Opportunities and Challenges of Multi-Ion, Dual-Ion and Single-Ion Intercalation in Phosphate-Based Polyanionic Cathodes for Zinc-Ion Batteries
Abstract
1. Introduction
2. Ion Insertion Mechanism of Rechargeable Zinc Batteries
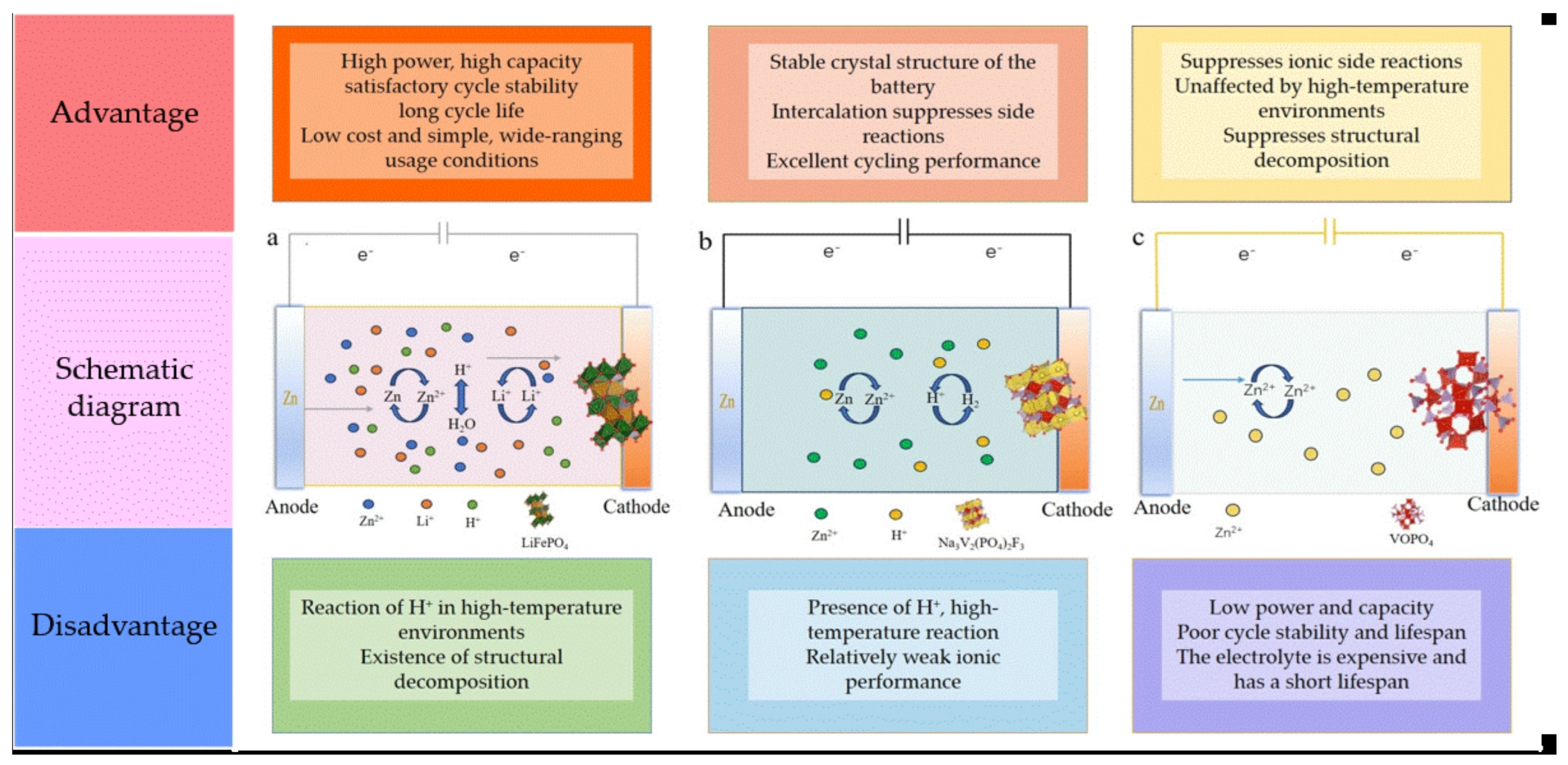
2.1. Multi-Ion Insertion Mechanism of Rechargeable Zinc Batteries
2.2. Dual-Ion Insertion Mechanism of Rechargeable Zinc Batteries
2.3. Mechanism of Single-Ion Insertion in Rechargeable Zinc Batteries
3. Ionic Properties of Rechargeable Zinc Batteries
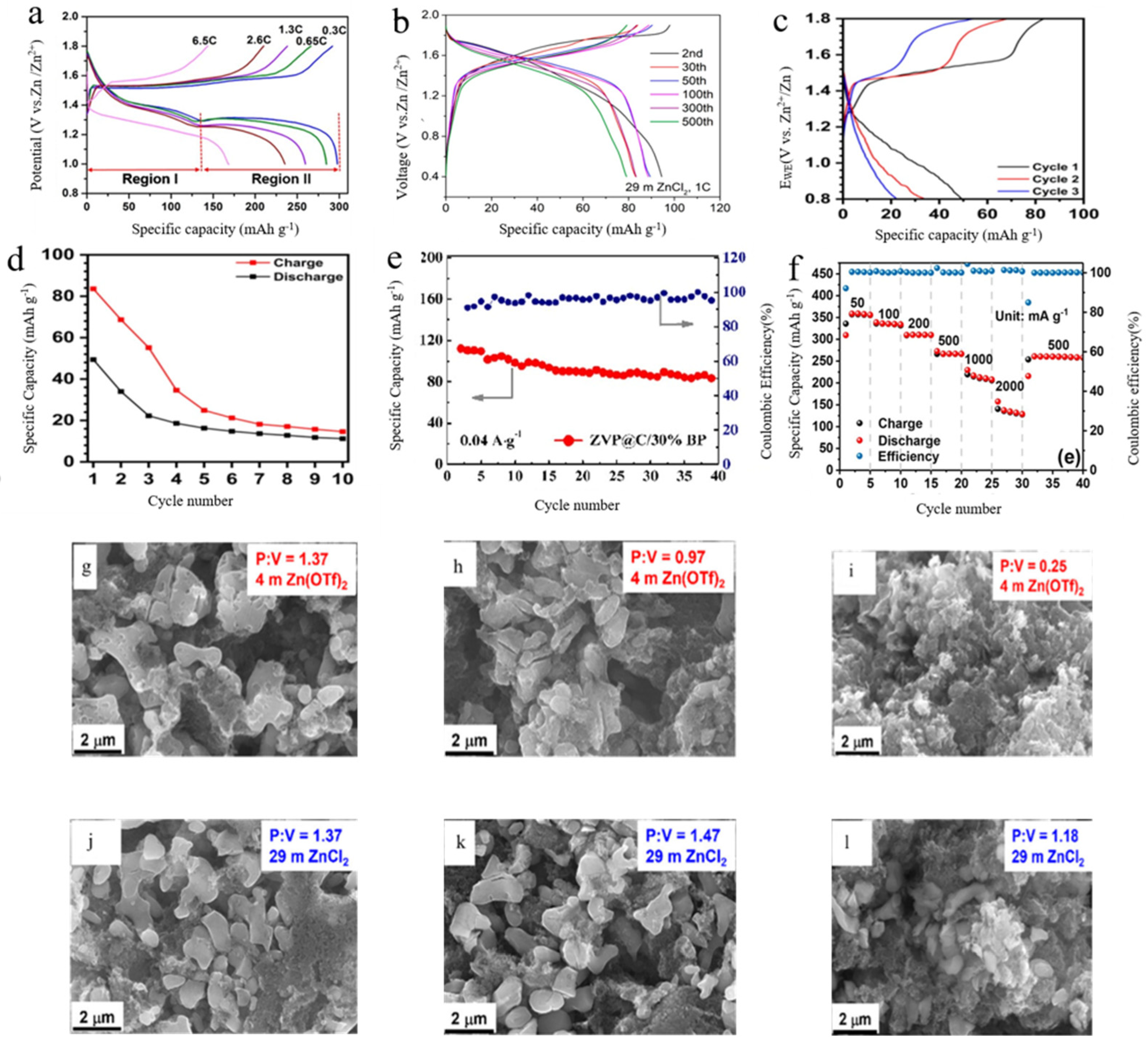
3.1. Multi-Ion Interaction Properties
3.2. Dual Ionization Properties
3.3. Single-Ion Performance
4. Opportunities and Challenges
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pan, X.; Shao, T.; Zheng, X.; Zhang, Y.; Ma, X.; Zhang, Q. Energy and Sustainable Development Nexus: A Review. Energy Strategy Rev. 2023, 47, 101078. [Google Scholar] [CrossRef]
- Ding, K.; Jiang, T.; Peng, J.; Wang, P.; Gou, W.; Xu, Q.; Fan, Q.; Wang, W.; Sun, Y. Recent Advances of Na3V2(PO4)3 as Cathode for Rechargeable Zinc-Based Batteries. Carbon Lett. 2023, 33, 989–1012. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Yang, T.; Liang, S.; Yang, X. Cooperation Behavior between Heterogeneous Cations in Hybrid Batteries. Chem. Commun. 2013, 49, 9977–9979. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Dai, S.; Yu, J.; Wu, S.; Wang, J. Research on Status and Prospects of Battery Energy Storage Stations on Energy Internet. In Proceedings of the 2019 IEEE 3rd Information Technology, Networking, Electronic and Automation Control Conference (ITNEC), Chengdu, China, 15–17 March 2019. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable Batteries: Technological Advancement, Challenges, Current and Emerging Applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.; Wang, W.; Bayhan, Z.; Alshareef, H.N. Status of Rechargeable Potassium Batteries. Nano Energy 2021, 83, 105792. [Google Scholar] [CrossRef]
- He, J.; Tao, T.; Yang, F.; Sun, Z. Unravelling Li+ Intercalation Mechanism and Cathode Electrolyte Interphase of Na3V2(PO4)3 and Na3(VOPO4)2F Cathode as Robust Framework Towards High-Performance Lithium-Ion Batteries. ChemSusChem 2022, 15, e202200817. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 Battery Chemistry With H+ and Zn2+ Coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Bin, D.; Du, Y.; Yang, B.; Lu, H.; Liu, Y.; Xia, Y. Progress of Phosphate-Based Polyanion Cathodes for Aqueous Rechargeable Zinc Batteries. Adv. Funct. Mater. 2023, 33, 2211765. [Google Scholar] [CrossRef]
- Yi, X.; Feng, Y.; Rao, A.M.; Zhou, J.; Wang, C.; Lu, B. Quasi-Solid Aqueous Electrolytes for Low-Cost Sustainable Alkali-Metal Batteries. Adv. Mater. 2023, 35, 2302280. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, Q.; Zhou, M.; Huang, X.; Sun, Y.; Duan, X.; Zhang, L.; Li, H.; Su, D.; Jia, B.; et al. Synergy of Dendrites-Impeded Atomic Clusters Dissociation and Side Reactions Suppressed Inert Interface Protection for Ultrastable Zn Anode. Adv. Mater. 2024, 36, 2400237. [Google Scholar] [CrossRef]
- Kundu, D.; Vajargah, S.H.; Wan, L.; Adams, B.; Prendergast, D.; Nazar, L.F. Aqueous vs. Nonaqueous Zn-Ion Batteries: Consequences of the Desolvation Penalty at the Interface. Energy Environ. Sci. 2018, 11, 881–892. [Google Scholar] [CrossRef]
- Gao, Y.; Li, G.; Wang, F.; Chu, J.; Yu, P.; Wang, B.; Zhan, H.; Song, Z. A High-Performance Aqueous Rechargeable Zinc Battery Based on Organic Cathode Integrating Quinone and Pyrazine. Energy Storage Mater. 2021, 40, 31–40. [Google Scholar] [CrossRef]
- Zhao, L.N.; Zhang, T.; Zhao, H.L.; Hou, Y.L. Polyanion-Type Electrode Materials for Advanced Sodium-Ion Batteries. Mater. Today Nano 2020, 10, 100072. [Google Scholar] [CrossRef]
- Lv, Z.; Ling, M.; Yue, M.; Li, X.; Song, M.; Zheng, Q.; Zhang, H. Vanadium-Based Polyanionic Compounds as Cathode Materials for Sodium-Ion Batteries: Toward High-Energy and High-Power Applications. J. Energy Chem. 2021, 55, 361–390. [Google Scholar] [CrossRef]
- Guo, J.-Z.; Gu, Z.-Y.; Du, M.; Zhao, X.-X.; Wang, X.-T.; Wu, X.-L. Emerging Characterization Techniques for Delving Polyanion-Type Cathode Materials of Sodium-Ion Batteries. Mater. Today 2023, 66, 221–244. [Google Scholar] [CrossRef]
- Li, W.; Jing, X.; Jiang, K.; Wang, D. Observation of Structural Decomposition of Na3V2(PO4)3 and Na3V2(PO4)2F3 as Cathodes for Aqueous Zn-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 2797–2807. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, C.; Ye, F.; Zhang, L.; Jiang, L.; Liu, Q.; Dong, H.; Sun, Z.; Hu, L. Bilayered VOPO4⋅2H2O Nanosheets with High-Concentration Oxygen Vacancies for High-Performance Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2106816. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Jiang, C.; Tang, Y. Multi-Ion Strategies towards Emerging Rechargeable Batteries with High Performance. Energy Storage Mater. 2019, 23, 566–586. [Google Scholar] [CrossRef]
- Liu, T.; Peng, N.; Zhang, X.; Zheng, R.; Xia, M.; Zhang, J.; Yu, H.; Zhang, L.; Shu, J. Insight into Anion Storage Batteries: Materials, Properties and Challenges. Energy Storage Mater. 2021, 42, 42–67. [Google Scholar] [CrossRef]
- Hao, J.; Long, J.; Li, B.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Yang, Z.; Pang, W.K.; Guo, Z. Toward High-Performance Hybrid Zn-Based Batteries via Deeply Understanding Their Mechanism and Using Electrolyte Additive. Adv. Funct. Mater. 2019, 29, 1903605. [Google Scholar] [CrossRef]
- Olbasa, B.W.; Huang, C.-J.; Fenta, F.W.; Jiang, S.-K.; Chala, S.A.; Tao, H.-C.; Nikodimos, Y.; Wang, C.-C.; Sheu, H.-S.; Yang, Y.-W.; et al. Highly Reversible Zn Metal Anode Stabilized by Dense and Anion-Derived Passivation Layer Obtained from Concentrated Hybrid Aqueous Electrolyte. Adv. Funct. Mater. 2022, 32, 2103959. [Google Scholar] [CrossRef]
- Zhao, H.B.; Hu, C.J.; Cheng, H.W.; Fang, J.H.; Xie, Y.P.; Fang, W.Y.; Doan, T.N.L.; Hoang, T.K.A.; Xu, J.Q.; Chen, P. Novel Rechargeable M3V2(PO4)3//Zinc (M = Li, Na) Hybrid Aqueous Batteries with Excellent Cycling Performance. Sci. Rep. 2016, 6, 25809. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, Z.; Jiang, Y.; Jin, C.; Huang, W.; Ding, X.; Huang, Y. Towards Polyvalent Ion Batteries: A Zinc-Ion Battery Based on NASICON Structured Na3V2(PO4)3. Nano Energy 2016, 25, 211–217. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, T.; Wang, X.; Zhou, X.; Wei, X.; Yao, X.; Luo, W.; Shi, C.; Owusu, K.A.; Zhou, L.; et al. Aqueous Zn//Zn(CF3SO3)2//Na3V2(PO4)3 Batteries with Simultaneous Zn2+/Na+ Intercalation/de-Intercalation. Nano Energy 2019, 58, 492–498. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Cheng, S.; Jiang, K. A Long-Life Aqueous Zn-Ion Battery Based on Na3V2(PO4)2F3 Cathode. Energy Storage Mater. 2018, 15, 14–21. [Google Scholar] [CrossRef]
- Bi, X.; Peng, Y.; Liu, S.; Liu, Y.; Yang, X.; Feng, K.; Hu, J. Na3(VO)2(PO4)2F Coated Carbon Nanotubes: A Cathode Material with High-Specific Capacity for Aqueous Zinc-Ion Batteries. Electrochim. Acta 2024, 475, 143657. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, Y.; Zhang, L.; Liu, D.; Wang, C.; Song, L.; Niu, Z.; Chen, J. Reversible Oxygen Redox Chemistry in Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Edit. 2019, 58, 7062–7067. [Google Scholar] [CrossRef]
- Shi, H.-Y.; Song, Y.; Qin, Z.; Li, C.; Guo, D.; Liu, X.-X.; Sun, X. Inhibiting VOPO4·x H2O Decomposition and Dissolution in Rechargeable Aqueous Zinc Batteries to Promote Voltage and Capacity Stabilities. Angew. Chem. Int. Edit. 2019, 58, 16057–16061. [Google Scholar] [CrossRef]
- Cao, L.; Li, D.; Pollard, T.; Deng, T.; Zhang, B.; Yang, C.; Chen, L.; Vatamanu, J.; Hu, E.; Hourwitz, M.J.; et al. Fluorinated Interphase Enables Reversible Aqueous Zinc Battery Chemistries. Nat. Nanotechnol. 2021, 16, 902–910. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Xiong, S.; Tian, F.; Feng, Z.; Jia, Y.; Feng, J.; Xi, B. A High-Rate and Ultrastable Aqueous Zinc-Ion Battery with a Novel MgV2O6·1.7H2O Nanobelt Cathode. Small 2021, 17, 2100318. [Google Scholar] [CrossRef]
- Oberholzer, P.; Tervoort, E.; Bouzid, A.; Pasquarello, A.; Kundu, D. Oxide versus Nonoxide Cathode Materials for Aqueous Zn Batteries: An Insight into the Charge Storage Mechanism and Consequences Thereof. ACS Appl. Mater. Interfaces 2019, 11, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kingsbury, R.; Zhou, L.; Shyamsunder, A.; Persson, K.A.; Nazar, L.F. Tuning the Solvation Structure in Aqueous Zinc Batteries to Maximize Zn-Ion Intercalation and Optimize Dendrite-Free Zinc Plating. ACS Energy Lett. 2022, 7, 533–540. [Google Scholar] [CrossRef]
- Wang, F.; Blanc, L.E.; Li, Q.; Faraone, A.; Ji, X.; Chen-Mayer, H.H.; Paul, R.L.; Dura, J.A.; Hu, E.; Xu, K.; et al. Quantifying and Suppressing Proton Intercalation to Enable High-Voltage Zn-Ion Batteries. Adv. Energy Mater. 2021, 11, 2102016. [Google Scholar] [CrossRef]
- Park, M.J.; Manthiram, A. Unveiling the Charge Storage Mechanism in Nonaqueous and Aqueous Zn/Na3V2(PO4)2F3 Batteries. ACS Appl. Energ. Mater. 2020, 3, 5015–5023. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Liu, J.-H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.-L.; Dou, S.-X.; et al. Vanadium-Based Cathodes for Aqueous Zinc-Ion Batteries: Mechanism, Design Strategies and Challenges. Energy Storage Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Tao, Y.; Huang, D.; Chen, H.; Luo, Y. Electrochemical Generation of Hydrated Zinc Vanadium Oxide with Boosted Intercalation Pseudocapacitive Storage for a High-Rate Flexible Zinc-Ion Battery. ACS Appl. Mater. Interfaces 2021, 13, 16576–16584. [Google Scholar] [CrossRef]
- Kadam, N.; Sarkar, A. A System for Recharging Zn-Air Battery with High Reversibility Using a Water-in-Salt Electrolyte. J. Energy Storage 2022, 54, 105265. [Google Scholar] [CrossRef]
- Wang, Z.; Diao, J.; Burrow, J.N.; Brotherton, Z.W.; Lynd, N.A.; Henkelman, G.; Mullins, C.B. Chaotropic Salt-Aided “Water-In-Organic” Electrolyte for Highly Reversible Zinc-Ion Batteries Across a Wide Temperature Range. Adv. Funct. Mater. 2024, 34, 2311271. [Google Scholar] [CrossRef]
- Nie, C.; Wang, G.; Wang, D.; Wang, M.; Gao, X.; Bai, Z.; Wang, N.; Yang, J.; Xing, Z.; Dou, S. Recent Progress on Zn Anodes for Advanced Aqueous Zinc-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300606. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiang, Y.; Zou, Q.; Liu, B.; Liu, S.; Zeng, H.; Chen, L.; Li, J.; Wu, X.; Xiong, L. Improving Li3V2(PO4)3 Cathode Performance by Mn2+ Doping for High-Rate Aqueous Zinc Ion Hybrid Batteries. Ionics 2022, 28, 3855–3864. [Google Scholar] [CrossRef]
- Li, C.; Yuan, W.; Li, C.; Wang, H.; Wang, L.; Liu, Y.; Zhang, N. Boosting Li3V2(PO4)3 Cathode Stability Using a Concentrated Aqueous Electrolyte for High-Voltage Zinc Batteries. Chem. Commun. 2021, 57, 4319–4322. [Google Scholar] [CrossRef] [PubMed]
- Kainat, S.; Anwer, J.; Hamid, A.; Gull, N.; Khan, S.M. Electrolytes in Lithium-Ion Batteries: Advancements in the Era of Twenties (2020’s). Mater. Chem. Phys. 2024, 313, 128796. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Jiang, J.-C.; Huang, A.-C.; Tang, Y.; Xie, L.-J.; Zhai, J.; Xing, Z.-X. A Novel Multifunctional Additive Strategy Improves the Cycling Stability and Thermal Stability of SiO/C Anode Li-Ion Batteries. Process Saf. Environ. Prot. 2022, 164, 555–565. [Google Scholar] [CrossRef]
- Fang, C.; Tran, T.-N.; Zhao, Y.; Liu, G. Electrolyte Decomposition and Solid Electrolyte Interphase Revealed by Mass Spectrometry. Electrochim. Acta 2021, 399, 139362. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Wang, C.; Xia, Y. Graphene Oxide Assisted Solvothermal Synthesis of LiMnPO4 Naonplates Cathode Materials for Lithium Ion Batteries. Electrochim. Acta 2014, 146, 8–14. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, Y.; Liu, S.; Liu, Y.; He, T.; Jiang, X.; Yang, X.; Feng, K.; Hu, J. Zn3V4(PO4)6: A New Rocking-Chair-Type Cathode Material with High Specific Capacity Derived from Zn2+/H+ Cointercalation for Aqueous Zn-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 32066–32074. [Google Scholar] [CrossRef]
- Liu, X.; Euchner, H.; Zarrabeitia, M.; Gao, X.; Elia, G.A.; Gross, A.; Passerini, S. Operando pH Measurements Decipher H+/Zn2+ Intercalation Chemistry in High-Performance Aqueous Zn/δ-V2O5 Batteries. ACS Energy Lett. 2020, 5, 2979–2986. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Wang, Y.; Rong, X.; Sun, W.; Fan, X.; Xu, S.; Schroeder, M.A.; Cresce, A.V.; Wang, F.; et al. “Water-in-Salt” Electrolyte Makes Aqueous Sodium-Ion Battery Safe, Green, and Long-Lasting. Adv. Energy Mater. 2017, 7, 1701189. [Google Scholar] [CrossRef]
- Jin, Y.; Han, K.S.; Shao, Y.; Sushko, M.L.; Xiao, J.; Pan, H.; Liu, J. Stabilizing Zinc Anode Reactions by Polyethylene Oxide Polymer in Mild Aqueous Electrolytes. Adv. Funct. Mater. 2020, 30, 2003932. [Google Scholar] [CrossRef]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Edit. 2018, 57, 3943–3948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yin, X.; Saadoune, I.; Wei, Y.; Wang, Y. Zwitterion Intercalated Manganese Dioxide Nanosheets as High-Performance Cathode Materials for Aqueous Zinc Ion Batteries. Small 2024, 2402811. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Wang, Y.; Liang, M.; Lu, K.; Xiong, C.; Hu, W.; Liu, B. A Comprehensive Review of Single Ion-Conducting Polymer Electrolytes as a Key Component of Lithium Metal Batteries: From Structural Design to Applications. Energy Storage Mater. 2023, 63, 102955. [Google Scholar] [CrossRef]
- Demir-Cakan, R.; Rosa Palacin, M.; Croguennec, L. Rechargeable Aqueous Electrolyte Batteries: From Univalent to Multivalent Cation Chemistry. J. Mater. Chem. A 2019, 7, 20519–20539. [Google Scholar] [CrossRef]
- Vazquez, D.G.; Pollard, T.P.; Mars, J.; Yoo, J.M.; Steinrück, H.-G.; Bone, S.E.; Safonova, O.V.; Toney, M.F.; Borodin, O.; Lukatskaya, M.R. Creating Water-in-Salt-like Environment Using Coordinating Anions in Non-Concentrated Aqueous Electrolytes for Efficient Zn Batteries. Energy Environ. Sci. 2023, 16, 1982–1991. [Google Scholar] [CrossRef]
- Jumare, I.A. Energy Storage with Salt Water Battery: A Preliminary Design and Economic Assessment. J. Energy Storage 2020, 27, 101130. [Google Scholar] [CrossRef]
- Feng, J.; Gao, Z.; Sheng, L.; Hao, Z.; Wang, F.R. Progress and Perspective of Interface Design in Garnet Electrolyte-based All-solid-state Batteries. Carbon Energy 2021, 3, 385–409. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, Z.; Zhou, J.; Liu, D.; Song, J.; Zhu, Y. A Minireview of the Solid-State Electrolytes for Zinc Batteries. Polymers 2023, 15, 4047. [Google Scholar] [CrossRef]
- Duggal, I.; Venkatesh, B. Short-Term Scheduling of Thermal Generators and Battery Storage with Depth of Discharge-Based Cost Model. IEEE Trans. Power Syst. 2015, 30, 2110–2118. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, F.; Huang, R.; Dong, N.; Jiao, S.; Cao, R.; Pan, H. Hierarchical Porous Separator with Excellent Isotropic Modulus Enabling Homogeneous Zn2+ Flux for Stable Aqueous Zn Battery. Sci. China Mater. 2023, 66, 982–991. [Google Scholar] [CrossRef]
- Lv, C.; Zhou, X.; Zhong, L.; Yan, C.; Srinivasan, M.; Seh, Z.W.; Liu, C.; Pan, H.; Li, S.; Wen, Y.; et al. Machine Learning: An Advanced Platform for Materials Development and State Prediction in Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2101474. [Google Scholar] [CrossRef] [PubMed]


| Working Ion | Ionic Radii (Å) | Electrode Potential vs. SHE (V) | Specific Gravimetric Capacity (mAh·g−1) | Specific Volumetric Capacity (mAh·g−1) |
|---|---|---|---|---|
| Li+ | 0.76 | −3.04 | 3862 | 2066 |
| Na+ | 1.02 | −2.71 | 1166 | 1129 |
| K+ | 1.38 | −2.93 | 685 | 586 |
| Mg2+ | 0.72 | −2.37 | 2205 | 3832 |
| Zn2+ | 0.74 | −0.76 | 820 | 5855 |
| Al3+ | 0.535 | −1.66 | 2980 | 8046 |
| Cathode | Electrolyte | Voltage/V | Capacity/mAh·g−1 | Retention%/Cycles | Number (Acting Ions) | Ref. |
|---|---|---|---|---|---|---|
| LiFePO4 | 1 M LiOTf + 1 M Zn (OTf)2 + SDBS | 0.9–1.4 V | 158 (0.5 C) | 88.6% at 1 C (100) | 3 (Zn2+, Li+, H+) | [20] |
| LiFePO4 | 4 M Zn(OTf)2 + 2 M LiClO4 | 0.9–1.4 V | 165 (0.2 C) | 90% at 0.2 C (285) | 2 (Zn2+, H+) | [21] |
| Li3V2(PO4)3 | 1 M Li2SO4 + 2 M ZnSO4 | 0.7–2.1 V | 131 (0.2 C) | 85.4% at 0.2 C (200) | 3 (Zn2+, Li+, H+) | [22] |
| Na3V2(PO4)3 | 0.5 M Zn(CH3COO)2 | 0.8–1.7 V | 92 (0.5 C) | 74.0% at 0.5 C (100) | 3 (Zn2+, Na+, H+) | [23] |
| Na3V2(PO4)3 | 2 M Zn(OTf)2 | 0.6–1.8 V | 114 (0.05 A·g−1) | 75.0% at 0.5 A·g−1 (200) | 3 (Zn2+, Na+, H+) | [24] |
| Na3V2(PO4)2F3 | 2 M Zn(OTf)2 | 0.8–1.9 V | 65 (0.08 A·g−1) | 98.0% at 0.2 A·g−1 (600) | 2 (Zn2+, H+) | [25] |
| Na3V2(PO4)2F3 | 3 M Zn(OTf)2 | 0.2–2.0 V | 100 (0.2C) | 90.0% at 0.2C (600) | 2 (Zn2+, H+) | [26] |
| VOPO4·2H2O | 21 M LiTFSI + 1 M Zn(Tr)2 | 0.8–2.1 V | 139 (0.1 A·g−1) | 93.0% at 1 A·g−1 (1000) | 1 (Zn2+) | [27] |
| VOPO4·xH2O | 13 M ZnCl2 + 0.8 M H3PO4 | 0.7–1.9 V | 170 (0.1 A·g−1) | 91.8% at 2 A·g−1 (500) | 1 (Zn2+) | [28] |
| VOPO4 | 4 M Zn(OTf)2 + 0.5 M Me3EtOTf | 0.2–1.9 V | 163 (0.05 A·g−1) | 88.7% at 2 A·g−1 (6000) | 1 (Zn2+) | [29] |
| MgV2O6·1.7H2O | 0.1 M Zn(OTf)2 in anhydrous acetonitrile + 1% vol water | 0.3–1.4 V | 425.7 mAh·g−1 at 0.2 A·g−1 | 97% at 0.2 A·g−1 (50) | 2 (Zn2+, H+) | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Du, T.; Wang, H.; Cheng, Z.-Y.; Wang, Y.-S.; Zhou, L.-F. Opportunities and Challenges of Multi-Ion, Dual-Ion and Single-Ion Intercalation in Phosphate-Based Polyanionic Cathodes for Zinc-Ion Batteries. Molecules 2024, 29, 4929. https://doi.org/10.3390/molecules29204929
Cao L, Du T, Wang H, Cheng Z-Y, Wang Y-S, Zhou L-F. Opportunities and Challenges of Multi-Ion, Dual-Ion and Single-Ion Intercalation in Phosphate-Based Polyanionic Cathodes for Zinc-Ion Batteries. Molecules. 2024; 29(20):4929. https://doi.org/10.3390/molecules29204929
Chicago/Turabian StyleCao, Lei, Tao Du, Hao Wang, Zhen-Yu Cheng, Yi-Song Wang, and Li-Feng Zhou. 2024. "Opportunities and Challenges of Multi-Ion, Dual-Ion and Single-Ion Intercalation in Phosphate-Based Polyanionic Cathodes for Zinc-Ion Batteries" Molecules 29, no. 20: 4929. https://doi.org/10.3390/molecules29204929
APA StyleCao, L., Du, T., Wang, H., Cheng, Z.-Y., Wang, Y.-S., & Zhou, L.-F. (2024). Opportunities and Challenges of Multi-Ion, Dual-Ion and Single-Ion Intercalation in Phosphate-Based Polyanionic Cathodes for Zinc-Ion Batteries. Molecules, 29(20), 4929. https://doi.org/10.3390/molecules29204929





