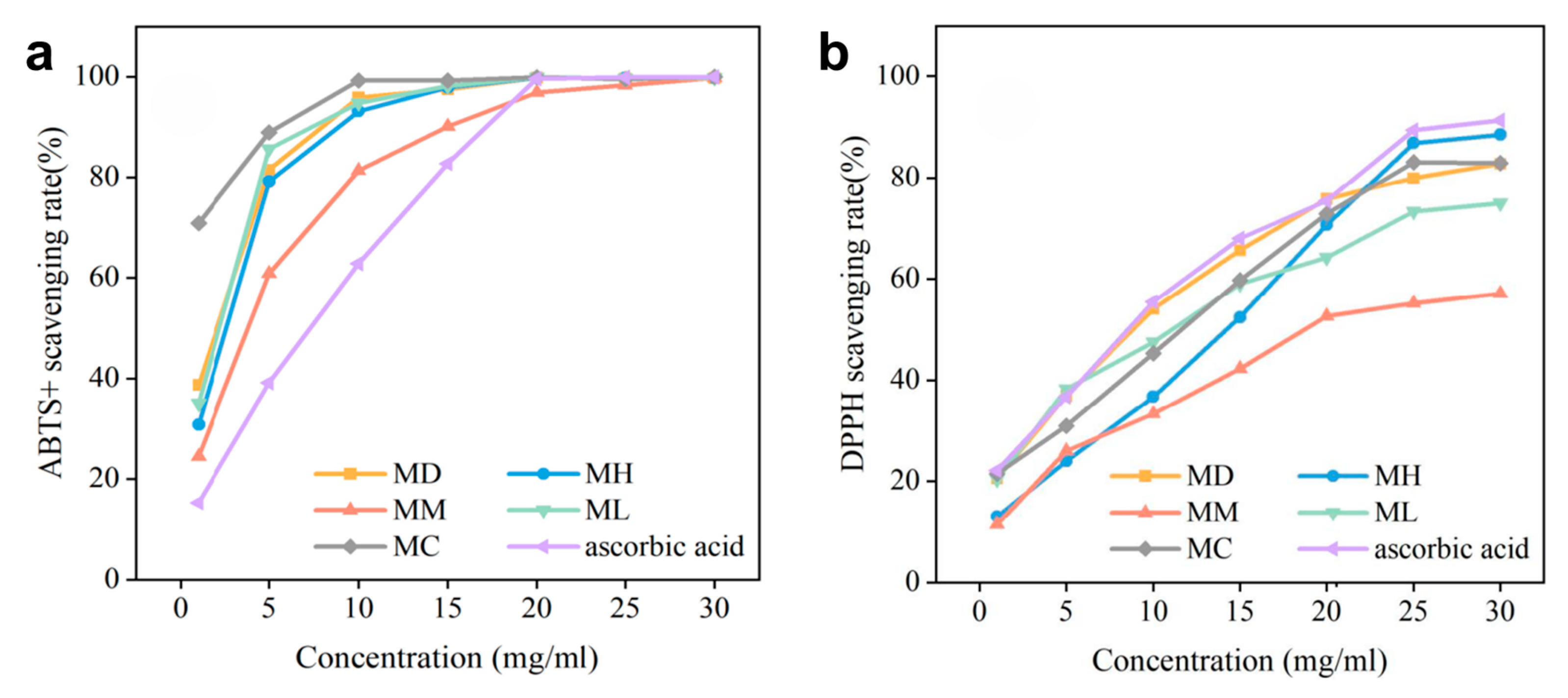
2.3. Antioxidant Activities of Different EOs
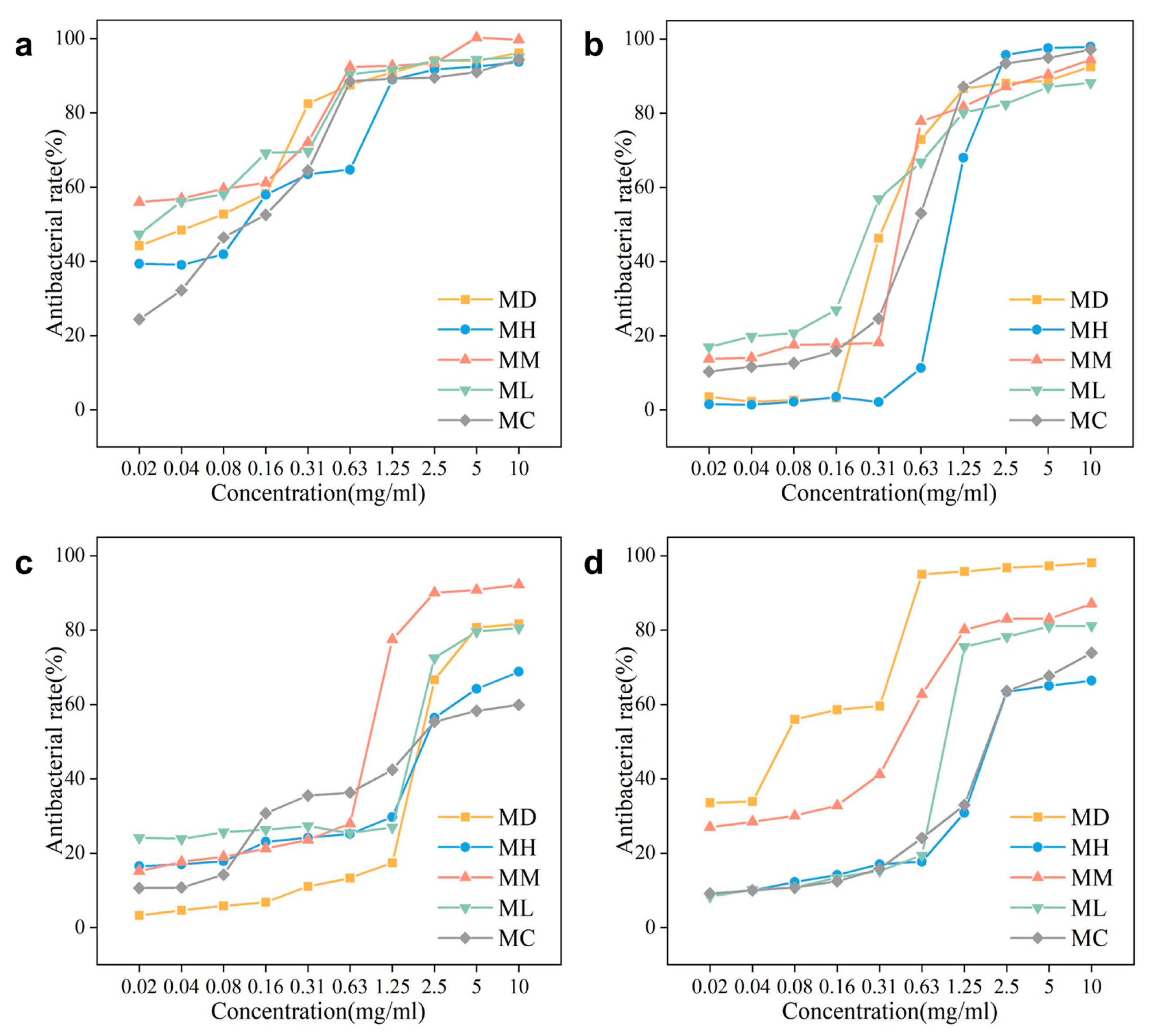
2.4. Antibacterial Activities of Different EOs
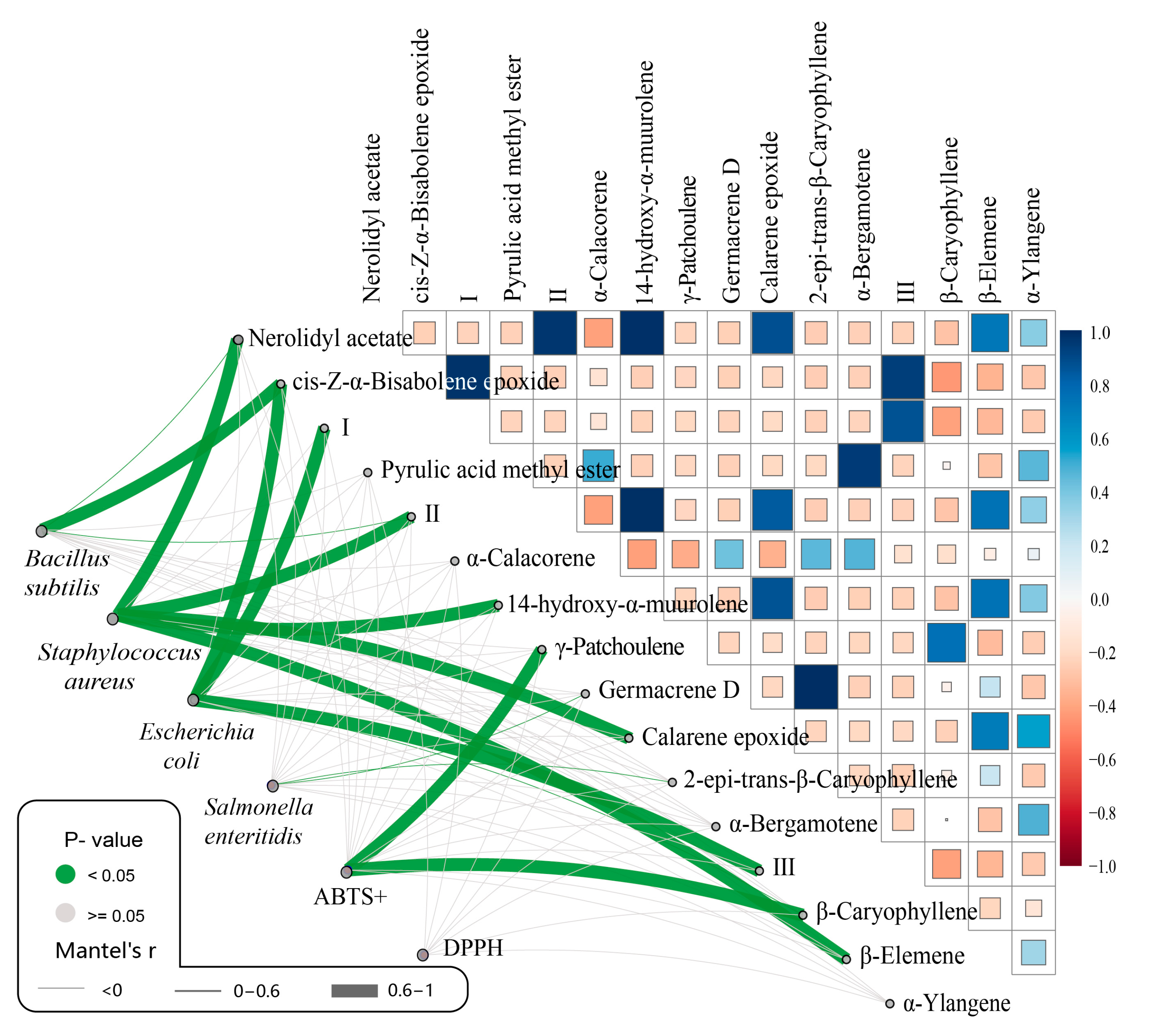
2.5. Correlation Analysis
3. Discussion
3.1. Compound Analysis of EOs from Five Magnoliaceae Species
3.2. Exploration of the Antioxidant Activity
3.3. Exploration of the Antibacterial Activity
4. Materials and Methods
4.1. Test Site and Materials
4.2. EO Extraction and Compound Identification
4.3. Antioxidant Assay
4.4. Antibacterial Assay
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azuma, H.; Toyota, M.; Asakawa, Y. Intraspecific variation of floral scent chemistry in Magnolia kobus DC. (Magnoliaceae). J. Plant Res. 2001, 114, 411–422. [Google Scholar] [CrossRef]
- Fengmao, Y.; Lei, C.; Zhiling, D.; Weibang, S. Genomic Data Reveals Population Genetic and Demographic History of Magnolia fistulosa (Magnoliaceae), a Plant Species with Extremely Small Populations in Yunnan Province, China. Front. Plant Sci. 2022, 13, 811312. [Google Scholar]
- Er-Qi, F.; Yun-Hua, W.; Ye, G.; Chun-Lian, Y.; Xin-Chun, L. Chemical components of essential oils from leaves of six Magnoliaceae species using GC-MS. J. Zhejiang A F Univ. 2012, 29, 307–312. [Google Scholar]
- Sun, L.; Nie, T.; Chen, Y.; Li, J.; Yang, A.; Yin, Z. Gene identification and tissue expression analysis inform the floral organization and color in the basal angiosperm Magnolia polytepala (Magnoliaceae). Planta 2023, 257, 4. [Google Scholar] [CrossRef] [PubMed]
- Schühly, W.; Khan, I.; Fischer, N.H. The ethnomedicinal uses of magnoliaceae from the southeastern United States as leads in drug discovery. Pharm. Biol. 2001, 39 (Suppl. S1), 63–69. [Google Scholar]
- Yu, C.; Yang, W.; Jiang, S.; Wang, T.; Yang, Z. Effects of star anise (Illicium verum Hook.f.) essential oil administration under three different dietary energy levels on growth performance, nutrient, and energy utilization in broilers. Anim. Sci. J. 2021, 92, e13496. [Google Scholar] [CrossRef]
- Hu, R.; Ning, L.; Nong, R.; Lu, S.; Liu, Y.; Tang, H. Chemical constituents of the essential oil extracted from Manglietia glauca. Chem. Nat. Compd. 2019, 55, 1133–1134. [Google Scholar] [CrossRef]
- Songsamoe, S.; Koomhin, P.; Matan, N. The effects of Michelia alba oil against mould on brown rice and assessing the brain response using electroencephalogram (EEG). J. Food Sci. Technol. 2021, 58, 1776–1787. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cabral, C.; Evora, L.; Ferreira, I.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical composition, anti-inflammatory activity and cytotoxicity of Thymus zygis L. subsp. sylvestris (Hoffmanns. & Link) Cout. essential oil and its main compounds. Arab. J. Chem. 2019, 12, 3236–3243. [Google Scholar]
- Silalahi, M.; Sutoyo, M.; Timur, C.J. Michelia alba DC (Botany, benefits and its essential oils). GSC Biol. Pharm. Sci. 2023, 22, 365–370. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, K.; Hou, M.; Li, Y.; Hou, X.; Dai, W.; Li, Z.; Zhao, J.; Liu, W.; Bai, Z. Schisandra chinensis essential oil attenuates acetaminophen-induced liver injury through alleviating oxidative stress and activating autophagy. Pharm. Biol. 2022, 60, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Chaudhary, N.; Uma Kumari, K.; Singh, J.; Tripathi, P.; Meena, A.; Luqman, S.; Yadav, A.; Chanotiya, C.S.; Pandey, G. Diversity of essential oil-secretory cells and oil composition in flowers and buds of Magnolia sirindhorniae and its biological activities. Chem. Biodivers. 2021, 18, e2000750. [Google Scholar] [CrossRef]
- Ha, C.T.; Thai, T.H.; Hien, N.T.; Anh, H.T.; Diep, L.N.; Thuy, D.T.; Nhat, D.D.; Setzer, W.N. Chemical composition and antimicrobial activity of the leaf and twig essential oils of Magnolia hypolampra growing in Na Hang Nature Reserve, Tuyen Quang Province of Vietnam. Nat. Prod. Commun. 2019, 14, 1934578X19860370. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, J.; Li, X.; Ning, Y.; Dai, C.-C. Endophytic bacterium-triggered reactive oxygen species directly increase oxygenous sesquiterpenoid content and diversity in Atractylodes lancea. Appl. Environ. Microbiol. 2016, 82, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Lan, S.; Peng, M.; Wang, Z.; Zhuang, L. The diversity of Ferula species and environmental factors on metabolite composition using untargeted metabolomics. Food Biosci. 2023, 56, 103075. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Banadka, A.; Nandhini, M.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.J.M. Essential oil from Coriandrum sativum: A review on its phytochemistry and biological activity. Molecules 2023, 28, 696. [Google Scholar] [CrossRef]
- Xianjin, S.; Sha, S.; Qi, M.; Zhang, Z.; Yang, Y.; Wu, H.; Li, L.; Wang, W.; Huang, A. Terpenoids from the barks of Magnolia maudiae (Dunn) Figlar. Nat. Prod. Res. 2018, 32, 1518–1524. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed MB, K.; Ezzat, M.O.; Majid AS, A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Da Silva Mota, J.; De Souza, D.S.; Boone, C.V.; Lima Cardoso, C.A.; Bastos Caramão, E. Identification of the volatile compounds of leaf, flower, root and stem oils of Piper amalago (Piperaceae). J. Essent. Oil Bear. Plants 2013, 16, 11–16. [Google Scholar] [CrossRef]
- Guobin, D.; Hanbo, Z.; Hongfen, X.; Shanna, C.; Xiaolan, C. Chemical Composition and Biological Activities of Essential Oil from the Rhizomes of Iris bulleyana. Agric. Sci. China 2009, 8, 691–696. [Google Scholar]
- Trindade, R.; Almeida, L.; Xavier, L.; Andrade, E.H.; Maia, J.G.; Mello, A.; Setzer, W.N.; Ramos, A.; Da Silva, J.K.R. Influence on Secondary Metabolism of Piper nigrum L. by Co-Inoculation with Arbuscular Mycorrhizal Fungi and Fusarium solani f. sp. piperis. Microorganisms 2021, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Gfeller, V.; Erb, M. Root volatiles in plant–plant interactions II: Root volatiles alter root chemistry and plant–herbivore interactions of neighbouring plants. Plant Cell Environ. 2019, 42, 1964–1973. [Google Scholar] [CrossRef]
- Cruz, L.; Santos, M.; Gama, B.; Araújo, L.; Terezan, A.; Oliveira Neto, J.; Cunha, L.; Oliveira, A.; Matos Da Silva, M.; Coelho, C.; et al. Profile of volatile compounds released by Waitea circinata against Magnaporthe oryzae under different periods and temperatures. Pesqui. Agropecuária Trop. 2023, 53, e75038. [Google Scholar] [CrossRef]
- Song, B.; Wang, W.; Liu, R.; Cai, J.; Jiang, Y.; Tang, X.; Wu, H.; Ao, H.; Chen, L. Geographic differentiation of essential oil from rhizome of cultivated Atractylodes lancea by using GC-MS and chemical pattern recognition analysis. Molecules 2023, 28, 2216. [Google Scholar] [CrossRef] [PubMed]
- Maral, H. Chemical and antioxidant diversity of essential oils of some Salvia species from Turkey. Biochem. Syst. Ecol. 2023, 106, 104575. [Google Scholar] [CrossRef]
- Ban, P.H.; Linh, L.D.; Huong, L.T.; Hoi, T.M.; Hung, N.H.; Dai, D.N.; Ogunwande, I.A. Mosquito larvicidal activity on Aedes albopictus and constituents of essential oils from Manglietia dandyi (Gagnep.) Dandy. Rec. Nat. Prod. 2020, 14, 201–206. [Google Scholar] [CrossRef]
- Könen, P.P.; Wüst, M. Analysis of sesquiterpene hydrocarbons in grape berry exocarp (Vitis vinifera L.) using in vivo-labeling and comprehensive two-dimensional gas chromatography–mass spectrometry (GC× GC–MS). Beilstein J. Org. Chem. 2019, 15, 1945–1961. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Kloukina, C.; Vassiliou, R.; Tomou, E.-M.; Skaltsa, H.; Tzortzakis, N. Cultivation strategy to improve chemical profile and antioxidant activity of Sideritis perfoliata L. subsp. perfoliata. Ind. Crops Prod. 2019, 140, 111694. [Google Scholar] [CrossRef]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Hairong, G.; Yaxin, W.; Chen, X.; Qing, L. Comparison and Analysis of Chemical Components and Their Antimicrobial Activity of Essential Oils from Three Artemisia Plants. Mod. Food Sci. Technol. 2020, 36, 262–268. [Google Scholar]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic studies on cancer cell interaction and its chemosensitization effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Phiri, N.; Serame, E.L.; Pheko, T. Extraction, chemical composition and antioxidant activity analysis of essential oil from Schinus molle medicinal plant found in Botswana. Am. J. Essent Oil Nat. Prod. 2021, 9, 1–9. [Google Scholar] [CrossRef]
- Sanna, D.; Delogu, G.; Mulas, M.; Schirra, M.; Fadda, A. Determination of free radical scavenging activity of plant extracts through DPPH assay: An EPR and UV–Vis study. Food Anal. Methods 2012, 5, 759–766. [Google Scholar] [CrossRef]
- Generalić, I.; Skroza, D.; Ljubenkov, I.; Katalinić, A.; Burčul, F.; Katalinić, V. Influence of the phenophase on the phenolic profile and antioxidant properties of Dalmatian sage. Food Chem. 2011, 127, 427–433. [Google Scholar] [CrossRef]
- Nonato CD, F.A.; Camilo, C.J.; Leite DO, D.; Da Nobrega MG, L.A.; Ribeiro-Filho, J.; De Menezes IR, A.; Tavares, J.F.; Da Costa, J.G.M. Comparative analysis of chemical profiles and antioxidant activities of essential oils obtained from species of Lippia L. by chemometrics. Food Chem. 2022, 384, 132614. [Google Scholar] [CrossRef]
- Parki, A.; Chaubey, P.; Prakash, O.; Kumar, R.; Pant, A.K. Seasonal variation in essential oil compositions and antioxidant properties of Acorus calamus L. accessions. Medicines 2017, 4, 81. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Sudipta, J.; Asit, R.; Ambika, S.; Bhusan, C.B.; Mohan, P.B.; Biswabhusan, D.; Sanghamitra, N.; Chandra, P.P. Chemical Composition and Antioxidant Activities of Essential oil from Leaf and Stem of Elettaria cardamomum from Eastern India. J. Essent. Oil Bear. Plants 2021, 24, 538–546. [Google Scholar]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Bisht, B.S.; Karki, G.; Padalia, R.C. Comparative analysis of the essential oil composition of wild and cultivated Valeriana jatamansi Jones. from Uttarakhand Himalaya. J. Essent. Oil Plant Compos. 2024. [Google Scholar] [CrossRef]
- Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant Therapy in Inflammatory Bowel Diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef]
- Císarová, M.; Tancinová, D.; Brodová, M. Antifungal activity of volatile components generated by essential oils against the genus penicillium isolated from bakery products. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 1. [Google Scholar] [CrossRef]
- Marzlan, A.A.; Muhialdin, B.J.; Abedin, N.H.Z.; Manshoor, N.; Ranjith, F.H.; Anzian, A.; Hussin, A.S.M. Incorporating torch ginger (Etlingera elatior Jack) inflorescence essential oil onto starch-based edible film towards sustainable active packaging for chicken meat. Ind. Crops Prod. 2022, 184, 115058. [Google Scholar] [CrossRef]
- Siroua, K.; El Ghallab, Y.; Mouss, R.A.; Kadiri, F.; Belamine, H.; El Kouali, M.; Kenz, A. Chemical composition of essential oil from invasive Moroccan Cyperus rotundus L., in vitro antimicrobial and antiradical activities, and in silico molecular docking of major compounds on drug efflux pumps. S. Afr. J. Bot. 2022, 147, 782–789. [Google Scholar] [CrossRef]
- Pu, Z.-H.; Zhang, Y.-Q.; Yin, Z.-Q.; Jiao, X.; Jia, R.-Y.; Yang, L.; Fan, Y. Antibacterial activity of 9-octadecanoic acid-hexadecanoic acid-tetrahydrofuran-3, 4-diyl ester from neem oil. Agric. Sci. China 2010, 9, 1236–1240. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Al-Reza, S.M.; Choi, U.K.; Lee, J.H.; Kang, S.C. Chemical composition, antibacterial and antioxidant activities of leaf essential oil and extracts of Metasequioa glyptostroboides Miki ex Hu. Food Chem. Toxicol. 2009, 47, 1876–1883. [Google Scholar] [CrossRef]
- Ogundajo, A.L.; Ewekeye, T.; Sharaibi, O.J.; Owolabi, M.S.; Dosoky, N.S.; Setzer, W.N. Antimicrobial Activities of Sesquiterpene-Rich Essential Oils of Two Medicinal Plants, Lannea egregia and Emilia sonchifolia, from Nigeria. Plants 2021, 10, 488. [Google Scholar] [CrossRef]
- Sieniawska, E.; Sawicki, R.; Golus, J.; Swatko-Ossor, M.; Ginalska, G.; Skalicka-Wozniak, K. Nigella damascena L. essential oil—A valuable source of β-elemene for antimicrobial testing. Molecules 2018, 23, 256. [Google Scholar] [CrossRef]
- Han, F.; Ma, G.Q.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.C.; Zhao, Z.D.; Xu, H.L. Chemical composition and antioxidant activities of essential oils from different parts of the oregano. J. Zhejiang Univ. 2017, 18, 79. [Google Scholar] [CrossRef]
- Alessandra, G.; Massimo, T.; Ilaria, C.; Alessandro, G.; Matteo, R.; Immacolata, M.; Guglielmo, P.; Gianni, S. A Comparative Study on Chemical Compositions and Biological Activities of Four Amazonian Ecuador Essential Oils: Curcuma longa L. (Zingiberaceae), Cymbopogon citratus (DC.) Stapf, (Poaceae), Ocimum campechianum Mill. (Lamiaceae), and Zingiber officinale Roscoe (Zingiberaceae). Antibiotics 2023, 12, 177. [Google Scholar] [CrossRef]
- Shen, X.; Chen, W.; Zheng, Y.; Lei, X.; Tang, M.; Wang, H.; Song, F. Chemical composition, antibacterial and antioxidant activities of hydrosols from different parts of Areca catechu L. and Cocos nucifera L. Ind. Crops Prod. 2017, 96, 110–119. [Google Scholar] [CrossRef]
- Song, F.; Tang, M.; Wang, H.; Zhang, Y.; Zhu, K.; Chen, X.; Chen, H.; Zhao, X. UHPLC-MS/MS identification, quantification of flavonoid compounds from Areca catechu L. extracts and in vitro evaluation of antioxidant and key enzyme inhibition properties involved in hyperglycemia and hypertension. Ind. Crops Prod. 2022, 189, 115787. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, X.; Luo, Z.; Cao, Y.; Liu, S.; Liu, Y. Metabolomic and transcriptomic analyses reveal comparisons against liquid-state fermentation of primary dark tea, green tea and white tea by Aspergillus cristatus. Food Res. Int. 2023, 172, 113115. [Google Scholar] [CrossRef] [PubMed]
| Free Radical | Sample | |||||
|---|---|---|---|---|---|---|
| M-maudiae | M-hedyosperma | M-macclurei | M-lucida | M-conifera | Ascorbic Acid | |
| ABTS+ | 1283.58 | 1694.57 | 2918.61 | 1387.45 | 69.42 | 4761.25 |
| DPPH | 6258.32 | 14,028.60 | 21,341.98 | 7988.74 | 12,279.51 | 19,486.27 |
| Microbial Strain | Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|---|
| M. maudiae | M. hedyosperma | M. macclurei | M. lucida | M. conifera | |
| Salmonella enteritidis | 26.92 ± 0.46 Aa | 16.71 ± 0.43 Ad | 22.92 ± 0.46 Ab | 23.63 ± 0.55 Ab | 18.04 ± 0.41 Ac |
| Escherichia coli | 7.61 ± 0.02 Cc | 6.96 ± 0.40 Bc | 8.55 ± 0.93 Db | 10.29 ± 0.28 Ba | 7.33 ± 0.30 Cc |
| Staphylococcus aureus | 7.87 ± 0.27 Cc | 6.57 ± 0.37 Bd | 15.78 ± 0.18 Ba | 6.57 ± 0.37 Dd | 8.61 ± 0.12 Bb |
| Bacillus subtilis | 15.03 ± 0.74 Ba | 6.41 ± 0.46 Be | 12.91 ± 0.26 Cb | 9.52 ± 0.22 Cc | 8.38 ± 0.73 Bd |
| Microbial strain | LC50(mg/mL) | ||||
| M. maudiae | M. hedyosperma | M. macclurei | M. lucida | M. conifera | |
| Salmonella enteritidis | 0.03 | 0.09 | 0.02 | 0.02 | 0.11 |
| Escherichia coli | 0.64 | 1.48 | 0.41 | 0.32 | 0.67 |
| Staphylococcus aureus | 2.28 | 2.62 | 0.78 | 1.79 | 2.28 |
| Bacillus subtilis | 0.07 | 2.46 | 0.28 | 1.00 | 2.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Ma, D.; Song, Z.; Yang, M.; Xu, Y. The Composition, Antioxidant and Antibacterial Activity of Essential Oils from Five Species of the Magnoliaceae Family. Molecules 2024, 29, 5182. https://doi.org/10.3390/molecules29215182
Yang D, Ma D, Song Z, Yang M, Xu Y. The Composition, Antioxidant and Antibacterial Activity of Essential Oils from Five Species of the Magnoliaceae Family. Molecules. 2024; 29(21):5182. https://doi.org/10.3390/molecules29215182
Chicago/Turabian StyleYang, Dandan, Daocheng Ma, Ziqi Song, Mei Yang, and Yuanyuan Xu. 2024. "The Composition, Antioxidant and Antibacterial Activity of Essential Oils from Five Species of the Magnoliaceae Family" Molecules 29, no. 21: 5182. https://doi.org/10.3390/molecules29215182
APA StyleYang, D., Ma, D., Song, Z., Yang, M., & Xu, Y. (2024). The Composition, Antioxidant and Antibacterial Activity of Essential Oils from Five Species of the Magnoliaceae Family. Molecules, 29(21), 5182. https://doi.org/10.3390/molecules29215182







