Saponin Molecules from Quinoa Residues: Exploring Their Surfactant, Emulsifying, and Detergent Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction and Characterization of Quinoa Saponin
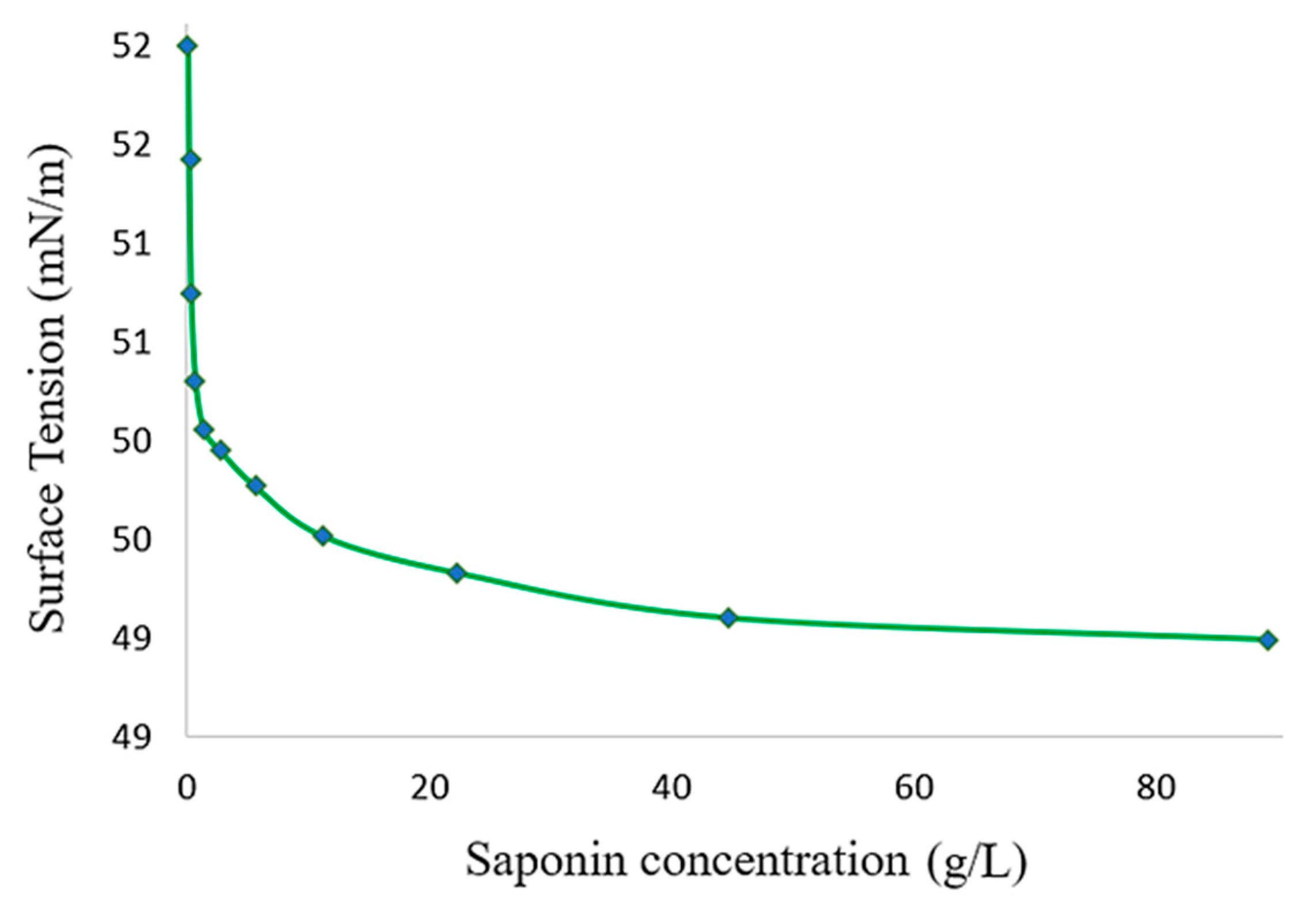
2.2. Determination of Critical Micelle Concentration (CMC)
2.3. Determination of Emulsifying Index
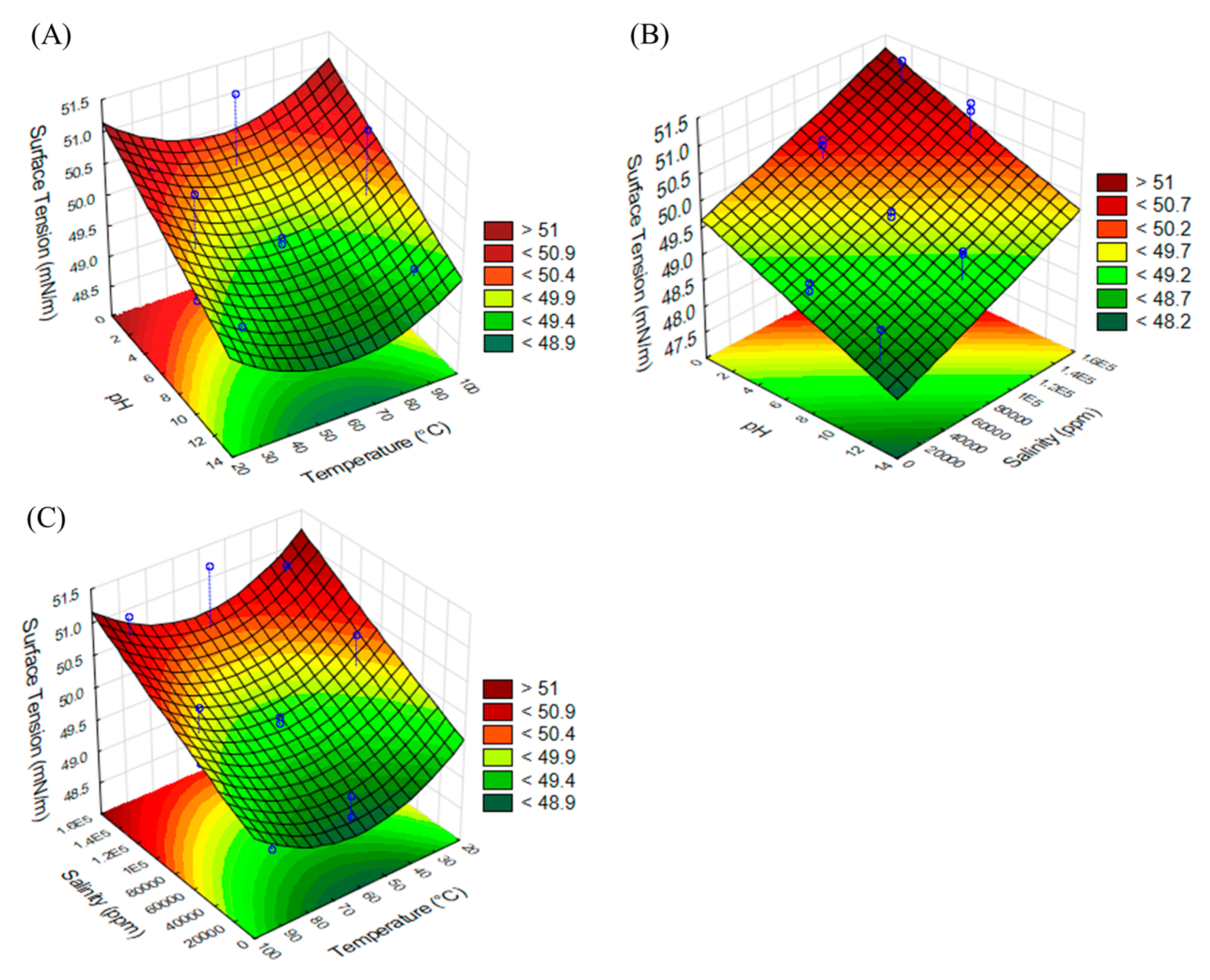
2.4. Experimental Design for Optimization of Emulsifying and Tensioactive Properties
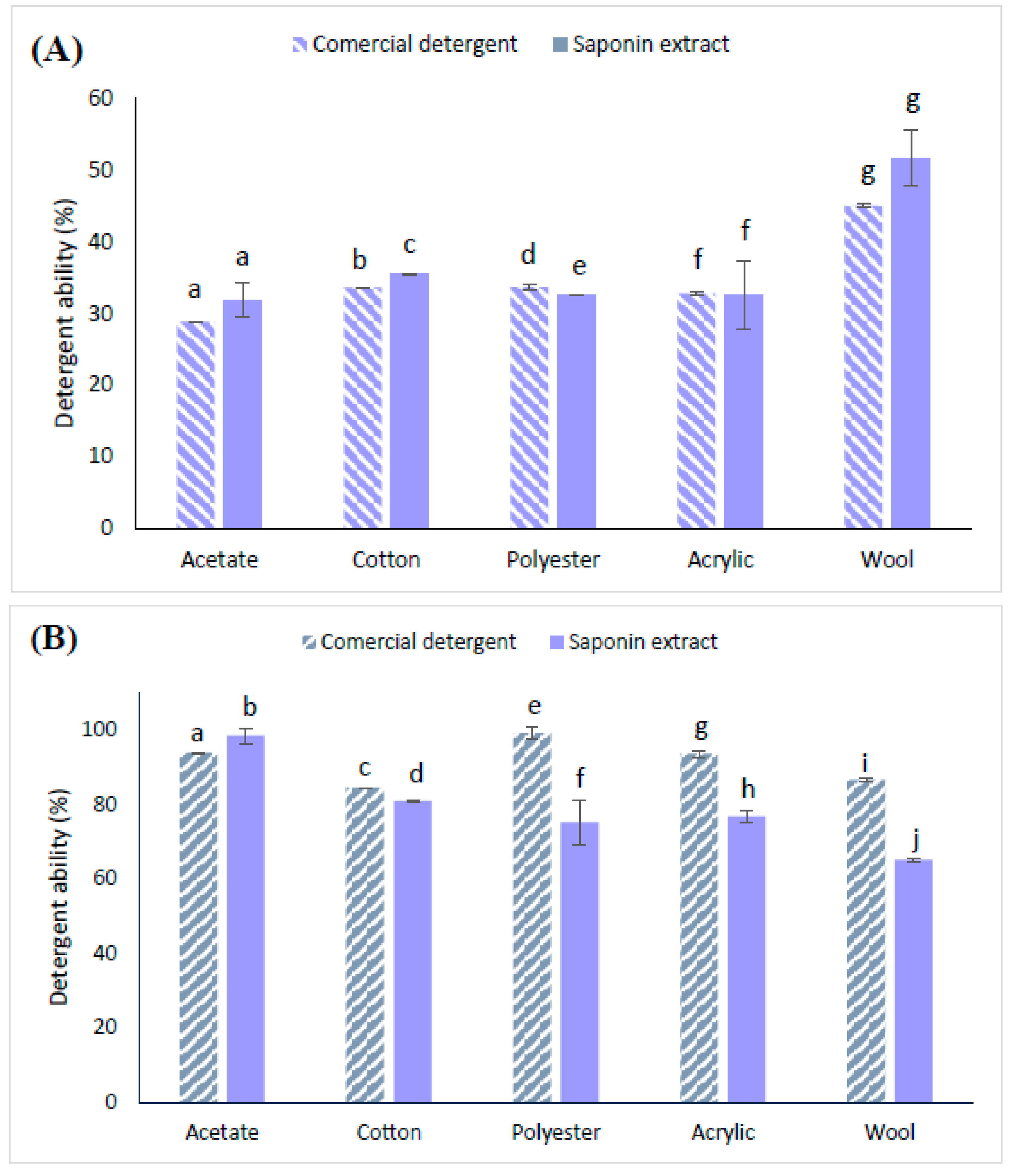
2.5. Results of the Detergency Test
3. Materials and Methods
3.1. Material and Chemicals
3.2. Saponin Extract from Quinoa Powder
3.3. Analysis of Saponin Extract
3.4. Surface Tension and Critical Micelle Concentration (CMC)
3.5. Emulsifying Properties of Saponin Extract
3.6. Effect of Temperature, pH, and Salinity on Emulsifying and Tensioactive Properties of Saponin Extract
3.7. Detergency Test
3.8. AI-Assisted Tool
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camacho-Muñoz, D.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of Surfactants in Wastewater: Hourly and Seasonal Variations in Urban and Industrial Wastewaters from Seville (Southern Spain). Sci. Total Environ. 2014, 468–469, 977–984. [Google Scholar] [CrossRef]
- Bautista-Toledo, M.I.; Rivera-Utrilla, J.; Méndez-Díaz, J.D.; Sánchez-Polo, M.; Carrasco-Marín, F. Removal of the Surfactant Sodium Dodecylbenzenesulfonate from Water by Processes Based on Adsorption/Bioadsorption and Biodegradation. J. Colloid Interface Sci. 2014, 418, 113–119. [Google Scholar] [CrossRef]
- Mungray, A.K.; Kumar, P. Fate of Linear Alkylbenzene Sulfonates in the Environment: A Review. Int. Biodeterior. Biodegrad. 2009, 63, 981–987. [Google Scholar] [CrossRef]
- Moura, A.G.L.; Centurion, V.B.; Okada, D.Y.; Motteran, F.; Delforno, T.P.; Oliveira, V.M.; Varesche, M.B.A. Laundry Wastewater and Domestic Sewage Pilot-Scale Anaerobic Treatment: Microbial Community Resilience Regarding Sulfide Production. J. Environ. Manag. 2019, 251, 109495. [Google Scholar] [CrossRef]
- Makkar, R.S.; Cameotra, S.S.; Banat, I.M. Advances in Utilization of Renewable Substrates for Biosurfactant Production. AMB Express 2011, 1, 5. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential Commercial Applications of Microbial Surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Carnevale Miino, M.; Baldi, M.; Manzi, S.; Abbà, A.; Bertanza, G. Removal of Non-Ionic and Anionic Surfactants from Real Laundry Wastewater by Means of a Full-Scale Treatment System. Process Saf. Environ. Prot. 2019, 132, 105–115. [Google Scholar] [CrossRef]
- Ramprasad, C.; Philip, L. Surfactants and Personal Care Products Removal in Pilot Scale Horizontal and Vertical Flow Constructed Wetlands While Treating Greywater. Chem. Eng. J. 2016, 284, 458–468. [Google Scholar] [CrossRef]
- Hao, M.; Qiu, M.; Yang, H.; Hu, B.; Wang, X. Recent Advances on Preparation and Environmental Applications of MOF-Derived Carbons in Catalysis. Sci. Total Environ. 2021, 760, 143333. [Google Scholar] [CrossRef]
- Yao, L.; Yang, H.; Chen, Z.; Qiu, M.; Hu, B.; Wang, X. Bismuth Oxychloride-Based Materials for the Removal of Organic Pollutants in Wastewater. Chemosphere 2021, 273, 128576. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Wang, H.; Yang, H.; Wen, T.; Wang, S.; Hu, B.; Wang, X. Removal of Organic Compounds by Nanoscale Zero-Valent Iron and Its Composites. Sci. Total Environ. 2021, 792, 148546. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent Developments of Doped G-C3N4 Photocatalysts for the Degradation of Organic Pollutants. Crit. Rev. Environ. Sci. Technol. 2020, 51, 751–790. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T.A. Environmental Risks and Toxicity of Surfactants: Overview of Analysis, Assessment, and Remediation Techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef] [PubMed]
- Jarzębski, M.; Smułek, W.; Siejak, P.; Kobus-Cisowska, J.; Pieczyrak, D.; Baranowska, H.M.; Jakubowicz, J.; Sopata, M.; Białopiotrowicz, T.; Kaczorek, E. Aesculus hippocastanum L. Extract as a Potential Emulsion Stabilizer. Food Hydrocoll. 2019, 97, 105237. [Google Scholar] [CrossRef]
- Clien, S.Y.; Lu, W.B.; Wei, Y.H.; Chen, W.M.; Chang, J.S. Improved Production of Biosurfactant with Newly Isolated Pseudomonas aeruginosa S2. Biotechnol. Prog. 2007, 23, 661–666. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Patel, M.K.; Theiß, A.; Worrell, E. Surfactant Production and Use in Germany: Resource Requirements and CO2 Emissions. Resour. Conserv. Recycl. 1999, 25, 61–78. [Google Scholar] [CrossRef]
- Saravanakumari, P.; Mani, K. Structural Characterization of a Novel Xylolipid Biosurfactant from Lactococcus Lactis and Analysis of Antibacterial Activity against Multi-Drug Resistant Pathogens. Bioresour. Technol. 2010, 101. [Google Scholar] [CrossRef]
- Böttcher, S.; Drusch, S. Interfacial Properties of Saponin Extracts and Their Impact on Foam Characteristics. Food Biophys. 2016, 11, 91–100. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Wink, M. Biological Activities and Chemistry of Saponins from Chenopodium quinoa Willd. Phytochem. Rev. 2009, 8. [Google Scholar] [CrossRef]
- Gordillo-Bastidas, E.; Díaz-Rizzolo, D.A.; Roura, E.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd), from Nutritional Value to Potential Health Benefits: An Integrative Review. J. Nutr. Food Sci. 2016, 6, 3. [Google Scholar] [CrossRef]
- Kimura, E.; Islam, M.A. Seed Scarification Methods and Their Use in Forage Legumes. Res. J. Seed Sci. 2012, 5. [Google Scholar] [CrossRef]
- Ciaciuch, A.; Wejnerowska, G.; Gozdecka, G. Mechanical Scarification of Quinoa Seeds (Chenopodium quinoa Willd.) and Obtaining Oil from the Seed Coat by Supercritical CO2 Extraction. Carpathian J. Food Sci. Technol. 2021, 13. [Google Scholar] [CrossRef]
- Gavrila, A.I.; Tatia, R.; Seciu-Grama, A.M.; Tarcomnicu, I.; Negrea, C.; Calinescu, I.; Zalaru, C.; Moldovan, L.; Raiciu, A.D.; Popa, I. Ultrasound Assisted Extraction of Saponins from Hedera helix L. and an In Vitro Biocompatibility Evaluation of the Extracts. Pharmaceuticals 2022, 15, 1197. [Google Scholar] [CrossRef]
- Espinoza, C.R.; Ruiz, C.A.J.; Ramos, O.P.F.; Solano, M.A.Q.; Quiñonez, G.H.; Mallma, N.E.S. Optimization of the Ultrasoud-Assisted Extraction of Saponins from Quinoa (Chenopodium quinoa Wild) Using Response Surface Methodology. Acta Sci. Pol. Technol. Aliment. 2021, 20, 17–23. [Google Scholar] [CrossRef]
- Li, H.; Zhai, B.; Sun, J.; Fan, Y.; Zou, J.; Cheng, J.; Zhang, X.; Shi, Y.; Guo, D. Ultrasound-Assisted Extraction of Total Saponins from Aralia Taibaiensis: Process Optimization, Phytochemical Characterization, and Mechanism of α-Glucosidase Inhibition. Drug Des. Devel Ther. 2022, 16, 83. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M.; et al. Exceptional Antioxidant, Non-Cytotoxic Activity of Integral Lemon Pectin from Hydrodynamic Cavitation. ChemistrySelect 2020, 5, 5066–5071. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa Bean Shell Waste Valorisation; Extraction from Lab to Pilot-Scale Cavitational Reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Nascimento, L.B.d.S.; De Carlo, A.; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Terán Hilares, R.; dos Santos, J.G.; Shiguematsu, N.B.; Ahmed, M.A.; da Silva, S.S.; Santos, J.C. Low-Pressure Homogenization of Tomato Juice Using Hydrodynamic Cavitation Technology: Effects on Physical Properties and Stability of Bioactive Compounds. Ultrason. Sonochem. 2019, 54, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; More, P.R.; Das, T.; Hilares, R.T.; Pereira, B.; Arantes, V.; da Silva, S.S.; Santos, J.C. dos Effect of Hydrodynamic Cavitation Processing on Orange Juice Physicochemical and Nutritional Properties. J. Agric. Food Res. 2023, 14, 100781. [Google Scholar] [CrossRef]
- Lozano, M.; Tícona, E.; Carrasco, C.; Flores, Y.; Almanza, G.R. Cuantificación de Saponinas en Residuos de Quinua Real Chenopodium quinoa Willd. Rev. Boliv. Química 2012, 29, 131–138. [Google Scholar]
- Cazals, F.; Huguenot, D.; Crampon, M.; Colombano, S.; Betelu, S.; Galopin, N.; Perrault, A.; Simonnot, M.O.; Ignatiadis, I.; Rossano, S. Production of Biosurfactant Using the Endemic Bacterial Community of a PAHs Contaminated Soil, and Its Potential Use for PAHs Remobilization. Sci. Total Environ. 2020, 709, 136143. [Google Scholar] [CrossRef]
- Ramesh, M.; Sakthishobana, K. Significance of Biosurfactants in Oil Recovery and Bioremediation of Crude Oil. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Biosurfactants for the Bioremediation of Polluted Environments; Elsevier: Amsterdam, The Netherlands, 2021; pp. 211–226. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Mulligan, C.N. The Production of Surfactin by Bacillus Subtilis Grown on Peat Hydrolysate. Appl. Microbiol. Biotechnol. 1987, 27, 110–116. [Google Scholar] [CrossRef]
- Meshram, P.D.; Shingade, S.; Madankar, C.S. Comparative Study of Saponin for Surfactant Properties and Potential Application in Personal Care Products. Mater. Today Proc. 2021, 45, 5010–5013. [Google Scholar] [CrossRef]
- Mitra, S.; Dungan, S.R. Micellar Properties of Quillaja saponin. 1. Effects of Temperature, Salt, and PH on Solution Properties. J. Agric. Food Chem. 1997, 45. [Google Scholar] [CrossRef]
- Verza, S.G.; De Resende, P.E.; Kaiser, S.; Quirici, L.; Teixeira, H.F.; Gosmann, G.; Ferreira, F.; Ortega, G.G. Micellar Aggregates of Saponins from Chenopodium quinoa: Characterization by Dynamic Light Scattering and Transmission Electron Microscopy. Pharmazie 2012, 67, 288–292. [Google Scholar] [CrossRef]
- Chen, Y.F.; Yang, C.H.; Chang, M.S.; Ciou, Y.P.; Huang, Y.C. Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Durvala, I.J.B.; Silva, I.A.; Almeida, F.C.G.; Melo, Y.T.F.; Rufino, R.D.; Sarubbo, L.A. Emulsifying Capacity of Biosurfactants from Chenopodium quinoa and Pseudomonas aeruginosa Ucp 0992 with Focus of Application in the Cosmetic Industry. Chem. Eng. Trans. 2020, 79, 211–216. [Google Scholar] [CrossRef]
- Sotomayor-Gerding, D.; Morales, E.; Rubilar, M. Comparison between Quinoa and Quillaja saponins in the Formation, Stability and Digestibility of Astaxanthin-Canola Oil Emulsions. Colloids Interfaces 2022, 6, 43. [Google Scholar] [CrossRef]
- Ulaganathan, V.; Del Castillo, L.; Webber, J.L.; Ho, T.T.M.; Ferri, J.K.; Krasowska, M.; Beattie, D.A. The Influence of PH on the Interfacial Behaviour of Quillaja Bark Saponin at the Air-Solution Interface. Colloids Surf. B Biointerfaces 2019, 176. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Alviano, D.S.; Barreto, D.W.; Coelho, M.A.Z. Functional Properties of Saponins from Sisal (Agave sisalana) and Juá (Ziziphus joazeiro): Critical Micellar Concentration, Antioxidant and Antimicrobial Activities. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 736–743. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Water-Oil Interfacial Tension (IFT) Reduction and Wettability Alteration in Surfactant Flooding Process Using Extracted Saponin from Anabasis Setifera Plant. J. Pet. Sci. Eng. 2020, 189. [Google Scholar] [CrossRef]
- Yang, C.H.; Huang, Y.C.; Chen, Y.F.; Chang, M.H. Foam Properties, Detergent Abilities and Long-Term Preservative Efficacy of the Saponins from Sapindus mukorossi. J. Food Drug Anal. 2010, 18. [Google Scholar] [CrossRef]
- Flores Alarcón, M.A.D.; Arenas Jarro, R.Y.; Ahmed, M.A.; García Bustos, K.A.; Pacheco Tanaka, D.A.; Terán Hilares, R. Intensification of Red-G Dye Degradation Used in the Dyeing of Alpaca Wool by Advanced Oxidation Processes Assisted by Hydrodynamic Cavitation. Ultrason. Sonochem. 2022, 89, 106144. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Hamanaka, H.; Odaka, Y. A Color Reaction of Panaxadiol with Vanillin and Sulfuric Acid. Planta Med. 1975, 28, 131–138. [Google Scholar] [CrossRef]
- Makkar, H. Quantification of Tannins in Tree Foliage: A Laboratory Manual for the FAO/IAEA Co-Ordinated Research Project on Use of Nuclear and Related Techniques to Develop Simple Tannin Assays for Predicting and Improving the Safety and Efficiency of Feeding Ruminants on the Tanniferous Tree Foliage. Jt. FAO/IAEA Div. Nucl. Tech. Food Agric. 2000, 1–29. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Assay of Protein. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacillus Species. Appl. Environ. Microbiol. 1987, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhuniskenov, Y.; Sabirova, A.; Serikov, G.; Abbas, A.H.; Pourafshary, P. Impact of the Naturally Driven Surfactant in EOR Application: Experimental, Microscopic, and Numerical Analyses. ACS Omega 2024, 9, 1327. [Google Scholar] [CrossRef] [PubMed]
- Azdarpour, A.; Norouzpour, M.; Nabipour, M.; Santos, R.M.; Mohammadian, E. Efficiency of the Green Surfactant Derived from Avena Sativa Plant in the Presence of Different Salts for EOR Purposes. J. GeoEnergy 2023, 9998466. [Google Scholar] [CrossRef]
- Maurad, Z.A.; Ghazali, R.; Siwayanan, P.; Ismail, Z.; Ahmad, S. Alpha-Sulfonated Methyl Ester as an Active Ingredient in Palm-Based Powder Detergents. J. Surfactants Deterg. 2006, 9, 161–167. [Google Scholar] [CrossRef]
- Tanthakit, P.; Ratchatawetchakul, P.; Chavadej, S.; Scamehorn, J.F.; Sabatini, D.A.; Tongcumpou, C. Palm Oil Removal from Fabric Using Microemulsion-Based Formulations. J. Surfactants Deterg. 2010, 13, 485–495. [Google Scholar] [CrossRef]
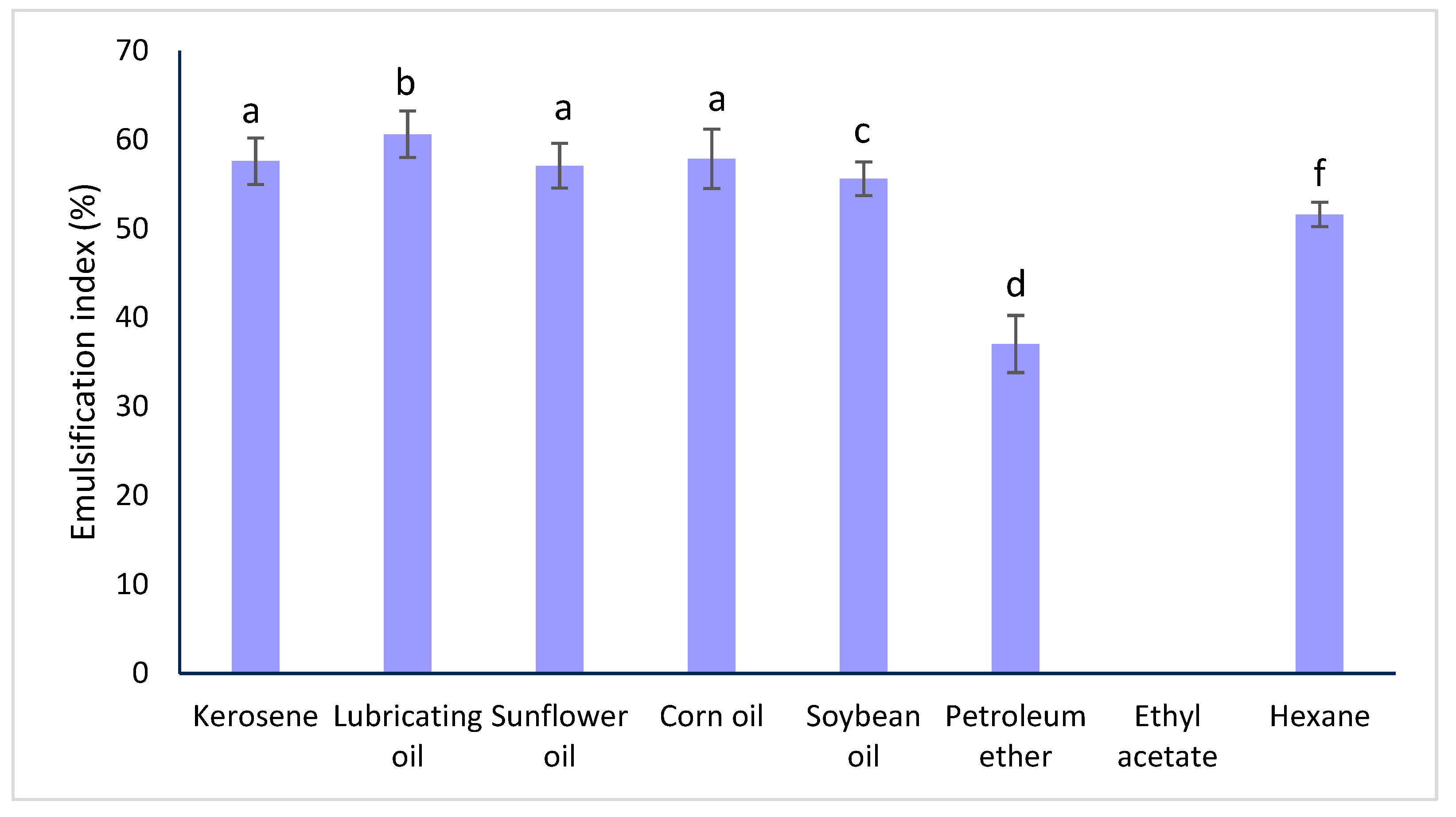

| Substrate | Emulsification Index After Different Times (h) | ||
|---|---|---|---|
| 24 | 48 | 72 | |
| Kerosene | 57.58 ± 2.62 a | 54.88 ± 0.58 a | 53.79 ± 1.31 a |
| Lubricating oil | 60.61 ± 2.62 b | 52.46 ± 2.50 b | 50.43 ± 0.74 b |
| Sunflower oil | 57.07 ± 2.52 a | 55.48 ± 1.85 c | 53.12 ± 2.41 a |
| Corn oil | 57.86 ± 3.32 a | 54.25 ± 1.74 a | 53.49 ± 2.33 a |
| Soybean oil | 55.63 ± 1.91 c | 54.32 ± 0.59 a | 53.22 ± 1.54 a |
| Petroleum ether | 37.04 ± 3.21 d | 53.82 ± 1.25 a | 37.52 ± 1.18 c |
| Ethyl acetate | 0.00 ± 0.00 e | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| Hexane | 51.59 ± 1.36 f | 49.07 ± 1.60 e | 58.75 ± 0.72 e |
| Run | Experimental Variables | Response Variables | |||
|---|---|---|---|---|---|
| pH | Temperature (°C) | Salinity (ppm) | Surface Tension (mN/m) | Emulsification Index (%) | |
| 1 | 12 | 90 | 80,000 | 49.57 | 67.50 |
| 2 | 2 | 90 | 80,000 | 50.42 | 34.00 |
| 3 | 7 | 30 | 10,000 | 49.22 | 61.00 |
| 4 | 7 | 60 | 80,000 | 49.49 | 63.50 |
| 5 | 7 | 90 | 150,000 | 51.04 | 49.50 |
| 6 | 7 | 30 | 150,000 | 50.9 | 66.30 |
| 7 | 2 | 60 | 150,000 | 51.33 | 53.10 |
| 8 | 7 | 60 | 80,000 | 49.66 | 64.00 |
| 9 | 7 | 60 | 80,000 | 49.76 | 59.00 |
| 10 | 7 | 90 | 10,000 | 49.08 | 58.70 |
| 11 | 2 | 30 | 80,000 | 50.52 | 54.70 |
| 12 | 2 | 60 | 10,000 | 49.33 | 61.00 |
| 13 | 12 | 60 | 150,000 | 49.01 | 64.70 |
| 14 | 12 | 30 | 80,000 | 49.61 | 30.00 |
| 15 | 12 | 60 | 10,000 | 49.01 | 60.80 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 7.96 | 5 | 1.59 | 28.85 | <0.0001 | Significant |
| A—pH | 2.42 | 1 | 2.42 | 43.83 | <0.0001 | |
| B—Temperature | 0.01 | 1 | 0.01 | 0.04 | 0.838 | |
| C—Salinity | 3.98 | 1 | 3.98 | 72.02 | <0.0001 | |
| AC | 1 | 1 | 1 | 18.11 | 0.002 | |
| B² | 0.57 | 1 | 0.57 | 10.25 | 0.011 | |
| Residual | 0.50 | 9 | 0.06 | |||
| Lack of Fit | 0.46 | 7 | 0.07 | 3.52 | 0.239 | not significant |
| Pure Error | 0.04 | 2 | 0.02 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 1525.75 | 9 | 169.53 | 4.93 | 0.0469 | significant |
| A—pH | 51 | 1 | 51 | 1.48 | 0.2778 | |
| B—Temperature | 0.66 | 1 | 0.66 | 0.019 | 0.8952 | |
| C—Salinity | 7.8 | 1 | 7.8 | 0.23 | 0.6541 | |
| AB | 846.81 | 1 | 846.81 | 24.61 | 0.0042 | |
| A² | 172.62 | 1 | 172.62 | 5.02 | 0.0752 | |
| B² | 228.25 | 1 | 228.25 | 6.63 | 0.0497 | |
| C² | 111.19 | 1 | 111.19 | 3.23 | 0.1322 | |
| Residual | 172.07 | 5 | 34.41 | |||
| Lack of Fit | 161.95 | 3 | 53.98 | 10.66 | 0.087 | not significant |
| Pure Error | 10.13 | 2 | 5.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos, K.A.G.; Muñoz, S.S.; da Silva, S.S.; Alarcon, M.A.D.F.; dos Santos, J.C.; Andrade, G.J.C.; Hilares, R.T. Saponin Molecules from Quinoa Residues: Exploring Their Surfactant, Emulsifying, and Detergent Properties. Molecules 2024, 29, 4928. https://doi.org/10.3390/molecules29204928
Bustos KAG, Muñoz SS, da Silva SS, Alarcon MADF, dos Santos JC, Andrade GJC, Hilares RT. Saponin Molecules from Quinoa Residues: Exploring Their Surfactant, Emulsifying, and Detergent Properties. Molecules. 2024; 29(20):4928. https://doi.org/10.3390/molecules29204928
Chicago/Turabian StyleBustos, Kiara A. García, Salvador Sanchez Muñoz, Silvio S. da Silva, Miguel A. D. Flores Alarcon, Júlio C. dos Santos, Gilberto J. Colina Andrade, and Ruly Terán Hilares. 2024. "Saponin Molecules from Quinoa Residues: Exploring Their Surfactant, Emulsifying, and Detergent Properties" Molecules 29, no. 20: 4928. https://doi.org/10.3390/molecules29204928
APA StyleBustos, K. A. G., Muñoz, S. S., da Silva, S. S., Alarcon, M. A. D. F., dos Santos, J. C., Andrade, G. J. C., & Hilares, R. T. (2024). Saponin Molecules from Quinoa Residues: Exploring Their Surfactant, Emulsifying, and Detergent Properties. Molecules, 29(20), 4928. https://doi.org/10.3390/molecules29204928










